Abstract
The importance of reactive oxygen intermediate (ROI) production in antimicrobial responses is demonstrated in human patients who suffer from chronic granulomatous disease (CGD) due to defective NADPH oxidase function. Exactly how bacterial products activating Toll-like receptors (TLRs) induce oxidative burst is unknown. Here, we identify the Vav family of Rho guanine nucleotide exchange factors (GEFs) as critical mediators of LPS-induced MyD88-dependent activation of Rac2, NADPH oxidase, and ROI production using mice deficient in Vav1, Vav2, and Vav3. Vav proteins are also required for p38 MAPK activation and for normal regulation of proinflammatory cytokine production, but not for other MyD88-controlled effector pathways such as those involving JNK, COX2, or iNOS and the production of reactive nitrogen intermediates (RNIs). Thus, our data indicate that Vav specifically transduces a subset of signals emanating from MyD88.
Introduction
Mammalian Toll-like receptors (TLRs) initiate innate host defenses and regulate adaptive immune responses by virtue of their ability to recognize conserved microbial components.1-3 Upon recognition of their ligands, TLRs transduce intracellular signals via associated Toll/interleukin-1 receptor (TIR) domain–containing adaptors, including MyD88, TIRAP, TRIF, and TRAM, which in turn recruit additional signaling molecules such as IRAK4, IRAK1, and TRAF6.1,3 In this manner, recognition of LPS by TLR4, or peptidoglycan (PGN) by TLR2, on macrophages and neutrophils activates MyD88-dependent inflammatory responses. These include the production of cytokines and prostaglandins as well as oxidative burst, which generates reactive oxygen intermediates (ROIs).4,5 The importance of oxidative burst for mounting effective antimicrobial responses is underscored by chronic granulomatous disease (CGD) in human patients bearing mutations in the NADPH oxidase complex. Phagocytes from these patients fail to efficiently generate superoxide (O2−) and secondary ROIs upon microbial challenge, thus rendering them susceptible to chronic and potentially life-threatening infections. However, excessive ROI generation in response to environmental stress or proinflammatory stimuli can cause widespread organ damage.6,7
Generation of ROIs is dependent on assembly of the plasma membrane-associated NADPH oxidase complex that catalyzes electron transfer from NADPH to FAD, heme, and O2, resulting in the production of superoxide (O2−) and secondary ROIs, such as hydrogen peroxide, hydroxyl radical, and hypochlorous acid. Given the importance of ROIs in controlling bacterial infections, the mechanism of NADPH oxidase activation has been extensively examined (for reviews, see Bokoch8 and Nathan9 ). Thus, the activation of NADPH oxidase complex involves the assembly of cytosolic regulatory components p47phox, p67phox, p40phox, and Rac2 and the recruitment of the transmembrane cytochrome b558 complex composed of 2 subunits, gp91phox and p22phox.8 In this regard, the activation of Rac2, which interacts with both the cytosolic p67phox and the membrane-bound cytochrome b558,8,10 is thought to be critical for NADPH oxidase induction. Accordingly, mutation of the RAC2 gene in humans leads to selective defects in neutrophil oxidative burst,11,12 whereas targeted disruption of the Rac2 gene in mice results in severely compromised oxidative burst in neutrophils, with more selective defects in macrophages.13-17 Notably, Rac1-deficient phagocytes do not exhibit any defects in neutrophil oxidative burst,18 even though Rac1 had been shown to contribute to NADPH oxidase activity in a cell-free system.19 Nonetheless, despite ample evidence pointing to the essential role of Rac2 in phagocyte oxidative burst, the upstream pathways regulating Rac2 in this process remain to be elucidated. Of particular interest is the identity of a putative guanine nucleotide exchange factor (GEF) catalyzing GTP loading and activation of the GDP-bound, or inactive, Rac2 in response to MyD88-dependent receptor TLR4 or TLR2.
The P-Rex1 protein has recently been identified as a specific GEF critical for Rac2 activation downstream of GPCRs; however, P-Rex1 is not required for Rac2 activation by LPS in neutrophils.20,21 In this context, among the plethora of mammalian Rho-GEFs, one candidate for linking TLR4/2 with Rac2 is the Vav family. Although Vav1 has been previously implicated in mediating LPS responses in phagocytes, clear genetic evidence is lacking.22-24 Furthermore, the combined contributions of the individual Vav family members (Vav1, Vav2, and Vav3) in mediating MyD88-dependent effector pathways remain unclear. Herein, we sought to elucidate the role of Vav in the induction of ROI in phagocytic cells. Using a combination of genetic and biochemical approaches, we identified the Vav family as critical in MyD88-dependent ROI production. Strikingly, Vav is also essential for normal p38 MAPK activation and proinflammatory cytokine production; however, it is not required in other LPS-induced signal transduction pathways, such as those involving JNK and NFκB, or in effector responses involving COX2 and iNOS and the production of reactive nitrogen intermediates (RNIs).
Materials and methods
Reagents
LPS (Sigma, St Louis, MO) was purified by phenol extraction from Escherichia coli serotype 0127:B8. Peptidoglycan and fMLP were obtained from Sigma.
Mice
Germline Vavnull and MyD88−/− mice have been previously described25,26 and were maintained in the SPF facility of Washington University School of Medicine. All protocols were approved by the Institutional Animal Care and Use Committee at Washington University and were carried out in accordance with institutional guidelines and regulations.
Flow cytometry
Cell suspensions were prepared, counted, and stained with antibodies according to standard procedures. The following antibody conjugates were used: fluorescein (FITC)–GL1 (anti-CD86), -16-10A1 (anti-CD80), -25-9-17 (anti–I-Ab), phycoerythrin (PE)–MEL-14 (anti-CD62L), and biotinylated-RP/14 (anti-RP105/CD180), all from BD Biosciences (San Jose, CA; FITC-BM8 (anti-F4/80), from Caltag (Burlingame, CA); biotinylated-MTS510 (anti-TLR4/MD2), from eBioscience (San Diego, CA); and FITC-M1/70 (anti-CD11b) and biotinylated-RB6-8C5 (anti-GR1), from Southern Biotechnologies (Birmingham, AL). Biotinylated antibodies were detected with streptavidin (SAV)–PE (BD Biosciences). All samples were analyzed on a FACSCalibur flow cytometer (BD Biosciences) with FlowJo software (TreeStar, Ashland, OR). Data are displayed as histograms with logarithmic scale. Each plot represents analysis of 5 × 105 or more events collected as listmode files.
Generation of murine bone marrow–derived macrophages
Bone marrow (BM) cells from the long bones of 4- to 8-week-old mice were cultured in large (150 ×) non–tissue culture–treated dishes (Fisher Scientific, Pittsburgh, PA) in macrophage media (DMEM supplemented with 15% FCS and 20% L cell-conditioned media [LCM], as a source of M-CSF) for 5 to 7 days.
Isolation of peritoneal exudate cells
Mice were injected intraperitoneally with 2 mL of 4% thioglycollate. Four hours after injection, peritoneal exudate cells (PECs) were harvested from mice by peritoneal lavage and immediately used in experiments.
Isolation of bone marrow neutrophils
BM cells were flushed from the long bones of 4- to 8-week-old mice and resuspended in 45% Percoll (Sigma) HBSS. Cells were overlaid on a step gradient composed of 50%, 55%, 60%, and 65% Percoll HBSS fractions prior to separation by centrifugation at 1273g for 30 minutes. Neutrophils were recovered between the 60% and 65% Percoll fractions.
Oxidative burst assay
BM-derived macrophages (BMDMs) or PECs were washed in PBS and resuspended in DMEM at a concentration of 2.5-5 × 105 cells/mL. Cells were then distributed into 1-mL aliquots in 5-mL polystyrene luminometer tubes. Lucigenin (Molecular Probes, Eugene, OR) was added to each sample to achieve a final concentration of 150 μM, and cells were allowed to equilibrate for 10 minutes. At this point (designated time 0), baseline luminescence was measured in each sample for 10 seconds in an Opto-compII luminometer (MGM Instruments, Hamden, CT). Immediately after the baseline reading, cells were stimulated with 10 μg/mL LPS (Sigma) or 50 ng/mL PMA (Sigma), at which point the time course began. Subsequently, luminescence was measured in each sample at the indicated time points. Luminescence is expressed as relative light units (RLUs) detected over 10 seconds. For each reading, the increase in RLU over background signal (unstimulated cells) was plotted versus time.
Phagocytosis assays
E coli were heat killed at 65°C for 30 minutes and washed 3 times in PBS. E coli were then stained with 1 μM CFDA (Molecular Probes) for 15 minutes at room temperature and washed thoroughly in PBS. BMDMs (250 000 cells) were stimulated with CFSE-labeled E coli (40 million cells) for the indicated time points. Free bacteria that had not been phagocytosed by macrophages were washed away, and macrophages were stained with DAPI and mounted on slides for microscopic analysis. Cells were mounted using Vectashield medium with DAPI (Vector Laboratories, Burlingame, CA) and were visualized using a Nikon Eclipse E400 microscope (Tokyo, Japan) equipped with a 60×/1.4 numerical aperture oil objective. Images were captured using an Optronics Magnafire camera (Optronics, Goleta, CA) and Adobe Photoshop CS software (Adobe Systems, San Jose, CA).
Reconstitution of murine bone marrow-derived macrophages
Reconstitution of BMDMs was achieved by culturing 4-5 × 106 BM cells in small (p100) non–tissue cultured–treated dishes (Fisher Scientific) in macrophage media for 2 days, followed by 24-hour incubation in retroviral supernatant containing GFP-Vav1WT, GFP-Vav1GEF-MUT, or GFP only, supplemented with 20% LCM. Following 24-hour culture, cells were harvested, split 1:2, and allowed to differentiate for 3 days. The Vav1GEF-MUT mutant contains a GEF-inactivating point mutation (L278Q) in the Dbl-homology domain.27
Rac2 assay
BMDMs were plated in 10-cm tissue culture-treated dishes and starved for 36 hours in media lacking M-CSF and serum. Cells were treated with 10 μg/mL LPS for the indicated time points, and Rac2 assay was performed using the Rac assay kit (Pierce, Rockford, IL) according to the manufacturer's instructions. In short, BMDMs were lysed, and clarified supernatants were incubated with PBD-GST fusion protein and glutathione beads at 4°C for 1 hour with gentle rocking. Following incubation, beads were centrifuged at 7200g for 10 to 30 seconds and washed with lysis buffer. Then 2 × SDS loading buffer was added to the samples, and the samples were boiled for 5 minutes followed by spinning at 7200g for 2 minutes. Samples were then run on 10% gel and Western blotted for Rac2 (Santa Cruz Biotechnology, Santa Cruz, CA).
Macrophage stimulations and immunoblotting
BMDMs were harvested from large non–tissue culture–treated dishes by removing media and adding 10 mL cold PBS, followed by incubation at 4°C for 5 minutes. BMDMs were gently scraped with a cell scraper and collected following repetitive pipetting. For biochemistry, 5 × 105 cells were plated in each well of a 6-well plate. Following overnight starvation in media lacking MCSF and FCS, cells were stimulated with LPS for the indicated time points and lysed on ice with RIPA buffer (PBS, 1% NP40, 0.5% deoxycholate, 0.1% SDS, 10 mM EDTA) supplemented with a protease inhibitor cocktail (Boehringer Mannheim, Mannheim, Germany), 10 mM NaF, and 1 mM Na3VO4. Crude lysates were cleared by centrifugation at 13 000g for 10 minutes at 4°C. Postnuclear lysates were prepared and resolved by SDS-PAGE followed by Western blotting with antibodies to the indicated proteins. Primary antibodies were developed with HRP-conjugated secondary antibodies (antimouse from Zymed, San Francisco, CA; antirabbit from Amersham Biosciences, Freiburg, Germany; antigoat from Upstate Biotechnology, Lake Placid, NY). Immune complexes were detected by enhanced chemiluminescence (Amersham Biosciences). Antibodies raised against p38, phospho-p38 T180/Tyr182, phospho-ASK1 T845, phospho-p44/42 MAPK (ERK) T202/Y204, JNK, phospho-JNK T183/Y185, Akt, and phospho-Akt S473 were obtained from Cell Signaling Technology (Beverly, MA). Anti-pTyr (4G10) was obtained from Upstate Biotechnology. Antibodies raised against ASK1, Vav, ERK2, IκB-α, COX2, and iNOS (NOS2) were purchased from Santa Cruz Biotechnology. Mouse anti-Vav1 was obtained from Chemicon (Temecula, CA).
Cytokine assays
BMDMs were plated in 12-well plates with 2.5 × 105 cells/well. Cells were starved overnight in media containing 5% FCS and stimulated for 24 hours with the indicated concentrations of LPS. Supernatants were collected and cytometric bead array assays performed using the Murine Inflammation Cytometric Bead Array kit (Becton Dickinson, Mountain View, CA) according to the manufacturer's instructions.
Nitric oxide assays
BMDMs were harvested and plated at 5 × 105 cells/well in 6-well plates. Cells were starved overnight in DMEM lacking FCS and M-CSF and stimulated for 18 hours with LPS (10 μg/mL) and/or IFN-γ (50 μm/mL). Following stimulation, culture supernatant was harvested and analyzed for nitrite content using the Greiss reagent, as previously described.28
Results
Defective LPS- and PGN-induced oxidative burst in Vavnull macrophages and neutrophils.
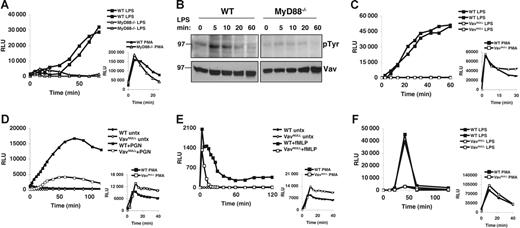
A recent study showed a requirement for MyD88 in ROI production upon phagocytosis of opsonized Gram-negative bacteria.29 However, since this was independent of TLR4, multiple innate immune receptors could have been triggered, and it remains unclear how MyD88 induces NADPH activation and ROI production in response to soluble endotoxin. To examine this, we first decided to confirm that soluble LPS-induced oxidative burst depends on MyD88. Indeed, neutrophils from peritoneal exudates (PECs) of MyD88−/− mice show a profound block in ROI production in response to soluble LPS, as compared to wild-type (WT) cells (Figure 1A). We note that in this in vitro assay, soluble LPS induction of ROI burst requires relatively high doses (more than 1 μg/mL) of purified LPS (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article), which also induce the greatest activation of MAPKs, such as p38 or ERK. Importantly, MyD88−/− PECs produce ROIs similar to WT in response to PMA, indicating that the lack of ROI generation in response to LPS is not due to intrinsic defects in NADPH oxidase function.
Vav is phosphorylated in a MyD88-dependent fashion and is required for LPS-induced oxidative burst. (A) Oxidative burst in MyD88−/− and WT PECs stimulated with LPS (10 μg/mL) or PMA (50 ng/mL) was measured by lucigenin chemiluminescence. (B) WT and MyD88−/− BMDMs were stimulated with LPS (10 μg/mL) for the indicated time points. Cells were lysed immediately following stimulation, and lysates were analyzed by sequential Western blot for phosphotyrosine (pTyr) and Vav. (C) Oxidative burst in Vavnull and WT PECs stimulated with LPS (10 μg/mL) or PMA (50 ng/mL) was measured by lucigenin chemiluminescence. (D-E) Oxidative burst was measured by lucigenin chemiluminescence in BM neutrophils stimulated with (D) PGN (10 μg/mL) or (E) fMLP (10 μM). (F) Oxidative burst in BMDMs stimulated with LPS (10 μg/mL) or PMA (50 ng/mL) was measured with lucigenin. Panels A, C, and F show 2 mice from each genotype that were analyzed within the same experiment. All panels show data representative of more than 5 independent experiments.
Vav is phosphorylated in a MyD88-dependent fashion and is required for LPS-induced oxidative burst. (A) Oxidative burst in MyD88−/− and WT PECs stimulated with LPS (10 μg/mL) or PMA (50 ng/mL) was measured by lucigenin chemiluminescence. (B) WT and MyD88−/− BMDMs were stimulated with LPS (10 μg/mL) for the indicated time points. Cells were lysed immediately following stimulation, and lysates were analyzed by sequential Western blot for phosphotyrosine (pTyr) and Vav. (C) Oxidative burst in Vavnull and WT PECs stimulated with LPS (10 μg/mL) or PMA (50 ng/mL) was measured by lucigenin chemiluminescence. (D-E) Oxidative burst was measured by lucigenin chemiluminescence in BM neutrophils stimulated with (D) PGN (10 μg/mL) or (E) fMLP (10 μM). (F) Oxidative burst in BMDMs stimulated with LPS (10 μg/mL) or PMA (50 ng/mL) was measured with lucigenin. Panels A, C, and F show 2 mice from each genotype that were analyzed within the same experiment. All panels show data representative of more than 5 independent experiments.
Given that Vav can activate Rac230 and is tyrosine phosphorylated in LPS-stimulated macrophages,22,31 we hypothesized that Vav could link MyD88 to Rac2 and the NADPH oxidase complex. Indeed, while tyrosine phosphorylation is thought to activate Vav's intrinsic GEF activity,30 we find that LPS-induced tyrosine phosphorylation of Vav requires MyD88 (Figure 1B). To determine whether Vav proteins are required for ROI induction by TLRs, we measured oxidative burst induced by LPS in neutrophils and macrophages from mice lacking individual Vav proteins (Vav1−/−, Vav2−/−, and Vav3−/−) or the entire family (Vavnull). Importantly, peritoneal exudates isolated from various Vav-deficient mice yield similar numbers of neutrophils that are phenotypically indistinguishable from WT, indicating that Vav proteins are not required for neutrophil recruitment (Figure S2). Strikingly however, while Vav1−/−, Vav2−/−, and Vav3−/− PECs all produce ROIs at levels similar to WT in response to LPS, Vavnull PECs show virtually no ROI production under the same conditions (Figure 1C; data not shown). Nevertheless, Vavnull PECs produce ROIs similar to WT in response to PMA, indicating that the lack of ROI generation in response to LPS is not due to defects in NADPH oxidase component expression, assembly, and/or function (Figure 1C).
To rule out that the LPS-induced respiratory burst defects we observed in Vavnull PECs were due to priming defects or inflammatory cells other than neutrophils, we examined purified, unprimed bone marrow neutrophils (BMNs) for their ability to generate ROIs in response to peptidoglycan (PGN), a TLR2 ligand that signals through a MyD88-dependent pathway but, unlike LPS, does not require priming of resting BMNs. Strikingly, Vavnull BMNs show a profound defect in ROI production in response to PGN, as compared to WT BMN (Figure 1D). We conclude from these experiments that MyD88-dependent ROI production shows a strict dependence on Vav.
In contrast to these TLR responses, our data indicate that PMA-induced oxidative burst is Vav independent, whereas GPCR-induced ROI response to formylated peptides, such as fMLP, is mildly affected in the absence of all Vav proteins (Figure 1E). This is in agreement with recent reports indicating that P-Rex1 can act as a Rac2-specific GEF activated by fMLP.20,21 However, in contrast to our findings, another report suggested that Vav1−/− neutrophils show defective oxidative burst in response to PMA or fMLP.32 At present, we do not understand the exact reasons for these discrepancies as we find no defects in ROI production in response to PMA in neutrophils lacking individual, or all, Vav proteins (Figure 1; data not shown), whereas fMLP response, which is not affected by the loss of any individual Vav, is only moderately reduced in neutrophils lacking all Vav proteins (Vavnull) (Figure 1E; data not shown).
In addition to neutrophils, macrophages generate ROIs in response to LPS stimulation.5,33 Consistent with our findings in neutrophils, Vavnull BMDMs also fail to generate oxidative burst in response to LPS, while WT BMDM or BMDM lacking individual Vav proteins respond vigorously (Figure 1F; data not shown). Importantly, BMDMs from Vavnull and WT mice are phenotypically indistinguishable and generate similar amounts of ROIs when stimulated with PMA (Figures 1F, S3). Thus, we conclude from these experiments that Vav proteins are critical in LPS-induced oxidative burst in murine phagocytes, although individual Vav family members appear to be functionally redundant in this context.
As an extension of these findings, we observed that Vavnull BMDM also fail to generate ROIs in response to unopsonized, heat-killed E coli (Figure 2A). This does not appear to result from defective phagocytosis as Vavnull BMDMs internalize E coli with similar efficiency, as compared to WT BMDMs (Figure 2B-C). Thus, these data further highlight the importance of Vav in regulating oxidative burst triggered by Gram-negative bacteria.
Vav is required for ROI production in macrophages stimulated with E coli. (A) Oxidative burst was measured by lucigenin chemiluminescence in BMDMs stimulated with unopsonized, heat-killed E coli. Three doses of E coli (40, 80, and 160 million cells) were used to stimulate macrophages. (B) BMDMs were stimulated with CFSE-labeled E coli (40 million cells) for the indicated time points. Free bacteria that had not been phagocytosed by macrophages were washed away, and macrophages were stained with DAPI and mounted on slides for microscopic analysis. (C) Quantitation of the number of E coli internalized by macrophages at 30 minutes. Data represent the mean ± SD E coli ingested per macrophage. Fifty macrophages were scored.
Vav is required for ROI production in macrophages stimulated with E coli. (A) Oxidative burst was measured by lucigenin chemiluminescence in BMDMs stimulated with unopsonized, heat-killed E coli. Three doses of E coli (40, 80, and 160 million cells) were used to stimulate macrophages. (B) BMDMs were stimulated with CFSE-labeled E coli (40 million cells) for the indicated time points. Free bacteria that had not been phagocytosed by macrophages were washed away, and macrophages were stained with DAPI and mounted on slides for microscopic analysis. (C) Quantitation of the number of E coli internalized by macrophages at 30 minutes. Data represent the mean ± SD E coli ingested per macrophage. Fifty macrophages were scored.
Vav GEF activity is required for LPS-induced oxidative burst
Vav proteins regulate multiple downstream signaling pathways that may underlie the requirement for Vav in LPS-induced oxidative burst. Given the known requirement for Rac2 in NADPH oxidase induction,19,34 we tested the hypothesis that Vav family Rho-GEFs are necessary for Rac2 activation in response to LPS. To this end, we assayed for active GTP-loaded Rac2 in WT and Vavnull BMDMs and found that while WT cells show Rac2 activation in response to LPS, Vavnull BMDMs essentially fail to activate Rac2 under the same conditions (Figure 3A). To determine whether the intrinsic Vav GEF activity is indeed required for oxidative burst, Vavnull BMDMs were infected with retroviruses encoding GFP-tagged WT Vav1 (Vav1WT) or a Vav1 mutant lacking GEF activity (Vav1GEF-MUT), and ROI production was assayed in response to LPS. These experiments show that reconstitution of the expression of Vav1WT, but not Vav1GEF-MUT, in Vavnull BMDMs rescues LPS-induced ROI production (Figure 3B). These results indicate that MyD88-induced oxidative burst in phagocytes requires the catalytic activity of the Vav DH domain, which mediates GTP exchange on Rac2.
Vav regulates Rac2 activation in response to LPS, and Vav GEF activity is required for LPS-induced oxidative burst. (A) Vavnull and WT BMDMs were stimulated with LPS (10 μg/mL) for the indicated time points. Rac2 activation was assayed by pull-down of GTP-Rac2 from cell lysates with PBD-GST fusion protein, followed by Western blotting with antibodies specific to Rac2. Total cell lysates (TCLs) were analyzed for Rac2 content by Western blotting. Densitometry was performed to quantitate GTP-bound Rac2 normalized to total Rac2. (B) Vavnull BMDMs were reconstituted by retroviral transduction with WT Vav1 (Vav1WT), a GEF-inactive Vav1 (Vav1GEF-MUT), or GFP control vector. Cells were stimulated with LPS (10 μg/mL), and oxidative burst was determined by lucigenin chemiluminescence. Data represent the mean ± SEM RLU measured at 15 minutes for triplicate samples. Results are representative of 3 independent experiments.
Vav regulates Rac2 activation in response to LPS, and Vav GEF activity is required for LPS-induced oxidative burst. (A) Vavnull and WT BMDMs were stimulated with LPS (10 μg/mL) for the indicated time points. Rac2 activation was assayed by pull-down of GTP-Rac2 from cell lysates with PBD-GST fusion protein, followed by Western blotting with antibodies specific to Rac2. Total cell lysates (TCLs) were analyzed for Rac2 content by Western blotting. Densitometry was performed to quantitate GTP-bound Rac2 normalized to total Rac2. (B) Vavnull BMDMs were reconstituted by retroviral transduction with WT Vav1 (Vav1WT), a GEF-inactive Vav1 (Vav1GEF-MUT), or GFP control vector. Cells were stimulated with LPS (10 μg/mL), and oxidative burst was determined by lucigenin chemiluminescence. Data represent the mean ± SEM RLU measured at 15 minutes for triplicate samples. Results are representative of 3 independent experiments.
Requirement for Vav proteins in LPS-induced activation of p38 MAPK
Having established that Vav proteins are critical for LPS induction of oxidative burst, we next examined the requirement for Vav in the activation of p38 MAPK, which is thought to be dependent, at least in part, on ROI-induced ASK1 activity.33,35 As expected based on previously published results,36 MyD88−/− BMDMs show impaired p38 MAPK activation in response to LPS (Figure 4A). Strikingly, we find that Vavnull BMDMs also show defective activation of p38 in response to LPS, indicating that Vav is critical for the regulation of p38 MAPK downstream of MyD88 (Figure 4A). This defect is observed with various doses of LPS, as stimulation of Vavnull BMDMs with lower doses (0.1 μg/mL LPS) also fails to efficiently induce p38 phosphorylation (Figure 4B).
Vav is required for LPS-induced activation of p38 MAPK. (A) BMDMs from WT, Vavnull, and MyD88−/− mice were stimulated with LPS (10 μg/mL) for the indicated time points. Activation of p38 MAPK was assayed by probing of immunoblots with antibodies recognizing phosphorylated p38 (p-p38). Protein loading was verified by stripping and reprobing of blots with antibodies against total p38. (B) BMDMs from WT and Vavnull mice were stimulated with LPS (0.1 μg/mL), and activation of p38 MAPK was assayed as in panel A. (C) Unmanipulated WT and Vavnull BMDMs were treated with 1 μM H2O2 for the indicated time points, and p38 MAPK activation was assayed as in panel A. (D) BMDMs from WT and Vavnull mice were stimulated with LPS (10 μg/mL) and analyzed for activated ASK1 by Western blotting, with phosphospecific antibodies recognizing threonine residue 845 in murine ASK1 (pASK1). Equal protein loading was demonstrated by stripping and reprobing blots with anti-ERK2. Densitometry was performed in all panels to quantitate the indicated phosphorylated protein relative to total levels of the protein. Data are representative of 3 experiments.
Vav is required for LPS-induced activation of p38 MAPK. (A) BMDMs from WT, Vavnull, and MyD88−/− mice were stimulated with LPS (10 μg/mL) for the indicated time points. Activation of p38 MAPK was assayed by probing of immunoblots with antibodies recognizing phosphorylated p38 (p-p38). Protein loading was verified by stripping and reprobing of blots with antibodies against total p38. (B) BMDMs from WT and Vavnull mice were stimulated with LPS (0.1 μg/mL), and activation of p38 MAPK was assayed as in panel A. (C) Unmanipulated WT and Vavnull BMDMs were treated with 1 μM H2O2 for the indicated time points, and p38 MAPK activation was assayed as in panel A. (D) BMDMs from WT and Vavnull mice were stimulated with LPS (10 μg/mL) and analyzed for activated ASK1 by Western blotting, with phosphospecific antibodies recognizing threonine residue 845 in murine ASK1 (pASK1). Equal protein loading was demonstrated by stripping and reprobing blots with anti-ERK2. Densitometry was performed in all panels to quantitate the indicated phosphorylated protein relative to total levels of the protein. Data are representative of 3 experiments.
We hypothesized that if defective p38 MAPK activation in LPS-stimulated Vavnull BMDMs is due to a block in ROI production (Figure 1), the requirement for Vav should be bypassed by the introduction of exogenous ROIs. Indeed, treatment of Vavnull BMDMs with hydrogen peroxide leads to robust p38 MAPK activation, suggesting that the defects in p38 activation in LPS-activated Vavnull BMDMs may be due, at least in part, to defective ROI production (Figure 4C). Given a recent report implicating the redox-sensitive MAP3K, ASK1, in p38 activation by ROIs,33 we examined ASK1 phosphorylation in LPS-stimulated Vavnull BMDMs and found reduced levels of phospho-ASK1, as compared to WT (Figure 4D). Thus, Vav may play a role in a positive feedback loop in which ROI production leads to ASK1 and p38 activation that in turn activates ROI production by activation of NADPH oxidase components.29 Together, these data indicate that while Vav proteins are critical for ROI generation in response to LPS, p38 phosphorylation is controlled by both ROI-independent and ROI-dependent pathways.
Differential requirement for Vav in the activation of MAPK cascades and NFκB in response to LPS
TLR4 engagement by LPS induces several kinase cascades, including MAPKs (p38, ERK, and JNK), phospholipid-kinases (PI3Ks), phospholipid-dependent kinases (such as Akt), as well as IKK leading to NFκB activation.37,38 In particular, LPS stimulation leads to the rapid activation of Akt, presumably via PI3K,39 which in turn phosphorylates the p47phox subunit of NADPH oxidase.40,41 In this context, we find that Vavnull BMDMs show a severe impairment in the activation of Akt in response to LPS (Figure 5A), which may be the result of defective Rac activation and subsequent PI3K activity. Thus, Vav has the potential to regulate oxidative burst at several levels, including by activating Rac2 and p38 and also by regulating phosphoinositol production and Akt activation, which may then act in concert to promote NADPH oxidase function.
Vav is differentially required for activation of LPS-induced kinase cascades. WT and Vavnull BMDMs were stimulated with LPS (10 μg/mL) and lysed at the indicated time points. Lysates were separated by SDS-PAGE and analyzed by Western blotting for (A) phosphorylated Akt (p-Akt) and total Akt, (B) phosphorylated ERK1/2 (p-ERK1/2) and total ERK2, (C) phosphorylated JNK1/2 (p-JNK1/2) and total JNK2, and (D) IκBα. Results are representative of 3 or more experiments for each protein analyzed. Densitometry was performed to quantitate the indicated phosphorylated protein relative to total levels of the protein (A-C). In addition, total levels of IκBα were quantitated by densitometry (D).
Vav is differentially required for activation of LPS-induced kinase cascades. WT and Vavnull BMDMs were stimulated with LPS (10 μg/mL) and lysed at the indicated time points. Lysates were separated by SDS-PAGE and analyzed by Western blotting for (A) phosphorylated Akt (p-Akt) and total Akt, (B) phosphorylated ERK1/2 (p-ERK1/2) and total ERK2, (C) phosphorylated JNK1/2 (p-JNK1/2) and total JNK2, and (D) IκBα. Results are representative of 3 or more experiments for each protein analyzed. Densitometry was performed to quantitate the indicated phosphorylated protein relative to total levels of the protein (A-C). In addition, total levels of IκBα were quantitated by densitometry (D).
Having shown that Vav regulates Akt and p38 MAPK activation in response to LPS, we examined the ability of WT and Vavnull macrophages to activate ERK and JNK MAPKs. In the absence of Vav, ERK induction in response to various doses of LPS is attenuated and delayed (Figures 5B, S4). Strikingly, however, JNK activation in Vavnull cells is indistinguishable from WT (Figure 5C), indicating that Vav proteins are not required for the regulation of JNK by LPS stimulation. These results are consistent with observations that while ROIs contribute to the induction of p38, activation of JNK is independent of oxidant production33 (Figures 4, 5). Moreover, Vav proteins do not appear to be required for the activation of MyD88-dependent effector pathways leading to NFκB activation,37,42 since LPS-induced degradation of IκBα in Vavnull BMDM appears normal, which contrasts with MyD88−/− BMDMs (Figure 5D).
Vav is essential for normal regulation of cytokine production in response to LPS
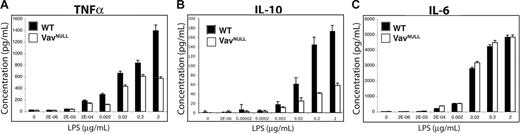
Downstream of TLR4, Vav proteins control effector pathways regulated by PI3K, p38, and ERK but not JNK and NFκB. Since these pathways control proinflammatory cytokine production,37 we next sought to determine whether Vavnull BMDMs exhibit defects in cytokine production. Indeed, Vavnull BMDMs stimulated with various doses of LPS reveal impaired production of TNF-α and IL-10, as compared to WT BMDM (Figure 6A-B). However, LPS-induced IL-6 production is comparable between WT and Vavnull BMDMs (Figure 6C). These data indicate a differential requirement for Vav proteins in the regulation of proinflammatory cytokine production by macrophages in response to endotoxin. In this regard, since Vav contributes to LPS-induced TNF-α but not IL-6 production, Vav may be involved in the regulation of IL-10 production either directly, such as by activating ERK or p38, or indirectly by promoting TNF-α production.
Dysregulated cytokine production in Vavnull BMDMs. WT and Vavnull BMDMs were starved overnight in serum-free medium and stimulated with the indicated concentrations of LPS for 24 hours. Supernatants were collected and analyzed for (A) TNF-α, (B) IL-6, and (C) IL-10 by cytometric bead array. Data shown are mean ± SD of triplicate samples and are representative of more than 5 independent experiments.
Dysregulated cytokine production in Vavnull BMDMs. WT and Vavnull BMDMs were starved overnight in serum-free medium and stimulated with the indicated concentrations of LPS for 24 hours. Supernatants were collected and analyzed for (A) TNF-α, (B) IL-6, and (C) IL-10 by cytometric bead array. Data shown are mean ± SD of triplicate samples and are representative of more than 5 independent experiments.
Vav is not essential for prostaglandin and RNI production in response to LPS
Given specific defects in endotoxin-induced responses of Vavnull BMDMs, we examined whether Vav is required for LPS induction of COX2 expression, which regulates the production of prostaglandins in macrophages.43 Surprisingly, we find that, following stimulation with LPS, expression of COX2 is similar in WT and Vavnull BMDMs (Figures 7A, S5). Thus, Vav regulates the production of inflammatory mediators such as cytokines yet is not required for COX2 up-regulation. Since the expression of COX2 is thought to be regulated in part by NFκB signaling,43 these results are consistent with our observation that Vav proteins are not required for NFκB activation by LPS (Figure 5D).
Vav is not required for COX2 or iNOS induction in response to LPS stimulation. (A) WT and Vavnull BMDMs were stimulated with LPS (10 μg/mL) and lysed at the indicated time points. Lysates were subsequently analyzed by Western blotting for COX2. Protein loading was verified by stripping and reprobing blots with antibodies against total ERK2. (B) WT and Vavnull BMDMs were stimulated with LPS (10 μg/mL), LPS and IFN-γ (50 U/mL), or LPS and PMA (50 ng/mL) for the indicated time points (18 hours, unless otherwise indicated). Cells were immediately lysed and analyzed by Western blotting for iNOS. Protein loading was verified by stripping and reprobing of blots with antibodies against total Erk-2. Densitometry was performed to quantitate iNOS and COX2 protein levels relative to total Erk-2. (C) BMDMs were stimulated with LPS (10 μg/mL) or LPS and IFN-γ (50 U/mL) for 18 hours. RNI production was analyzed by measuring nitrite content in culture supernatant using the Greiss reagent. For RNI experiments, data shown are mean ± SD of triplicate samples and are representative of 2 independent experiments. COX2 and iNOS expression was examined in more than independent experiments.
Vav is not required for COX2 or iNOS induction in response to LPS stimulation. (A) WT and Vavnull BMDMs were stimulated with LPS (10 μg/mL) and lysed at the indicated time points. Lysates were subsequently analyzed by Western blotting for COX2. Protein loading was verified by stripping and reprobing blots with antibodies against total ERK2. (B) WT and Vavnull BMDMs were stimulated with LPS (10 μg/mL), LPS and IFN-γ (50 U/mL), or LPS and PMA (50 ng/mL) for the indicated time points (18 hours, unless otherwise indicated). Cells were immediately lysed and analyzed by Western blotting for iNOS. Protein loading was verified by stripping and reprobing of blots with antibodies against total Erk-2. Densitometry was performed to quantitate iNOS and COX2 protein levels relative to total Erk-2. (C) BMDMs were stimulated with LPS (10 μg/mL) or LPS and IFN-γ (50 U/mL) for 18 hours. RNI production was analyzed by measuring nitrite content in culture supernatant using the Greiss reagent. For RNI experiments, data shown are mean ± SD of triplicate samples and are representative of 2 independent experiments. COX2 and iNOS expression was examined in more than independent experiments.
Reactive nitrogen intermediates (RNIs) generated by iNOS have antimicrobial properties and are critical for innate immune responses.44 Strikingly, we find that although Vavnull macrophages exhibit severe defects in ROI production, LPS-induced expression of iNOS and production of the RNI nitric oxide are comparable between WT and Vavnull macrophages (Figure 7B-C). These results indicate a differential requirement for Vav during activation of the ROI pathway and the RNI pathway. Thus, Vav proteins appear to transmit only a subset of signals emanating from MyD88-dependent receptors that control the production of proinflammatory mediators and antimicrobial compounds.
Discussion
Evolutionarily conserved bacterial components such as endotoxins and peptidoglycans promote inflammation by engaging TLRs on cells of the innate immune system.1,3 Although the inflammatory response is critical for the resolution of infection, severe pathology can arise in the host as a result of rampant TLR signaling in phagocytes.6 In this context, phagocytes produce inflammatory mediators such as cytokines and prostaglandins and antimicrobial compounds such as defensins, proteases, ROIs, and RNIs.45 ROIs, in particular, can cause severe tissue damage and can exacerbate numerous pathological conditions such as atherosclerosis7 and acute respiratory distress syndrome (ARDS).45
In phagocytes, the primary source of ROIs produced during oxidative burst is the NADPH oxidase complex containing Nox2 (gp91); however, phagocytes also express additional Nox family members that can generate ROIs in response to inflammatory stimuli.46 The mechanism of ROI production by phagocyte NADPH oxidase Nox2 has been relatively well defined and involves several points of regulation: GTP exchange on Rac2 as well as phosphorylation and phosphatidylinositol binding of cytosolic phox subunits.8 In the present study, we characterize the mechanism of MyD88-dependent oxidative burst and identify Vav proteins as critical activators for Rac, p38, and Akt, all of which have been implicated in the activation of NADPH oxidase Nox2 (gp91). However, since Rac is also implicated in the regulation of additional Nox homologues, it is possible that Vav may play a more global role in ROI production by Nox enzymes.
A variety of innate immune receptors expressed on phagocytes activate different GEFs capable of activating Rac2. Although Vav1 was implicated in oxidative burst in response to fMLP,32 2 recent reports identify P-Rex1 as the primary GEF for Rac2 in the context of fMLP-induced oxidative burst.20,21 Importantly, LPS-induced oxidative burst in neutrophils does not require P-Rex1.20 In this context, we demonstrate an essential requirement for Vav in LPS- or PGN-induced ROI production. Thus, oxidative burst is regulated by multiple Rac2-specific GEFs that can be differentially activated by various innate immune receptors.
As an important extension to our finding that Vav regulates MyD88-dependent oxidative burst, our data indicate that Vav proteins play a critical role in a ROI-p38 positive feedback loop. Specifically, Vav activity is required for the production of ROI, which is thought to activate the redox-sensitive MAP3K ASK1.33 In turn, ASK1 phosphorylates MKK3/6, leading to p38 activation.47 Signaling through this pathway is amplified and perpetuated by p38, which contributes to activation of NADPH oxidase,29 thus augmenting ROI production. Notably, JNK, MAPK, and NFκB do not require Vav to become activated in response to LPS stimulation. Thus, Vav is differentially involved in the control of specific pathways downstream of TLR4 that regulate inflammation.
Given the differential requirements for Vav proteins in TLR4-induced signaling cascades, it was not surprising that Vav is differentially required for macrophage cytokine production in response to LPS. Consistent with the defects in p38 and ERK1/2 activation that we observed, Vavnull macrophages stimulated with LPS produced less TNFα and IL-10 than WT macrophages. Our observation that IL-6 production was not compromised in response to LPS stimulation in Vavnull macrophages suggests that the defects in MAPKs were not sufficient to result in global cytokine defects, such as in MyD88-deficient macrophages. After the initial burst in production of inflammatory cytokines by LPS-stimulated macrophages, IL-10 is produced. Strikingly, Vavnull macrophages produced significantly less IL-10 in response to LPS than did WT macrophages, which is consistent with our previous observation that p38 activity was diminished in Vavnull macrophages stimulated with LPS. In fact, numerous reports have shown that inhibition of p38 results in impaired IL-10 production.48-51 In addition to regulating the production of inflammatory cytokines and ROS, LPS stimulation induces macrophages to express COX-2, which regulates prostaglandin production and iNOS, which generates RNI.43,52 Here, using mice lacking Vav1, Vav2, and Vav3, we clearly demonstrate that Vav proteins are not required for iNOS expression or nitric oxide production. In addition, LPS-induced COX2 expression appears unperturbed in Vavnull macrophages. Both these observations are consistent with the role for NFκB in transcriptional regulation of iNOS and COX-2,43,52 which appears to be regulated independently of Vav downstream of TLR4.
Our data indicate that the Vav family of Rho GEFs selectively regulates a subset of signals emanating from MyD88 and is differentially involved in the control of its downstream effector pathways. In this context, while we have previously shown that Vav proteins are implicated in integrin signaling and phagocytosis,53,54 here we provide evidence that Vav is specifically required for LPS- or PGN-induced production of ROI and respiratory burst but not for the production of RNI. In addition, Vav regulates LPS-induced inflammatory responses, such as cytokine production, but not COX-2 expression. Mechanistically, the selective effects of Vav on MAPK activation are likely responsible for the cytokine defects observed in Vavnull macrophages. However, our observations also delineate a MyD88-dependent pathway in which Vav acts as a GEF to activate Rac2 and the NADPH oxidase complex. By regulating the activity of MAPKs and Rac2 in response to LPS, Vav integrates diverse signaling pathways critical in innate immunity and inflammation.
Authorship
Contribution: A.V.M. and D.B.G. performed and designed research, contributed to data analysis, and wrote the manuscript. V.M. and B.W. performed experiments. K.F. provided vital reagents. T.K. and K.B. assisted with research. R.S. was involved in data analysis. R.X. contributed to data analysis and research design. W.S. designed research, performed data analysis, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A.V.M. and D.B.G. contributed equally to this study.
Correspondence: Wojciech Swat, Department of Pathology and Immunology, Washington University School of Medicine, 660 S Euclid Ave, St Louis, MO 63110; e-mail: swat@wustl.edu.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by National Institutes of Health grants AI06107703 and AI06302402 (W.S.).
We thank Dr Roberta Faccio for technical advice and Drs Marco Colonna and Daniel Schuster for critical reading of the manuscript and helpful discussions.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal