Abstract
Bruton tyrosine kinase (Btk) and phospholipase Cγ2 (PLCγ2) are 2 key molecules involved in B-cell receptor (BCR) signaling. Biochemical studies have placed them in a linear signaling pathway, with Btk acting upstream of PLCγ2. Consistent with this, mice lacking either molecule display a leaky but similar block in B-cell development. Here, we generated Btk−/−PLCγ2−/− mice and showed that combined deficiencies in Btk and PLCγ2 severely arrested B lymphopoiesis at the large pre–B-cell stage. In contrast to either single mutant, Btk−/−PLCγ2−/− pre–B cells expressed high levels of pre-BCR on their cell surfaces and exhibited reduced immunoglobulin light chain gene rearrangements. Pre-BCR–induced calcium signaling was also drastically compromised in Btk−/−PLCγ2−/− pre–B cells compared with wild-type and single-mutant cells. Interestingly, immunoglobulin heavy chain allelic exclusion remained intact in the absence of Btk and PLCγ2. Overall, our results suggest that Btk and PLCγ2 have combinatorial roles in regulating pre–B cell differentiation.
Introduction
Proper expression of a functional pre–B-cell receptor (pre-BCR) represents an important checkpoint in early B-cell development.1,2 The pre-BCR is made up of the immunoglobulin (Ig) heavy (H) chain and the surrogate light (L) chains λ5 and VpreB, and associates with the signal-transducing subunits Igα and Igβ.3 The critical role played by the pre-BCR in B-cell maturation is evident in various gene knock-out studies, in which disruptions of the structural components of the pre-BCR complex, such as the μ H chain,4 surrogate L chains,5,6 and Igα7 or Igβ,8 severely disrupt pre–B-cell transition. In later stages of B-cell differentiation, the pre-BCR is replaced by the BCR, which is composed of the same Ig H chain but has the Ig L chain replacing λ5 and VpreB
Signals propagated by the pre-BCR, together with those triggered by cytokines in the bone marrow milieu, are essential for pre–B-cell transition. Biochemically, the cross-linking of pre-BCR/BCR activates multiple downstream signaling molecules, among them the cytoplasmic tyrosine kinases, Syk, Lyn, and Bruton tyrosine kinase (Btk), as well as the adaptor protein B-cell linker (BLNK) and phospholipase Cγ2 (PLCγ2).9,10 Following the engagement of BCR, Syk phosphorylates BLNK, which in turn recruits Btk and PLCγ2 into a macromolecular complex.11 One of the important biochemical outcomes of the formation of this complex is the activation of PLCγ2 by Syk and Btk, which leads to the production of second messengers, inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). As a result, pre-BCR/BCR proximal signaling events are translated into calcium (Ca2+) mobilization and the activation of protein kinase C (PKC), which lead subsequently to the activation of nuclear factor-κB (NF-κB).12
The importance of the Syk/BLNK/Btk/PLCγ2 complex (also known as the BCR signalosome) in B lymphopoiesis is underscored by studies of mice lacking each of these molecules.13 Syk−/− mice suffered from embryonic lethality and a block in early B-cell development at the pro–B cell stage.14,15 BLNK−/−, Btk−/−, and PLCγ2−/− mice had milder but similar xid-like phenotypes,13 namely a reduction in peripheral B-cell population, absence of CD5+ B-1 cells, and a failure to respond to T-cell–independent type II antigens. However, compared with Btk−/− and PLCγ2−/− mice, BLNK−/− mice appeared to have more severe defects in early B-cell development. In particular, they had a significant fraction of cells expressing surface pre-BCR,16,17 a phenomenon that was not reported in wild-type, Btk−/−, or PLCγ2−/− mice.
The more severe phenotype of Syk−/− mice is consistent with the idea that Syk is proximal in the pre-BCR/BCR signal transduction hierarchy and could be involved in more branches of signaling pathways. The milder phenotypes of BLNK−/−, Btk−/−, and PLCγ2−/− mice indicate that these proteins are downstream of Syk and could act along a linear signaling pathway.13 However, recent studies indicate that these molecules might have additional independent functions during pre-BCR/BCR signaling, as Btk−/−BLNK−/−,18 and BLNK−/−PLCγ2−/−19 mice had a more severe B-cell developmental arrest at the pre–B-cell stage compared with each single mutants. These double mutants raise the possibility that Btk and PLCγ2 could also participate in signaling pathways independent of BLNK and probably outside of the BCR signalosome.
Biochemical studies have placed Btk upstream of PLCγ2 in the signaling cascade because Btk can directly phosphorylate PLCγ2.20,21 Btk can also activate phosphatidylinositol-4-phosphate 5-kinase (PIP5K), thereby generating PI(4,5)P2, which is the substrate for PLCγ2.22 Both Btk−/− and PLCγ2−/− B cells exhibit defective Ca2+ flux and NF-κB activation.23-28 Moreover, detailed analyses of Btk−/− and PLCγ2−/− mice have indicated that these 2 mutants are much more similar to each other than to BLNK−/− mice, namely a mild defect in early B-cell differentiation and lack of surface pre-BCR–expressing cells.24,25,29-31 Thus, it remains to be determined whether Btk and PLCγ2 could also participate in signaling pathways independent of each other during B-cell development.
In this report, we investigated the effect of combined deficiencies in Btk and PLCγ2 on early B-cell development. Strikingly, B-cell development in Btk/PLCγ2 double-mutant mice was severely arrested at a surface pre-BCR+ large pre–B-cell stage, and these mutant B cells have more drastic impairment of pre-BCR–induced Ca2+ signaling. Thus, our results provided genetic evidence that Btk and PLCγ2 could also participate in nonoverlapping and independent signaling pathways outside of their roles in the BCR signalosome, and cooperatively signal pre–B-cell transition.
Materials and methods
Mice
BLNK−/−,32 PLCγ2−/−,24 and VHB1-8 knock-in33 mice were described previously. Btk−/− mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Btk−/−PLCγ2−/− mice were generated by crossing Btk−/− and PLCγ2−/− mice. All mice were predominantly in the C57BL/6 background and used between 6 to 12 weeks of age in accordance to national guidelines.
Flow cytometry and cell sorting
Cell preparation and flow cytometry were performed as described.32 The following antibodies were purchased from BD PharMingen (San Diego, CA): anti-Igκ, anti-Igλ, anti-preBCR, anti-IgM, anti-CD25, anti-CD43, anti-B220, and anti–MHC II. For cell-cycle analysis, bone marrow cells were incubated at 37°C for 90 minutes with Hoechst 33342 (Molecular Probes, Eugene, OR) in DMEM containing 2% FCS and analyzed on a LSR II flow cytometer equipped with a UV laser (Becton Dickinson,). To purify CD19+IgL− cells, bone marrow cells were stained with biotin–anti-Igκ and anti-Igλ antibodies and later, incubated with streptavidin-conjugated magnetic beads before passage through a magnetic column (Miltenyi Biotec, Bergisch Gladbach, Germany). Unbound cells were further positively selected with anti-CD19 magnetic beads. B220+IgL− pre-BCR− bone marrow cells were sorted on a FACSVantage flow cytometer (Becton Dickinson, San Jose, CA) from mice of different genotypes except for Btk−/−PLC−/− mice, in which both pre-BCR+ and pre-BCR− cells were purified.
Examination of immunoglobulin gene rearrangement and κ germ-line transcription
In vitro culture of pre–B cells and analysis of intracellular calcium flux
Purified CD19+IgL− bone marrow cells were cultured for up to 14 days in OptiMEM (Invitrogen, Carlsbad, CA) medium containing 10% FCS, 100 U/mL penicillin-streptomycin, 2 mM l-glutamine, 50 μM β-mercaptoethanol, and 1 to 5 ng/mL recombinant IL-7 (R&D Systems, Minneapolis, MN). For analysis of calcium signaling, cells were loaded with Indo-1 am (2 μM; Molecular Probes) as described previously,36 followed by staining for B220 and Igκ/λ expression. Calcium flux in gated B220+Igκ−/λ− cells was monitored on a LSR II flow cytometer in real time for 10 minutes after stimulation with different doses of goat anti–mouse Igμ F(ab′)2 antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA).
Immunoblotting
Bone marrow–derived pre–B cells were expanded in 5 ng/mL IL-7 for 8 to 12 days. After exclusion of dead cells using Ficoll-Paque PLUS (Amersham Biosciences, Uppsala, Sweden), 5 × 106 cells were resuspended in OptiMEM medium and incubated with 10 μg/mL goat anti–mouse Igμ F(ab′)2 antibody at 37°C for various periods of time. Western blotting was performed as described.19 Anti–phospho-PLCγ1 (Y783) and anti–phospho-PLCγ2 (Y759) antibodies were obtained from Cell Signaling Technology (Beverly, MA); all other antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Results
Btk/PLCγ2 double-deficient mice exhibit more severe B-cell developmental defects compared with single mutants
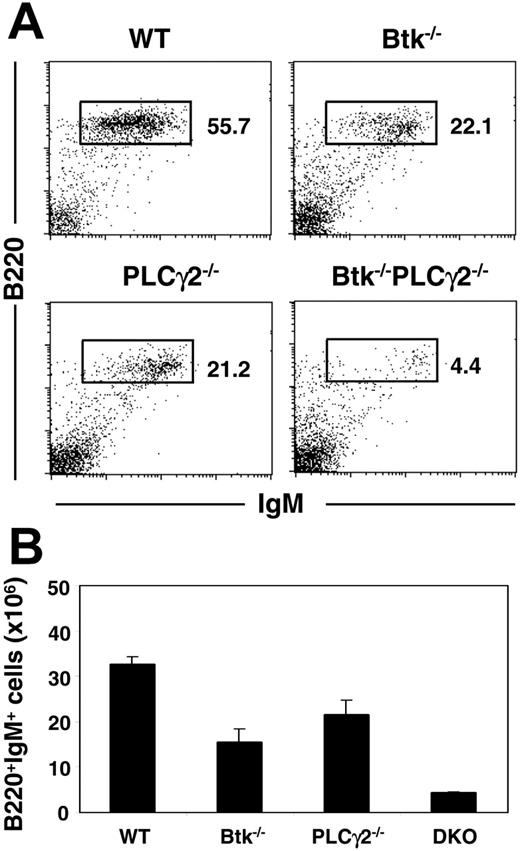
Previous analyses indicated that mice lacking either Btk29-31 or PLCγ224,25 have similar phenotypes in terms of B-cell development and function. This is consistent with the notion that upon engagement of the pre-BCR/BCR, Btk acts together with PLCγ2 in signaling Ca2+ flux and NF-κB activation. To determine if these 2 molecules could also participate in other independent pathways to regulate B-cell development and activation, we generated Btk/PLCγ2 double-mutant mice and examined the effect of their combined deficiencies on B lymphopoiesis. As shown in Figure 1A, both Btk and PLCγ2 single mutants had reduced mature B cells in their spleens, as there was approximately 2-fold reduction in the fractions of B220+IgM+ B cells in these mutant animals compared with wild-type mice. Interestingly, the fraction of splenic B cells in Btk−/−PLCγ2−/− mice was further reduced by 5-fold compared with the 2 single mutants. In addition, most of the B cells from Btk−/−PLCγ2−/− mice manifested IgMhigh phenotype (Figure 1A), indicating that these cells were more immature than those from wild-type and single-mutant mice. The severely reduced B-cell population in the double mutant was also reflected by the significant decrease in the absolute number of B cells in the spleens of these animals compared with those in control animals (Figure 1B). Taken together, the data indicate that Btk−/−PLCγ2−/− mice have more severe B-cell lymphopenia compared with single mutants, suggesting that Btk and PLCγ2 may have independent signaling functions during B-cell development.
Btk−/−PLCγ2−/− mice have reduced peripheral B-cell populations compared with single mutants. (A) Splenocytes from mice of various genotypes were analyzed via fluorescence-activated cell sorter (FACS) using fluorochrome-conjugated anti-B220 and anti-IgM antibodies. Numbers indicate percentage of cells in lymphocyte gate. Figures shown were representative of at least 4 independent experiments. (B) Enumeration of splenic B cells (mean ± SEM) in various mice. Number of cells is calculated based on total cell count and fraction of B220+IgM+ cells present in the tissues as revealed by FACS staining. Wild-type (WT), n = 6; Btk−/−, n = 6; PLCγ2−/−, n = 5; and Btk−/−PLCγ2−/− (DKO), n = 4.
Btk−/−PLCγ2−/− mice have reduced peripheral B-cell populations compared with single mutants. (A) Splenocytes from mice of various genotypes were analyzed via fluorescence-activated cell sorter (FACS) using fluorochrome-conjugated anti-B220 and anti-IgM antibodies. Numbers indicate percentage of cells in lymphocyte gate. Figures shown were representative of at least 4 independent experiments. (B) Enumeration of splenic B cells (mean ± SEM) in various mice. Number of cells is calculated based on total cell count and fraction of B220+IgM+ cells present in the tissues as revealed by FACS staining. Wild-type (WT), n = 6; Btk−/−, n = 6; PLCγ2−/−, n = 5; and Btk−/−PLCγ2−/− (DKO), n = 4.
Developing Btk/PLCγ2-deficient B cells are arrested at a surface pre-BCR+ stage
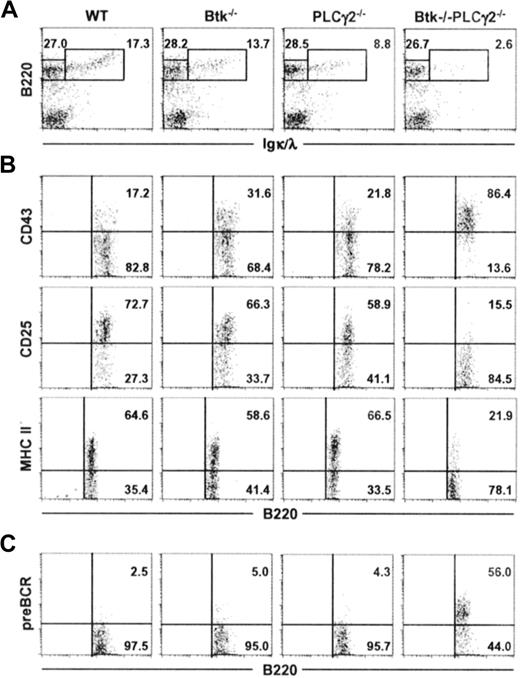
We next examined the development of B cells in the bone marrow of Btk−/−PLCγ2−/− mice. As shown in Figure 2A, and in comparison to wild-type and single-mutant animals, Btk−/−PLCγ2−/− mice had significantly decreased fraction of B220+IgL+ immature or mature B cells in their bone marrow, indicating that the severe peripheral B-cell lymphopenia seen in the double mutant was due to a defect in primary B lymphopoiesis in the bone marrow. This defect was also reflected by changes in the absolute number of B220+ IgL− and B220+ IgL+ cells as well as the ratio of these 2 populations (Table 1).
Combined deficiencies in Btk and PLCγ2 arrest B lymphopoiesis at a pre-BCR+ stage. Bone marrow cells from mice of various genotypes were characterized for their expression of B220 and Igκ/λ (IgL) (A). B220+IgL− cells were further analyzed for the expression of CD43, CD25, and MHC class II antigens (B), as well as the cell-surface expression of pre-BCR (C). Numbers indicate percentage of cells in the lymphocyte gate (A), and percentage of B220+IgL− cells (B-C). Figures shown are representative of at least 4 independent analyses.
Combined deficiencies in Btk and PLCγ2 arrest B lymphopoiesis at a pre-BCR+ stage. Bone marrow cells from mice of various genotypes were characterized for their expression of B220 and Igκ/λ (IgL) (A). B220+IgL− cells were further analyzed for the expression of CD43, CD25, and MHC class II antigens (B), as well as the cell-surface expression of pre-BCR (C). Numbers indicate percentage of cells in the lymphocyte gate (A), and percentage of B220+IgL− cells (B-C). Figures shown are representative of at least 4 independent analyses.
B-cell populations in the bone marrow of various mice
| Genotype . | No. of mice . | Bone marrow cells, × 106 . | ||
|---|---|---|---|---|
| B220+IgL− . | B220+IgL+ . | B220+IgL−B220+IgL+ . | ||
| Btk+/+PLCγ2+/+ | 6 | 2.8 ± 0.4 | 1.9 ± 0.2 | 1.5 ± 0.1 |
| Btk−/−PLCγ2+/+ | 6 | 3.2 ± 0.6 | 1.5 ± 0.2 | 2.1 ± 0.1 |
| Btk+/+PLCγ2−/− | 5 | 2.1 ± 0.6 | 0.9 ± 0.2 | 2.3 ± 0.3 |
| Btk−/−PLCγ2−/− | 4 | 1.4 ± 0.4*† | 0.2 ± 0.1‡§ | 7.0 ± 1.4‖¶ |
| Genotype . | No. of mice . | Bone marrow cells, × 106 . | ||
|---|---|---|---|---|
| B220+IgL− . | B220+IgL+ . | B220+IgL−B220+IgL+ . | ||
| Btk+/+PLCγ2+/+ | 6 | 2.8 ± 0.4 | 1.9 ± 0.2 | 1.5 ± 0.1 |
| Btk−/−PLCγ2+/+ | 6 | 3.2 ± 0.6 | 1.5 ± 0.2 | 2.1 ± 0.1 |
| Btk+/+PLCγ2−/− | 5 | 2.1 ± 0.6 | 0.9 ± 0.2 | 2.3 ± 0.3 |
| Btk−/−PLCγ2−/− | 4 | 1.4 ± 0.4*† | 0.2 ± 0.1‡§ | 7.0 ± 1.4‖¶ |
Cells were obtained from 1 femur and tibia of 8- to 12-week-old mice of various indicated genotypes. The absolute numbers of B220+IgL− (Igκ−/λ−) and B220+IgL+ (Igκ+/λ+) cells as well as the ratio of these 2 populations were calculated based on total cell counts and fractions of bone marrow cells as determined by FACS analyses. Statistical significance was established by Student t test.
*P = .002 from Btk−/−.
†P = .001 from PLCγ2−/−.
‡P = .002 from Btk−/−.
§P = .001 from PLCγ2−/−.
‖P = .02 from Btk−/−.
¶P = .002 from PLCγ2−/−.
The B220+ IgL− B cells in the bone marrow comprise both pro–B and pre–B cells and as pro–B cells differentiate into large pre–B and later into small pre–B cells, they modify the expression of certain cell-surface markers. Specifically, they down-regulate the expression of CD43 and up-regulate the expression of CD25 (IL-2Rα) and MHC class II antigens.37 As shown in Figure 2B, most of the B220+ IgL− cells in Btk−/−, PLCγ2−/−, or wild-type mice were CD43−CD25+ MHC II+, indicating that the B220+ IgL− cells in these animals were comparable in phenotype and comprised mainly small pre-B cells. This suggests that there is no drastic block in B-cell differentiation at the pre–B cell transition stage in either Btk or PLCγ2 single-mutant mice. By contrast, the phenotype of B220+ IgL− cells in Btk−/−PLCγ2−/− mice were quite different, as most of these cells expressed high levels of CD43 and did not express CD25 and MHC class II antigens, suggesting that they were arrested at the stage where pro–B cells differentiate into pre–B cells.
During pre–B cell transition, pre–B cells transiently express the pre-BCR after successful gene rearrangement and expression of the μ H chain. However, it is difficult to detect pre-BCR on the cell surface of normal developing pre–B cells, as the cell surface expression of pre-BCR is down-regulated rapidly.12 As shown in Figure 2C, B220+ IgL− cells from wild-type as well as Btk−/− and PLCγ2−/− mice had little or nondetectable levels of cell-surface expression of pre-BCR. Interestingly, more than 50% of the developing Btk−/−PLCγ2−/− pre–B cells expressed pre-BCR on their cell surface (Figure 2C). Taken together, our data indicate that Btk−/−PLCγ2−/− B cells are arrested at the pre–B-cell transition stage and express high levels of pre-BCR on their cell surface.
Early B lymphopoiesis is more severely affected in Btk−/−PLCγ2−/−than BLNK−/− mice
The phenotype of Btk−/−PLCγ2−/− mice is reminiscent of the developmental defects found in BLNK−/− mice, namely a severe block in early B lymphopoiesis at the pre–B-cell stage. In particular, they both possess a significant fraction of surface pre-BCR+ cells in bone marrow (Figure 2C; Flemming et al16 and Hayashi et al17 ). BLNK is the adaptor protein that brings Btk and PLCγ2 together to form the BCR signalosome complex.13 Thus, in the absence of BLNK, one would envisage that Btk and PLCγ2 are not efficiently activated since the signaling complex is not appropriately formed without the scaffolding function of BLNK. This could be equivalent to a situation whereby BLNK is present but Btk and PLCγ2 are absent as in the case of Btk−/−PLCγ2−/− mice.
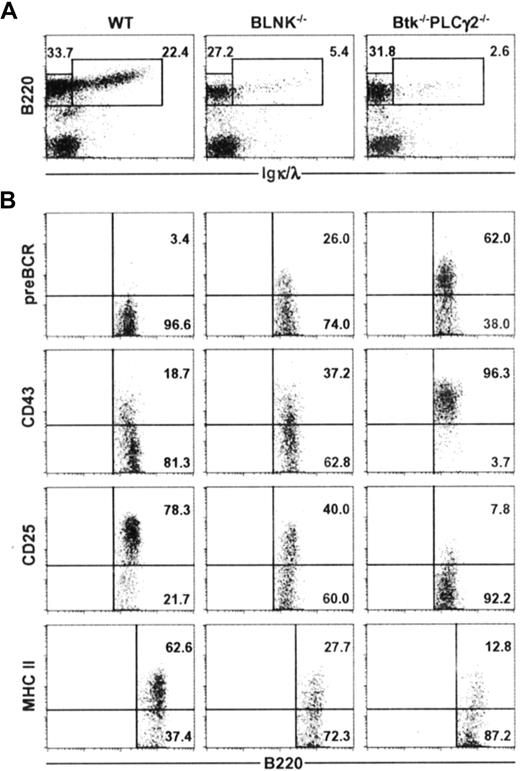
To determine if the primary roles of Btk and PLCγ2 in early B-cell development is solely within the BLNK/Btk/PLCγ2 signaling complex or if the 2 molecules have additional roles outside of this complex, we directly compared BLNK−/− mice and Btk−/−PLCγ2−/− mice. As shown in Figure 3A, BLNK−/− mice had 4 to 5 times less B220+ IgL+ immature and mature B cells compared with wild-type mice, and Btk−/−PLCγ2−/− mice had a further 50% decrease in the fraction of immature and mature B cells compared with BLNK−/− mice. This is the first indication that Btk−/−PLCγ2−/− mice may have a more drastic arrest of B-cell development. Analyses of the B220+ IgL− cells in BLNK−/− and Btk−/−PLCγ2−/− mice further supported the notion that B lymphopoiesis is more severely affected in the latter. As shown in Figure 3B, most of the B220+ IgL− cells in Btk−/−PLCγ2−/− mice were pre-BCR+, CD43high, CD25− and express low levels of MHC class II antigens. In contrast, most of the B220+ IgL− cells in BLNK−/− mice have started to down-regulate their expression of CD43 and up-regulate their expressions of CD25 and MHC class II antigens. More importantly, a smaller fraction of the B220+ IgL− pre-B cells (approximately 20%) in BLNK−/− mice expressed pre-BCR on their cell surface compared with most Btk−/−PLCγ2−/− pre–B cells (approximately 60%). Thus, it would appear that BLNK−/− pre–B cells are more differentiated than Btk−/−PLCγ2−/− pre–B cells. Our data thus suggest that Btk and PLCγ2 might function both within and outside of the BLNK/Btk/PLCγ2 signaling complex; hence, combined deficiencies in Btk and PLCγ2 arrest early B-cell development more severely than single BLNK deficiency.
Btk−/−PLCγ2−/− B cells are arrested at an earlier developmental stage compared with BLNK−/− B cells. Bone marrow cells from WT, BLNK−/−, and Btk−/−PLCγ2−/− mice were stained for the expression of B220 and Igκ/λ (IgL) (A), and B220+IgL− cells were further analyzed for the expression of pre-BCR, CD43, CD25, and MHC class II antigens (B). Numbers indicate percentage of cells in the lymphocyte gate (A), and percentage of B220+IgL− cells (B). Figures shown are representative of 4 independent analyses.
Btk−/−PLCγ2−/− B cells are arrested at an earlier developmental stage compared with BLNK−/− B cells. Bone marrow cells from WT, BLNK−/−, and Btk−/−PLCγ2−/− mice were stained for the expression of B220 and Igκ/λ (IgL) (A), and B220+IgL− cells were further analyzed for the expression of pre-BCR, CD43, CD25, and MHC class II antigens (B). Numbers indicate percentage of cells in the lymphocyte gate (A), and percentage of B220+IgL− cells (B). Figures shown are representative of 4 independent analyses.
Pre-BCR+Btk−/−PLCγ2−/− pre–B cells are in cell cycle and have reduced Ig L chain gene rearrangements
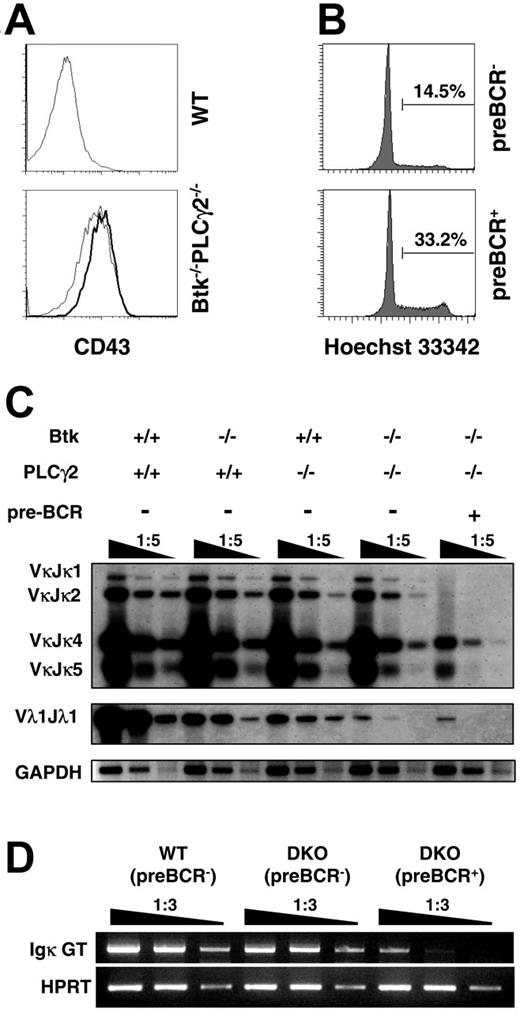
In normal mice, the expression and signaling of the pre-BCR lead to the initial expansion of pre–B cells. These cycling pre–B cells are also known as large pre–B cells.2 To determine more precisely the developmental stage where pre–B cells were arrested in the double mutant, we examined the cell-cycle status of Btk−/−PLCγ2−/− pre-BCR+ cells. We found that Btk−/−PLCγ2−/− pre-BCR+ cells were bigger in size (data not shown) and expressed higher levels of CD43 on their cell surface compared with their pre-BCR− counterparts (Figure 4A). In addition, Btk−/−PLCγ2−/− pre-BCR+ cells were more actively cycling than pre-BCR− cells from the same mouse as determined by Hoechst staining, which revealed the cellular DNA content of these cells (Figure 4B). These results indicate that double deficiencies in Btk and PLCγ2 impede B-cell development at the large pre–B cell stage where the cells are cycling.
Btk−/−PLCγ2−/− pre-B cells are in cell cycle and exhibit reduced immunoglobulin light chain gene rearrangement. (A) CD43 expression on pre–B cells from WT and Btk−/−PLCγ2−/− mice. Bone marrow cells were stained with anti-B220, Igκ/Igλ (IgL), pre-BCR, and CD43 antibodies. Histogram profiles showed CD43 expression on B220+IgL− preBCR− (thin line) versus B220+IgL− preBCR+ cells (bold line). (B) Cell-cycle analysis of Btk−/−PLCγ2−/− pre–B cells. Cell-cycle status of bone marrow cells was determined by Hoechst 33342 staining of DNA. Data displayed Hoechst 33342 staining in gated B220+IgL− preBCR− and B220+IgL− pre-BCR+ cells from Btk−/−PLCγ2−/− mice. Numbers indicate percentage of cells in cell cycle. (C) Analysis of Ig κ and λ chain gene rearrangements in WT, Btk−/−, PLCγ2−/−, and Btk−/−PLCγ2−/− pre–B cells. DNA was isolated from FACS-sorted bone marrow B220+IgL− pre-BCR− cells of various mice and from sorted B220+IgL− pre-BCR+ cells of double-mutant mice, and serially diluted for analysis of Vκ (top panels) and Vλ1 (middle panel) light chain gene rearrangements by PCR. PCR products were visualized by Southern blotting using Jκ- and Cλ1-specific probes. The GADPH gene was also amplified via PCR as control for the loading of DNA template (bottom panels). Results shown are representative of 3 independent experiments. (D) Examination of Ig κ germ-line transcription by reverse transcription (RT)–PCR. Total RNA was purified from FACS-sorted bone marrow B220+IgL− pre-BCR− cells of WT and Btk−/−PLCγ2−/− mice and B220+IgL− pre-BCR+ cells of Btk−/−PLCγ2−/− (DKO) mice. Reverse-transcribed cDNA was serially diluted for analysis of Ig κ germ-line transcription by PCR. HPRT gene expression was included as cDNA template control. Results shown are representative of 2 independent experiments.
Btk−/−PLCγ2−/− pre-B cells are in cell cycle and exhibit reduced immunoglobulin light chain gene rearrangement. (A) CD43 expression on pre–B cells from WT and Btk−/−PLCγ2−/− mice. Bone marrow cells were stained with anti-B220, Igκ/Igλ (IgL), pre-BCR, and CD43 antibodies. Histogram profiles showed CD43 expression on B220+IgL− preBCR− (thin line) versus B220+IgL− preBCR+ cells (bold line). (B) Cell-cycle analysis of Btk−/−PLCγ2−/− pre–B cells. Cell-cycle status of bone marrow cells was determined by Hoechst 33342 staining of DNA. Data displayed Hoechst 33342 staining in gated B220+IgL− preBCR− and B220+IgL− pre-BCR+ cells from Btk−/−PLCγ2−/− mice. Numbers indicate percentage of cells in cell cycle. (C) Analysis of Ig κ and λ chain gene rearrangements in WT, Btk−/−, PLCγ2−/−, and Btk−/−PLCγ2−/− pre–B cells. DNA was isolated from FACS-sorted bone marrow B220+IgL− pre-BCR− cells of various mice and from sorted B220+IgL− pre-BCR+ cells of double-mutant mice, and serially diluted for analysis of Vκ (top panels) and Vλ1 (middle panel) light chain gene rearrangements by PCR. PCR products were visualized by Southern blotting using Jκ- and Cλ1-specific probes. The GADPH gene was also amplified via PCR as control for the loading of DNA template (bottom panels). Results shown are representative of 3 independent experiments. (D) Examination of Ig κ germ-line transcription by reverse transcription (RT)–PCR. Total RNA was purified from FACS-sorted bone marrow B220+IgL− pre-BCR− cells of WT and Btk−/−PLCγ2−/− mice and B220+IgL− pre-BCR+ cells of Btk−/−PLCγ2−/− (DKO) mice. Reverse-transcribed cDNA was serially diluted for analysis of Ig κ germ-line transcription by PCR. HPRT gene expression was included as cDNA template control. Results shown are representative of 2 independent experiments.
Down-regulation of pre-BCR expression and successful rearrangements of IgL chain genes are hallmark events of successful pre–B-cell transition.12 We next examined IgL gene rearrangements in Btk−/−, PLCγ2−/−, and Btk−/−PLCγ2−/− pre–B cells. It was previously shown that Btk−/− pre–B cells have a defect in initiating Igλ gene rearrangements.38 However, it is not known if PLCγ2−/− pre–B cells and, more interestingly, Btk−/−PLCγ2−/− pre–B cells, have a similar defect in either Igκ or Igλ gene rearrangements. As shown in Figure 4C, Igκ gene arrangements were largely normal in Btk−/− and PLCγ2−/− pre–B cells that have already down-regulated the cell-surface expression of pre-BCR. Interestingly, pre-BCR+ cells from Btk−/−PLCγ2−/− mice had approximately a 5-fold reduction in Igκ gene rearrangement compared with their pre-BCR− counterparts, which only had slightly reduced Igκ gene rearrangement compared with pre-BCR− cells from wild-type and single-mutant mice. These results indicate that Btk−/−PLCγ2−/− pre-BCR− cells might be more differentiated than pre-BCR+ cells from the same mouse. We further used the presence of Igκ germ-line transcripts as an additional molecular marker to directly compare the differentiation status of B220+IgL− pre-BCR− and B220+IgL− pre-BCR+ cells from Btk−/−PLCγ2−/− mice. As shown in Figure 4D, Btk−/−PLCγ2−/− pre-BCR+ cells had approximately 10-fold less Igκ germ-line transcripts, while their pre-BCR− counterparts had only a 2- to 3-fold reduction compared with wild-type control, suggesting that most Btk−/−PLCγ2−/− pre-BCR− cells are more differentiated than the pre-BCR+ cells from the same mouse and likely have down-regulated their surface pre-BCR expression. However, we could not exclude the possibility that a small fraction of these Btk−/−PLCγ2−/− pre-BCR− cells could be pro–B cells that have not yet expressed surface pre-BCR. Nevertheless, the fact that Btk−/−PLCγ2−/− pre-BCR− cells have lower levels of CD43 expression (Figure 4A) and higher levels of Igκ gene rearrangement and Igκ germ-line transcription than pre-BCR+ cells (Figure 4C-D) would suggest that a large portion of these pre-BCR− cells are more differentiated than the pre-BCR+ cells from the same mouse. Taken together, these results further support the argument that Btk−/−PLCγ2−/− pre–B cells are arrested at an earlier differentiation stage, most likely at a large pre–B-cell stage in which they just express pre-BCR and are about to initiate Igκ gene rearrangements.
Other than gene rearrangements in the Igκ locus, pre–B cells can also rearrange the Ig λ locus when they fail to functionally rearrange a κ L chain. We thus investigated Igλ gene arrangement in single- and double-mutant pre–B cells by examining Vλ1 rearrangement. Consistent with previous results,38 Vλ1 gene rearrangement was significantly reduced in Btk−/− pre–B cells compared with wild-type cells (Figure 4C). In addition, we demonstrated here for the first time that PLCγ2 deficiency also affected Igλ gene rearrangement to a similar extent as Btk deficiency. Interestingly, the combined absence of Btk and PLCγ2 further reduced Vλ1 gene rearrangement by 25- to 30-fold in both pre-BCR+ and pre-BCR− cells compared with the single mutants (Figure 4C). These results indicate that both Btk and PLCγ2 act cooperatively to effect Igλ gene rearrangements; the concurrent absence of these 2 molecules leads to a more drastic impairment of Igλ gene rearrangement than either single mutation.
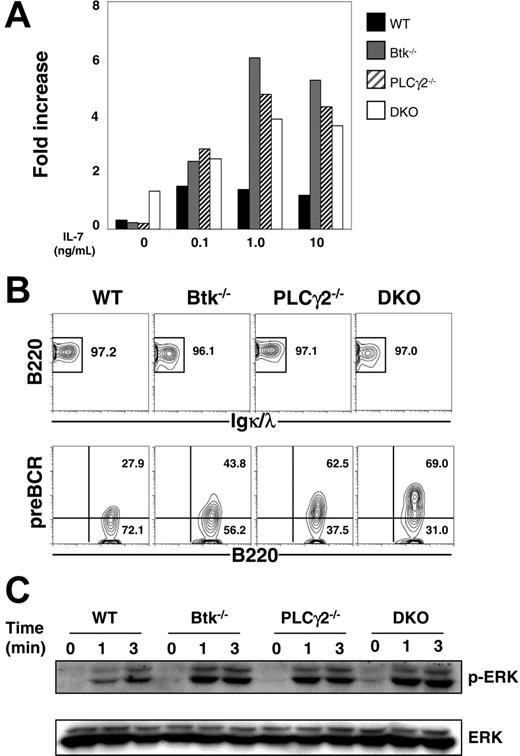
Btk−/−PLCγ2−/− pre-B cells expand in the presence of IL-7
Pre–B cells can grow and expand in vitro in the presence of IL-7,39 and the expression of pre-BCR is able to significantly increase the responsiveness of pre–B cells to IL-7.40 Previous studies indicated that BLNK−/− pre–B cells, which have high levels of surface pre-BCR expression, proliferated robustly in the presence of IL-7.16 Here, we examined the IL-7–driven proliferation of Btk−/−, PLCγ2−/−, and Btk−/−PLCγ2−/− pre–B cells. As shown in Figure 5A, single- and double-mutant pre–B cells expanded faster than wild-type control, though the composition of CD19+IgL− pre–B-cell population varied in these mice. After 8 days of culture, more than 95% of cells in the various cultures were B220+ and IgL− (Figure 5B; top panel), and a considerable fraction of cultured B220+IgL− cells were pre-BCR+, with the double-mutant cells having the highest level and the wild-type cells having the lowest level of surface expression of pre-BCR (Figure 5B; bottom panel).
Expansion of Btk−/−PLCγ2−/− pre–B cells in the presence of IL-7. (A) Proliferation of pre–B cells in IL-7 culture. CD19+ B cells from bone marrow of WT, Btk−/−, PLCγ2−/− and Btk−/−PLCγ2−/− (DKO) mice were cultured in the presence of various doses of IL-7, and the number of cells was counted after 4 days of treatment. The proliferative capacity was defined as the increase in cell number relative to the input number of cells at day 0. (B) FACS analysis of cultured pre–B cells. Cells in a 9-day culture were analyzed for the expression of B220, Igκ/λ, and pre-BCR. Numbers indicate percentage of cells in the live cell gate (top panels) and percentage of B220+IgL− cells (bottom panels). (C) Western blot analysis of pre-BCR–induced ERK activation. Cultured pre–B cells were stimulated with 10 μg/mL anti-Igμ F(ab′)2 antibody for various periods of time, and whole-cell lysates were probed with anti–phospho-ERK antibody. Blots were reprobed with anti-ERK2 antibody to determine protein loading. Results shown are representative of 4 and 3 independent experiments for panel A and panels B-C, respectively.
Expansion of Btk−/−PLCγ2−/− pre–B cells in the presence of IL-7. (A) Proliferation of pre–B cells in IL-7 culture. CD19+ B cells from bone marrow of WT, Btk−/−, PLCγ2−/− and Btk−/−PLCγ2−/− (DKO) mice were cultured in the presence of various doses of IL-7, and the number of cells was counted after 4 days of treatment. The proliferative capacity was defined as the increase in cell number relative to the input number of cells at day 0. (B) FACS analysis of cultured pre–B cells. Cells in a 9-day culture were analyzed for the expression of B220, Igκ/λ, and pre-BCR. Numbers indicate percentage of cells in the live cell gate (top panels) and percentage of B220+IgL− cells (bottom panels). (C) Western blot analysis of pre-BCR–induced ERK activation. Cultured pre–B cells were stimulated with 10 μg/mL anti-Igμ F(ab′)2 antibody for various periods of time, and whole-cell lysates were probed with anti–phospho-ERK antibody. Blots were reprobed with anti-ERK2 antibody to determine protein loading. Results shown are representative of 4 and 3 independent experiments for panel A and panels B-C, respectively.
The cell-surface expression of pre-BCR in these IL-7–cultured pre–B cells allowed us to directly examine pre-BCR signaling in these cells. We first studied pre-BCR–induced activation of extracellular signal–regulated kinase (ERK), which is essential for B-cell proliferation and survival.41 When triggered by anti-μ antibody, wild-type, Btk−/−, PLCγ2−/−, and Btk−/−PLCγ2−/− pre–B cells all manifested similar kinetics of ERK1/2 activation (Figure 5C). Interestingly, the magnitude of ERK1/2 phosphorylation appeared to correlate with the surface expression levels of pre-BCR in these cells such that Btk−/−PLCγ2−/− pre–B cells that had the highest level of pre-BCR expression showed the strongest activation of ERK1/2. These results indicate that single Btk, PLCγ2, or combined Btk/PLCγ2 deficiencies do not affect pre-BCR–induced ERK activation, suggesting that ERK signaling in response to pre-BCR engagement might be independent of these molecules.
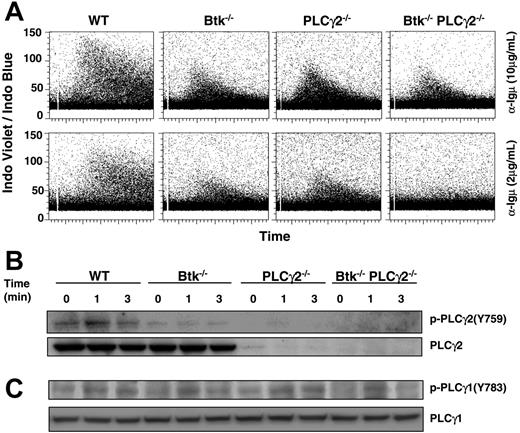
Pre-BCR–induced Ca2+ flux is more severely compromised by the combined absence of Btk and PLCγ2
Another important signal transduction pathway triggered by BCR/pre-BCR is intracellular Ca2+ signaling, and single mutation in either Btk or PLCγ2 caused significant reduction in BCR-induced Ca2+ flux.23-25 However, the effect of Btk or PLCγ2 single mutation and Btk/PLCγ2 double mutation on pre-BCR–triggered Ca2+ flux has so far not been reported. As shown here, and in contrast to the sustained Ca2+ flux seen in wild-type pre–B cells, pre-BCR–induced Ca2+ signaling was more transient and reduced in Btk−/− and PLCγ2−/− pre–B cells in response to both high (10 μg/mL) and low (2 μg/mL) doses of anti-μ stimulation (Figure 6A). Interestingly, the ability of Btk−/−PLCγ2−/− pre–B cells to elicit Ca2+ flux was further reduced in response to high-dose anti-μ stimulation (10 μg/mL) and virtually abolished in response to low-dose (2 μg/mL) anti-μ engagement compared with wild-type and single-mutant cells.
Btk−/−PLCγ2−/− pre–B cells have reduced intracellular calcium signaling in response to pre-BCR engagement. (A) Analysis of pre-BCR–induced calcium flux in WT and various mutant pre–B cells. Cultured pre–B cells were loaded with Indo-1 and treated with high (10 μg/mL) and low (2 μg/mL) doses of anti-Igμ F(ab′)2 antibody. Intracellular calcium influx in gated B220+IgL− cells was shown as a ratio of Indo-1 violet/blue fluorescence versus time. (B-C) Western blot analysis of pre-BCR–induced activation of PLCγ2 and PLCγ1. Cultured pre–B cells were stimulated for various periods of time with anti-Igμ F(ab′)2 antibody, and whole-cell lysates were probed with anti–phospho-PLCγ2(Y759) and anti–phospho-PLCγ1(Y783) antibodies. Blots were reprobed with anti-PLCγ2 and anti-PLCγ1 antibodies to determine protein loading. Results shown are representative of 5 and 3 independent experiments for panels A and B, respectively.
Btk−/−PLCγ2−/− pre–B cells have reduced intracellular calcium signaling in response to pre-BCR engagement. (A) Analysis of pre-BCR–induced calcium flux in WT and various mutant pre–B cells. Cultured pre–B cells were loaded with Indo-1 and treated with high (10 μg/mL) and low (2 μg/mL) doses of anti-Igμ F(ab′)2 antibody. Intracellular calcium influx in gated B220+IgL− cells was shown as a ratio of Indo-1 violet/blue fluorescence versus time. (B-C) Western blot analysis of pre-BCR–induced activation of PLCγ2 and PLCγ1. Cultured pre–B cells were stimulated for various periods of time with anti-Igμ F(ab′)2 antibody, and whole-cell lysates were probed with anti–phospho-PLCγ2(Y759) and anti–phospho-PLCγ1(Y783) antibodies. Blots were reprobed with anti-PLCγ2 and anti-PLCγ1 antibodies to determine protein loading. Results shown are representative of 5 and 3 independent experiments for panels A and B, respectively.
Although PLCγ2 is the major isoform of PLCγ found in B cells, it has been reported that PLCγ1, which is ubiquitously expressed and plays a critical role in T-cell receptor–mediated Ca2+ flux, is also expressed in B cells42,43 and involved in BCR-induced Ca2+ flux as well as pre–B-cell development.44 To determine the reason for the reduced Ca2+ flux in Btk−/− and PLCγ2−/− pre–B cells as well as the more drastically impaired Ca2+ flux in Btk−/−PLCγ2−/− pre–B cells, we examined pre-BCR–induced activation of PLCγ2 and PLCγ1 in these cells.
Upon BCR engagement, PLCγ2 is phosphorylated on Tyr759, which is important for PLCγ2 activation.45 In Btk−/− pre–B cells, the phosphorylation of Tyr759 was significantly reduced compared with that in wild-type cells (Figure 6B), suggesting that Btk is required for PLCγ2 activation in pre-BCR signaling, which is similar to its involvement in BCR signaling. Interestingly, PLCγ1 phosphorylation on Tyr783, which is critical for the activation of PLCγ1,46 was also less sustained in Btk−/− and Btk−/−PLCγ2−/− pre–B cells compared with wild-type cells (Figure 6C; lanes 4-6, 10-12). In contrast, the kinetics of PLCγ1 activation in PLCγ2−/− pre–B cells was largely comparable to that of wild-type cells (Figure 6C; lanes 1-3, 7-9), suggesting that the sustained activation of PLCγ1 is dependent on Btk, but activation still occurs in the absence of Btk. Therefore, in Btk−/− pre–B cells, the concurrent defects in PLCγ2 and PLCγ1 activation would contribute to the compromised pre-BCR–triggered Ca2+ flux; in PLCγ2−/− pre–B cells, the deficiency of PLCγ2, which is the major isoform in B cells, results in defective Ca2+ flux though the apparently normal function of PLCγ1 could compensate to a certain extent and lead to residual Ca2+ signaling. Last, the complete loss of PLCγ2 and defective PLCγ1 activation in Btk−/−PLCγ2−/− cells further diminish the magnitude and kinetics of the pre-BCR–induced Ca2+ flux in these cells. It is interesting to note that although Btk−/−, PLCγ2−/−, and Btk−/−PLCγ2−/− pre–B cells expressed higher levels of pre-BCR on their cell surface compared with wild type pre–B cells, these high levels of pre-BCR triggering could not increase or overcome the defect in intracellular Ca2+ signaling.
Ig H chain allelic exclusion is maintained in Btk−/−PLCγ2−/− mice
It was reported that reduced PLCγ1 expression, in the absence of PLCγ2 expression (PLCγ1+/−PLCγ2−/−), impeded pre–B-cell development and impaired Ig H chain allelic exclusion.44 Given that Btk−/−PLCγ2−/− mice had major defects in pre–B-cell transition, and that double-mutant pre–B cells had a complete loss of PLCγ2 and a partially defective activation of PLCγ1, we next examined if Ig H chain allelic exclusion was compromised in Btk−/−PLCγ2−/− mice.
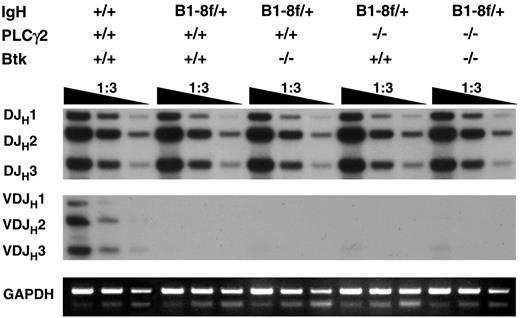
To address this, a rearranged VHB1-8 transgene targeted into 1 of the 2 Ig H chain gene loci by homologous recombination33 was introduced into Btk−/−, PLCγ2−/−, and Btk−/−PLCγ2−/− mice. Genomic DNA were isolated from sorted CD19+IgL− bone marrow cells and subjected to polymerase chain reaction (PCR) to detect endogenous DH-to-JH and VH-to-DHJH gene rearrangements. It has been shown that the expression of a transgenic Ig H chain in pro–B cells leads to the early assembly of a pre-BCR that signals allelic exclusion by suppressing VH-to-DHJH joining at the other H chain gene locus.47 As shown in Figure 7, single- or double-mutant mice showed normal levels of DH-to-JH rearrangements at the endogenous Ig H chain gene locus, similar to the situation in wild-type mice. However, in contrast to wild-type mice, where the rearrangements of VHJ558 gene family members to DH and to downstream JH1, JH2, and JH3 gene segments could be detected, such rearrangements at the endogenous Ig H chain gene locus were completely suppressed in wild-type, Btk−/−, PLCγ2−/−, and Btk−/−PLCγ2−/− mice bearing the VHB1-8 transgene, suggesting that the mechanism maintaining Ig H chain allelic exclusion is largely intact in these animals. Thus, Btk- and PLCγ2-mediated pre-BCR signaling appears not to be essential for the maintenance of Ig H chain allelic exclusion.
Immunoglobulin heavy chain allelic exclusion is maintained in the absence of Btk and PLCγ2. Genomic DNA was extracted from bone marrow CD19+ IgL− cells of various mice and was serially diluted and subjected to PCR using primers to amplify DH-to-JH and VHJ558-to-DHJH gene arrangements. The PCR products were analyzed by Southern blotting using a DNA fragment spanning JH3 to JH4 as probe. The GADPH gene was amplified as control for the amount of genomic DNA used. Data shown are representative of 2 independent experiments.
Immunoglobulin heavy chain allelic exclusion is maintained in the absence of Btk and PLCγ2. Genomic DNA was extracted from bone marrow CD19+ IgL− cells of various mice and was serially diluted and subjected to PCR using primers to amplify DH-to-JH and VHJ558-to-DHJH gene arrangements. The PCR products were analyzed by Southern blotting using a DNA fragment spanning JH3 to JH4 as probe. The GADPH gene was amplified as control for the amount of genomic DNA used. Data shown are representative of 2 independent experiments.
Discussion
We report here that combined deficiencies in Btk and PLCγ2 dramatically arrest B-cell development at the large pre–B-cell transition stage. This is in contrast to Btk or PLCγ2 single-mutant mice that show milder defects in B lymphopoiesis. In addition, we also showed that Btk−/−PLCγ2−/− pre–B cells expressed high levels of pre-BCR on their cell surfaces and were actively in cell cycle. These pre-BCR+Btk−/−PLCγ2−/− pre–B cells exhibited reduced Ig L chain gene rearrangements, although they could maintain Ig H chain allelic exclusion. Despite the high expression of pre-BCR on their cell surfaces, these Btk−/−PLCγ2−/− pre–B cells displayed a more drastic reduction in their capacity to elicit Ca2+ flux upon pre-BCR stimulation compared with wild-type and individual single-mutant cells.
It has been shown that Btk and PLCγ2 function as part of the BCR signalosome.13 Btk both directly phosphorylates PLCγ220,21 and indirectly provides the substrate, PI(4,5)P2, through the activation of PIP5K.22 Earlier analyses of Btk−/− and PLCγ2−/− mice also supported the notion of a linear signaling relationship between Btk and PLCγ2 as these 2 single-mutant mice had similar B-cell defects, namely reduced mature B-cell populations in spleen and lymph nodes, absence of CD5+ B-1 B cells in peritoneal cavities, and defective BCR-induced Ca2+ flux13 and NF-κB activation.26-28 Although early B-cell development was only mildly affected in Btk−/− or PLCγ2−/− mice, our current work indicates a more drastic B-cell developmental block at the pre–B-cell transition stage in Btk−/−PLCγ2−/− mice. This suggests that these 2 molecules might also participate in other signaling conduits, such that these signaling pathways synergize with the linear Btk-PLCγ2 pathway to regulate B-cell differentiation. In addition, our findings suggest that Btk and PLCγ2 might have some BLNK-independent roles that are outside of the BLNK/Btk/PLCγ2 signaling complex, as Btk−/−PLCγ2−/− mice revealed a more severe pre–B-cell developmental block than BLNK−/− mice in which the absence of scaffolding protein BLNK is supposed to disrupt the whole signaling complex.
It is interesting to note that Btk−/−PLCγ2−/− B cells were mainly arrested at the large pre–B-cell transition stage, and that most of these mutant pre–B cells express pre-BCR on their cell surfaces. Previously, it was shown that BLNK−/− pre–B cells had high levels of cell-surface expression of pre-BCR,16 and this was further augmented by the concomitant absence of Btk,48 CD19,17 and PLCγ2,19 suggesting that BLNK is a critical regulator of the cell-surface expression of pre-BCR. Here, we reported that Btk−/−PLCγ2−/− pre–B cells expressed even higher levels of cell-surface pre-BCR than BLNK−/− pre–B cells (Figure 3B), suggesting that the down-regulation of pre-BCR might be determined by the overall strength of pre-BCR signaling rather than signaling involving BLNK itself.
In line with the failed down-regulation of pre-BCR, pre-BCR+Btk−/−PLCγ2−/− cells were actively cycling and had reduced Ig κ gene rearrangements. Such a phenomenon was again not seen in Btk−/− or PLCγ2−/− mice but in BLNK−/− mice (Figure 4C; Flemming et al16 and Hayashi et al17 ). Thus, it appears that a certain threshold of signaling via the BLNK/Btk/PLCγ2 macromolecular complex is required for the down-regulation of cell-surface expression of pre-BCR in large cycling pre–B cells, followed by the transition of large cycling pre–B cells to small quiescent pre–B cells and the initiation of Ig κ gene rearrangement. In this current report, we also confirmed previous finding that Btk is required for the activation of Ig λ gene rearrangements38 and further implicated a role for PLCγ2 in signaling Ig λ gene rearrangements. In addition, we demonstrated a more severe reduction of Ig λ gene rearrangements in both pre-BCR+ and pre-BCR−Btk−/−PLCγ2−/− pre–B cells. This suggests that Btk and PLCγ2 cooperatively signal Ig λ gene rearrangements. It is thus tempting to speculate that a higher signaling threshold might be required to regulate Ig λ compared to Ig κ gene rearrangements.
Interestingly, even though Btk−/−PLCγ2−/− pre–B cells have defective Ig L chain gene rearrangements, they could still maintain Ig H chain allelic exclusion, which requires membrane-expression of μ heavy chain49 and signaling molecules Syk and ZAP-70.50 It could be that the signaling threshold for maintaining Ig H chain allelic exclusion is relatively low, or that other signaling components downstream of Syk and ZAP-70 but not BLNK/Btk/PLCγ2 are involved in this process.
In conclusion, our current study supports a model of pre-BCR/BCR signaling in which components of the BCR signalosome, namely BLNK, Btk, and PLCγ2, have roles both within and outside of the signaling complex. Since the various combination of the double-mutant mice have a more drastic arrest of B-cell development compared with single-mutant mice, it will be interesting to examine the phenotype of mice simultaneously lacking BLNK, Btk, and PLCγ2.
Authorship
Contribution: K.-P.L. and S.X. designed the research and wrote the paper. S.X., K.-G.L., J.H. performed the research, and K.P.-L. and S.X. analyzed data. T.K. contributed vital reagents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kong-Peng Lam and Shengli Xu, Laboratory of Molecular and Cellular Immunology, 6-15, Proteos, 61 Biopolis Dr, Singapore 138673; e-mail: mcblamkp@imcb.a-star.edu.sg and shengli@imcb.a-star.edu.sg.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Motomi Osato and Mr Weng Keong Chew for assistance with cell sorting and mice genotyping, respectively, and members of the LAM laboratory for insightful discussion.
This work was supported by the Biomedical Research Council of the Agency for Science, Technology and Research (A*STAR), Singapore.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal