Abstract
The Lutheran (Lu) blood group and basal cell adhesion molecule (BCAM) antigens are both carried by 2 glycoprotein isoforms of the immunoglobulin superfamily representing receptors for the laminin α5 chain. In addition to red blood cells, Lu/BCAM proteins are highly expressed in endothelial cells. Abnormal adhesion of red blood cells to the endothelium could potentially contribute to the vaso-occlusive episodes in sickle cell disease. Considering the presence of integrin consensus-binding sites in Lu/BCAM proteins, we investigated their potential interaction with integrin α4β1, the unique integrin expressed on immature circulating sickle red cells. Using cell adhesion assays under static and flow conditions, we demonstrated that integrin α4β1 expressed on transfected cells bound to chimeric Lu-Fc protein. We showed that epinephrine-stimulated sickle cells, but not control red cells, adhered to Lu-Fc via integrin α4β1 under flow conditions. Antibody-mediated activation of integrin α4β1 induced adhesion of sickle red cells to primary human umbilical vein endothelial cells; this adhesion was inhibited by soluble Lu-Fc and vascular cell adhesion molecule-1 (VCAM-1)–Fc proteins. This novel interaction between integrin α4β1 in sickle red cells and endothelial Lu/BCAM proteins could participate in sickle cell adhesion to endothelium and potentially play a role in vaso-occlusive episodes.
Introduction
The Lutheran (Lu) blood group and basal cell adhesion molecule (BCAM) antigens are both carried by 2 glycoprotein (gp) isoforms of the immunoglobulin (Ig) superfamily, Lu and Lu(v13). Lu (85 kDa) and Lu(v13) (78 kDa) exhibit 5 extracellular Ig-like domains, a single transmembrane domain, and a cytoplasmic tail of 59 and 19 amino acids, respectively. Lu/BCAM gp's bind to the laminin α5 chain (present in laminin-10/11 isoforms) and represent the unique receptors for this laminin in normal (AA) and sickle (SS) red blood cells (RBCs) of patients with sickle cell anemia.1-3 These gp's have been also recognized as laminin α5 receptors in kidney epithelial cells, in smooth muscle cells, and endothelial cell lines.4-6 Lu/BCAM gps bind to the G3 domain of laminin α57 by their first 3 Ig-like domains.3,8 The specific cytoplasmic domain of Lu gp isoform includes serine phosphorylation sites, which is consistent with a receptor signaling function.9
In sickle cell disease, RBCs are 2 to 10 times more adherent to components that line the blood vessel wall or circulate in the plasma than normal RBCs, and this may impair the blood flow and dramatically impact vaso-occlusion.10-12 Lu/BCAM gp's are overexpressed in SS RBCs; this correlates with increased adhesion to laminin α5.1,2 Lee and collaborators suggested that SS RBCs adhere to endothelial basement membrane by binding to laminin α5.13 More recently, Hines and collaborators showed that the physiologic stress mediator epinephrine, acting through the β2-adrenergic receptor, increased the Lu/BCAM-mediated adhesion of SS RBCs to laminin α5 via a cAMP and protein kinase A (PKA)–dependent signaling pathway.14 Accordingly, we recently demonstrated that the long isoform of Lu/BCAM proteins was phosphorylated in epinephrine-stimulated SS RBCs. Our results indicated that PKA-mediated phosphorylation of the Lu gp positively regulates the adhesion function to laminin and suggested that enhanced adhesion of epinephrine-stimulated RBCs to laminin α5 is mediated by a PKA-phosphorylated serine of Lu gp.9
Abnormal adhesion of SS RBCs to endothelial cells could potentially contribute to the vaso-occlusive crises in sickle cell disease.10,15 It has been recently shown that human SS RBCs adhered in vivo to activated rat mesocecum vasculature, leading to frequent blockage of small-diameter venules, and that this adhesion was inhibited by blocking the interaction between erythroid ICAM-4 and endothelial integrin αvβ3.16 Considering the expression of Lu/BCAM on the surface of endothelial cells in blood vessels,17 we postulated that they could be involved in the binding of ligands on SS RBCs, contributing to their reinforced adhesiveness to vascular endothelium. Integrins are expressed on erythrocyte precursors and play a role in normal erythrocyte development.18-23 Integrin α4β1 (CD49d/CD29), or very late antigen 4 (VLA-4), is the only integrin maintained in a population of circulating SS RBCs, mainly in the population of young reticulocytes.24,25 A pathologic role for this integrin has been suggested in sickle RBC adhesion, through its interaction with vascular cell adhesion molecule-1 (VCAM-1), fibronectin, and thrombospondin.12,24,26,27 Interaction of integrin α4β1 with its endothelial and/or matrix ligands could play a role in the adhesion of SS RBCs to the blood vessel wall and could slow or stop blood flow.12 As several members of the Ig superfamily are known to bind integrins, we hypothesized that endothelial Lu/BCAM might interact with erythroid integrin α4β1. Our hypothesis is supported by the presence of α4β1 consensus-binding motifs28 in the extracellular domain of Lu/BCAM gp's.
In the present study, we investigated the potential interaction between integrin α4β1 and Lu/BCAM proteins using adhesion assays under static and flow conditions. We provide evidence for a novel interaction between α4β1 integrin expressed in SS RBCs and endothelial Lu/BCAM proteins that could promote SS RBC adhesion to endothelium and potentially contribute to vaso-occlusive episodes.
Patients, materials, and methods
Blood samples, antibodies, proteins, cell lines, and endothelial cells
All adhesion assays were performed with 5 mL freshly drawn EDTA-anticoagulated venous blood from 2 healthy donors (AA1 and AA2), 7 adult patients with sickle cell disease (SS1-SS7) who were diagnosed by standard methods and family testing, and 3 patients with a high reticulocyte count (C1 to C3) suffering from different types of hemoglobinopathy: C1 is a woman with unsplenectomized hemoglobin H (HbH) disease; C2 is a man with splenectomized Heinz body hemolytic anemia due to an unstable α globin variant; and C3 is a man with splenectomized and untransfused β thalassemia intermedia. Blood was collected more than 3 months after either transfusion or hydroxyurea treatments, at least 1 month after and 2 weeks before a clinical vaso-occlusive event, and after informed consent was obtained in accordance with the Declaration of Helsinki. The study has been approved by the Scientific Committee of the Institut National de la Transfusion Sanguine.
Monoclonal antibodies (mAbs) used were mouse anti–human β1 integrin (clone TS2/16; Endogen, Woburn MA), rat anti–human β1 integrin (clone mAb 13; BD/Pharmingen, San Diego, CA), mouse anti–human α3 integrin (clone ASC-1; Chemicon, Temecula, CA), mouse anti–human α4 integrin (clone HP2/1; Serotec, Oxford, United Kingdom), rat anti–human α6 integrin (clone GoH3; BD/Pharmingen, San Diego, CA), and mouse anti–human VCAM-1 (clone 4B2; R&D Systems, Minneapolis, MN). Mouse anti–human Lu mAb, clone F241, was produced in our institute (in collaboration with Dr D. Blanchard, Etablissement Français du Sang, Nantes, France).
Soluble human Lu-Fc and ICAM-1-Fc were obtained as described.2,29 Lu-Fc was also purchased with human VCAM-1–Fc from R&D Systems Europe (Lille, France).
Wild-type (WT) L929 cells and L929 cells expressing human recombinant integrin α4β1 (10 200 copies per cell), αLβ2 (32 000 copies per cell), or αMβ2 (46 000 copies per cell) were obtained and grown as previously described.29,30 Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical vein cords (Dr Marie-Christine Boulanger, Centre Hospitalier Robert Ballanger, Aulnay sous Bois, France) as described.31
Flow cytometry analyses
The percentage of reticulocytes in whole-blood samples and expression of integrin α4β1 were determined using thiazole orange dye (Retic-Count; Becton Dickinson, San Jose, CA), anti–integrin α4 mAb HP2/1, and a FACScan flow cytometer (Becton Dickinson) as described.32 Expression of Lu/BCAM, integrin α4, and VCAM-1 in HUVECs was tested using F241 anti-Lu, HP2/1 anti-α4, and 4B2 anti–VCAM-1 mAbs as described.2 For VCAM-1 analysis, HUVECs were incubated for 16 hours with recombinant human tumor necrosis factor α (TNF-α; Sigma, St Louis, MO) at 5 ng/mL. Specific antibody-binding capacity (SABC) for Lu/BCAM (on HUVECs) and integrin α4 (on RBCs) was determined by flow cytometry using Qifikit with the appropriate antibodies (Dako, Trappes, France).
Immunoprecipitation of Lu/BCAM proteins from HUVECs
Lu/BCAM proteins were immunoprecipitated from HUVECs after biotinylation of cell-surface proteins. Cells were biotinylated with 0.3 mg/mL NHS-LC-biotin (Pierce, Brebières, France) diluted in 10 mM HEPES, 150 mM NaCl, 0.2 mM CaCl2, and 0.2 mM MgCl2 (pH 7.5) at 4°C for 30 minutes. Cells were lysed in situ with 750 μL of lysing buffer (150 mM NaCl, 20 mM Tris [pH8.5], 2 mM EDTA, and 1% Triton X-100) containing protease inhibitor cocktail (Roche, Indianapolis, IN) and 1 mM PMSF (Sigma) at 4°C for 40 minutes. After a preclearing step with goat serum, Lu gp's were immunoprecipitated with anti-Lu mouse mAb F241 and analyzed by SDS-PAGE, membrane transfer, and chemoluminescence after incubation with horseradish-biotinylated streptavidin (Amersham Biosciences. Freiburg, Germany).
Adhesion assays under static conditions
Purified Lu-Fc or fibronectin (Sigma) diluted in 25 mM Tris (pH 8.0), 150 mM NaCl, 2 mM MgCl2, and 2 mM CaCl2 were adsorbed onto flat-bottomed 96-well microtiter plates overnight at 4°C at different concentrations (50 μL/well in duplicate). Adhesion of L929 WT and recombinant cells (105 cells/well) was carried as described2 with a 30- to 45-minute incubation time at 22°C in serum-free RPMI 1640 medium (Invitrogen, Carlsbad, CA) with or without 1 mM EDTA. Each experiment was performed at least 3 times.
Cell-substrate adhesion under flow conditions
Adhesion of RBCs and L929 cells to Lu-Fc, VCAM-1–Fc, or intercellular adhesion molecule-1 (ICAM-1)–Fc was measured under physiologic flow conditions using a plate flow chamber as described.33 Soluble proteins (0.1-0.3 mg/mL) were immobilized into rectangular glass capillaries (microslides; 50 mm length, 3 mm internal width, 0.3 mm depth, and 3.5 cm2 surface; Cam Lab, Cambridge, United Kingdom) at 4°C overnight and microslides were mounted as described.9 L929 cells (107 cells/mL) or RBCs (hematocrit, .005 [0.5%]) were washed 3 times and suspended in Hanks buffer, 1 mM MgCl2, 1 mM CaCl2, and 0.4% human albumin. When 2 populations of L929 cells were injected simultaneously, 1 population was labeled with calcein. The cell suspension was injected at a final concentration of 107 cells/mL (1:1). Cells were injected for 10 minutes at shear stress of 0.2 dyne/cm2, and 5-minute washouts were carried out with Hanks buffer at 0.2, 0.4, 1, 2, and 4 dyne/cm2. After each washing step, adherent cells were quantified in 6 representative areas along the centerline of the microslide by microscopy using the Optimas 6.1 image analysis system (Media Cybernetics, Silver Spring, MD). When mAb anti–β1 TS2/16 was used, cells were incubated for 20 minutes at room temperature with 1 μg/mL antibody before the injection step. For inhibition assays, cells were preincubated for 15 minutes on ice with 10 μg/mL blocking or isotype-matched control antibody. Activation with epinephrine was obtained by preincubating the cells for 1 minute at room temperature with 20 nM of epinephrine. Each adhesion experiment using L929 cells was performed a minimum of 3 times.
Cell-cell adhesion under flow conditions
HUVECs (4.5 × 106 cells/mL) were grown on microslides coated with 2% gelatin as described.33 Growing endothelial cells on gelatin does not modify the surface expression of endothelial markers.34,35 Adhesion of RBCs (.005 [0.5%] hematocrit) on the HUVEC monolayer under flow conditions was analyzed as described for Lu-Fc–coated miscroslides. RBCs were either untreated or incubated with 1 μg/mL anti–β1 TS2/16 at room temperature for 20 minutes. For inhibition assays, RBCs were preincubated or not for 5 minutes at room temperature with 1 μg/mL anti–β1 TS2/16 followed by incubation with soluble Lu-Fc or VCAM-1–Fc at 10 μg/mL for 20 minutes.
Statistical analysis
Results are presented as the mean ± standard deviation. Statistical significance was determined using an unpaired Wilcoxon rank-sum test.
Results
Adhesion of L-α4β1 cells to Lu-Fc recombinant protein under static conditions
To determine whether Lu/BCAM gp's could interact with integrin α4β1, we performed static adhesion assays using Lu-Fc protein coated on plastic wells and stably transfected L929 mouse fibroblasts expressing human integrin α4β1. L-WT cells were used as negative control. As shown in Figure 1A, L-α4β1 cells, but not L-WT cells, adhered to coated Lu-Fc in the presence of Ca2+ and Mg2+ (21 600 ± 2700 cells/mm2 vs 1800 ± 900 cells/mm2). Adhesion was totally abolished when 1 mM EDTA was added, indicating that the interaction is dependent on divalent cations. As a positive control, L-α4β1 cells adhered to fibronectin, a known ligand for integrin α4β1.
L-α4β1 cells adhere to Lu-Fc in a divalent cation dose-dependent manner under static conditions. (A) Lu-Fc or fibronectin (Fn) (1 μg) were adsorbed onto flat-bottomed 96-well microtiter plates and 105 L-WT or L-α4β1 cells were added in the presence or absence of 1 mM EDTA and incubated for 40 minutes. (B) Adhesion assay using L-α4β1 cells and increasing amounts of Lu-Fc protein. (C) Adhesion assay using 0.5 μg of Lu-Fc and control L-WT, L-αLβ2, and L-αMβ2 cells. Error bars represent SD.
L-α4β1 cells adhere to Lu-Fc in a divalent cation dose-dependent manner under static conditions. (A) Lu-Fc or fibronectin (Fn) (1 μg) were adsorbed onto flat-bottomed 96-well microtiter plates and 105 L-WT or L-α4β1 cells were added in the presence or absence of 1 mM EDTA and incubated for 40 minutes. (B) Adhesion assay using L-α4β1 cells and increasing amounts of Lu-Fc protein. (C) Adhesion assay using 0.5 μg of Lu-Fc and control L-WT, L-αLβ2, and L-αMβ2 cells. Error bars represent SD.
The adhesion assays using increasing amounts of coated Lu-Fc protein demonstrated that L-α4β1 cells adhered to coated Lu-Fc in a dose-dependent manner (Figure 1B). When 2 μg or more of Lu-Fc were immobilized, a plateau was reached as the totality of the added cells adhered to the well (100% of adhesion). L cells expressing 2 other integrins, αLβ2 or αMβ2, were used to test the specificity of the interaction between Lu-Fc and L-α4β1 cells. As shown in Figure 1C, adhesion experiments using L-WT, L-α4β1, L-αLβ2 and L-αMβ2 cells indicated that only L-α4β1 cells interact with Lu-Fc.
Adhesion of L-α4β1 cells to Lu-Fc under flow conditions
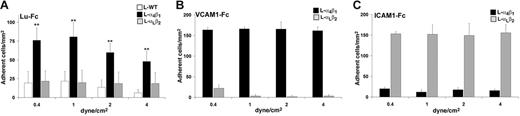
To approach physiologic conditions, we examined the interaction between L-α4β1 cells and Lu-Fc under flow conditions. Lu-Fc protein was immobilized on a glass microslide and cells were injected over the coated surface. L-α4β1 cells were injected at 0.2 dyne/cm2 simultaneously with calcein-labeled L-WT or L-αLβ2 cells. After this adhesion step, washouts were performed at increasing shear stress of 0.4, 1, 2, and 4 dyne/cm2, and the number of adherent cells was determined after each wash. As shown in Figure 2A, L-α4β1 cells adhered to Lu-Fc and resisted to high levels of shear stress, exceeding the physiologic postcapillary shear stress of 1 dyne/cm2,36 in contrast to L-WT and L-αLβ2 cells (P < .01). Human VCAM-1–Fc and ICAM-1–Fc were used as positive controls for adhesion of L-α4β1 and L-αLβ2 cells, respectively. L-α4β1 but not L-αLβ2 cells adhered to VCAM-1–Fc, indicating that integrin α4β1 was functional at the L-α4β1 cell surface (Figure 2B). Similarly, L-αLβ2 but not L-α4β1 cells adhered to the αLβ2 ligand ICAM-1–Fc, indicating that the L-αLβ2 cell line also expressed a functional integrin; labeling these cells with calcein did not impede their adhesive function (Figure 2C). Furthermore, adhesion of L-α4β1 cells to Lu-Fc or to VCAM-1–Fc was not affected by calcein labeling. These data indicated that adhesion of L-α4β1 cells in the presence of immobilized Lu-Fc was due to a specific interaction of these cells with the extracellular domain of Lu/BCAM gp's.
L-α4β1 cells adhere to Lu-Fc under flow conditions. Lu-Fc (0.3 mg/mL) (A), VCAM-1-Fc (0.1 mg/mL) (B), or ICAM-1-Fc (0.3 mg/mL) (C) were immobilized on a microslide. A cell suspension including L-α4β1 and calcein-labeled L-WT or L-αLβ2 cells (107 cells/mL; 1:1) was injected in the microslide at shear stress of 0.2 dyne/cm2 for 10 minutes. Washouts (5 minutes) were applied sequentially from 0.4 to 4 dyne/cm2, and cells were counted in 6 different areas along the microslide centerline after each wash (**P < .01). Error bars represent SD.
L-α4β1 cells adhere to Lu-Fc under flow conditions. Lu-Fc (0.3 mg/mL) (A), VCAM-1-Fc (0.1 mg/mL) (B), or ICAM-1-Fc (0.3 mg/mL) (C) were immobilized on a microslide. A cell suspension including L-α4β1 and calcein-labeled L-WT or L-αLβ2 cells (107 cells/mL; 1:1) was injected in the microslide at shear stress of 0.2 dyne/cm2 for 10 minutes. Washouts (5 minutes) were applied sequentially from 0.4 to 4 dyne/cm2, and cells were counted in 6 different areas along the microslide centerline after each wash (**P < .01). Error bars represent SD.
Adhesion of L-α4β1 cells to Lu-Fc is specifically inhibited by anti-α4β1 antibodies
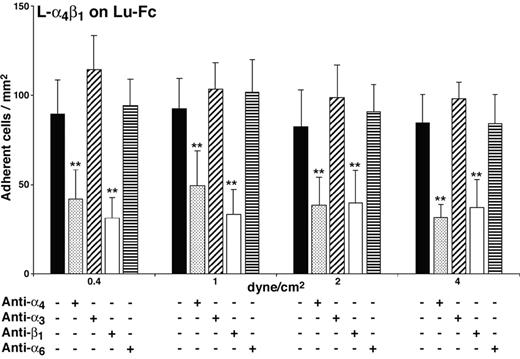
Adhesion of L-α4β1 cells to Lu-Fc could be due either to the interaction of Lu-Fc with recombinant integrin α4β1 or to its interaction with an endogenous surface protein that is expressed or activated by the presence of recombinant human integrin α4β1 in L cells. To explore this issue, adhesion assays were performed under flow conditions using blocking antibodies directed against α4 or β1 integrin chains. A mixture of L-α4β1 and calcein-labeled L-αLβ2 cells was incubated with anti–integrin α4 (HP2/1; mouse IgG1)– or anti–integrin β1 (mAb 13; rat IgG2a)–blocking mAbs, before injection into the Lu-Fc–coated microslide at 0.2 dyne/cm2. Isotype-matched control antibodies anti–integrin α3 (ASC-1; mouse IgG1) and anti–integrin α6 (GoH3; rat IgG2a) were used separately. Anti–integrin α6 mAb GoH3 cross-reacts with mouse α6 and binds to the L-α4β1 cell surface as determined by flow cytometry. Anti-α4 antibody strongly inhibited the adhesion of L-α4β1 cells to Lu-Fc, while isotype-matched control anti-α3 antibody did not have any effect (Figure 3; 32 ± 8 versus 98 ± cells/mm2 at 4 dyne/cm2) (P < .01). The background adhesion level of control L-αLβ2 cells was not modified by either of the 2 antibodies (data not shown). Similarly, anti–integrin β1 mAb 13 inhibited L-α4β1 cell adhesion to Lu-Fc compared with the adhesion of L-α4β1 cells incubated with isotype-matched control antibody anti-α6 (Figure 3; 37 ± 16 vs 84 ± 17 cells/mm2 at 4 dyne/cm2) (P < .01). All these results indicated that the inhibition of L-α4β1 cell adhesion to Lu-Fc was due to the specific interaction of anti-α4 and anti-β1 mAbs with α4β1 and that adhesion of L-α4β1 cells to Lu-Fc was the consequence of a direct interaction between recombinant integrin α4β1 and the extracellular domain of Lu gp's.
Adhesion of L-α4β1 cells to Lu-Fc is specifically inhibited by anti-α4β1 antibodies. L-α4β1 cells (107 cells/mL) were either untreated or incubated with 10 μg/mL blocking antibodies anti–integrin α4 (HP2/1) or anti–integrin β1 (mAb 13), or isotype-matched control antibodies anti–integrin α3 (ASC-1) or anti–integrin α6 (GoH3) for 15 minutes, then injected into a Lu-Fc–coated microslide (0.3 mg/mL) for 10 minutes at 0.2 dyne/cm2. Washouts (5 minutes) were applied sequentially from 0.4 to 4 dyne/cm2, and cells were counted in 6 different areas along the microslide centerline after each wash (**P < .01). Error bars represent SD.
Adhesion of L-α4β1 cells to Lu-Fc is specifically inhibited by anti-α4β1 antibodies. L-α4β1 cells (107 cells/mL) were either untreated or incubated with 10 μg/mL blocking antibodies anti–integrin α4 (HP2/1) or anti–integrin β1 (mAb 13), or isotype-matched control antibodies anti–integrin α3 (ASC-1) or anti–integrin α6 (GoH3) for 15 minutes, then injected into a Lu-Fc–coated microslide (0.3 mg/mL) for 10 minutes at 0.2 dyne/cm2. Washouts (5 minutes) were applied sequentially from 0.4 to 4 dyne/cm2, and cells were counted in 6 different areas along the microslide centerline after each wash (**P < .01). Error bars represent SD.
Activation of integrin α4β1 enhances L-α4β1 cell adhesion to Lu-Fc
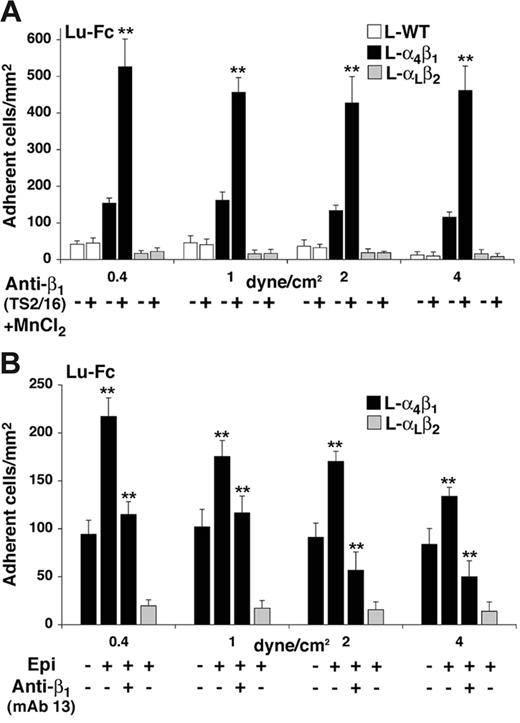
Integrin α4β1 is known to exhibit multiple activation states37,38 that may be regulated by several factors, including divalent cation concentration and agonist-induced “inside-out” signaling events.39,40 We examined the adhesion state of L-α4β1 cells to Lu-Fc in the presence of 1 mM Mn2+ and 1 μg/mL of the activating anti-β1 mAb TS2/16.41,42 L-WT, L-α4β1, and L-αLβ2 cells were incubated for 20 minutes with TS2/16 and Mn2+, then injected into an Lu-Fc–coated microslide at 0.2 dyne/cm.2 L-α4β1 cells were injected simultaneously with either L-WT or L-αLβ2 cells. L-α4β1 cells showed enhanced adhesion to Lu-Fc upon incubation with TS2/16/Mn2+ when compared with untreated cells, with a stimulation factor of 3 to 4 depending on the shear stress (Figure 4A, P < .01). Conversely, incubation of control L-WT or L-αLβ2 cells with the same components did not lead to any adhesion above background.
Increase of L-α4β1 cell adhesion to Lu-Fc by TS2/16 anti-β1–activating antibody and by epinephrine under flow conditions. (A) A cell suspension including L-α4β1 and calcein-labeled L-α4β1 or L-WT cells (107 cells/mL; 1:1) was incubated with 1 μg/mL activating antibody anti–integrin β1 (TS2/16) and 1 mM MnCl2 for 20 minutes at room temperature, then injected into a Lu-Fc–coated microslide (0.3 mg/mL) for 10 minutes at 0.2 dyne/cm2. Washouts (5 minutes) were applied sequentially from 0.4 to 4 dyne/cm2, and cells were counted in 6 different areas along the microslide centerline after each wash. Untreated cells were injected separately as control. (B) A cell suspension, including L-α4β1 and calcein-labeled L-αLβ2 cells (107 cells/mL; 1:1) was either untreated, or incubated with 20 nM epinephrine (Epi) for 1 minute at room temperature with or without preincubation with 10 μg/mL blocking antibody anti–integrin β1 (mAb 13). Injection and washes were carried out as for panel A. (**P < .01). Error bars represent SD.
Increase of L-α4β1 cell adhesion to Lu-Fc by TS2/16 anti-β1–activating antibody and by epinephrine under flow conditions. (A) A cell suspension including L-α4β1 and calcein-labeled L-α4β1 or L-WT cells (107 cells/mL; 1:1) was incubated with 1 μg/mL activating antibody anti–integrin β1 (TS2/16) and 1 mM MnCl2 for 20 minutes at room temperature, then injected into a Lu-Fc–coated microslide (0.3 mg/mL) for 10 minutes at 0.2 dyne/cm2. Washouts (5 minutes) were applied sequentially from 0.4 to 4 dyne/cm2, and cells were counted in 6 different areas along the microslide centerline after each wash. Untreated cells were injected separately as control. (B) A cell suspension, including L-α4β1 and calcein-labeled L-αLβ2 cells (107 cells/mL; 1:1) was either untreated, or incubated with 20 nM epinephrine (Epi) for 1 minute at room temperature with or without preincubation with 10 μg/mL blocking antibody anti–integrin β1 (mAb 13). Injection and washes were carried out as for panel A. (**P < .01). Error bars represent SD.
The effects of agonist induced “inside-out” signaling events on L-α4β1 cell adhesion to Lu-Fc were investigated by incubating the cells with epinephrine before injection. Epinephrine induces several signaling pathways, including PKA and small G protein–dependent pathways known to activate integrin α4β1.43,44 When L-α4β1 and L-αLβ2 cells were incubated with 20 nM epinephrine for 1 minute, only L-α4β1 cells showed an enhanced adhesion to Lu-Fc by 1.5- to 2-fold compared with that of untreated cells (Figure 4B; P < .01). L-αLβ2 cells did not adhere when incubated with epinephrine, suggesting that the increase of L-α4β1 cell adhesion to Lu-Fc was not due to the activation of an endogenous surface protein in these cells (Figure 4B). To test whether the increased adhesion of epinephrine-treated L-α4β1 cells was directly mediated by integrin α4β1, cells were incubated with anti-β1 blocking mAb 13 before stimulation with epinephrine. As shown in Figure 4B, incubation with mAb 13 prevented the increase of cell adhesion to Lu-Fc in the presence of epinephrine (P < .01). Isotype-matched control antibody anti–integrin α6 (GoH3) was used and did not influence adhesion of epinephrine-treated L-α4β1 cells (data not shown). Thus, blocking the extracellular domain of integrin α4β1 with mAb 13 impeded the “inside-out” effect of the signaling events triggered by epinephrine, indicating that the increase of cell adhesion induced by epinephrine was mediated by integrin α4β1.
Adhesion of AA and SS RBCs to Lu-Fc under flow
Integrin α4β1 is the only integrin expressed on circulating immature RBCs of patients with sickle cell disease.24,25 To determine whether SS RBCs could bind to Lu-Fc through α4β1 interaction, adhesion experiments were performed under flow conditions as described in “Adhesion of L-α4β1 cells to Lu-Fc under flow conditions.” When AA and SS RBCs were used without any prior treatment, no significant difference was detected between the 2 populations (Figure 5A). AA and SS RBCs were treated with epinephrine, as activation of L-α4β1 cells with epinephrine stimulated the integrin α4β1–mediated adhesion to Lu-Fc (Figure 4B). Incubation of RBCs with 20 nM epinephrine induced SS but not AA RBC adhesion (Figure 5A; P < .01). RBCs from 6 independent patients with sickle cell disease, SS1 to SS6 (Table 1), were analyzed. Figure 5B shows the number of adherent RBCs at the physiologic shear stress of 1 dyne/cm2. A significant activation of cell adhesion was obtained with all SS RBCs after incubation with epinephrine (1.5- to 2.8-fold; P < .01). In order to test whether integrin α4β1 was involved in the interaction of epinephrine-stimulated SS RBCs with Lu-Fc, cells from patients SS3, SS4, SS5, and SS6 were preincubated with blocking anti-β1 mAb 13 before adding epinephrine. Blocking integrin α4β1 with mAb 13 significantly decreased the activating effect of epinephrine, as the number of adherent RBCs was diminished for all patients when compared with RBCs treated with epinephrine alone (Figure 5B; P < .01). On the other hand, selective activation of integrin α4β1 by anti–β1 TS2/16 antibody induced SS RBC adhesion to Lu-Fc for all patients (Figure 5B; P < .01). These results clearly indicated that activation of α4β1 either by epinephrine or by anti–β1 TS2/16 antibody stimulates SS RBCs adhesion to Lu/BCAM proteins. Adhesion to Lu-Fc of 3 control RBCs with high reticulocyte count (C1, C2, and C3) was tested in order to determine whether the adhesion of SS RBCs to Lu-Fc was “sickle-hemoglobin”–dependent or a consequence of any circulating reticulocyte expressing α4β1. Control RBCs with high reticulocyte counts showed the same adhesion level when compared with AA RBCs, and no activation was detected after incubation with epinephrine.
Epinephrine-treated SS RBCs adhere to Lu-Fc in an integrin α4β1–dependent manner under flow conditions. (A) AA and SS RBCs (.005 [0.5%] hematocrit) were either untreated or incubated with 20 nM epinephrine (Epi) for 1 minute at room temperature, then injected into a Lu-Fc–coated microslide (0.3 mg/mL) at 0.2 dyne/cm2 for 10 minutes before applying 5-minute washout steps at 0.4, 1, 2, and 4 dyne/cm2. Cells were counted after each wash in 6 areas along the microslide centerline. (B) AA (AA1 and AA2), SS (SS1-SS6), and high-reticulocyte control (C1-C3) RBC samples (.005 [0.5%] hematocrit) were either untreated or incubated with 20 nM epinephrine with or without preincubation with 10 μg/mL blocking antibody anti–integrin β1 (mAb 13), or with 1 μg/mL activating antibody anti–integrin β1 TS2/16. Injection and washouts were carried out as for panel A. The graph shows results at 1 dyne/cm2. Each value is a mean of 6 measures. (**P < .01; in panel B, ▧ and ▪ are compared with □, and ⊡ is compared with ▪). Error bars represent SD.
Epinephrine-treated SS RBCs adhere to Lu-Fc in an integrin α4β1–dependent manner under flow conditions. (A) AA and SS RBCs (.005 [0.5%] hematocrit) were either untreated or incubated with 20 nM epinephrine (Epi) for 1 minute at room temperature, then injected into a Lu-Fc–coated microslide (0.3 mg/mL) at 0.2 dyne/cm2 for 10 minutes before applying 5-minute washout steps at 0.4, 1, 2, and 4 dyne/cm2. Cells were counted after each wash in 6 areas along the microslide centerline. (B) AA (AA1 and AA2), SS (SS1-SS6), and high-reticulocyte control (C1-C3) RBC samples (.005 [0.5%] hematocrit) were either untreated or incubated with 20 nM epinephrine with or without preincubation with 10 μg/mL blocking antibody anti–integrin β1 (mAb 13), or with 1 μg/mL activating antibody anti–integrin β1 TS2/16. Injection and washouts were carried out as for panel A. The graph shows results at 1 dyne/cm2. Each value is a mean of 6 measures. (**P < .01; in panel B, ▧ and ▪ are compared with □, and ⊡ is compared with ▪). Error bars represent SD.
Reticulocyte count and copy number of integrin α4β1 in reticulocytes of patients with sickle cell disease (SS) and high-reticulocyte controls (C)
| Patient . | Reticulocytes, × 109/L . | α4β1 SABC/cell . |
|---|---|---|
| SS1 | 165 | 720 |
| SS2 | 180 | 660 |
| SS3 | 244 | 1735 |
| SS4 | 272 | 2260 |
| SS5 | 285 | 1720 |
| SS6 | 249 | 1570 |
| SS7 | 281 | 1425 |
| C1 | 350 | 1410 |
| C2 | 50 | 2470 |
| C3 | 238 | 1530 |
| Patient . | Reticulocytes, × 109/L . | α4β1 SABC/cell . |
|---|---|---|
| SS1 | 165 | 720 |
| SS2 | 180 | 660 |
| SS3 | 244 | 1735 |
| SS4 | 272 | 2260 |
| SS5 | 285 | 1720 |
| SS6 | 249 | 1570 |
| SS7 | 281 | 1425 |
| C1 | 350 | 1410 |
| C2 | 50 | 2470 |
| C3 | 238 | 1530 |
Activation of α4β1 induces SS RBC adhesion to endothelial cells through Lu/BCAM binding
Since epinephrine-stimulated SS RBCs interacted with Lu-Fc proteins in an integrin α4β1–dependent manner, we analyzed the interaction of RBCs with primary HUVECs to approach physiologic conditions. Lu gp's expression in HUVECs was tested by flow cytometry and immunoprecipitation. Lu/BCAM proteins were immunoprecipitated from HUVECs using F241 anti-Lu mAb after biotinylation of cell-surface proteins. Epithelial A498 kidney cells were used as control since they express both Lu and Lu(v13) isoforms. As shown in Figure 6A, HUVECs express only the Lu gp isoform. Flow cytometry analysis (Figure 6B) showed that HUVECs exhibited a high copy number of Lu gp compared with RBCs (SABC per cell: 22 000 vs 1000-4000, respectively). It has been previously shown that integrin α4β1 was not expressed in endothelial cells of quiescent vessels.45 This absence of expression was confirmed on HUVECs by flow cytometry (Figure 6B), thus excluding any interaction between Lu/BCAM expressed on SS RBCs and potential α4β1 on HUVECs. VCAM-1 expression was also tested in HUVECs and was close to α4β1 background level (16% of positive cells; mean fluorescence intensity [MFI] = 21.8) (Figure 6B). This was not due to the inability of the cells to express VCAM-1, as activation of HUVECs by TNF-α induced a strong expression of this protein (90% of positive cells; MFI = 466) (Figure 6B). This result was consistent with our previous study using radiolabeled antibodies in specific binding assays and showed that following our isolation procedure, resting HUVECs do not express significant amounts of VCAM-1.46
Expression of Lu/BCAM proteins, integrin α4β1, and VCAM-1 in HUVECs. (A) HUVECs and A498 cell-surface proteins were biotinylated, and Lu/BCAM proteins were immunoprecipitated after cell lysis using anti-Lu mAb F241. Immunoprecipitates were run on 8% polyacrylamide gel, and the proteins were revealed using streptavidin–biotinylated HRP. (B) Flow cytometry analysis of Lu/BCAM, integrin α4, and VCAM-1 expression in HUVECs. VCAM-1 expression was tested in HUVECs before and after stimulation by TNF-α. Solid lines represent cells positive for each protein; dashed lines, cells incubated with isotype-matched control antibodies.
Expression of Lu/BCAM proteins, integrin α4β1, and VCAM-1 in HUVECs. (A) HUVECs and A498 cell-surface proteins were biotinylated, and Lu/BCAM proteins were immunoprecipitated after cell lysis using anti-Lu mAb F241. Immunoprecipitates were run on 8% polyacrylamide gel, and the proteins were revealed using streptavidin–biotinylated HRP. (B) Flow cytometry analysis of Lu/BCAM, integrin α4, and VCAM-1 expression in HUVECs. VCAM-1 expression was tested in HUVECs before and after stimulation by TNF-α. Solid lines represent cells positive for each protein; dashed lines, cells incubated with isotype-matched control antibodies.
Control AA RBCs and SS RBCs were injected over a HUVEC monolayer at 0.2 dyne/cm2 before washing at 0.4, 1, and 2 dyne/cm2. AA RBCs did not show significant adhesion to HUVECs, while SS RBCs adhered to these cells at low shear stress of 0.4 dyne/cm2 but were detached at shear stress exceeding 1 dyne/cm2 (Figure 7A). AA and SS RBCs were then preincubated with the activating mAb anti–β1 TS2/16. Adhesion of AA RBCs to HUVECs was not activated by TS2/16. Conversely, SS RBCs showed a significant adhesion at all shear stresses when compared with untreated RBCs, indicating that activation of integrin α4β1 was capable of stimulating SS RBCs adhesion to endothelial cells (Figure 7A; P < .01). In order to test the potential role of Lu gp's expressed on HUVECs in this adhesion, SS RBCs activated by TS2/16 were incubated with soluble Lu-Fc. Lu-Fc significantly inhibited adhesion of TS2/16-activated SS RBCs to HUVECs (P < .01), indicating that the increase of adhesion after activation of integrin α4β1 was in part mediated by the interaction of SS RBCs with Lu/BCAM gp's on the surface of endothelial cells. RBCs from 5 patients (SS3-SS7; Table 1) were analyzed in order to confirm these results. Adhesion of all SS RBCs was activated by TS2/16 (1.4- to 2.7-fold; P < .01) and inhibited after incubation with soluble Lu-Fc (P < .01 and P < .05 depending on the sample; Figure 7B). We then used soluble human VCAM-1–Fc, a specific ligand for α4β1, as a competitor to endothelial Lu/BCAM. Activated RBCs from all patients were incubated with VCAM-1–Fc. This treatment significantly inhibited their adhesion to HUVECs (P < .01 and P < .05 depending on the sample), indicating that the adhesion increase of activated SS RBCs to HUVECs was mediated by integrin α4β1 (Figure 7B). The adhesion level of 3 control RBCs with high reticulocyte counts (C1, C2, and C3) was similar to the level of AA RBCs; no activation was detected after incubation with activating antibody TS2/16.
SS RBCs activated by anti–β1 TS2/16 antibody adhere to HUVECs in a Lu/BCAM-dependent manner under flow conditions. (A) SS RBCs (.005 [0.5%] hematocrit) were either untreated or incubated with 1 μg/mL activating antibody anti–integrin β1 TS2/16 with or without 10 μg/mL of soluble Lu-Fc for 20 minutes at room temperature. Red cells were then injected for 10 minutes over a HUVEC monolayer at 0.2 dyne/cm2. Washouts (5 minutes) were applied sequentially from 0.4 to 4 dyne/cm2, and cells were counted in 6 different areas along the microslide centerline after each wash. AA RBCs were used as control. (B) SS RBCs from 5 patients (SS3-SS7; .005 [0.5%] hematocrit) were either untreated or activated by 1 μg/mL activating antibody anti–integrin β1 TS2/16. Activated RBCs were incubated with 10 μg/mL of Lu-Fc or VCAM-1–Fc, and RBC adhesion was analyzed as in panel A. The figure shows the number of adherent RBCs at 1 dyne/cm2. Each value is a mean of 6 measures. (*P < .05; **P < .01; in panel B ▪ is compared with □, and ▧; and ⊡ are compared with ▪). Error bars represent SD.
SS RBCs activated by anti–β1 TS2/16 antibody adhere to HUVECs in a Lu/BCAM-dependent manner under flow conditions. (A) SS RBCs (.005 [0.5%] hematocrit) were either untreated or incubated with 1 μg/mL activating antibody anti–integrin β1 TS2/16 with or without 10 μg/mL of soluble Lu-Fc for 20 minutes at room temperature. Red cells were then injected for 10 minutes over a HUVEC monolayer at 0.2 dyne/cm2. Washouts (5 minutes) were applied sequentially from 0.4 to 4 dyne/cm2, and cells were counted in 6 different areas along the microslide centerline after each wash. AA RBCs were used as control. (B) SS RBCs from 5 patients (SS3-SS7; .005 [0.5%] hematocrit) were either untreated or activated by 1 μg/mL activating antibody anti–integrin β1 TS2/16. Activated RBCs were incubated with 10 μg/mL of Lu-Fc or VCAM-1–Fc, and RBC adhesion was analyzed as in panel A. The figure shows the number of adherent RBCs at 1 dyne/cm2. Each value is a mean of 6 measures. (*P < .05; **P < .01; in panel B ▪ is compared with □, and ▧; and ⊡ are compared with ▪). Error bars represent SD.
Discussion
In this study we identified integrin α4β1 as a new ligand for Lu/BCAM proteins. We used recombinant human integrin α4β1 expressed in L929 mouse fibroblasts and immobilized chimeric Lu-Fc proteins to demonstrate that α4β1 binds specifically to Lu-Fc, leading to cell adhesion under static and flow conditions. Since L929 cells were cotransfected with human integrin α4 and β1, we cannot exclude that mouse α5, α6, or αv subunits could pair with β1 and lead to interaction with Lu-Fc. Nevertheless, L-α4β1 cell adhesion was similarly inhibited by human anti-α4 and anti-β1 antibodies, indicating that it was mainly mediated by the human heterodimer α4β1. “Outside-in” signaling by integrin α4β1 can be initiated by clustering α4β1 with mAb, which triggers a tyrosine phosphorylation pathway.47,48 Indeed, integrin α4β1 is known to present activation states37,38 that can be regulated by extracellular factors such as divalent cations or antibodies,42 or by “inside-out” cell signaling induced by agonists.39,40 We investigated both pathways, using TS2/16 anti-β1 mAb and epinephrine, and showed that activation of α4β1 led to an enhanced adhesion of L-α4β1 cells to Lu-Fc. The adhesion increase mediated by α4β1 in epinephrine-treated L-α4β1 cells illustrates an “inside-out” cell-signaling mechanism that will be further investigated.
Integrin α4β1 and several other integrins are expressed in erythroid precursors and disappear during normal erythroid maturation. Integrin α4β1 is the unique integrin expressed in a population of circulating sickle RBCs.24,25 To investigate the biological relevance of Lu/BCAM–integrin α4β1 interaction, we explored adhesion of normal and sickle RBCs to Lu-Fc and HUVECs under flow conditions. Untreated SS as well as AA RBCs did not adhere to Lu-Fc under flow conditions, but a significant adhesion was observed when SS RBCs were treated with epinephrine. This adhesion was inhibited by the anti-β1–blocking antibody, indicating that it was mainly mediated by a direct interaction of activated α4β1 and Lu-Fc through an “inside-out” activation of α4β1. On the other hand, the increase of SS RBCs adhesion after incubation with the anti-β1–activating antibody TS2/16 indicated that this adhesion can also be stimulated by an “outside-in” signaling mechanism. Vaso-occlusion is associated with various types of physiologic stress, and epinephrine is known to elevate cAMP in SS RBCs through stimulation of adrenergic receptors.14 This elevation in intracellular cAMP leads to activation of signaling pathways involving PKA and small GTPases.14,49 Adhesion of epinephrine-treated SS RBCs to Lu-Fc was most likely induced by PKA and/or small GTPases like Rap-1, as they are known to activate integrin α4β1.43,44
There was a clear difference in the adhesion level between untreated L-α4β1 cells and SS RBCs. This could arise from the difference in the percentage of cells expressing α4β1 and from its density at the cell surface. All (100%) L-α4β1 cells express 20 to 25 copies/μm2 of integrin α4β1 (10 200 copies/cell in average, 450 μm2 of cell-surface area) compared with only 4.5% to 10% of SS RBCs expressing 4 to 16 copies/μm2 (660-2260 copies/RBC, 145 μm2 of RBC surface area; Table 1). In addition, L929 are adherent cells that express a wide range of adhesion molecules that could reinforce the adhesion of L-α4β1 cells once they attach to the Lu-Fc substrate.
To approach physiologic conditions we tested the adhesion of RBCs to HUVECs under flow. In contrast to AA RBCs, untreated SS RBCs showed a basal adhesion to HUVECs, but this adhesion was independent of endothelial Lu/BCAM as it was not inhibited by soluble Lu-Fc (data not shown). It is well documented that resting HUVECs do not express VCAM-1 in the absence of stimulating factors like TNF-α, phorbol esters, or epinephrine, yet we cannot totally exclude that this expression could be induced by the flow during the adhesion assay. However, adhesion of untreated SS RBCs to HUVECs at low shear stress was not diminished by soluble VCAM-1 (data not shown), suggesting that the observed adhesion was most likely not due to a potential α4β1–VCAM-1 interaction. A similar adhesion, called “transport-controlled,” has been reported to occur at low shear stress in the absence of stimulating factors, and is partially due to terminal sialic acid residues on erythrocyte membranes and to endothelial fibronectin.50 In addition, this basal adhesion could be due to the interaction of erythroid Lu/BCAM with the laminin α5 chain that is secreted and present on HUVECs during cell culture.51 Our results indicated that SS RBCs from all patients showed significant adhesion to HUVECs after selective activation of erythroid α4β1 by the specific TS2/16 mAb. We chose to target α4β1 activation by TS2/16 and not by epinephrine, as epinephrine activates other adhesion molecules on SS RBCs, like LW/ICAM-4, which interacts with integrin αvβ3 on endothelial cells,52 as well as Lu/BCAM gp's, which enhance RBC adhesion by binding to the laminin α5 chain.9,14 After activation with TS2/16 mAb, we used the highly avid α4β1 ligand VCAM-1 as a competitor and showed that it inhibited the activated SS RBCs adhesion to HUVECs. On the endothelial side, we could state that this adhesion was mainly mediated by Lu/BCAM proteins, as it was inhibited by incubating the activated SS RBCs with soluble Lu-Fc. Finally, using the high-reticulocyte controls, we showed that the adhesion of SS RBCs to Lu-Fc and to HUVECs was dependent on the sickle phenotype and was not the result of the presence of any circulating reticulocyte expressing integrin α4β1.
We recently showed that the Lu/BCAM long isoform named Lu gp was phosphorylated by glycogen synthase 3 beta, casein kinase II, and PKA, and that its adhesion to the laminin α5 chain was modulated by PKA.9 In the present study, we show that the cytoplasmic tail of Lu gp was not necessary for the interaction with α4β1 since it was absent from the Lu-Fc protein. Nevertheless, Lu gp binding to integrin α4β1 could also be modulated by phophorylation in a cellular context, as is the case for binding to laminin. One can assume that under stress conditions, elevation of intracellular cAMP by epinephrine could activate both endothelial Lu and SS RBC α4β1 and reinforce their interaction. This hypothesis, as well as the signaling pathway that activates integrin α4β1 binding to Lu proteins, will be investigated.
The interaction between Lu/BCAM proteins and integrin α4β1 could occur independently of the sickle cell disease context, opening new fields of investigation. For example, endothelial Lu/BCAM proteins could participate in the transendothelial leukocyte migration during the inflammatory process where integrin α4β1 on leukocytes plays a key role by interacting with its endothelial ligand VCAM-1.
In summary, we describe a novel interaction between SS RBC α4β1 and endothelial Lu/BCAM proteins that could contribute to the abnormal adhesion of SS RBCs to the endothelium either by tethering RBCs to the endothelium or by reinforcing their adhesion along with the well-known interactions of α4β1 with its other ligands. This is the first report pointing out a potential role for endothelial Lu/BCAM in a pathologic process. Taken together with the established role of erythroid Lu/BCAM in the enhanced adhesion of SS RBCs to subendothelial laminin α5, this suggests that Lu/BCAM proteins could be appropriate targets for development of antiadhesive agents, such as peptides or antibodies, in sickle cell disease treatment.
Authorship
Contributions: W.E.N. designed all the phases of the research, performed all adhesion assays, analyzed the data, and wrote the manuscript; M.P.W. isolated HUVECs, supervised flow adhesion experiments, and critically reviewed the manuscript; C.R. produced and purified the soluble chimeric proteins; P.G. performed the flow cytometry analyses; P.H. provided cell lines expressing recombinant integrins; F.G. provided blood samples and clinical advice and critically reviewed the manuscript; J.L.W. critically reviewed the manuscript; J.P.C. provided scientific advice and critically reviewed the manuscript; Y.C. directed the overall study and critically reviewed and revised the manuscript; and C.L.V.K. directed the overall study, critically reviewed the experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Caroline Le Van Kim, INSERM U665–Institut National de la Transfusion Sanguine, 6 rue Alexandre Cabanel, 75015 Paris, France; e-mail: levankim@idf.inserm.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Ms Sarah Descraques from the Centre Hospitalier Robert Ballanger for providing the umbilical cords for HUVEC preparation. We wish to thank Ms Eliane Vera from Centre National de Référence pour les Groupes Sanguins (CNRGS) for blood sample management and Dr Dominique Goossens for proofreading the manuscript.
This work was supported in part by the Institut National de la Transfusion Sanguine (INTS) and INSERM.


![Figure 5. Epinephrine-treated SS RBCs adhere to Lu-Fc in an integrin α4β1–dependent manner under flow conditions. (A) AA and SS RBCs (.005 [0.5%] hematocrit) were either untreated or incubated with 20 nM epinephrine (Epi) for 1 minute at room temperature, then injected into a Lu-Fc–coated microslide (0.3 mg/mL) at 0.2 dyne/cm2 for 10 minutes before applying 5-minute washout steps at 0.4, 1, 2, and 4 dyne/cm2. Cells were counted after each wash in 6 areas along the microslide centerline. (B) AA (AA1 and AA2), SS (SS1-SS6), and high-reticulocyte control (C1-C3) RBC samples (.005 [0.5%] hematocrit) were either untreated or incubated with 20 nM epinephrine with or without preincubation with 10 μg/mL blocking antibody anti–integrin β1 (mAb 13), or with 1 μg/mL activating antibody anti–integrin β1 TS2/16. Injection and washouts were carried out as for panel A. The graph shows results at 1 dyne/cm2. Each value is a mean of 6 measures. (**P < .01; in panel B, ▧ and ▪ are compared with □, and ⊡ is compared with ▪). Error bars represent SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/8/10.1182_blood-2006-07-035139/4/m_zh80080711120005.jpeg?Expires=1769196351&Signature=L7~jAurOlwryfM~YRIZlThzAiZ2mym0w~4e1pDDg6qJSswwAVoBjs4n0eO2zYeJh2XqwhKob1pbNgwtS5h3nPQT-ieo-8ZUTbOOoCka4WZay34UJRbaO8rDi5vQchvbp7-gQG~oemaP5JPoVN~d2Z3u59Jn-zwVk~y2TqvCG5I5uxYiN-iwqvGzTst1HgwN-zznuJyU-HwMA~-SZ91Yr76LnBQrj-jiGeU-vswlfNUNU8S1mGeYetBuj0js68ErgyR8taVtpZujDvFw2wM39R51V8MUAYDDsdc4OYZ~tGQ8-XL1LIgaqUPLq~RyFt-TuNIYF1-a-xxky1a8WABxjaA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 7. SS RBCs activated by anti–β1 TS2/16 antibody adhere to HUVECs in a Lu/BCAM-dependent manner under flow conditions. (A) SS RBCs (.005 [0.5%] hematocrit) were either untreated or incubated with 1 μg/mL activating antibody anti–integrin β1 TS2/16 with or without 10 μg/mL of soluble Lu-Fc for 20 minutes at room temperature. Red cells were then injected for 10 minutes over a HUVEC monolayer at 0.2 dyne/cm2. Washouts (5 minutes) were applied sequentially from 0.4 to 4 dyne/cm2, and cells were counted in 6 different areas along the microslide centerline after each wash. AA RBCs were used as control. (B) SS RBCs from 5 patients (SS3-SS7; .005 [0.5%] hematocrit) were either untreated or activated by 1 μg/mL activating antibody anti–integrin β1 TS2/16. Activated RBCs were incubated with 10 μg/mL of Lu-Fc or VCAM-1–Fc, and RBC adhesion was analyzed as in panel A. The figure shows the number of adherent RBCs at 1 dyne/cm2. Each value is a mean of 6 measures. (*P < .05; **P < .01; in panel B ▪ is compared with □, and ▧; and ⊡ are compared with ▪). Error bars represent SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/8/10.1182_blood-2006-07-035139/4/m_zh80080711120007.jpeg?Expires=1769196351&Signature=ZHS1SshwzLcQQM0Rq6~iVOj3Tt~6u8QMoWWZmH0~SCZIesm9gm2Tf7zRIt0cHWklag~oQhVf2NmT2vtYWXBQeqDZg5hYMZrAdLzN-Ru9x66kCQ8q~6lggD28iRja8jOvAnWIa5fLn2mNXt5QZHiGMQOtaU7516nEehidKw51pdlASCuTMLlUtYKWeZysTZrwqCbUhT5850lYvrvJuxewGNEIL2bgBl3EPPECINlKItAjx~yJG907lgU2Zqv-O7E58eMqtUGwzyzvLRybycKg17w~mv6ZIap~TjrCKmNA8DpY96Vwae9fJifn9uAzaWEQ6HXedL-RbeYG37i-J6mweQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal