Abstract
Dendritic cells (DCs) are believed to regulate T cell-mediated immunity primarily by directing differentiation of naive T cells. Here, we show that a large fraction of CD4+ memory cells produce IL-10 within the first hours after interaction with plasmacytoid DCs (PDCs). In contrast, CD11c+ DCs induce IFN-γ and little IL-10. IL-10–secreting T cells isolated after 36 hours of culture with PDCs suppressed antigen-induced T-cell proliferation by an IL-10–dependent mechanism, but were distinct from natural and type 1 regulatory T cells. They proliferated strongly and continued to secrete IL-10 during expansion with PDCs, and after restimulation with immature monocyte-derived DCs or CD11c+ DCs. The IL-10–producing T cells acquired the ability to secrete high levels of IFN-γ after isolation and subsequent coculture with PDCs or CD11c+ DCs. Compared to CD11c+ DCs, PDCs were superior in their ability to selectively expand T cells that produced cytokines on repeated antigenic challenge. The DC-dependent differences in cytokine profiles were observed with viral recall antigen or staphylococcal enterotoxin B and were independent of extracellular type I interferon or IL-10. Our results show that DCs can regulate memory responses and that PDCs rapidly induce regulatory cytokines in effector T cells that can suppress bystander activity.
Introduction
Dendritic cells (DCs) have been thought to regulate T cell-mediated immunity primarily by directing differentiation of naive T cells. DCs sense the nature of an offending pathogen via pattern recognition receptors (PRRs).1,2 Engagement of PRRs triggers DC maturation and induces expression of costimulatory molecules and secretion of cytokines that determine T-cell cytokine production.3-5 During the first 4 to 6 divisions of naive T cells, the cytokine profile is imprinted by chromatin remodeling of relevant gene loci. These changes are transferred to daughter cells and perpetuated through the generation of long-lived memory T cells, which rapidly secrete the same cytokines on rechallenge with the antigen.6-8
The notion that cytokine profiles are determined at the level of naive T cells is supported by studies of cloned memory T cells and short-term cultures of naive T cells. Nevertheless, a substantial fraction of memory T cells remain unpolarized in vivo.8 Their undifferentiated state can be retained for several cell generations by manipulating the antigen dose or the cytokine environment.9,10 Even differentiated subsets such as effector T cells, show dual IL-4 and IFN-γ production, suggesting lack of full Th1 or Th2 polarization.11 Cells cloned under Th1-polarizing conditions can remodel chromatin and produce IL-4 when cultured under Th2 conditions.12 Freshly isolated memory T cells, moreover, show a wide range of thresholds for reactivation.13 Memory responses are therefore subject to extensive regulation.
Human blood contains 2 major subsets of DCs: plasmacytoid DCs (PDCs),14,15 which can be identified by their high levels of CD123,15 and CD11c+ DCs.16 The cells differ in their PRRs, cytokine profiles, and ability to induce polarization of naive T cells.3,17-20 CD11c+ DCs produce IL-12 and are potent inducers of Th1 cytokine secretion.21 Human PDCs do not secrete IL-1221 and can drive Th2 development in the absence of microbial PRR engagement.17 In response to viruses and TLR ligands, such as CpG DNA or dsRNA, PDCs produce large amounts of type I interferons and stimulate Th1 differentiation.22-28 Under certain conditions, or in specific anatomic locations, PDCs may have tolerogenic functions (for reviews, see Colonna et al18 and Tang and Bluestone29 ). Naive T cells activated by PDCs acquire features of certain regulatory T (Tr) cells,30,31 including the combined secretion of IFN-γ and IL-10, characteristic of so-called Tr1 cells.32,33 PDCs activated by CpG nucleotides induce CD25+FoxP3+ Tr cells from naive T cells.5 When stimulated by CTLA4-Ig or OX2(CD200)-Ig, PDCs produce indoleamine 2,3-dioxygenase, which may contribute to their tolerogenic potential.1,34 Furthermore, PDCs prevent sensitization to harmless antigens in a mouse asthma model.35 The presence of large numbers of PDCs in sterile human fetal lymph nodes suggested a role in tolerance development,15 and PDCs located in peripheral lymph nodes, but not spleen, were recently shown to mediate tolerance to vascularized grafts in a murine allograft transplantation model.36
PDCs and CD11c+ DCs accumulate at inflammatory foci.24,37,38 During recall responses, the DCs are likely to interact with memory T cells and could influence those that are not yet terminally differentiated. A recent study in mice suggests that PDCs may be more important as antigen-presenting cells (APCs) in recall responses than as inducers of naive T cells.39 Similar to CD8α+ DCs, PDCs had the ability to stimulate Th1 development of unpolarized memory T cells.39
In the present study, we investigated whether human PDCs and CD11c+ DCs activate memory T cells differently. Human cytomegalovirus (HCMV) was chosen as a recall antigen. This virus is well suited for studies of memory responses due to the high frequencies of antigen-specific T cells that exist in peripheral blood of healthy, seropositive individuals.40,41 In addition, responses to the polyclonal stimulus staphylococcal enterotoxin B (SEB) were investigated in isolated memory T cells. The results showed that PDCs and CD11c+ DCs induce different cytokine profiles in CD4+ memory T cells.
Materials and methods
Virus
A low passage (< 5) clinical isolate of HCMV (HCMV2006) was propagated in human embryonic fibroblasts and purified by gradient centrifugation, as previously described.42 Mock preparations were treated in the same way.
Cell isolation
This study was approved by the Regional Ethics Committee and written consent was obtained from subjects in accordance with the Declaration of Helsinki. Buffy coats were obtained from healthy blood donors or volunteers with a known HCMV status as determined by serology and polymerase chain reaction (PCR). Peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation (Lymphoprep; Axis-Shield, Oslo, Norway). PDCs and CD11c+ DCs were then isolated by BDCA-4 and BDCA-1 DC isolation kits (Miltenyi Biotec, Bergish Gladbach, Germany) to a purity of more than 98% as previously described.42
To generate immature monocyte-derived DCs (imoDCs), monocytes were isolated with CD14 Microbeads (Miltenyi Biotec) (> 96% pure) and cultured (106 cells/mL) for 7 days in RPMI containing 10% FCS, GM-CSF (100 ng/mL, Leukomax; Sandoz, Basel, Switzerland), and IL-4 (50 ng/mL, R&D Systems, Minneapolis, MN) as previously described.42
CD4+ T cells were isolated from PBMCs with CD4 Dynabeads in combination with Detachabead reagent (Dynal Biotech, Oslo, Norway) to more than 99% purity as determined by flow cytometry. CD45RA+ T cells and CD25+ T cells were isolated from purified CD4+ T cells by positive selection with CD45RA Microbeads and CD25 Microbeads, respectively (Miltenyi Biotec). The fractions were confirmed to express the expected phenotypes by flow cytometry, after staining with CD45RA FITC or CD25 PE (BD Biosciences, San Diego, CA), respectively. IL-10–producing T cells were isolated with the IL-10 Secretion Assay-Cell Enrichment and Detection Kit (PE), according to the manufacturer's recommendations (Miltenyi Biotec), to a purity of more than 98% and were confirmed negative for CD123 by flow cytometry.
DC–T-cell cocultures
For proliferation assays, purified CD4+ T cells were labeled with 1.5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) in protein-free, phospate-buffered saline (PBS). CFSE-labeled CD4+ T cells (105) were cultured in triplicate with either PDCs or CD11c+ DCs (104) in the presence of HCMV2006 (multiplicity of infection [MOI] of 5), mock, SEB (1 μg/mL, Sigma Chemical, St Louis, MO), or medium alone in round-bottomed 96-well culture plates for 6.5 days before harvesting. The medium for all cocultures was RPMI 1640 containing l-glutamine (PAA Laboratories, Pasching, Austria), 10% heat-inactivated pooled human serum (PS) seronegative for HCMV, 50 U/mL penicillin, and 50 μg/mL streptomycin (Sigma Chemical), referred to as RPMI 10% PS, supplemented with 10 ng/mL IL-3 (PeproTech, Rocky Hill, NJ).
For some cytokine studies, PDCs or CD11c+ DCs were cultured with either CD4+ T cells (105; 12-48 hours) or CFSE-labeled CD4+ T cells (105; 6.5-day primary culture followed by 16-hour restimulation) in triplicate in the presence of HCMV2006 (MOI = 5), mock, SEB (1 μg/mL), or medium only. For restimulation experiments, cells were washed on day 6, and imoDCs (104) and HCMV2006 (MOI = 5) or mock were added 16 hours before harvesting. Cytokine production depended on the addition of imoDCs and virus. Where indicated, neutralizing antibodies were added to the primary cultures, namely, anti–IL-10 (10 μg/mL; clone 12G8, American Type Culture Collection [ATCC], Manassas, VA), anti–IL-18 (clone 125-2H; MBL International, Woburn, MA), polyclonal anti–IL-1β (Ab9722; Abcam, Cambridge, United Kingdom), anti–IL-12 (clone 24910; R&D Systems Europe, Abingdon, Oxon, United Kingdom), or a combination of anti–IFN-α, anti–IFN-β, and anti–IFN-Rαβ (CD118; clone MMHAR-2), referred to as anti–type I IFN (all from PBL Biomedical Laboratories, Piscataway, NJ), at final concentrations of 5000 neutralization units/mL, 2000 neutralization units/mL, and 20 μg/mL, respectively.42 Blocking was confirmed by cytokine measurements in supernatants (see “Cytokine detection”).
Flow cytometry and T-cell proliferation
Cells were harvested and washed before surface immunostaining with monoclonal antibodies (mAbs; 30 minutes; 4°C). Accumulated T-cell proliferation was assessed by flow cytometry as the percentage of CFSElow cells among cells positive for CD3 conjugated to peridinin chlorophyll protein (PerCP; BD Biosciences), and negative for either CD11c conjugated to allophycocyanin (AlPC; BD Biosciences) or CD123 AlPC (Miltenyi Biotec). Dead cells were excluded on the basis of low values for forward and side scatter. Intracellular staining for Foxp3 protein was performed after initial cell surface staining with CD3 PerCP and CD25 AlPC (BD Biosciences), using anti–human FoxP3 PE included in the FoxP3 Staining Buffer Set (eBiosciences, San Diego, CA). All immunostained cells were analyzed with a FACSCalibur (BD Biosciences) flow cytometer.
Cytokine detection
Flow cytometric measurements of cytokines in culture supernatants were performed with Th1/Th2 (IL-10 and IFN-γ) or inflammation (IL-12p70) cytokine cytometric bead arrays (CBAs) and BD CBA software (BD PharMingen, San Diego, CA). Analyses of IL-1β and IL-10 were also performed using a 27-plex bead kit (Bio-Rad Laboratories, Hercules, CA) with a Bio-Plex Workstation (Bio-Rad Laboratories) according to the manufacturer's instruction. Secretion of IL-10 and IFN-γ was measured by flow cytometry on a single-cell basis with the IL-10 and IFN-γ secretion assays (Miltenyi Biotec) according to the manufacturer's recommendations. T cells were distinguished from DCs by scatter values and positive staining with CD3 PerCP (BD Biosciences). Enzyme-linked immunosorbent assay (ELISA) was used to measure levels of IFN-α (sensitivity 4.8 pg/mL) and instant ELISA was used to measure levels of IL-18 (Bender Medsystems, Vienna, Austria).
Restimulation of IL-10–producing cells and suppression assays
IL-10–producing T cells were immunomagnetically isolated from cocultures of CD4+ T cells and PDCs following 36 hours of SEB stimulation. After a 5-day resting period in RPMI 10% PS supplemented with IL-15 (2 ng/mL; PeproTech), the cells were restimulated with either autologous PDCs or CD11c+ DCs in the absence or presence of SEB for 48 hours. Cytokines were then measured in the culture supernatants with Th1/Th2 CBA. Isolated IL-10–producing cells were also added at graded doses to freshly isolated autologous CFSE-labeled PBMCs and incubated for 6.5 days in the absence or presence of SEB and neutralizing mAbs to IL-10 or TGF-β-1, clone 27235.1 (R&D Systems). Proliferation was assessed by flow cytometry among CD3+CD4+ T cells.
Statistics
The paired t test was performed using GraphPad Prism version 4.03 (GraphPad Software, San Diego, CA). Data are presented as mean values and standard error of the mean (SEM). A 2-tailed P value below .05 was considered significant.
Results
PDCs and CD11c+ DCs both induce extensive HCMV-dependent proliferation but different cytokine profiles in CD4+ memory T cells
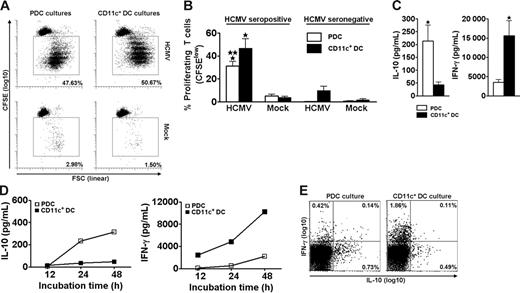
Freshly isolated CD4+ T cells proliferated extensively when cocultured for 6.5 days with autologous PDCs or CD11c+ DCs in the presence of live HCMV (Figure 1A). This response was observed for all 7 seropositive individuals tested (Figure 1B). Only negligible responses were observed for HCMV seronegative individuals (Figure 1B). These results showed that PDCs and CD11c+ DCs have a similar ability to present HCMV to memory T cells and that the experimental conditions primarily measured memory-associated activities.
PDCs and CD11c+ DCs both induce extensive HCMV-dependent proliferation but different cytokine profiles in CD4+ memory T cells. PDCs or CD11c+ DCs were cocultured with autologous CFSE-labeled purified CD4+ T cells in the presence of HCMV (MOI of 5) or mock, as indicated. On day 6.5, accumulated proliferation was measured by flow cytometry as the percentage of CFSElow cells, gated on CD3+CD123− (PDC cultures) or CD3+CD11c− (CD11c+ DC cultures) cells. A representative experiment is shown in panel A. Proliferation was HCMV-dependent in cells from HCMV seropositive individuals (A-B) and was lower in cocultures with PDCs than with CD11c+ DCs (B; n = 7, *P < .001 relative to mock, **P < .05 relative to CD11c+ DC cultures). Only minor responses were observed in seronegative individuals (B; n = 3). Supernatants from the cocultures described in panels A and B were harvested following stimulation with HCMV on day 6.5 (C), or at 12, 24, and 48 hours (D). Cytokines were measured with CBA. (C) The IL-10 levels were 8.8 ± 2.2-fold higher in cultures with PDCs than with CD11c+ DCs, whereas the amounts of IFN-γ were 5.7 ± 1.6-fold higher in the cocultures containing CD11c+ DCs as opposed to PDCs (n = 12, *P < .05). (D) IL-10 was detected at 24 hours in the PDC cocultures, whereas IFN-γ was detected at 12 hours in the CD11c+ DC cocultures. One representative experiment of 2 is shown. (E) Cytokine production was measured by flow cytometry at the single-cell level in CD3+ T cells from DC–T-cell cocultures 24 hours subsequent to HCMV stimulation. One representative experiment of 3 is shown. Means and SEM are indicated in panels B and C.
PDCs and CD11c+ DCs both induce extensive HCMV-dependent proliferation but different cytokine profiles in CD4+ memory T cells. PDCs or CD11c+ DCs were cocultured with autologous CFSE-labeled purified CD4+ T cells in the presence of HCMV (MOI of 5) or mock, as indicated. On day 6.5, accumulated proliferation was measured by flow cytometry as the percentage of CFSElow cells, gated on CD3+CD123− (PDC cultures) or CD3+CD11c− (CD11c+ DC cultures) cells. A representative experiment is shown in panel A. Proliferation was HCMV-dependent in cells from HCMV seropositive individuals (A-B) and was lower in cocultures with PDCs than with CD11c+ DCs (B; n = 7, *P < .001 relative to mock, **P < .05 relative to CD11c+ DC cultures). Only minor responses were observed in seronegative individuals (B; n = 3). Supernatants from the cocultures described in panels A and B were harvested following stimulation with HCMV on day 6.5 (C), or at 12, 24, and 48 hours (D). Cytokines were measured with CBA. (C) The IL-10 levels were 8.8 ± 2.2-fold higher in cultures with PDCs than with CD11c+ DCs, whereas the amounts of IFN-γ were 5.7 ± 1.6-fold higher in the cocultures containing CD11c+ DCs as opposed to PDCs (n = 12, *P < .05). (D) IL-10 was detected at 24 hours in the PDC cocultures, whereas IFN-γ was detected at 12 hours in the CD11c+ DC cocultures. One representative experiment of 2 is shown. (E) Cytokine production was measured by flow cytometry at the single-cell level in CD3+ T cells from DC–T-cell cocultures 24 hours subsequent to HCMV stimulation. One representative experiment of 3 is shown. Means and SEM are indicated in panels B and C.
The supernatants of day 6.5 cocultures contained high levels of IFN-γ and IL-10 (Figure 1C-D) but only low levels of IL-2, IL-4, and IL-5 (not shown). We were intrigued to observe that the contents of IL-10 and IFN-γ varied with the type of stimulating DC. Cocultures with PDCs (n = 12) contained on average 8.8-fold higher levels of IL-10 than those with CD11c+ DCs (Figure 1C). Conversely, cocultures with CD11c+ DCs (n = 12) produced on average 5.7-fold higher levels of IFN-γ than those with PDCs (Figure 1C). The cytokines were only detected when T cells and DCs were cocultured (not shown). Furthermore, only negligible levels were seen in cultures from HCMV seronegative individuals (n = 3, not shown), indicating memory T cell-dependent production. IL-10 was readily detected in supernatants from cocultures with PDCs as early as 24 hours after adding HCMV, whereas the kinetics for IFN-γ were slower (Figure 1D). The results were confirmed by 5 additional experiments with cytokine measurements at 48 hours. Measurements at the single-cell level moreover demonstrated mainly IL-10 single-positive T cells in the PDC cultures, and mainly IFN-γ+ T cells in the CD11c+ DC cultures at 24 hours (Figure 1E). The levels of IFN-α were high in cocultures with PDCs, as expected,42 whereas IL-12p70 was produced only in cocultures with CD11c+ DCs, as previously reported (n = 3, not shown).21 No IL-18 was detected, and only low levels of IL-1β were found in both cocultures (n = 3, not shown).
PDCs selectively expand T cells that produce cytokines on rechallenge with antigen
These results suggested that PDCs and CD11c+ DCs polarize memory responses similarly to what has been described for naive T cells.5,17,30,43 To further address this question, we stimulated and restimulated T cells with separate types of DCs. T cells loaded with CFSE were first cultured with PDCs or CD11c+ DCs and stimulated with HCMV to expand antigen-specific cells. On day 6, the cells were washed and imoDCs and HCMV were added for secondary stimulation. After 16 hours, extensive IL-10 production was observed in T cells expanded (ie, CFSElow cells) by PDCs, but not in those expanded by CD11c+ DCs (Figure 2A-B).
PDCs selectively expand T cells that produce cytokines on rechallenge with antigen. Cocultures of CFSE-labeled CD4+ T cells and PDCs or CD11c+ DCs were stimulated with HCMV in the absence or presence of neutralizing anti–IL-10 or anti–type I IFN, as indicated. On day 6, expanded T cells were restimulated for 16 hours with autologous imoDCs and HCMV. The production of IL-10 and IFN-γ was measured in CD3+ T cells with the cytokine secretion assay and was almost exclusively found in the CD3+ T cells that had divided (CFSElow), as shown for IL-10 in the upper plots of panel A. The lower 6 plots are gated on dividing cells, as indicated, and show the secretion of IL-10 and IFN-γ in one experiment representative of 4 performed. Percentages of single and double cytokine-producing cells are indicated. In panel B the results from 4 different experiments are shown, with means and SEM indicated. All cytokine-producing populations were higher in PDC-expanded as compared with CD11c+ DC-expanded cultures (*P < .05). Addition of neutralizing anti–IL-10 resulted in significantly higher levels of double cytokine-producing cells in the PDC cocultures (**P < .02), whereas the levels were nonsignificantly changed in the presence of anti–type I IFN. (C) Blocking of IL-12, IL-18, or IL-1β did not reduce the levels of IL-10 produced in PDC cocultures relative to control (stippled line), as measured in the culture supernatants at 48 hours following stimulation with HCMV. Two separate experiments are indicated by distinct symbols.
PDCs selectively expand T cells that produce cytokines on rechallenge with antigen. Cocultures of CFSE-labeled CD4+ T cells and PDCs or CD11c+ DCs were stimulated with HCMV in the absence or presence of neutralizing anti–IL-10 or anti–type I IFN, as indicated. On day 6, expanded T cells were restimulated for 16 hours with autologous imoDCs and HCMV. The production of IL-10 and IFN-γ was measured in CD3+ T cells with the cytokine secretion assay and was almost exclusively found in the CD3+ T cells that had divided (CFSElow), as shown for IL-10 in the upper plots of panel A. The lower 6 plots are gated on dividing cells, as indicated, and show the secretion of IL-10 and IFN-γ in one experiment representative of 4 performed. Percentages of single and double cytokine-producing cells are indicated. In panel B the results from 4 different experiments are shown, with means and SEM indicated. All cytokine-producing populations were higher in PDC-expanded as compared with CD11c+ DC-expanded cultures (*P < .05). Addition of neutralizing anti–IL-10 resulted in significantly higher levels of double cytokine-producing cells in the PDC cocultures (**P < .02), whereas the levels were nonsignificantly changed in the presence of anti–type I IFN. (C) Blocking of IL-12, IL-18, or IL-1β did not reduce the levels of IL-10 produced in PDC cocultures relative to control (stippled line), as measured in the culture supernatants at 48 hours following stimulation with HCMV. Two separate experiments are indicated by distinct symbols.
Surprisingly, significantly higher frequencies of cytokine-producing T cells were expanded by PDCs than by CD11c+ DCs. Among PDC-stimulated T cells, a large proportion produced high levels of both IL-10 and IFN-γ (Figure 2A-B). This cytokine profile is characteristic for so-called type 1 regulatory T (Tr1) cells, generated by culture of naive CD4+ T cells with IL-10 and IFN-α.32 However, addition of blocking antibodies to these cytokines during the first 6 days of coculture with PDCs neither prevented the generation of IL-10+IFN-γ+ T cells nor altered the DC-dependent differences in cytokine profiles (Figure 2A-B). In fact, the fraction of double-cytokine–producing cells was significantly increased when neutralizing IL-10 (Figure 2B). Additional experiments simultaneously neutralizing IL-10 and type I IFN did not show a reduction in the percentage of IL-10–producing T cells (single- or double-producing) in HCMV-stimulated PDC cocultures restimulated with imoDCs and HCMV (not shown, n = 3). Moreover, the IL-10 production was not inhibited in the presence of antibodies blocking IL-12, IL-1β, or IL-18 (Figure 2C). The cytokine production was detected almost exclusively in the cell population that had undergone division (ie, CFSElow cells), as shown for IL-10 (Figure 2A top). Analysis revealed that the fraction of IL-10–producing cells increased for each division, demonstrating that these antigen-experienced cells did not have a low proliferative capacity, in contrast to natural and certain adaptive Tr cells.32,44,45
Collectively, these data showed that the memory response to HCMV is highly dependent on the type of APC, and that PDCs have a prominent ability to expand antigen-specific T cells secreting a combination of proinflammatory and suppressive cytokines. The low frequencies of cytokine-producing T cells in the cultures containing CD11c+ DCs suggested that many of these T cells were bystanders.
A large fraction of memory T cells produce IL-10 but not IFN-γ within hours after interaction with freshly isolated PDCs and antigen
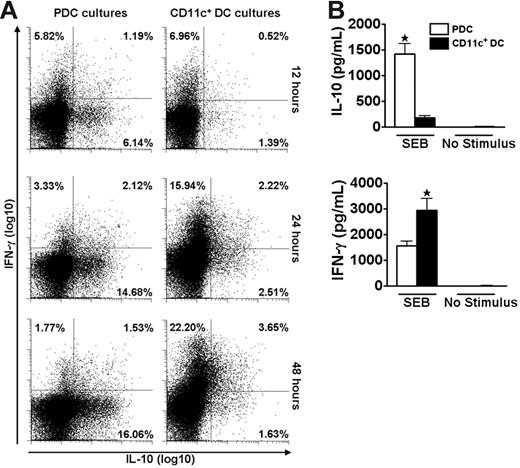
We speculated that the disparate cytokine profiles induced by PDCs and CD11c+ DCs could represent a general phenomenon independent of the antigenic stimulus. The polyclonal stimulus SEB was used to test this hypothesis and the striking DC-dependent difference in production of IL-10 and IFN-γ by memory T cells was maintained under this stimulatory condition (Figure 3). The use of a polyclonal stimulus further allowed reliable studies of DC–T-cell interactions at early time points. During the first 48 hours, responses to SEB were dominated by T cells producing either IL-10 or IFN-γ (Figure 3A). A large population of IL-10–producing cells was observed as early as 12 hours after coculture of freshly isolated T cells and PDCs. In contrast, cultures with CD11c+ DCs were dominated by IFN-γ–producing cells. These results were confirmed by measurements of cytokine contents in the medium (Figure 3B). No IFN-α was detected in the cocultures stimulated with SEB (n = 3, not shown).
PDCs and CD11c+ DCs induce different cytokine profiles in CD4+ T cells as early as 12 hours after SEB stimulation. Cocultures of CFSE-labeled CD4+ T cells and PDCs or CD11c+ DCs were stimulated with SEB. (A) At 12, 24, and 48 hours the secretion of IL-10 and IFN-γ was measured in CD3+CD123− (PDC cultures) or CD3+CD11c− (CD11c+ DC cultures) cells with the cytokine secretion assay. One experiment representative of 3 is shown. (B) IL-10 and IFN-γ were measured with CBA in supernatants harvested at 48 hours from the cocultures described in panel A. Levels of IL-10 were 14.2 ± 6.7-fold higher in cultures with PDCs than with CD11c+ DCs (n = 7, *P = .001), whereas the amounts of IFN-γ were 2.0 ± 0.2-fold higher in the cultures containing CD11c+ DCs as opposed to PDCs (n = 7, *P = .007). Means and SEM are indicated in panel B.
PDCs and CD11c+ DCs induce different cytokine profiles in CD4+ T cells as early as 12 hours after SEB stimulation. Cocultures of CFSE-labeled CD4+ T cells and PDCs or CD11c+ DCs were stimulated with SEB. (A) At 12, 24, and 48 hours the secretion of IL-10 and IFN-γ was measured in CD3+CD123− (PDC cultures) or CD3+CD11c− (CD11c+ DC cultures) cells with the cytokine secretion assay. One experiment representative of 3 is shown. (B) IL-10 and IFN-γ were measured with CBA in supernatants harvested at 48 hours from the cocultures described in panel A. Levels of IL-10 were 14.2 ± 6.7-fold higher in cultures with PDCs than with CD11c+ DCs (n = 7, *P = .001), whereas the amounts of IFN-γ were 2.0 ± 0.2-fold higher in the cultures containing CD11c+ DCs as opposed to PDCs (n = 7, *P = .007). Means and SEM are indicated in panel B.
To verify that PDCs induced IL-10 in memory T cells, isolated naive (CD4+CD45RA+) or memory (CD4+CD45RA−) T cells were cocultured with PDCs and SEB. Figure 4A shows a high frequency of IL-10–producing cells among CD45RA− memory cells. When taking into account the relative frequency of each cell type, more than 82% of IL-10–producing cells derived from the CD45RA− population. The results were verified by analysis of cell culture supernatants, showing that more than 90% of total IL-10 produced was derived from the CD45RA− population (Figure 4B). Cytokine production was SEB-dependent, and at 48 hours there was no cell proliferation in the cultures (not shown). The activated cells, however, showed extensive proliferative capacity, with similar differences observed between the cocultures on day 6 as for HCMV (56%/42% proliferating T cells in PDC cocultures versus 88%/74% in CD11c+DC cocultures, respectively, n = 2). Collectively, these results showed that freshly isolated PDCs rapidly stimulate a large fraction of memory T cells to produce IL-10 and that this ability is not shared by CD11c+ DCs.
PDC-induced IL-10 is almost exclusively produced by CD4+CD45RA− T cells. CD4+ T cells were immunomagnetically separated into CD45RA+ and CD45RA− fractions, combined with PDCs, and then compared as to SEB-induced cytokine production at 36 hours by 2 different methods. (A) IL-10 and IFN-γ were measured with the cytokine secretion assay. One of 2 similar experiments is shown. (B) IL-10 was measured in the supernatants with CBA, and the contribution of IL-10 from each subset in percentage of total IL-10 was calculated, taking into account the relative frequency of the 2 cell populations. Two separate experiments are shown, as indicated by distinct symbols.
PDC-induced IL-10 is almost exclusively produced by CD4+CD45RA− T cells. CD4+ T cells were immunomagnetically separated into CD45RA+ and CD45RA− fractions, combined with PDCs, and then compared as to SEB-induced cytokine production at 36 hours by 2 different methods. (A) IL-10 and IFN-γ were measured with the cytokine secretion assay. One of 2 similar experiments is shown. (B) IL-10 was measured in the supernatants with CBA, and the contribution of IL-10 from each subset in percentage of total IL-10 was calculated, taking into account the relative frequency of the 2 cell populations. Two separate experiments are shown, as indicated by distinct symbols.
PDCs induce IL-10 production in CD4+CD25− T cells and expand T cells that express intermediate levels of FoxP3
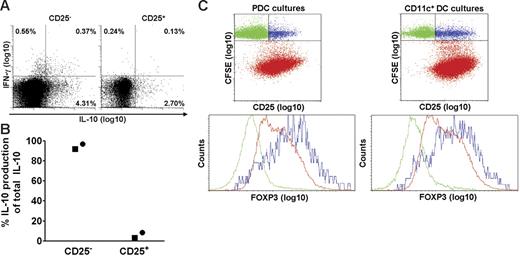
Human blood contains natural CD4+CD25+ Tr cells that produce IL-10,45-47 and we investigated whether PDCs selectively activated this subset. The results obtained after SEB stimulation of isolated CD25+ and CD25− T-cell subsets showed, on the contrary, that most of the IL-10–producing cells belonged to the CD25− subset (Figure 5A). When considering that the frequency of CD25+ cells among the CD4+ T cells was less than 10%, the calculated proportion of IL-10–producing cells that were CD25− prior to activation, was at least 92%. The results were confirmed by cytokine measurements in culture supernatants, as more than 92% of the IL-10 production was maintained after depleting for CD25+ cells (Figure 5B). Induction of cytokines depended on addition of stimulus. Therefore, PDC-stimulated IL-10 production in memory T cells did not reflect selective activation of natural Tr cells.
PDCs induce IL-10 in CD4+CD25− T cells and expand T cells expressing intermediate levels of FoxP3. CD4+ T cells were separated into CD25+ and CD25− fractions, combined with PDCs, and compared with regard to SEB-induced cytokine production at 36 hours. (A) IL-10 and IFN-γ were measured with the cytokine secretion assay. One of 2 similar experiments is shown. (B) IL-10 was measured in the supernatants with CBA, and the relative contribution of IL-10 from each subset is shown. Two separate experiments are indicated by distinct symbols. (C) PDCs or CD11c+ DCs were cocultured with autologous CFSE-labeled CD4+ T cells in the presence of HCMV (MOI of 5), and intracellular expression of FoxP3 was measured on day 6.5. The upper 2 plots are gated on CD3+ cells. The lower plots are gated on T cells that have not proliferated (CFSEhigh), being CD25− (green) or CD25+ (blue), or on cells that have proliferated (CFSElow; red). One representative experiment of 3 is shown.
PDCs induce IL-10 in CD4+CD25− T cells and expand T cells expressing intermediate levels of FoxP3. CD4+ T cells were separated into CD25+ and CD25− fractions, combined with PDCs, and compared with regard to SEB-induced cytokine production at 36 hours. (A) IL-10 and IFN-γ were measured with the cytokine secretion assay. One of 2 similar experiments is shown. (B) IL-10 was measured in the supernatants with CBA, and the relative contribution of IL-10 from each subset is shown. Two separate experiments are indicated by distinct symbols. (C) PDCs or CD11c+ DCs were cocultured with autologous CFSE-labeled CD4+ T cells in the presence of HCMV (MOI of 5), and intracellular expression of FoxP3 was measured on day 6.5. The upper 2 plots are gated on CD3+ cells. The lower plots are gated on T cells that have not proliferated (CFSEhigh), being CD25− (green) or CD25+ (blue), or on cells that have proliferated (CFSElow; red). One representative experiment of 3 is shown.
Natural Tr cells, but also certain adaptive Tr cells, express the transcription factor FoxP3.48,49 We compared the expression of FoxP3 in T cells activated by PDCs and CD11c+ DCs, respectively (Figure 5C), and found in both cocultures that this marker was selectively expressed by the CD25+ subset among T cells that had not undergone division (ie, CFSEhi; Figure 5C blue dots). These cells most likely corresponded to natural Tr cells, thus attesting to the specificity of the immunostaining. FoxP3 was also detected in HCMV-stimulated T cells that had undergone division (ie, CFSElo; Figure 5C red dots) during coculture with PDCs or CD11c+ DCs. Here, however, the expression levels were intermediate, and no differences were observed between the 2 cocultures (Figure 5C). Thus, differential IL-10 production did not reflect selective induction of FoxP3+ Tr cells by PDCs.
IL-10–secreting T cells are activated both by PDCs and CD11c+ DCs and acquire the ability to produce IFN-γ during culture
These results showed that PDCs induce rapid IL-10 production in memory T cells. Furthermore, the DC-dependent T-cell cytokine profiles were maintained during a second round of stimulation with imoDCs (Figure 2), which could suggest that a particular DC imprints lasting skewing of the cytokine production. However, the experiments did not exclude the possibility that PDCs selectively activate an IL-10–producing subpopulation of memory T cells that is not engaged by CD11c+ DCs.
To address this question, we sorted IL-10–secreting T cells following activation with PDCs and determined whether CD11c+ DCs could activate these T cells. The purified IL-10–producing cells were first rested for 5 days, and CD11c+ DCs or PDCs were then added together with SEB. The 2 DC subsets were equally effective in stimulating the selected T cells (Figure 6); regardless of the DC type used for restimulation, the T cells produced high levels of IL-10 and IFN-γ in response to SEB. These data showed that CD11c+ DCs effectively activated the IL-10–secreting memory T cells. However, these experiments could not exclude the possibility that CD11c+ DCs can only stimulate the IL-10–producing T-cell subpopulation subsequent to primary interaction with PDCs, potentially altering the T cells. The high production of IL-10 observed during restimulation with CD11c+ DCs suggested that PDCs leave a lasting imprint on the memory T-cell cytokine profile during the first 36 hours of activation. However, as the cells are cultured they acquire the ability to produce IFN-γ.
IL-10–secreting T cells are activated both by PDCs and CD11c+ DCs and acquire the ability to produce IFN-γ during culture. IL-10–producing cells were immunomagnetically isolated from cocultures of CD4+ T cells and PDCs following 36 hours of SEB stimulation. After a 5-day resting period, the cells were restimulated for 48 hours with autologous PDCs or CD11c+ DCs in the absence or presence of SEB, as indicated. IL-10 and IFN-γ were measured in the supernatants with CBA. Two separate experiments are shown, indicated by distinct symbols.
IL-10–secreting T cells are activated both by PDCs and CD11c+ DCs and acquire the ability to produce IFN-γ during culture. IL-10–producing cells were immunomagnetically isolated from cocultures of CD4+ T cells and PDCs following 36 hours of SEB stimulation. After a 5-day resting period, the cells were restimulated for 48 hours with autologous PDCs or CD11c+ DCs in the absence or presence of SEB, as indicated. IL-10 and IFN-γ were measured in the supernatants with CBA. Two separate experiments are shown, indicated by distinct symbols.
IL-10–producing memory T cells suppress proliferation of autologous PBMCs in an IL-10–dependent manner
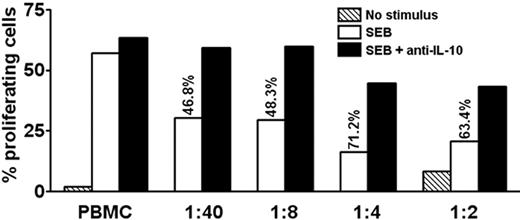
We examined whether the levels of IL-10 secreted by PDC-activated T cells were sufficient to suppress T-cell proliferation. Isolated IL-10–producing cells were added to autologous PBMCs. As few as a 1000 cells suppressed SEB-induced proliferation of CD3+CD4+ T cells by 46.8% in day 6 cultures (Figure 7). This inhibition was reversed by addition of antibodies neutralizing IL-10 (Figure 7) but not TGF-β (not shown). Thus, the levels of IL-10 secreted by these cells were sufficient to inhibit the antigen-presenting function of PBMCs.
IL-10–producing memory T cells suppress proliferation of autologous PBMCs in an IL-10–dependent manner. CFSE-labeled PBMCs were stimulated with SEB or medium only in the absence or presence of graded amounts of purified autologous IL-10–producing cells, followed by culture for 6.5 days. The ratio of added IL-10–producing cells to PBMCs is indicated. Adding IL-10–producing cells caused inhibition, as indicated in percentage of original proliferation. Where indicated, neutralizing antibodies to IL-10 were added at the beginning of the experiment, which preserved most of the proliferation. Proliferation was assessed by flow cytometry as CFSElow cells in percentage of total CFSE+CD3+CD4+ T cells. One representative experiment of 3 is shown.
IL-10–producing memory T cells suppress proliferation of autologous PBMCs in an IL-10–dependent manner. CFSE-labeled PBMCs were stimulated with SEB or medium only in the absence or presence of graded amounts of purified autologous IL-10–producing cells, followed by culture for 6.5 days. The ratio of added IL-10–producing cells to PBMCs is indicated. Adding IL-10–producing cells caused inhibition, as indicated in percentage of original proliferation. Where indicated, neutralizing antibodies to IL-10 were added at the beginning of the experiment, which preserved most of the proliferation. Proliferation was assessed by flow cytometry as CFSElow cells in percentage of total CFSE+CD3+CD4+ T cells. One representative experiment of 3 is shown.
Discussion
The present study shows that PDCs and CD11c+ DCs regulate immune responses at the level of memory T cells. During 6 days of culture with PDCs and HCMV, T cells from seropositive donors proliferated extensively and developed into cells producing both IL-10 and IFN-γ. In contrast, HCMV-specific T cells expanded by CD11c+ DCs produced IFN-γ and only low amounts of IL-10. The measured responses reflected activation of memory cells because cells from seronegative donors were unresponsive. This was confirmed by demonstrating similar cytokine patterns when purified memory T cells were stimulated with the 2 DC subsets and SEB. Differences in cytokine profiles were observed within hours of coculture of freshly isolated cells and could therefore not be explained on the basis of DC exhaustion. Finally, the differences were maintained when T cells were rechallenged with different DC types. Our results therefore indicate a difference in the way PDCs and CD11c+ DCs activate memory T cells and suggest that the initial interaction can leave a lasting imprint on cytokine production similar to that observed in naive T cells.
Previous studies have shown that naive T cells produce IL-10 when activated for 6 to 8 days in the presence of PDCs.5,17,25,33 Early observations suggested that this reflected a specialized function for PDCs as Th2 inducers, because IL-3–stimulated PDCs induced a complete set of Th2 cytokines.17,43 Later, PDCs were found to induce Th1 development in the presence of viruses or ligands for TLR7 and TLR9. Remarkably, these T cells also secreted IL-10.5,25,33,43 Combined secretion of IL-10 and IFN-γ has been coined as a characteristic of Tr1 cells.32 Two recent studies showed that naive T cells acquire several features of Tr1 cells when activated by PDCs, including hyporesponsiveness and the ability to suppress T-cell activation.5,33 One of the studies also reported high levels of the Tr-associated transcription factor FoxP3.5 In contrast, the IL-10 secretion in memory T cells observed here was not secondary to Tr development or Th2 differentiation. The memory T cells activated by PDCs in response to live virus or a polyclonal stimulus proliferated strongly, and their capacity to produce cytokines increased during proliferation (Figure 2). Furthermore, the cells were CD25− when encountering the PDCs, expressed only intermediate levels of FoxP3 after coculture with PDCs and, unlike Tr1 cells, produced IL-10 independently of exogenous IL-10 or IFN-α. Finally, they acquired the ability to secrete large amounts of IFN-γ, but this was not secondary to the production of IL-12, which is important for the generation of IL-10+IFN-γ+ T cells in persistent infections,50 or to the production of IL-1β or IL-18.
The rapid kinetics of the response show that a large number of memory T cells are capable of immediate IL-10 secretion. Regardless of the mechanism, the interaction leaves a lasting imprint; the CD4+ T cells continued to secrete IL-10 when isolated after 36 hours and rechallenged by CD11c+ DCs or imoDCs after multiple divisions in culture (Figures 2 and 6). It is tempting to speculate that a similar mechanism operates in naive cells, because also a proportion of the CD45RA+ cells rapidly secreted IL-10 in response to PDCs and SEB (Figure 4A). Previously published results showing that Tr development of naive CD8 cells was completely inhibited when IL-10 was blocked, supports the view that IL-10 induction precedes Tr development.30 Collectively these results suggest that PDCs have an intrinsic ability to induce immediate IL-10 production in T cells. In naive T cells this might lead to anergy and Tr development, whereas memory T cells expand and retain effector functions.
The different kinetics of IL-10 and IFN-γ induction could point to a mechanism by which PDCs regulate recall responses. PDCs accumulate at inflammatory sites,24,51,52 where they play an important role in the first line of defense against viruses by secreting large amounts of type I interferon.22-26 Accordingly, memory T cells are likely to encounter large numbers of PDCs at sites of viral infection. Our results suggest that most activated T cells produce IL-10, whereas only little IFN-γ is secreted during the early phases of this response. IL-10 is a potent regulator of DC maturation and function, effectively suppressing upregulation of costimulatory molecules, secretion of proinflammatory cytokines, such as IL-12 and TNF-α, and the ability to stimulate T cells.53-55 Thus, secretion of IL-10 may result in a general inhibition of antigen presentation, resulting in an elevated threshold for T-cell activation, allowing memory T cells with high receptor affinity to be selectively activated. This idea is supported by our data, showing that T cells stimulated in the presence of PDCs were more responsive to secondary stimulation than cells cocultured with CD11c+ DCs (Figure 2).
A study performed in mice showed that naive T cells failed to proliferate in response to antigen presented on virally activated PDCs. In contrast, antigen-specific memory T cells could be fully activated by the very low levels of MHC-peptide complexes present on unstimulated PDCs.39 Our results demonstrating a delayed IFN-γ response in memory T cells activated by PDCs as compared with CD11c+ DCs could reflect requirements for maturation signals that license PDCs for IFN-γ induction. Viral components that engage TLR7 or TLR9 induce maturation of PDCs and production of IFN-α.22-26 Ligands expressed by activated T cells, such as CD40L, might be equally important and sufficient since SEB induced secretion of IFN-γ in PDC-activated T cells but not IFN-α in PDCs. This view is supported by the results showing that murine unstimulated PDCs were able to induce Th1 development of T cells that had recently been expanded by antigenic stimulation.39
Thus, PDCs might selectively activate high-affinity memory T cells, which initially secrete IL-10. Rather than inducing anergy in the antigen-specific memory T cells, continuous secretion of IL-10 by PDC-activated T cells could serve to limit bystander activation as the levels of proinflammatory cytokines rise during the immune response. Once induced by the PDCs, the memory T cells might in turn license the PDCs to activate T-cell production of IFN-γ. At this point in the immune response the antigen-specific T cells have a proliferative advantage over bystander T cells. Rapid secretion of IFN-α by PDCs is likely to serve a similar purpose as IL-10. A recent study showed that antigen-specific CD8+ cells escaped the antiproliferative effects of type I interferons by TCR-mediated down-regulation of STAT1.56 Thus, PDCs may regulate recall responses both directly by secreting IFN-α and indirectly by inducing IL-10 production in T cells.
Previous studies have shown that memory T cells can be maintained in an unpolarized state for prolonged periods.8-10 Yet, the frequency of unpolarized cells among memory T cells that recognize human recall antigens is largely unknown. Our results show that a large number of HCMV-specific T cells can be polarized to produce IL-10. The high frequency of HCMV-specific T cells is probably related to repeated stimulation during multiple events with subclinical reactivation of the virus.57,58 The fact that these cells can be induced to produce IL-10 by PDCs suggests that the ability to polarize memory T cells extends even to highly antigen-experienced cells.
Altogether, our study demonstrates that established immune responses can be manipulated by differential engagement of DCs. This may have important implications for understanding and treating hypersensitivity and autoimmunity. The frequency of memory T cells is several orders of magnitude higher than that of naive precursors. Development of Tr cells from naive cells may be limited by induction of a nonproliferative anergic state in the T cells. In contrast, PDCs do not induce anergy in memory T cells, but rather allow them to expand and differentiate into antiviral Th1 effector cells with the potential to suppress bystander activation in an IL-10–dependent manner. Thus, PDCs may effectively secure antimicrobial responses as well as limit autoimmunity and tissue damage. Our results thus point to a novel homeostatic role for PDCs in adaptive immune responses.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Espen O. Kvale, Laboratory for Immunohistochemistry and Immunopathology, Institute of Pathology, University of Oslo, Rikshospitalet-Radiumhospitalet Medical Center, N-0027 Oslo, Norway; e-mail: e.o.kvale@medisin.uio.no; and Johanna Olweus, Institute of Immunology, University of Oslo, Rikshospitalet-Radiumhospitalet Medical Center, N-0027 Oslo, Norway; e-mail: johanna.olweus@medisin.uio.no.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the University of Oslo, the Research Council of Norway, and Medinnova.
The authors are greatly indebted to Solveig Beck for providing excellent technical assistance.
S.G. is an employee of BD Biosciences.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal