Abstract
The secretory granules (SGs) of secretory cells of the hematopoietic lineage, such as the mast cells, are lysosome-related organelles whose membrane proteins travel through the plasma membrane and the endocytic system. Therefore, a mechanism must exist to prevent proteins destined to recycling or to the trans-Golgi network (TGN) from reaching the SGs. We now show that synaptotagmin (Syt) IX, a Syt homologue that is required for recycling from the endocytic recycling compartment (ERC) in rat basophilic leukemia (RBL-2H3) cultured mast cells, is involved in segregating recycling proteins from the SGs. By using as a marker the recycling protein TGN38, which cycles between the TGN, plasma membrane, and the ERC, we show that knock-down of Syt IX results in mistargeting of HA-tagged TGN38 to the SGs. We further demonstrate that Syt IX binds directly the small GTPase ARF1 and associates with the clathrin adaptor complex AP-1. These results therefore implicate Syt IX as an essential factor for the correct sorting of SGs proteins. Moreover, they place Syt IX as part of the machinery that is involved in the formation of transport carriers that mediate SGs protein sorting.
Introduction
Mast cells belong to immune cells of the hematopoietic lineage, where an intimate connection exists between the endocytic and exocytic systems.1 The secretory granules (SGs) of mast cells include, in addition to their specific secretory cargo of vasoactive amines (ie, histamine), lysosomal enzymes such as β-hexosaminidase, β-glucuronidase, arylsulfatase, and carboxypeptidases,2 as well as lysosomal integral membrane proteins (LIMPs).3 These SGs, therefore, belong to the subset of regulated exocytic granules collectively referred to as lysosome-related organelles (LROs).4 Morphologic analyses have documented the presence of a heterogeneous population of SGs in mast cells, consisting of 3 types of ultrastructurally defined granules.5 Type 1 and type 2 granules contain MHC class II molecules and can be labeled by a fluid-phase endocytic marker; type 2 and type 3 contain serotonin, whereas type 3 cannot internalize fluid-phase endocytic markers nor does it contain MHC class II.5 The precise relationship between these 3 types of granules remained obscure. Recently, we have demonstrated the involvement of synaptotagmins (Syts) II and III, members of the Syt family of traffic controlling proteins, that are endogenously expressed in mast cells,6-8 in regulating the exocytic competence and size of mast cell SGs. Specifically, our studies have implicated Syt II as a negative regulator, responsible for the retention of lysosomal amine-free granules during cell activation6 and Syt III as playing a role in SG maturation.8 By using an antisense RNA approach to selectively reduce the expression level of Syt III in the mast cell line rat basophilic leukemia (RBL-2H3), we have identified 2 cellular processes, which were strongly affected consequently to Syt III suppression. First, formation of the pericentriolar endocytic recycling compartment (ERC) was strongly impaired. Second, a significant increase in the number of enlarged SGs was detected.8 These results have therefore suggested a possible link between the ERC and the biogenesis of SGs, presumably by removal and recycling of proteins from the immature granule during the process of granule maturation.
Recently, we identified Syt IX, as an endogenously expressed and ERC-localized Syt homologue in mast cells.9,10 Moreover, we have demonstrated that Syt IX regulates recycling from the ERC to the plasma membrane.9 In the present work, we investigated whether Syt IX influences the SGs. Here we show that knock-down of Syt IX is associated with mistargeting of TGN38, a protein that normally cycles between the trans-Golgi network (TGN), the ERC, and the plasma membrane,11-13 and its delivery to the SGs. We further identify the small GTPase ARF1 and the clathrin adaptor complex AP-1 as effectors of Syt IX, suggesting their involvement in controlling the intercommunication between the endocytic and exocytic transport pathways in mast cells.
Materials and methods
Antibodies
Antibodies used included monoclonal anti-HA antibodies (clone 16B12, Babco, Berkeley Antibodies, Richmond, CA); polyclonal anti-HA antibodies (Y-11, Santa Cruz Biotechnology, Santa Cruz, CA); monoclonal anti-T7 antibodies (Novagen, Darmstadt, Germany); monoclonal anti-Syt IX antibodies (clone 46, Transduction Laboratories, Lexington, KY); polyclonal anti-Syt IX antibodies9,10 ; polyclonal anti–mannosidase II antibodies (a generous gift from Dr J. Donaldson, National Institutes of Health, Bethesda, MD); monoclonal anti-GFP antibodies (Roche Diagnostics, Indianapolis, IN); polyclonal anti-ARF1 antibodies (a generous gift from Dr D. Cassel, Technion-Israel Institute of Technology, Haifa, Israel); monoclonal anti-TGN38 antibodies (Transduction Laboratories); polyclonal anti–γ-adaptin antibodies (a generous gift from Dr M. S. Robinson, University of Cambridge, Cambridge, United Kingdom); monoclonal antiserotonin antibodies (Dako, Glostrup, Denmark); and horseradish-peroxidase (HRP)–conjugated goat anti–rabbit or anti–mouse IgG and rhodamine- or FITC-conjugated donkey anti–rabbit or anti–mouse IgG (Jackson Research Laboratories, West Grove, PA).
Reagents
Texas red-conjugated human transferrin (Tfn) was obtained from Molecular Probes (Eugene, OR). Brefeldin A, nocodazole, serotonin, and glutathione-Sepharose were from Sigma-Aldrich (St Louis, MO) and protein A-Sepharose was from Amersham Biosciences (Freiburg, Germany). Calcium ionophore A23187 and the phorbol ester, 12-O-tetradecanoyl-13-acetate (TPA), were from Calbiochem (La Jolla, CA).
Cell culture
RBL-2H3 (hereafter referred to as RBL) cells were maintained in adherent cultures in DMEM supplemented with 10% FCS in a humidified atmosphere of 5% CO2 at 37°C.
Cells lysates
RBL cells (1 × 107) were lysed in a lysis buffer made up of 50 mM HEPES, pH 7.4, 150 mM NaCl, 10 mM EDTA, 2 mM EGTA, 1% Triton X-100, 0.1% SDS, 50 mM NaF, 10 mM NaPPi, 2 mM NaVO4, 1 mM PMSF, and a cocktail of protease inhibitors (Roche Diagnostics). Lysates were cleared by centrifugation at 9000g for 15 minutes at 4°C. The cleared supernatants were mixed with 5 × Laemmli sample buffer, boiled for 5 minutes, and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting.
DNA constructs
GST-Syt_IX-C2A, GST-Syt_IX-C2B-CT, GST-Syt_IX-C2B-ΔCT, and recombinant plasmids of sense or antisense full-length Syt IX cDNAs were described previously.9 HA-TGN38 cDNA was a generous gift from Dr J. Bonifacino (National Institutes of Health). Rab 11-GFP was a generous gift from Dr M. Zerial (Max Planck Institute for Molecular Cell Biology and Genetics, Dresden, Germany). ARF1-GFP and ARF1(Q71L) were a generous gift from Dr J. Lippincott-Schwartz (National Institutes of Health) and pET 22b-ARF1 was a generous gift from Dr M. A. De Matteis (Istituto di Ricerche Farmacologiche Mario Negri, Sud, Santa Maria Imbaro Chieti, Italy).
Cell transfection
Stable RBL cell lines overexpressing or knocked-down in Syt IX expression were described previously.9 For transient transfection, RBL cells (6 × 107) were electroporated (400 V, 960 μF) in the presence of 40 μg plasmids expressing HA-TGN38, Rab 11-GFP, ARF1-GFP, HA-ARF1(Q71L), or T7-Syt IX. Cells were immediately replated in tissue culture dishes containing supplemented DMEM for the desired time periods.
Immunoprecipitation
Cells were lysed in buffer A 150 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM MgCl2, 1% Triton X-100, 1 mM PMSF, and cocktail of protease inhibitors (Boehringer Mannheim, Mannheim, Germany). After solubilization at 4°C for 10 minutes, supernatants were cleared by centrifugation at 9000g for 15 minutes at 4°C. Aliquots of cleared supernatants containing 500 μg protein were incubated for 18 hours at 4°C with the desired antibody and immune complexes were captured by adding a 25 μL protein A-Sepharose (50% vol/vol) and incubating for 1.5 hours at 4°C. Immune complexes were washed 4 times with lysis buffer A, suspended in Laemmli sample buffer, boiled for 5 minutes, resolved by 10% SDS-PAGE under reducing conditions, and transferred into nitrocellulose papers for Western blotting with the appropriate antibodies. Immunoreactive bands were visualized by the enhanced chemiluminescence method according to manufacturer's instructions.
Affinity chromatography on GST fusion proteins
GST, GST-Syt IX-C2A, GST-Syt IX-C2B-CT, or GST-Syt IX-C2BΔCT (20 μg) was incubated at 4°C with RBL cell lysates (500 μg) prepared in buffer A or with a partially purified preparation of recombinant ARF1 (500 μg). At the end of the incubation period, beads were sedimented by centrifugation at 5000g for 4 minutes at 4°C, washed 4 times with buffer A, and finally suspended in 1 × Laemmli sample buffer, boiled for 5 minutes, and subjected to SDS-PAGE and immunoblotting.
Isolation of ARF1
BL-21 cells were transformed with pET 22b-ARF1 cDNA and the protein induced by incubating the cells with IPTG (1 mM) for 90 minutes at 37°C. Cells were harvested and the cell pellet resuspended in 20 mL of 50 mM Tris-HCl, pH 8.0, 40 mM EDTA, 25% (wt/vol) sucrose, 1 mg/mL lysozyme, and incubated at room temperature for 15 minutes. Cells were subsequently lysed in 8 mL of 0.2% Triton X-100, 50 mM Tris-HCl, pH 8.0, and 100 mM MgCl2 for 10 minutes at 4°C and pelleted at 100 000g for 60 minutes at 4°C. The cleared supernatant was then loaded onto a DEAE Sephadex A25 column pre-equilibrated with 20 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1 mM dithiothreitol, and 50 mM NaCl. The flow-through and one column volume of wash were collected and concentrated to 8 mL by Centriplus (Millipore, Billerica, MA) YM-10 membranes and used as is.
Immunofluorescence microscopy
RBL cells (2 × 105 cells/mL) were grown on 12-mm round glass coverslips. For immunofluorescence processing, cells were washed twice with PBS and fixed for 15 minutes at room temperature in 3% paraformaldehyde/PBS. Cells were subsequently washed 3 times with PBS supplemented with 1 mM CaCl2 and 1 mM MgCl2 (PBSCM) and permeabilized on ice for 5 minutes with 100 μg/mL digitonin. After 2 washes with PBSCM, cells were permeabilized for additional 15 minutes at room temperature with 0.1% saponin in PBSCM. Cells were subsequently incubated for 1 hour at room temperature with the primary antibodies diluted in PBSCM/5% FCS/2% BSA, washed 3 times in PBSCM/0.1% saponin, and incubated for 30 minutes in the dark with the appropriate secondary antibody (rhodamine- or FITC-conjugated donkey anti–rabbit or anti–mouse IgG, at 1:200 dilution in PBSCM/5% FCS/2% BSA). Coverslips were subsequently washed in PBSCM/0.1% saponin and mounted with Gel Mount mounting medium (Biomedica, Foster City, CA). Samples were analyzed using a C-LSM-410 Zeiss laser confocal microscope (Oberkochen, Germany). All confocal images were taken with a 63× oil correction objective and operated by confocal software (LSM; Zeiss).
Tfn internalization
RBL cells were grown on glass coverslips, serum starved for 1 hour at 37°C in DMEM supplemented with 0.2% BSA and 50 mM HEPES, pH 7.4, followed by incubation at 37°C with Texas red-conjugated Tfn (50 μg/mL) to allow internalization. The reaction was stopped by placing the cells on ice and the cells were subsequently processed for immunofluorescence microscopy as described (see “Immunofluorescence microscopy”).
Antibody uptake
RBL cells were incubated with monoclonal anti HA antibodies (2 μg/mL) for 30 minutes on ice, followed by incubation at 37°C for the indicated time periods. Samples were subsequently processed for microscopy.
Stimulation of secretion from RBL cells
Cells were washed 3 times in Tyrode buffer (10 mM HEPES, pH 7.4, 130 mM NaCl, 5 mM KCl, 1.4 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, and 0.1% BSA) and stimulated for 30 minutes at 37°C in the same buffer with calcium ionophore A23187 and TPA. Samples were subsequently processed for microscopy.
Data presentation
Data represent one of at least 3 separate experiments.
Results
Syt IX is a limiting factor in the targeting of TGN38 to the TGN
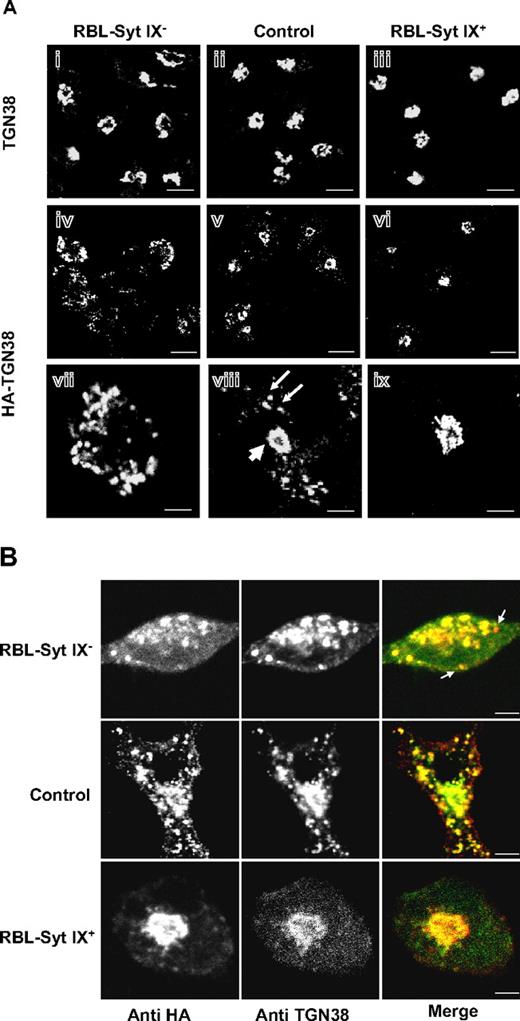
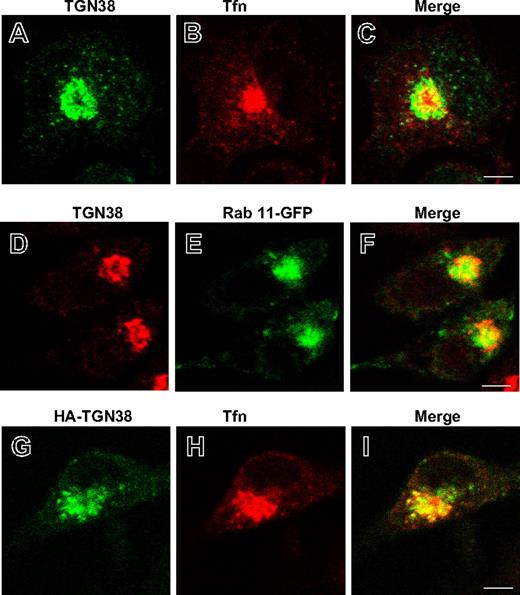
Previously, we described the generation of stable RBL cell lines that either overexpress (by ∼2-fold, RBL-Syt IX+) or are specifically knocked-down (by ∼90%, RBL-Syt IX−) in Syt IX expression.9 These clones maintain an intact Golgi complex as evidenced by staining for the Golgi marker mannosidase II and also express comparable levels of cellular proteins, including the Syt homologue Syt III, tubulin, the adaptor proteins γ-adaptin and epsinR, the TGN protein TGN38, and the small GTPase ARF1 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). We have shown that RBL-Syt IX− cells internalize Tfn normally but recycling from the ERC to the cell surface is slowed significantly.9 The ERC was also implicated as an intermediate station in the route of trafficking of TGN38, from the plasma membrane to the TGN.11,13 Therefore, to investigate whether Syt IX is also required for trafficking of TGN38, we have transiently transfected an HA-tagged version of TGN38 into control RBL, RBL-Syt IX−, or RBL-Syt IX+ cells and have compared its cellular distribution under steady-state conditions (48-72 hours after transfections). Strikingly, while staining the different clones for endogenous TGN38 reveled that in the absence of HA-TGN38 transfection, the endogenous protein has acquired a perinuclear localization reminiscent of the TGN in all clones (Figure 1Ai-iii), when overexpressed, both endogenous and exogenous TGN38 were mislocalized to vesicular structures in RBL-Syt IX− cells (Figure 1Aiv and vii,B). In control cells, the endogenous and exogenous HA-TGN38 distributed between a major perinuclear region and a few scattered peripheral vesicles (Figure 1Av,viii,B), whereas in RBL-Syt IX+, HA-TGN38 is completely and exclusively localized to a perinuclear localization (Figure 1Avi,ix,B). To better define the perinuclear site at which TGN38 resides, we compared its localization with regard to internalized Tfn, which marks the juxtanuclear ERC.14 Comparing the distribution of endogenous TGN38 with that of internalized Tfn in RBL-Syt IX+ cells revealed only partial overlap consistent with the notion that the major fraction of TGN38 resides at the TGN (Figure 2A-C). A similar segregation was also observed when comparing TGN38 distribution with that of Rab 11, which resides mainly at the ERC15,16 (Figure 2D-F). Performing a similar analysis on RBL-Syt IX+ cells expressing HA-TGN38 demonstrated a higher degree of colocalization between HA-TGN38 and internalized Tfn suggesting that overexpressed TGN38 was distributed between the TGN and the ERC (Figure 2G-I). These results have therefore suggested that deviation of TGN38 from the ERC/TGN coincides with the levels of Syt IX expression and occurs only when its level exceeds a certain cellular threshold. Importantly, mislocalization of TGN38 in RBL-Syt IX− cells was not a consequence of its increased expression in those cells because Western blot analysis has indicated that even lower amounts of protein were expressed in RBL-Syt IX− cells (Figure S2). To substantiate this notion further, we also monitored the biosynthetic route of HA-TGN38 in each clone following a 2-hour incubation period at 20°C to allow its accumulation in the Golgi.17,18 This analysis demonstrated that following its exit from the Golgi (up to 3.5 hours after transfection, as indicated by its complete sensitivity to brefeldin A, which redistributes the Golgi stack into the endoplasmic reticulum (ER),19,20 at this time period, Figure S3B) and up to 7.5 hours, HA-TGN38 localizes to the TGN in all 3 clones (Figure S3A). However, with increasing periods of time after transfection, mislocalization begins in Syt IX knocked-down, but not in Syt IX-overexpressing cells (Figure S3A). These findings therefore indicate that the divergence in the route of TGN38 trafficking occurs after exit from the Golgi.
Localization of endogenously and ectopically expressed HA-TGN38 in control, Syt IX knocked-down, or Syt IX-overexpressing RBL cells. (A) Control RBL (ii,v,viii) RBL-Syt IX− (i,iv,vii) and RBL-Syt IX+ (iii,vi,ix) cells were either labeled with monoclonal anti-TGN38 (i-iii) or were transiently transfected with HA-TGN38 cDNA (iv-ix), grown on glass coverslips for 48 hours, and subsequently labeled with monoclonal anti-HA antibodies followed by Cy3-conjugated donkey anti–mouse IgG. Bars equal 10 μm (i-vi) and 3 μm (vii-ix). (B) Control RBL, RBL-Syt IX−, and RBL-Syt IX+ cells were transiently transfected with HA-TGN38 cDNA and grown on glass coverslips for 48 hours. Cells were subsequently labeled with polyclonal anti-HA antibodies and monoclonal anti-TGN38 antibodies, followed by FITC- or Cy3-conjugated donkey anti–mouse or anti–rabbit IgG. Bars represent 3 μm.
Localization of endogenously and ectopically expressed HA-TGN38 in control, Syt IX knocked-down, or Syt IX-overexpressing RBL cells. (A) Control RBL (ii,v,viii) RBL-Syt IX− (i,iv,vii) and RBL-Syt IX+ (iii,vi,ix) cells were either labeled with monoclonal anti-TGN38 (i-iii) or were transiently transfected with HA-TGN38 cDNA (iv-ix), grown on glass coverslips for 48 hours, and subsequently labeled with monoclonal anti-HA antibodies followed by Cy3-conjugated donkey anti–mouse IgG. Bars equal 10 μm (i-vi) and 3 μm (vii-ix). (B) Control RBL, RBL-Syt IX−, and RBL-Syt IX+ cells were transiently transfected with HA-TGN38 cDNA and grown on glass coverslips for 48 hours. Cells were subsequently labeled with polyclonal anti-HA antibodies and monoclonal anti-TGN38 antibodies, followed by FITC- or Cy3-conjugated donkey anti–mouse or anti–rabbit IgG. Bars represent 3 μm.
Localization of endogenously and ectopically expressed HA-TGN38 in Tfn-loaded or Rab 11-GFP–expressing cells. RBL-Syt IX+ cells (A-C) or RBL-Syt IX+ cells transiently transfected with either Rab 11-GFP (D-F) or HA-TGN38 (G-I) cDNAs were grown on glass coverslips for 48 hours, serum starved for 1 hour, and allowed to internalize Texas red-conjugated Tfn (50 μg/mL) for 30 minutes (A-C,G-I). Cells were subsequently labeled with monoclonal anti-TGN38 (A-F) or anti-HA (G-I) antibodies followed by FITC- or Cy3-conjugated donkey anti–mouse IgG. Bars represent 4 μm.
Localization of endogenously and ectopically expressed HA-TGN38 in Tfn-loaded or Rab 11-GFP–expressing cells. RBL-Syt IX+ cells (A-C) or RBL-Syt IX+ cells transiently transfected with either Rab 11-GFP (D-F) or HA-TGN38 (G-I) cDNAs were grown on glass coverslips for 48 hours, serum starved for 1 hour, and allowed to internalize Texas red-conjugated Tfn (50 μg/mL) for 30 minutes (A-C,G-I). Cells were subsequently labeled with monoclonal anti-TGN38 (A-F) or anti-HA (G-I) antibodies followed by FITC- or Cy3-conjugated donkey anti–mouse IgG. Bars represent 4 μm.
HA-TGN38 is delivered to the SGs in RBL-Syt IX− cells
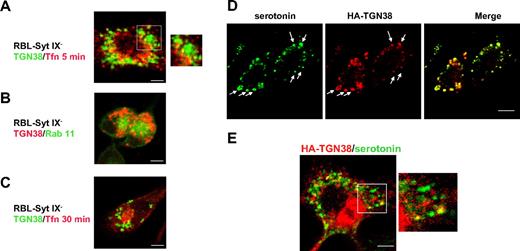
Early endosomes and the ERC are intermediates in the route of trafficking of TGN38 to the TGN.11,13 However, allowing HA-TGN38-transfected RBL-Syt IX− cells to internalize Texas red-conjugated Tfn for 5 minutes to label the early endosomal compartment demonstrated no significance colocalization between HA-TGN38 and internalized Tfn (Figure 3A). Notably, cotransfection of the RBL-Syt IX− cells with Rab 11 that normally localizes to the ERC15,16,21 and HA-TGN38 or loading the cells with Tfn for 30 minutes revealed that ectopically expressed Rab 11 as well as internalized Tfn localize to a perinuclear region in same cells in which HA-TGN38 is mistargeted to peripheral vesicles (Figure 3B-C). Hence, consistent with our previous results,9 demonstrating that RBL-Syt IX− cells could deliver internalized Tfn to the ERC, these data confirm that the ERC is formed in Syt IX knocked-down cells and elimination of the ERC is not the reason for the deviation of TGN38 from its normal route in RBL-Syt IX− cells.
Subcellular localization of HA-TGN38 in RBL-Syt IX− cells. RBL-Syt IX− (A-D) or control RBL cells (E) were transiently transfected with HA-TGN38 cDNA or cotransfected with both HA-TGN38 and Rab 11-GFP (B). Cells were grown on glass coverslips for 48 hours and either allowed to internalize Texas red-conjugated Tfn (50 μg/mL) for 5 minutes (A) or 30 minutes (C) prior to their processing for immunofluorescence or left untreated (B) or incubated with serotonin (200 μM) for the last 24 hours of growth to load the SGs (D-E). Cells were subsequently labeled with mouse anti-HA antibodies (A-E) followed by Cy3-conjugated anti–mouse IgG or double-labeled with mouse antiserotonin and rabbit anti-HA antibodies, followed by FITC-conjugated donkey anti–mouse and Cy3 anti–rabbit IgG (D-E). Bars represent 3 μm (A,D-E) and 5 μm (B-C). Arrows indicate colocalization between HA-TGN38 and serotonin.
Subcellular localization of HA-TGN38 in RBL-Syt IX− cells. RBL-Syt IX− (A-D) or control RBL cells (E) were transiently transfected with HA-TGN38 cDNA or cotransfected with both HA-TGN38 and Rab 11-GFP (B). Cells were grown on glass coverslips for 48 hours and either allowed to internalize Texas red-conjugated Tfn (50 μg/mL) for 5 minutes (A) or 30 minutes (C) prior to their processing for immunofluorescence or left untreated (B) or incubated with serotonin (200 μM) for the last 24 hours of growth to load the SGs (D-E). Cells were subsequently labeled with mouse anti-HA antibodies (A-E) followed by Cy3-conjugated anti–mouse IgG or double-labeled with mouse antiserotonin and rabbit anti-HA antibodies, followed by FITC-conjugated donkey anti–mouse and Cy3 anti–rabbit IgG (D-E). Bars represent 3 μm (A,D-E) and 5 μm (B-C). Arrows indicate colocalization between HA-TGN38 and serotonin.
Because of the close association that exists between the endocytic and the regulated secretory systems in mast cells and because endosomal cargo can reach the SGs of RBL cells,22-24 we next examined whether HA-TGN38 is delivered to SGs in RBL-Syt IX− cells. For this purpose, HA-TGN38–expressing RBL-Syt IX− cells were colabeled for HA and serotonin, a marker of SGs.5,23 Indeed, a substantial colocalization was detected between HA-TGN38 and serotonin (Figure 3D). Notably, double staining of control RBL cells for HA-TGN38 and serotonin confirmed that the fraction of mislocalized HA-TGN38 in these cells also resides on SGs (Figure 3E).
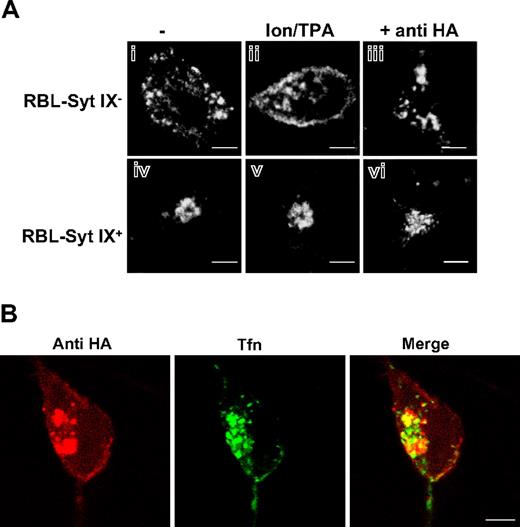
To further confirm the SGs as the site of localization of overexpressed TGN38 in RBL-Syt IX− cells, we examined whether trigger of exocytosis would result in translocation of the protein from its vesicular residence to the plasma membrane as a result of fusion of SGs with the plasma membrane. To this end, cells were exposed to Ca2+ ionophore and the phorbol ester TPA, a combination known to trigger regulated exocytosis in RBL cells.25 Indeed, in triggered cells, a clear translocation of HA-TGN38 from its vesicular localization to the plasma membrane was observed in RBL-Syt IX− cells (Figure 4Ai-ii). In contrast, such translocation could not be detected in RBL-Syt IX+ cells, where HA-TGN38 retained its perinuclear localization also in triggered cells (Figure 4Aiv-v).
Translocation of HA-TGN38 to the plasma membrane. RBL-Syt IX− (Ai-iii) or RBL-Syt IX+ (Aiv-vi,B) were either left untreated (Ai,iv) or triggered with the calcium ionophore A23187 (1 μM) and the phorbol ester TPA (50 nM) for 30 minutes at 37°C (Aii,v), or incubated with monoclonal anti-HA antibodies (2 μg/mL) for 30 minutes at 4°C and subsequently allowed to internalize the surface bound antibodies at 37°C for 30 minutes without (Aiii,vi) or with Alexa Fluor 488-conjugated Tfn (50 μg/mL) (B) Cells were then labeled with monoclonal anti-HA antibodies, followed by Cy3-conjugated donkey anti–mouse IgG (Ai-ii,iv-v) or labeled with Cy3-conjugated donkey anti–mouse IgG (Aiii,vi,B). Bars represent 3 μm (A) and 4 μm (B).
Translocation of HA-TGN38 to the plasma membrane. RBL-Syt IX− (Ai-iii) or RBL-Syt IX+ (Aiv-vi,B) were either left untreated (Ai,iv) or triggered with the calcium ionophore A23187 (1 μM) and the phorbol ester TPA (50 nM) for 30 minutes at 37°C (Aii,v), or incubated with monoclonal anti-HA antibodies (2 μg/mL) for 30 minutes at 4°C and subsequently allowed to internalize the surface bound antibodies at 37°C for 30 minutes without (Aiii,vi) or with Alexa Fluor 488-conjugated Tfn (50 μg/mL) (B) Cells were then labeled with monoclonal anti-HA antibodies, followed by Cy3-conjugated donkey anti–mouse IgG (Ai-ii,iv-v) or labeled with Cy3-conjugated donkey anti–mouse IgG (Aiii,vi,B). Bars represent 3 μm (A) and 4 μm (B).
TGN38 is a type 1 integral membrane protein. Therefore, when passing through the plasma membrane, TGN38 can bind and internalize exogenously added antibodies that are directed against its N-terminal domain. Therefore, to investigate whether trafficking of HA-TGN38 to the SGs in RBL-Syt IX− cells still involves trafficking through the plasma membrane and internalization, the ability of the cells to take up exogenously added antibodies to the HA tag was examined. Following 1 hour of endocytosis, anti-HA IgG was detected in a perinuclear structure in the RBL-Syt IX+ cells, whereas it localized to scattered vesicles in the RBL-Syt IX− cells (Figure 4 Aiii,vi). Allowing the cells to internalize Tfn as well has demonstrated a partial overlap (Figure 4B) suggesting that in a similar fashion to HA-TGN38, the internalizing antibodies were distributed between the ERC and the TGN. Incubation of nontransfected cells with the same antibodies failed to exhibit any signal (data not shown). Taken together, these results have indicated that HA-TGN38 is passing through the plasma membrane in both RBL-Syt IX+ as well as RBL-Syt IX− cells, therefore suggesting that mistargeting of TGN38 in RBL-Syt IX− cells occurs after internalization from the plasma membrane.
Syt IX associates with ARF1 and γ-adaptin
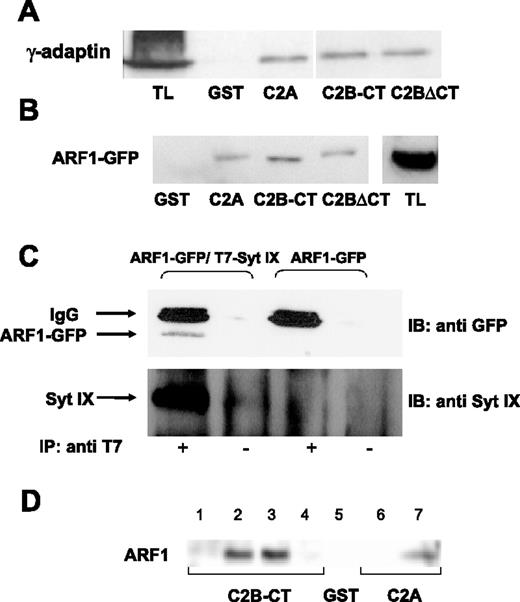
Taken together, our results have implicated Syt IX and the ERC as important determinants in shaping the correct composition of SGs. Because ARF1 and the adaptor complex AP-1 were implicated as playing a role in the removal of cargo from immature granules during their maturation process26,27 and because Syt homologues have been shown to bind clathrin adaptor proteins,28,29 we investigated whether Syt IX might interact with any of these proteins. As depicted in Figure 5, GST fusion proteins comprising either the C2A or the C2B-CT domains of Syt IX were sufficient to pull down γ-adaptin from RBL cell lysates (Figure 5A). Consistent with this notion, also the C2B lacking the carboxy terminus (C2BΔCT) was equally efficient (Figure 5A). These fusion proteins were also capable of ARF1 pull-down from ARF1-GFP–expressing RBL cell lysates (Figure 5B). Quantitative analysis of the results has indicated that 5% to 7% of total ARF1-GFP was pulled down by Syt IX fusion proteins. Moreover, an interaction between Syt IX and ARF1 could also be demonstrated in intact cells expressing ARF1-GFP and T7-tagged Syt IX (Figure 5C). This interaction was specific, as indicated by the finding that antibodies directed against the T7 tag could not immunoprecipitate ARF1-GFP from cells transfected with only ARF1-GFP (Figure 5C). To elucidate whether the interaction between Syt IX and ARF1 is direct, Syt IX fusion proteins were also incubated with recombinant bacteria-expressed ARF1. Consistently, both the C2B and C2A domains bound recombinant ARF1 (Figure 5D). Moreover, binding of recombinant ARF1 was significantly more efficient with 40% of total ARF1 pulled down by the fusion proteins. Because neither myristoylation nor GTP loading was required for this binding, we concluded that Syt IX directly binds inactive ARF1.
Binding of γ-adaptin and ARF1 by Syt IX. (A) Twenty micrograms of GST, GST-Syt IX-C2A, GST-Syt IX C2B-CT, or GST-Syt IX C2BΔCT was immobilized on glutathione-Sepharose beads and incubated for 18 hours at 4°C with RBL cell lysates (500 μg). Bound proteins were eluted in sample buffer; half of the total eluted proteins were resolved by SDS-PAGE and analyzed by Western blot using anti–γ-adaptin antibodies. Total lysate (TL; 100 μg) was loaded. (B) Twenty micrograms of GST, GST-Syt IX-C2A, GST-Syt IX-C2B-CT, or GST-Syt IX C2BΔCT, as indicated, was immobilized and incubated as described except that RBL cells lysates (500 μg) were derived from cells transiently transfected with ARF1-GFP cDNA. Bound proteins were eluted in sample buffer; half of the total eluted proteins were resolved by SDS-PAGE and analyzed by Western blot using anti-GFP antibodies. Total lysate (TL; 100 μg) was loaded. (C) Immunoprecipitation was performed as described in “Materials and methods,” using RBL cell lysates derived from cells cotransfected with T7-Syt IX and ARF1-GFP or only ARF1-GFP cDNAs as indicated and using anti-T7 antibodies (IP antibody). Immune complexes were separated by SDS-PAGE and analyzed by Western blot using anti-GFP or anti–Syt IX antibodies as indicated (IB antibody). (D) Twenty micrograms of GST, GST-Syt IX-C2A, or GST-Syt IX-C2B-CT, as indicated, was immobilized on glutathione-Sepharose beads and incubated for 18 hours at 4°C with buffer (lanes 1 and 6), RBL cell lysates (500 μg, lane 2) DEAE Sephadex A25 effluent derived from ARF1-transformed bacteria (500 μg; lanes 3, 5, and 7) or nontransformed bacteria (500 μg; lane 4). Bound proteins were processed as described.
Binding of γ-adaptin and ARF1 by Syt IX. (A) Twenty micrograms of GST, GST-Syt IX-C2A, GST-Syt IX C2B-CT, or GST-Syt IX C2BΔCT was immobilized on glutathione-Sepharose beads and incubated for 18 hours at 4°C with RBL cell lysates (500 μg). Bound proteins were eluted in sample buffer; half of the total eluted proteins were resolved by SDS-PAGE and analyzed by Western blot using anti–γ-adaptin antibodies. Total lysate (TL; 100 μg) was loaded. (B) Twenty micrograms of GST, GST-Syt IX-C2A, GST-Syt IX-C2B-CT, or GST-Syt IX C2BΔCT, as indicated, was immobilized and incubated as described except that RBL cells lysates (500 μg) were derived from cells transiently transfected with ARF1-GFP cDNA. Bound proteins were eluted in sample buffer; half of the total eluted proteins were resolved by SDS-PAGE and analyzed by Western blot using anti-GFP antibodies. Total lysate (TL; 100 μg) was loaded. (C) Immunoprecipitation was performed as described in “Materials and methods,” using RBL cell lysates derived from cells cotransfected with T7-Syt IX and ARF1-GFP or only ARF1-GFP cDNAs as indicated and using anti-T7 antibodies (IP antibody). Immune complexes were separated by SDS-PAGE and analyzed by Western blot using anti-GFP or anti–Syt IX antibodies as indicated (IB antibody). (D) Twenty micrograms of GST, GST-Syt IX-C2A, or GST-Syt IX-C2B-CT, as indicated, was immobilized on glutathione-Sepharose beads and incubated for 18 hours at 4°C with buffer (lanes 1 and 6), RBL cell lysates (500 μg, lane 2) DEAE Sephadex A25 effluent derived from ARF1-transformed bacteria (500 μg; lanes 3, 5, and 7) or nontransformed bacteria (500 μg; lane 4). Bound proteins were processed as described.
Syt IX, ARF1, and HA-TGN38 colocalize in Syt IX-overexpressing but not Syt IX knocked-down cells
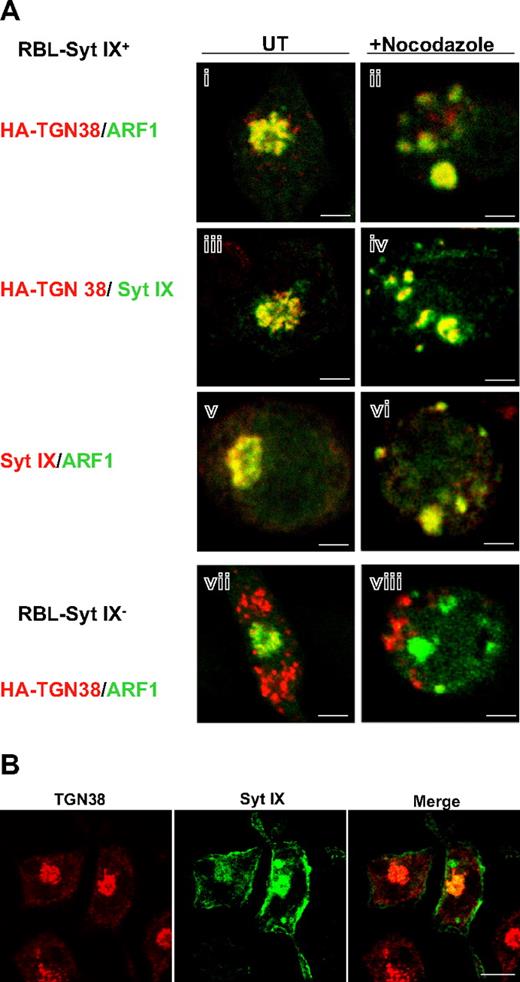
The next set of experiments was aimed at obtaining functional evidence to support the physiologic relevance of the interaction between Syt IX and ARF1. Previously, we found that Syt IX interacts with tubulin and remains associated with it in cells in which the microtubules are disrupted.9 Therefore, we compared the localization of ARF1, Syt IX, and HA-TGN38 in nocodazole-treated Syt IX-overexpressing cells. In those cells, Syt IX redistributed from its original perinuclear localization (Figure 6Aiii,v) to vesicular clusters scattered throughout the cytosol (Figure 6Aiv,vi). Both ARF1 (Figure 6Ai,vi) and HA-TGN38 (Figure 6Aiv) colocalized with Syt IX in these clusters. Furthermore, because colocalization could also be documented between HA-TGN38 and ARF1 (Figure 6Ai,ii), these data confirmed that all 3 proteins (Syt IX, ARF1, and TGN38) remain associated in nocodazole-treated cells. In contrast, in RBL-Syt IX− cells, ARF1 and HA-TGN38 clearly segregated into different structures in both untreated and nocodazole-treated cells (Figure 6Avii,viii). These results imply that Syt IX is required to maintain the colocalization of ARF1 and HA-TGN38. Moreover, because a substantial colocalization was also detected between Syt IX and HA-TGN38 in untreated cells (Figure 6Aiii), we postulated that Syt IX might also distribute between the ERC and the TGN. Indeed, double staining for Syt IX and endogenous TGN38 demonstrated a partial overlap between the 2 proteins (Figure 6B).
Cellular localization of Syt IX, ARF1, and TGN38. (A) RBL-Syt IX+ (i-vi) and RBL-Syt IX− (vii-viii) cells were transiently transfected with ARF1-GFP cDNA (i-ii,v-viii) or with HA-TGN38 cDNA (i-iv,vii,viii). Cells were grown on glass coverslips for 24 hours and either left untreated (UT) or treated with nocodazole (20 μM) for 2 hours, as indicated. Cells were subsequently labeled with monoclonal anti-HA antibodies (i-iv,vii-viii) or polyclonal anti–Syt IX antibodies (iii-vi) followed by Cy3- or FITC-conjugated donkey anti–mouse or anti–rabbit IgG as indicated. Bars represent 3 μm. (B) RBL-Syt IX+ cells were double labeled with polyclonal anti–Syt IX and monoclonal anti-TGN38 antibodies followed by FITC- or Cy3-conjugated donkey anti–mouse or anti–rabbit IgG. Bars represent 8 μm.
Cellular localization of Syt IX, ARF1, and TGN38. (A) RBL-Syt IX+ (i-vi) and RBL-Syt IX− (vii-viii) cells were transiently transfected with ARF1-GFP cDNA (i-ii,v-viii) or with HA-TGN38 cDNA (i-iv,vii,viii). Cells were grown on glass coverslips for 24 hours and either left untreated (UT) or treated with nocodazole (20 μM) for 2 hours, as indicated. Cells were subsequently labeled with monoclonal anti-HA antibodies (i-iv,vii-viii) or polyclonal anti–Syt IX antibodies (iii-vi) followed by Cy3- or FITC-conjugated donkey anti–mouse or anti–rabbit IgG as indicated. Bars represent 3 μm. (B) RBL-Syt IX+ cells were double labeled with polyclonal anti–Syt IX and monoclonal anti-TGN38 antibodies followed by FITC- or Cy3-conjugated donkey anti–mouse or anti–rabbit IgG. Bars represent 8 μm.
GTP-locked ARF1 rescues the defect in TGN38 trafficking in Syt IX knocked-down cells
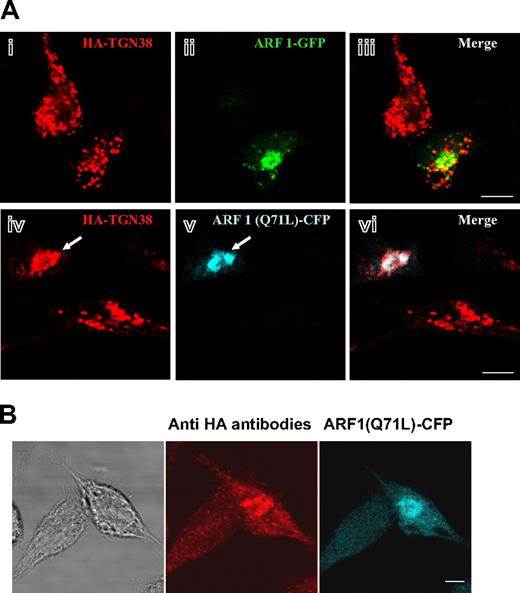
To establish further the functional link between Syt IX and ARF1 we assessed the ability of ARF1 to rescue the defect in HA-TGN38 trafficking in Syt IX knocked-down cells. Expression of ARF1-GFP in RBL-Syt IX− cells had no impact on the vesicular localization of HA-TGN38 (Figure 7Ai-iii), consistent with the notion that Syt IX is the limiting factor in the membrane recruitment of ARF1. However, in stark contrast, in cells expressing the GTPase-defective mutant of ARF1, ARF1(Q71L), HA-TGN38 has lost its vesicular appearance and has clearly acquired a perinuclear localization (Figure 7A,iv-vi). ARF1(Q71L) causes irreversible coating of membranes with COPI, therefore leading to the accumulation of newly synthesized proteins in pre-Golgi intermediates or between successive Golgi compartments.30 However, cells coexpressing ARF1(Q71L) and HA-TGN38 were able to internalize exogenous anti-HA antibodies (Figure 7B), therefore indicating that part of HA-TGN38 has reached the plasma membrane and internalized and yet none of it was delivered to SGs in cells expressing ARF1(Q71L).
Inhibition of ERC-to-MVBs/SGs transport in RBL-Syt IX− cells by a GTPase-deficient mutant of ARF1. (A) RBL-Syt IX− cells were cotransfected with HA-TGN38 and either ARF1-GFP (i-iii) or ARF1(Q71L)-CFP (iv-vi) cDNA and grown on glass coverslips for 24 hours. Cells were labeled with monoclonal anti-HA antibodies followed by Cy3-conjugated donkey anti–mouse IgG. Bars equal 5 μm. The arrow points to a cell double-transfected with HA-TGN38 and ARF1(Q71L)-CFP. (B) RBL-Syt IX− cells were double-transfected with HA-TGN38 and ARF1(Q71L)-CFP cDNA and grown on glass coverslips for 48 hours. Cells were then incubated with monoclonal anti-HA antibodies (2 μg/mL) for 30 minutes at 4°C, washed, and subsequently incubated at 37°C for 30 minutes. Cells were labeled with Cy3-conjugated donkey anti–mouse IgG. Bars represent 8 μm. The phase-contrast image is shown on the left.
Inhibition of ERC-to-MVBs/SGs transport in RBL-Syt IX− cells by a GTPase-deficient mutant of ARF1. (A) RBL-Syt IX− cells were cotransfected with HA-TGN38 and either ARF1-GFP (i-iii) or ARF1(Q71L)-CFP (iv-vi) cDNA and grown on glass coverslips for 24 hours. Cells were labeled with monoclonal anti-HA antibodies followed by Cy3-conjugated donkey anti–mouse IgG. Bars equal 5 μm. The arrow points to a cell double-transfected with HA-TGN38 and ARF1(Q71L)-CFP. (B) RBL-Syt IX− cells were double-transfected with HA-TGN38 and ARF1(Q71L)-CFP cDNA and grown on glass coverslips for 48 hours. Cells were then incubated with monoclonal anti-HA antibodies (2 μg/mL) for 30 minutes at 4°C, washed, and subsequently incubated at 37°C for 30 minutes. Cells were labeled with Cy3-conjugated donkey anti–mouse IgG. Bars represent 8 μm. The phase-contrast image is shown on the left.
Discussion
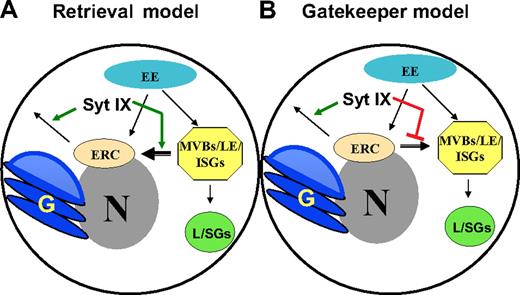
The tight linkage that exists between the SGs of mast cells and the endocytic system is well established. Both biochemical evidence demonstrating the delivery of internalized cargo to SGs and morphologic electron microscopic (EM) analyses have documented this relationship.5,22-24 In particular, EM analyses implicate multivesicular bodies (MVBs) and late endosomes as potential intermediates in the biogenesis process of these lysosome-related SGs.5,31 However, we have previously shown that in cells in which the level of Syt III is reduced and the formation of the pericentriolar ERC is hampered, the biogenesis of SGs is affected.8 The ERC, a distinct endocytic organelle, has been implicated in receptor and lipid recycling;14,32,33 as well as in the cycling of endogenous proteins such as TGN3811,13 from the plasma membrane to the TGN and in the retrograde transport of toxins, such as the Shiga toxin,34,35 from the plasma membrane to the ER. Therefore, our findings have imposed an additional role for the ERC, namely, in the biogenesis of the SGs, presumably by mediating the maturation of the immature granules, by removing nondestined proteins. In this study, we have challenged this concept by perturbing the recycling process from the ERC and analyzing its impact on the SGs. Previously, we demonstrated that in Syt IX knocked-down RBL cells, recycling of internalized Tfn from the ERC to the cell surface is inhibited.9 In this study we show that in Syt IX knocked-down RBL cells HA-tagged TGN38 travels through the plasma membrane; however, instead of reaching the TGN, it is mistargeted and delivered to SGs. Notably, expressing equal amounts of HA-TGN38 in Syt IX-overexpressing cells results in its exclusive targeting to the ERC and TGN. Therefore, the degree of ERC/TGN targeting coincides with the levels of Syt IX expression and therefore presumably with the efficiency of recycling through the ERC. Indeed, mistargeting of TGN38 in the RBL-Syt IX− cells occurs at a step distal to its internalization from the plasma membrane. This conclusion is based on the findings that HA-TGN38-expressing RBL-Syt IX− cells can internalize antibodies directed against the HA tag. Therefore, our results are compatible with 2 models (Figure 8), which are not mutually exclusive. According to the first model, Syt IX is needed for the retrieval of a fraction of overexpressed TGN38, which is delivered from early endosomes to MVBs/late endosomes, rather than to the ERC (model A). According to this model, perturbation of endocytic recycling by knock-down of Syt IX impairs the retrieval process, thus resulting in the lasting of TGN38 in the MVBs, which eventually mature to form the SGs. Consistent with this model, immunomicroscopy and EM analyses have demonstrated that recycling cargo, such as Tfn, is present in both the tubulovesicles of the recycling compartment and the tubular extensions or budding vesicles of MVBs,36 therefore indicating that maturing MVBs continue to function as sorting endosomes for a considerable time, until their maturation is almost complete. According to the alternative model, Syt IX plays a dual role both in facilitating export from the ERC to the plasma membrane and in preventing retrograde transport from the ERC to MVBs (model B). According to this model, TGN38 is exclusively targeted to the ERC from the early endosomes; however, the combination of inhibition of recycling and removal of the ERC to MVBs transport barrier, by the knock-down of Syt IX, results in the spillover of TGN38 to the MVBs leading to its subsequent localization in the lysosome-related SGs (model B). Notably, endogenous TGN38 maintains its TGN localization in RBL-Syt IX− cells, suggesting that the residual amount of Syt IX present in the knocked-down cells suffices to support its correct targeting. However, in HA-TGN38–expressing RBL-Syt IX− cells, both the endogenous and the exogenous proteins mislocalize, indicating that both proteins take similar routes. Interestingly, in sharp contrast to its delivery to SGs in mast cells, in HeLa cells, blocking of endosome to TGN transport results in the disappearance of TGN46, most likely due to its deviation to the endocytic degradative pathway and lysosomal degradation.37 Therefore, our results unveil a novel mechanism, which allows secretory cells of the immune system to recycle proteins back to the plasma membrane from the lysosomal endocytic system under conditions that the recycling system is impaired or overloaded.
Models suggesting possible roles for Syt IX in controlling composition of SGs. (A) According to the “Retrieval” model, Syt IX regulates the transport of carriers destined to exit the ERC and the retrieval of recycling cargo from the MVBs/ISGs to the ERC. (B) According to the “Gatekeeper” model, Syt IX regulates export from the ERC and prevents ERC to MVBs/ISGs transport. N indicates nucleus; G, Golgi apparatus; L, lysosome; ERC, endocytic recycling compartment; ISG, immature secretory granule; EE, early endosome; LE, late endosome; MVB, multivesicular body.
Models suggesting possible roles for Syt IX in controlling composition of SGs. (A) According to the “Retrieval” model, Syt IX regulates the transport of carriers destined to exit the ERC and the retrieval of recycling cargo from the MVBs/ISGs to the ERC. (B) According to the “Gatekeeper” model, Syt IX regulates export from the ERC and prevents ERC to MVBs/ISGs transport. N indicates nucleus; G, Golgi apparatus; L, lysosome; ERC, endocytic recycling compartment; ISG, immature secretory granule; EE, early endosome; LE, late endosome; MVB, multivesicular body.
Finally, we show that Syt IX interacts with the clathrin adaptor complex AP-1 and with ARF1. These adaptor proteins have been implicated in recycling from the ERC38,39 as well as in the maturation process of immature SGs.26,27 Therefore, Syt IX might play a role in the assembly of a complex that facilitates transport from immature SGs to the ERC (model A in Figure 8), export from the ERC to the plasma membrane (model B in Figure 8), or both. Strikingly, we show that in Syt IX knocked-down cells, which express the GTP-locked mutant of ARF1, ARF1(Q71L), HA-TGN38 acquires a perinuclear appearance. Thus, overexpression of the dominant active ARF1 in Syt IX knocked-down cells prevents HA-TGN38 from trafficking to SGs. These results favor the model whereby Syt IX, presumably by binding ARF1 and by coat recruitment, serves as a “gatekeeper” of the recycling arm of the endocytic system, preventing ERC-to-MVBs transport (model B in Figure 8). By this model, by stabilizing the coat, ARF1(Q71L) can replace Syt IX in preventing ERC-to-MVBs trafficking.
In conclusion, in the present work we demonstrate that in mast cells, Syt IX is an essential determinant in the biogenesis of SGs. Syt IX may thus play in mast cells a role homologous to the one played by Syt IV in SG maturation in PC12 cells.40,41 We further show that ARF1 and AP-1 serve as downstream effectors of Syt IX.
Authorship
Contribution: Y.H. designed many experiments, performed research, and analyzed data; I.Z. designed many experiments, performed research, and analyzed data; Y.G. was involved in ARF1 purification; K.H. contributed significantly to the microscopy studies; L.M. assisted in all confocal microscopy studies; M.F. contributed essential reagents; and R.S.-E. designed the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Y.H. and I.Z. contributed equally to this work.
Correspondence: Ronit Sagi-Eisenberg, Department of Cell and Developmental Biology, Sackler School of Medicine, Tel Aviv University Tel Aviv, 69978, Israel; e-mail: histol3@post.tau.ac.il.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants from the Israel Science Foundation, founded by the Israel Academy for Sciences and Humanities (R.S.-E.) and the Constantiner Institute (Y.H.).
We thank Drs J. Bonifacino, M. Zerial, J. Lippincott-Schwartz, M. A. De Matteis, M. S. Robinson, and J. Donaldson for their generous gifts of cDNAs and antibodies. We thank Dr D. Cassel for his help in ARF1 purification.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal