Abstract
Infection with the human immunodeficiency virus type-1 (HIV) results in acute and progressive numeric loss of CD4+ T-helper cells and functional impairment of T-cell responses. The mechanistic basis of the functional impairment of the surviving cells is not clear. Indoleamine 2,3-dioxygenase (IDO) is an immunosuppressive enzyme that inhibits T-cell proliferation by catabolizing the essential amino acid tryptophan (Trp) into the kynurenine (kyn) pathway. Here, we show that IDO mRNA expression is elevated in peripheral blood mononuclear cells (PBMCs) from HIV+ patients compared with uninfected healthy controls (HCs), and that in vitro inhibition of IDO with the competitive blocker 1-methyl tryptophan (1-mT) results in increased CD4+ T-cell proliferative response in PBMCs from HIV-infected patients. We developed an in vitro model in which exposure of PBMCs from HCs to either infectious or noninfectious, R5- or X4-tropic HIV induced IDO in plasmacytoid dendritic cells (pDCs). HIV-induced IDO was not inhibited by blocking antibodies against interferon type I or type II, which, however, induced IDO in pDCs when added to PBMC cultures. Blockade of gp120/CD4 interactions with anti-CD4 Ab inhibited HIV-mediated IDO induction. Thus, induction of IDO in pDCs by HIV may contribute to the T-cell functional impairment observed in HIV/AIDS by a non–interferon-dependent mechanism.
Introduction
The immunologic hallmark of the acquired immunodeficiency syndrome (AIDS), resulting from infection with the human immunodeficiency virus type-1 (HIV), is the depletion of CD4+ T cells.1 However, qualitative alterations of the function of circulating T cells are observed that do not appear to be related to the decline of CD4+ T-cell number.2-5 In vitro T-cell responses are impaired in peripheral blood mononuclear cells (PBMCs) from HIV-infected patients. Thus, proliferative responses to HIV epitopes are lost early during infection,6-8 followed by sequential impairment of T-helper cell responses to recall antigens and mitogens.9 This progressive loss of T-cell function during the course of HIV disease is predictive for the time of onset of AIDS and death.10
Tryptophan (Trp) catabolism mediated by indoleamine 2,3-dioxygenase (IDO) is an immunoregulatory mechanism that limits T-cell proliferation by depletion of the essential amino acid Trp and/or accumulation of catabolites with immune-suppressive activity (kynurenines, kyn).11 Alterations of this mechanism have been suggested to be involved in (1) development of autoimmune conditions, such as multiple sclerosis and autoimmune diabetes12,13 ; (2) failure of immune surveillance of tumor cells14 ; and (3) rejection of semiallogenic fetuses.15,16 The molecular basis for T-cell hyporesponsiveness in IDO-mediated Trp depletion has been recently clarified. The consequence of reduction in available Trp in the extracellular microenvironment is the accumulation of uncharged Trp-specific transfer RNA (free-tRNAtrp) in the cytoplasm.17 Free-tRNAtrp binds to the GCN2 kinase, a key enzyme of the cellular stress-response system.17 Once activated by ligation to free-tRNAtrp, the GCN2 kinase initiates a cascade of events leading to arrest of the cell cycle, which is, in turn, the ultimate effect of tryptophan starvation.17
Increased IDO-mediated tryptophan catabolism during HIV infection has been reported.18-21 IDO is induced in macrophages by HIV infection, and has been suggested to be involved in the induction of HIV encephalitis and AIDS-related dementia.20,22,23 Inhibition of HIV-induced IDO in brain macrophages enhanced HIV-specific cytotoxic T-lymphocyte (CTL) response and elimination of infected macrophages in a murine model.24 We recently reported that IDO mRNA expression is increased in the tonsils of HIV-infected patients in whom viral replication is not controlled by effective antiretroviral therapy (ART).25 A similar increase of IDO was found in lymphoid tissues of macaques during acute simian immunodeficiency virus (SIV) and chronic SIV/HIV infection, and correlated with reduced immune responses.26,27 However, the functional role of IDO in HIV-associated immunosuppression is unknown.
In the present study, we tested the effect of 1-methyl-tryptophan (1-mT), a competitive inhibitor of IDO, on the stimulation of PBMCs from HIV-infected (HIV+) patients with phytohemagglutinin (PHA) and the activating antibodies anti-CD3 and anti-CD28 (anti-CD3/28). We found that 1-mT restored T-cell responses, suggesting that IDO is involved in the impairment of T-cell function. Proliferation of CD4+ T cells, but not CD8+ T cells, was enhanced by 1-mT. We then developed an in vitro model of induction of IDO in PBMCs from HIV-uninfected donors by exposure to infectious or noninfectious (inactivated with aldrithiol-2 [AT-2]) HIV. We demonstrate that plasmacytoid dendritic cells (pDCs) are responsible for IDO expression under these conditions, and that HIV gp120-CD4 interaction is required for IDO induction.
Materials and methods
Blood donors and cell isolation
PBMCs were isolated by density centrifugation (Cambrex, Walkersville, MI) from citrate-anticoagulated peripheral blood obtained from healthy donors (HCs), under an NIH IRB-approved protocol developed by the Department of Transfusion Medicine, NIH (Bethesda, MD); and from HIV+ patients who were involved in the USAF Natural History Study, under protocols that were reviewed and approved by the institutional review boards of the USAF Wilford Hall Medical Center (Lackland AFB, TX) and of the National Cancer Institute (Bethesda, MD). Informed consent was provided according to the Declaration of Helsinki. Viral loads and CD4 counts are shown in Table 1 for patients who were or were not receiving antiretroviral therapy. None of the patients included in the study had received interleukin-2 as part of the treatment. Cells were cultured in RPMI medium (Invitrogen, Gaithersburg, MD) with 10% fetal bovine serum (Hyclone, Logan, UT). The culture medium is a source of all essential amino acids, including Trp at a concentration of 5mg/L.
Clinical data of HIV+ patients
| . | ART, 2 RTIs + 1 PI . | No ART . | P . |
|---|---|---|---|
| No. patients | 45 | 25 | |
| Viral load, copies/mL | < .001 | ||
| Min | < 50 | 2 780 | |
| Median | < 50 | 25 600 | |
| Max | 422 | 317 000 | |
| CD4 count, cells/mm3 | < .001 | ||
| Min | 342 | 321 | |
| Median | 751 | 542 | |
| Max | 1701 | 846 |
| . | ART, 2 RTIs + 1 PI . | No ART . | P . |
|---|---|---|---|
| No. patients | 45 | 25 | |
| Viral load, copies/mL | < .001 | ||
| Min | < 50 | 2 780 | |
| Median | < 50 | 25 600 | |
| Max | 422 | 317 000 | |
| CD4 count, cells/mm3 | < .001 | ||
| Min | 342 | 321 | |
| Median | 751 | 542 | |
| Max | 1701 | 846 |
N = 70.
RTI indicates reverse transcriptase inhibitors; PI, protease inhibitors.
Plasmacytoid dendritic cells (CD4+CD123+BDCA2+) were enriched from PBMCs using BDCA4 magnetic beads (Miltenyi Biotech, Auburn, CA) according to the manufacturer's instructions.
Preparation of AT-2–inactivated virions
All viruses used in this study were kindly provided by Dr Jeffrey D. Lifson (AIDS Vaccine Program, SAIC Frederick, NCI-Frederick, Frederick, MD). HIVMN (X4-tropic) and HIVAda (R5-tropic) were propagated as described previously.28 Virus-containing supernatants were treated with 1 mM 2,2′-dithiodipyridine (AT-2) for 18 hours at 4°C. This treatment has been shown to eliminate infectivity of retroviral virions by preferential covalent modification of internal virion proteins including the nucleocapsid protein, while preserving functionally intact envelope glycoproteins on retroviral virions.28-30 AT-2–treated virus preparations were concentrated and purified, and residual unreacted AT-2 was removed by sucrose gradient purification.30 Microvesicles (MVs), used as a control reagent, were isolated from supernatants of matched uninfected cell cultures in a manner identical to that used for virus preparation from infected cells.31
Incubation of PBMCs with viruses, CTLA-4-Fc, CD28-Fc, and blocking antibodies
PBMCs from HIV-uninfected donors were cultured with AT-2 HIV or the corresponding untreated infectious HIV at a final concentration of 500 ng/mL p24CA equivalent, in RPMI-1640 medium (Trp concentration = 5 mg/L). CTLA-4-Fc or CD28-Fc (R&D Systems, Minneapolis, MN) was added to the PBMC cultures at a final concentration of 4 μg/mL. Blocking anti–IFN-α (Invitrogen) and anti–IFN-β polyclonal Ab (Invitrogen) were used at a final concentration of 6 × 105 blocking units/mL and 2 × 105 blocking units/mL, respectively. Blocking anti–IFN-γ mAb (R&D Systems) was used at a final concentration of 5 μg/mL. Interaction between gp120 and CD4 was blocked with anti-CD4 mAb B432 (NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, Bethesda, MD; Cell Surface CD4 Complex Monoclonal B4 was from United Biomedical, Hauppauge, NY) at a final concentration of 1 μg/mL. 1-Methyl-d-tryptophan (1-mT; Sigma, St Louis, MO) was added to the culture at a final concentration of 200 μM.
Bioreduction cell proliferation assay
PBMCs isolated from HIV+ patients and HCs were cultured in presence or absence of 1-mT (200 μM). At the same time, cells were stimulated with 0.5% phytohemagglutinin A (PHA; Invitrogen) or with the OKT3 activating monoclonal antibodies anti-CD3 and anti-CD28 (both from eBioscience, San Diego, CA) at final concentration of 1 μg/mL each. After 72 hours, proliferation was assessed as the increase in the number of viable cells, using the CellTiter 96 AQueous One Solution Proliferation Assay (Promega, Madison, WI), according to the manufacturer's instructions. Titration curves made with serial dilution of cells were not modified by addition of 1-mT, demonstrating that the compound does not influence the assay.
CD4+ and CD8+ T-cell proliferation by flow cytometry
Total PBMCs from HIV+ patients were stained with 5 μM CFSE and cultured at a density of 2 × 106 cells/mL. CFSE-labeled cells were stimulated with anti-CD3/28 (1 μg/mL) or PHA (0.5%) in presence or absence of 1-mT (200 μM). Control experiments with unstimulated cells with or without 1-mT were performed. After 6 days of culture, cells were washed twice and incubated for 20 minutes at room temperature with peridinin chlorophyll (PerCP)–conjugated anti-CD8, phycoerythrin-cyanine (PE-Cy7)–conjugated anti-CD3, allophycocyanin (APC)–Cy7 anti-CD4 (BD Biosciences, San Jose, CA), or with isotype-matched control antibodies (at 5 μg/mL each; BD Biosciences) in PBS containing 2% mouse serum (Sigma). Cells were acquired on a FACSCanto flow cytometer using FACSDiva software (BD Biosciences). Fluorescence-activated cell sorter (FACS) analysis was preformed using FlowJo software (Treestar, Ashland, OR). Results are shown as histograms of CFSE mean florescence intensity (MFI) on the x-axis and distribution units of cell counts on the y-axis.
RNA extraction and reverse transcription
Total RNA was extracted from unseparated PBMCs using the guanidium thiocyanate–phenol–chloroform method, modified for TRIzol (Invitrogen). RNA (1 μg) was reverse transcribed into first-strand cDNA in a 20-μL reaction containing 1 μM random hexanucleotide primers, 1 μM oligo dT, and 200 U Moloney murine leukemia virus reverse transcriptase (Promega).
Real-time PCR
cDNA quantification for IDO, interferon-β1 (IFN-β), IFN-γ, and glyceraldehyde 3-phosphate dioxygenase (GAPDH) was obtained using a real-time polymerase chain reaction (PCR) technique. Real-time PCR was conducted with the ABI Prism 7900HT (Applied Biosystems, Foster City, CA). All reactions were performed using a SYBR green PCR mix (Qiagen, Valencia, CA), according to the following thermal profile: denaturation at 95°C for 15 seconds, annealing at 60°C for 15 seconds, and extension at 72°C for 15 seconds (data collection was performed during the extension step). The primer sequences were designed using the Primer Express Software v2.0 (Applied Biosystems) provided with the ABI Prism 7900HT (IDO: forward, 5-AGTCCGTGAGTTTGTCCTTTCAA-3 and reverse, 5-TTTCACACAGGCGTCATAAGCT-3; IFN-γ: forward, 5-TCATCCAAGTGATGGCTGAACT-3 and reverse, 5-CGAAACAGCATCTGACTCCTTGT-3; IFN-β: forward, 5-TTCACCAGGGGAAAACTCAT-3 and reverse, 5-TCCTTGGCCTTCAGGTAATG-3; GAPDH: forward, 5-CCACCCATGGCAAATTCC-3 and reverse, 5-TGGGATTTCCATTGATGACAAG-3). All reactions were performed in triplicate. Data analysis was performed with the SDS2.1 software (Applied Biosystems), provided with the ABI Prism 7900HT. The threshold level was determined by the software according to the optimization of the baseline and the standard curve. Results are presented as ratios between the target gene mRNA and the GAPDH mRNA.
Tryptophan and kynurenine concentration measurement by HPLC
Detection of tryptophan and kynurenine was performed by high-performance liquid chromatography (HPLC) on supernatants from PBMC culture as previously described.33
Detection of IDO in pDCs by flow cytometry
Total PBMCs from HIV+ patients or HCs cultured 24 hours with HIVMN or AT-2 HIVMN were tested for intracellular IDO expression in pDCs. After 2 washes, cells were incubated for 20 minutes at room temperature with fluorescein isothiocyanate (FITC)–conjugated monoclonal antibody anti–human CD123 (MBL International, Woburn, MA), PerCP-conjugated anti-CD8, PE-Cy7–conjugated anti-CD3, APC-conjugated anti-BDCA2 antibody (Miltenyi Biotech), APC-Cy7 anti-CD4 (BD Biosciences), or with isotype-matched control antibodies (at 5 μg/mL each; BD Biosciences) in PBS containing 2% mouse serum (Sigma). Cells were washed twice in ice-cold PBS, and intracellular staining was performed using Cytofix/Cytoperm (BD Biosciences) according to the manufacturer's instructions. Briefly, cells were fixed for 20 minutes at 4°C and then stained for 20 minutes with rabbit anti-IDO antibody (Axxora, San Diego, CA). The cells were then stained with PE-conjugated goat antirabbit antibody for 20 minutes and acquired on a FACSCanto flow cytometer using FACSDiva software (BD Biosciences). FACS analysis was preformed using FlowJo software (Treestar). Results are presented as histograms showing the MFI for the relevant staining on the x-axis and the cell count normalized on the maximum cell number detected (% of max cell count) on the y-axis.
Statistical analysis
Statistical analyses were performed using the SPSS 13.0 software (SPSS, Chicago, IL). Differences between treated and untreated cells were assessed using a 2-tailed paired Student t test. Differences between HIV-infected and uninfected donors were assessed using a nonparametric 2-tailed Mann-Whitney U test. Correlations were determined with Spearman rank correlation.
Results
Increased IDO expression impairs proliferative responses in HIV-infected patients
Increased tryptophan catabolism was shown to be associated with HIV infection, since the plasma of HIV+ patients exhibits an increase in kynurenine and a corresponding decrease in tryptophan.18,21 However, changes in expression of IDO in peripheral leukocytes of HIV+ patients have not been reported. We compared IDO mRNA expression in unfractionated PBMCs from 20 HIV+ patients and 6 HCs. Ten of the patients were receiving antiretroviral therapy and had undetectable viral load (HIV+ patients ART). We found a significant increase in IDO mRNA in PBMCs from HIV+ patients compared with HCs (Figure 1A). In contrast, HIV+ patients ART showed IDO mRNA levels comparable with HCs. Remarkably, we found that IDO mRNA levels directly correlated with plasma viral load in the HIV+ patients (Figure 1B). To test whether IDO overexpression could affect proliferative responses in HIV+ patients, we stimulated PBMCs from 38 HIV+ patients and 12 HCs with PHA or anti-CD3/28 in the presence or absence of 1-mT. After 3 days of culture, proliferation was evaluated for changes in the number of viable cells. PBMCs from HIV+ patients proliferated less than cells from HCs in response to PHA and to anti-CD3/28 (Figure 1C and D, respectively, open bars). The addition of 1-mT to the cultures increased the proliferation of PBMCs in HIV+ patients, in response to both PHA and anti-CD3/28, to levels comparable with HCs (Figure 1C and D, respectively, solid bars). Treatment with 1-mT showed no effect on PBMCs from HCs, suggesting that the IDO immunomodulatory effect is minimal in the blood of healthy individuals. These data indicate that increased IDO expression in PBMCs from HIV+ patients contributes to the impairment of T-cell proliferation in response to mitogenic stimuli, and that this effect can be reversed by 1-mT.
Increased IDO in PBMCs from HIV+ patients impairs T-cell proliferative responses. (A) IDO mRNA expression, measured by real-time quantitative PCR, in PBMCs from HIV+ patients who were or were not receiving ART (HIV+ ART and HIV+ no ART, respectively) and HCs; horizontal bars within boxes correspond to the median; box limits correspond to the 25th and 75th percentiles; vertical lines extend to the 10th and 90th percentiles. (B) Direct correlation between IDO mRNA expression and viral load (log10 copies/mL) in HIV+ patients; *= for patients with undetectable viral load a value of 50 copies/mL (detection limit) has been used. (C-D) Proliferative response to PHA (C) and activating antibodies recognizing CD3 and CD28 (D) in PBMCs from HIV+ patients and HCs in cultures without (open bars) and with (solid bars) 1-mT; number of viable cells was measured by bioreduction colorimetric assay; relative cell number was calculated for each sample as ratio between stimulated (with PHA or anti-CD3/CD28) and unstimulated culture, in presence or absence of 1-mT; mean values ± SE are shown. (E) The increase of proliferation was calculated as a ratio between proliferative response in presence and in absence of 1-mT; values higher than 1 indicate that proliferation was increased in presence of 1-mT. 1-mT–induced increase in proliferation in HIV+ patients receiving ART compared with HIV+ patients not receiving ART (no ART); horizontal bars within boxes correspond to the median; box limits correspond to the 25th and 75th percentiles; vertical lines extend to the 10th and 90th percentiles.
Increased IDO in PBMCs from HIV+ patients impairs T-cell proliferative responses. (A) IDO mRNA expression, measured by real-time quantitative PCR, in PBMCs from HIV+ patients who were or were not receiving ART (HIV+ ART and HIV+ no ART, respectively) and HCs; horizontal bars within boxes correspond to the median; box limits correspond to the 25th and 75th percentiles; vertical lines extend to the 10th and 90th percentiles. (B) Direct correlation between IDO mRNA expression and viral load (log10 copies/mL) in HIV+ patients; *= for patients with undetectable viral load a value of 50 copies/mL (detection limit) has been used. (C-D) Proliferative response to PHA (C) and activating antibodies recognizing CD3 and CD28 (D) in PBMCs from HIV+ patients and HCs in cultures without (open bars) and with (solid bars) 1-mT; number of viable cells was measured by bioreduction colorimetric assay; relative cell number was calculated for each sample as ratio between stimulated (with PHA or anti-CD3/CD28) and unstimulated culture, in presence or absence of 1-mT; mean values ± SE are shown. (E) The increase of proliferation was calculated as a ratio between proliferative response in presence and in absence of 1-mT; values higher than 1 indicate that proliferation was increased in presence of 1-mT. 1-mT–induced increase in proliferation in HIV+ patients receiving ART compared with HIV+ patients not receiving ART (no ART); horizontal bars within boxes correspond to the median; box limits correspond to the 25th and 75th percentiles; vertical lines extend to the 10th and 90th percentiles.
We calculated the ratio between the proliferative response of PBMCs in HIV+ patients to PHA in presence and in absence of 1-mT, an indicator of the effect of 1-mT in reversing IDO-mediated immune suppression. Of interest, we found that the increase in proliferation induced by 1-mT in PBMCs from HIV+ patients stimulated with PHA directly correlated with the CD4 count (R2 = 0.44, P = .001; data not shown), and was significantly higher in patients undergoing ART (n = 24/38) compared with therapy-free patients (no ART; n = 14/38) (Figure 1E). Thus, IDO-mediated impairment of T-cell responses in HIV+ patients can be corrected only in patients with higher number of CD4+ T cells, and ART favors 1-mT–mediated enhancement of immune response.
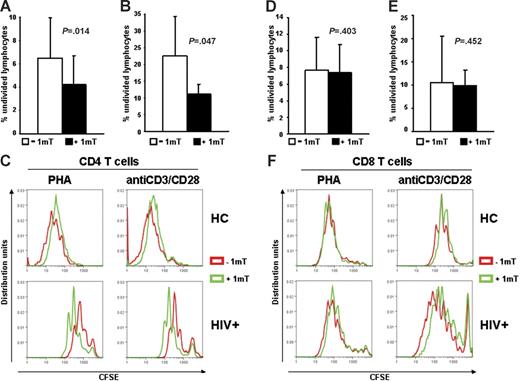
CD4+, but not CD8+, T-cell proliferation is inhibited by IDO in HIV+ patients
We tested whether increased IDO in HIV+ patients has different effects on CD4+ and CD8+ T cells. PBMCs from HIV+ patients were stimulated with PHA or anti-CD3/28 in presence or absence of 1-mT. Cells were stained with CFSE, anti-CD4, and anti-CD8 antibodies, and proliferation was evaluated by flow cytometry after 6 days. Proliferation of both CD4+ and CD8+ T cells from HIV+ patients was reduced compared with HCs (Figure 2C and F, respectively), as described by others.4 The average percentage of CD4+ T cells that had not undergone cell division was significantly reduced in PBMCs of HIV+ patients (Figure 2A–C), but not HCs (Figure 2C), stimulated in presence of 1-mT. Of interest, proliferation of CD8+ T cells from PBMCs of HIV+ patients in response to PHA or anti-CD3/28 was not affected by 1-mT (Figure 2D–F). These data suggest that HIV-induced IDO preferentially inhibits CD4+ T-cell proliferation and that other mechanisms, independent of IDO, may account for the functional defect of CD8+ T cells during HIV infection.
Proliferation of CD4+, but not CD8+ T cells from HIV+ patients is increased by 1-mT. (A-B) Frequency of nondividing cells, measured by CFSE dilution after 6 days of culture, in CD4+ T cells of PBMCs from HIV+ patients stimulated with PHA (A) or anti-CD3/28 (B) in presence or absence of 1-mT. (C) Individual results for 1 of 22 HIV+ patients and 1 of 3 HCs tested are shown for CD4+ T-cell proliferation in response to both PHA and anti-CD3/28 stimulation. (D-E) Frequency of nondividing cells, measured by CFSE dilution after 6 days of culture, in CD8+ T cells of PBMCs from HIV+ patients stimulated with PHA (D) or anti-CD3/28 (E) in presence or absence of 1-mT. (F) Individual results for 1 of 22 HIV+ patients and 1 of 3 HCs tested are shown for CD8+ T-cell proliferation in response to both PHA and anti-CD3/28 stimulation. For panels A-B and D-E, mean values (n = 22) ± SE are shown.
Proliferation of CD4+, but not CD8+ T cells from HIV+ patients is increased by 1-mT. (A-B) Frequency of nondividing cells, measured by CFSE dilution after 6 days of culture, in CD4+ T cells of PBMCs from HIV+ patients stimulated with PHA (A) or anti-CD3/28 (B) in presence or absence of 1-mT. (C) Individual results for 1 of 22 HIV+ patients and 1 of 3 HCs tested are shown for CD4+ T-cell proliferation in response to both PHA and anti-CD3/28 stimulation. (D-E) Frequency of nondividing cells, measured by CFSE dilution after 6 days of culture, in CD8+ T cells of PBMCs from HIV+ patients stimulated with PHA (D) or anti-CD3/28 (E) in presence or absence of 1-mT. (F) Individual results for 1 of 22 HIV+ patients and 1 of 3 HCs tested are shown for CD8+ T-cell proliferation in response to both PHA and anti-CD3/28 stimulation. For panels A-B and D-E, mean values (n = 22) ± SE are shown.
Infectious or noninfectious HIV increases IDO in vitro
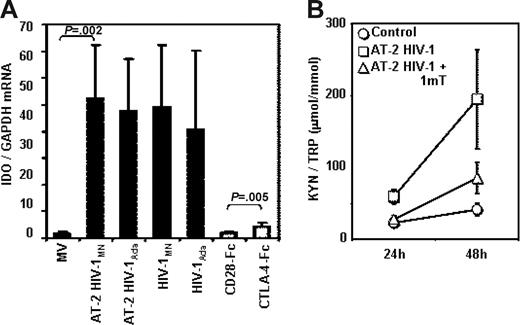
PBMCs from HCs were cultured in the presence of infectious HIV or AT-2 HIV to establish whether the overexpression of IDO that we observed in PBMCs from HIV+ patients is the result of their direct exposure to HIV particles. Figure 3A shows that exposure to AT-2 HIV or infectious HIV induced IDO mRNA expression in PBMCs. We used both an X4-tropic (HIVMN) and an R5-tropic (HIVAda) strain, and obtained similar levels of IDO induction. For comparative purposes, we cultured PBMCs in the presence of CTLA-4-Fc (CD28-Fc served as negative control), and found that both infectious and noninfectious HIVs are approximately 10-fold more potent than CTLA-4 in inducing IDO mRNA expression (Figure 3A). The IDO enzymatic activity, measured as kyn/Trp ratio, was significantly augmented in the supernatants of PBMCs cultured with AT-2 HIV compared with controls after 24 and 48 hours of culture (P = .005 and P = .01, respectively), and was reduced by 1-mT (P = .001 and P = .009 respectively, compared with AT-2 HIV) (Figure 3B). These results demonstrate that both infectious and noninfectious HIV increase degradation of Trp into kyn by increasing IDO gene expression. The observation that infectious and AT-2 HIV had similar effect on IDO expression indicates that productive infection is not required for HIV-induced IDO. Furthermore, the ability to induce IDO is not selective for CXCR4 or CCR5 coreceptor-using viruses, since both HIVMN and HIVAda isolates induced equal increases of IDO mRNA.
Infectious or AT-2 HIV induces IDO mRNA expression and enzymatic activity in PBMCs of HIV-uninfected donors. (A) IDO mRNA was measured by quantitative real-time PCR in PBMCs from HIV-uninfected donors cultured for 24 hours in presence of MVs, AT-2 HIVMN, AT-2 HIVAda, infectious HIVMN, and infectious HIVAda (solid bars); a series of experiments was performed using CTLA-4-Fc and CD28-Fc (open bars); mean values (n = 6) ± SE are shown. (B) Ratios between the concentration of kynurenine (μM) and tryptophan (mM) were measured by HPLC in supernatants from PBMCs cultured with microvesicles (control), AT-2 HIV, or AT-2 HIV and 1-mT for 24 and 48 hours; mean values (n = 5) ± SE are shown.
Infectious or AT-2 HIV induces IDO mRNA expression and enzymatic activity in PBMCs of HIV-uninfected donors. (A) IDO mRNA was measured by quantitative real-time PCR in PBMCs from HIV-uninfected donors cultured for 24 hours in presence of MVs, AT-2 HIVMN, AT-2 HIVAda, infectious HIVMN, and infectious HIVAda (solid bars); a series of experiments was performed using CTLA-4-Fc and CD28-Fc (open bars); mean values (n = 6) ± SE are shown. (B) Ratios between the concentration of kynurenine (μM) and tryptophan (mM) were measured by HPLC in supernatants from PBMCs cultured with microvesicles (control), AT-2 HIV, or AT-2 HIV and 1-mT for 24 and 48 hours; mean values (n = 5) ± SE are shown.
Plasmacytoid dendritic cells are responsible for HIV-induced tryptophan catabolism in PBMCs
Several cell types can express IDO in response to different stimuli.34 Dendritic cells and macrophages express high levels of IDO, which can be increased in response to IFN-γ, IFN-α/β, and/or by CTLA-4-B7 engagement.35-37 pDCs, which constitute approximately 0.5% to 1.0% of PBMCs in healthy individuals, express IDO and can downmodulate immune reactions through IDO-mediated tryptophan depletion.38,39 Therefore, we used intracellular staining in a 6-color cytofluorimetric assay to determine which cell types among PBMCs are responsible for IDO expression, and which cells respond to HIV exposure by further increasing IDO. A summary of our results is shown in Figure 4A. Only approximately 3% of PBMCs stained positive for IDO, and the IDO+ cells showed a predominant CD123+BDCA2+ phenotype, typical of pDCs (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). CD4+ T cells (CD4+/CD3+), CD8+ T cells (CD8+/CD3+), and monocytes (CD4+/CD14+) expressed variable and low baseline levels of IDO, but none of these cell types responded to HIV exposure with an increase of IDO protein expression (Figure 4A). In contrast, pDCs (CD4+/CD123+/BDCA2+) showed the highest IDO expression in control cultures (Figure 4A). Furthermore, 24-hour exposure to AT-2 HIV induced a 2-fold increase of IDO levels in pDCs (Figure 4A–B).
pDCs express IDO in PBMCs exposed to HIV. PBMCs from HIV-uninfected donors were cultured with microvesicles (control; open bars) or AT-2 HIV (solid bars) for 24 hours, and IDO protein expression was detected by flow cytometry in CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD4+), monocytes (CD4+CD3−CD8−BDCA2−), and pDCs (CD4+CD123+BDCA2+). (A) Mean IDO-PE MFI (n = 5) ± SE gated on each cell type is shown. (B) One representative experiment of 5 tested is shown; left panel shows gate on pDCs among CD4+ cells; right panel shows histograms for MFI on isotype control (black), and IDO PE on microvesicle-treated (control; green) and AT-2 HIV-treated (red) cells. (C) Intracellular staining for IDO in pDCs enriched by magnetic separation with anti-BDCA4–coated beads and cultured with or without AT-2 HIV.
pDCs express IDO in PBMCs exposed to HIV. PBMCs from HIV-uninfected donors were cultured with microvesicles (control; open bars) or AT-2 HIV (solid bars) for 24 hours, and IDO protein expression was detected by flow cytometry in CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD4+), monocytes (CD4+CD3−CD8−BDCA2−), and pDCs (CD4+CD123+BDCA2+). (A) Mean IDO-PE MFI (n = 5) ± SE gated on each cell type is shown. (B) One representative experiment of 5 tested is shown; left panel shows gate on pDCs among CD4+ cells; right panel shows histograms for MFI on isotype control (black), and IDO PE on microvesicle-treated (control; green) and AT-2 HIV-treated (red) cells. (C) Intracellular staining for IDO in pDCs enriched by magnetic separation with anti-BDCA4–coated beads and cultured with or without AT-2 HIV.
Exposure of pDC-enriched cell cultures to AT-2 HIV resulted in increased IDO protein expression (Figure 4C), suggesting that the induction of IDO is the direct consequence of HIV interaction with pDCs and does not require the presence of other leukocytes.
Infectious or noninfectious HIV induced mRNA for type I and type II IFNs in PBMCs, and recombinant IFN induced IDO in PBMCs
We previously reported that pDCs from HIV-uninfected donors produce high levels of IFN-α after exposure to infectious or noninfectious HIV and that blockade of the CD4-gp120 interaction prevented IFN-α induction.40 Furthermore, IDO induction can be mediated by type I and type II IFNs.35-37 Therefore, we investigated whether exposure to infectious or AT-2 HIV induced activation of IFN-β and IFN-γ gene expression. We observed significant increases in IFN-β and IFN-γ mRNA expression in PBMCs cultured for 24 hours in presence of infectious (data not shown) or AT-2 (Figure 5A and B, respectively) HIV. In both cases, addition of anti-CD4 mAb, which blocks the CD4-gp120 interaction, prevented HIV-induced IFN-β and IFN-γ mRNA expression (Figure 5A and B, respectively). These results, together with our previous findings showing HIV-induced IFN-α production,40 demonstrate that the major known inducers of IDO are augmented by direct exposure of PBMCs to infectious or noninfectious HIV through a CD4-gp120 interaction–dependent mechanism.
IFN-β and IFN-γ mRNA expression is increased in PBMCs exposed to AT-2 HIV. IFN-β (A) and IFN-γ (B) mRNA was measured by quantitative real-time PCR in PBMCs from HIV-uninfected donors cultured for 24 hours in presence of MVs, AT-2 HIV, or AT-2 HIV and mAb anti-CD4; mean values (n = 3) ± SE are shown. (C) Intracellular staining for IDO, gated on pDCs (BDCA2+CD123+), in PBMCs cultured with or without AT-2 HIV, rIFN-α, rIFN-β, or rIFN-γ.
IFN-β and IFN-γ mRNA expression is increased in PBMCs exposed to AT-2 HIV. IFN-β (A) and IFN-γ (B) mRNA was measured by quantitative real-time PCR in PBMCs from HIV-uninfected donors cultured for 24 hours in presence of MVs, AT-2 HIV, or AT-2 HIV and mAb anti-CD4; mean values (n = 3) ± SE are shown. (C) Intracellular staining for IDO, gated on pDCs (BDCA2+CD123+), in PBMCs cultured with or without AT-2 HIV, rIFN-α, rIFN-β, or rIFN-γ.
We tested whether recombinant type I or type II IFN induced IDO in pDCs. We found that pDCs from PBMCs cultured in presence of IFN-α, IFN-β, or IFN-γ had higher intracellular concentration of IDO compared with untreated cells (Figure 5C). These data show that either type I or type II IFN increases IDO expression in pDCs.
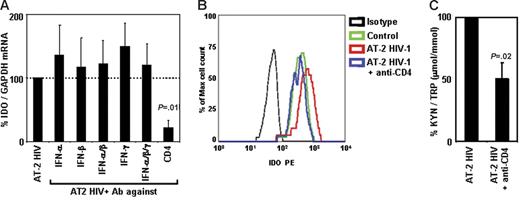
HIV-induced IDO is inhibited by anti-CD4 antibodies, but not by anti–IFN-α, anti–IFN-β, or anti–IFN-γ antibodies
Because we found that type I and type II IFNs are induced in PBMCs after exposure to HIV, and that recombinant type I and type II IFNs induced IDO in pDCs, we tested whether blockade of these cytokines would inhibit HIV-induced IDO expression and/or activity. PBMCs from HCs were cultured with AT-2 HIV in the presence or absence of blocking Ab of different specificities. Addition of anti–IFN-α, anti–IFN-β, and/or anti–IFN-γ did not affect HIV-induced IDO mRNA expression (Figure 6A). We previously reported that these antibodies effectively blocked induction of TNF-related apoptotic ligand (TRAIL)40,41 and tryptophanyl-tRNA synthetase (TTS).42 Therefore, in the current experiments, we verified that the anti-IFN Abs were effective at inhibiting expression of other genes regulated by interferons (66% ± 6% inhibition of TRAIL with anti–IFN-α and anti–IFN-β; 65% ± 13% inhibition of TTS with anti–IFN-γ), demonstrating that the lack of effect on IDO was not due to inefficient antibody blocking. Of interest, interference with the interaction between gp120 and CD4 by addition of anti-CD4 mAb resulted in a significant inhibition of HIV-induced IDO mRNA (78% ± 12% inhibition, P = .011, Figure 6A). Similar results were obtained when IDO protein expression was analyzed in pDCs by flow cytometry. Thus, addition of anti-CD4 completely abolished HIV-induced IDO expression in pDCs (Figure 6B), whereas no effect was observed when anti–IFN-α/β or anti–IFN-γ was added (data not shown). We tested whether blockade of CD4-gp120 interaction affected IDO enzymatic activity. Anti-CD4 mAb inhibited HIV-induced Trp degradation and production of kyn (Figure 6C). Blockade of IFN-α, IFN-β, and/or IFN-γ did not reduce HIV-induced conversion of Trp into kyn (data not shown). Surprisingly, anti-CD4 mAb appeared to be more potent in inhibiting IDO mRNA expression (78% ± 12% inhibition) than enzymatic activity (51% ± 13% inhibition). This difference could be due to (1) the lack of linearity in the correlation between gene expression and enzymatic activity, in that residual gene expression would result in production of enzymatically active proteins sufficient to alter the kyn/Trp ratio in the media; and/or (2) a delay in the blocking effect not detectable in mRNA and protein after 24 hours, which may have led to a transient induction of IDO that was rapidly blocked by anti-CD4 mAb, but sufficient to convert part of the Trp in the media into kyn.
Blockade of CD4, but not of type I or type II IFN, inhibits AT-2 HIV-induced IDO. (A) IDO mRNA expression was measured in PBMCs from HIV-uninfected donors after exposure to AT-2 HIV in presence or absence of Ab against IFN-α, IFN-β, and/or IFN-γ or CD4; expression was normalized on the AT-2 HIV-treated condition (100%) for each experiment; mean values (n = 3) ± SE are shown. (B) IDO protein was detected by flow cytometry in pDCs (CD4+CD123+BDCA2+) from PBMCs cultured in presence of microvesicles (control; green), AT-2 HIV (red), or AT-2 HIV + anti-CD4 mAb (blue); 1 representative experiment of 3 tested is shown. (C) Ratios between the concentration of kynurenine (μM) and tryptophan (mM) were measured by HPLC in supernatants from PBMCs cultured with AT-2 HIV in presence or absence of Ab against CD4; kyn/Trp ratios were normalized on the AT-2 HIV-treated condition (100%) for each experiment; mean values (n = 3) ± SE are shown.
Blockade of CD4, but not of type I or type II IFN, inhibits AT-2 HIV-induced IDO. (A) IDO mRNA expression was measured in PBMCs from HIV-uninfected donors after exposure to AT-2 HIV in presence or absence of Ab against IFN-α, IFN-β, and/or IFN-γ or CD4; expression was normalized on the AT-2 HIV-treated condition (100%) for each experiment; mean values (n = 3) ± SE are shown. (B) IDO protein was detected by flow cytometry in pDCs (CD4+CD123+BDCA2+) from PBMCs cultured in presence of microvesicles (control; green), AT-2 HIV (red), or AT-2 HIV + anti-CD4 mAb (blue); 1 representative experiment of 3 tested is shown. (C) Ratios between the concentration of kynurenine (μM) and tryptophan (mM) were measured by HPLC in supernatants from PBMCs cultured with AT-2 HIV in presence or absence of Ab against CD4; kyn/Trp ratios were normalized on the AT-2 HIV-treated condition (100%) for each experiment; mean values (n = 3) ± SE are shown.
Considered together, these results suggest that although type I and type II IFNs are induced by HIV and can induce IDO expression when added to PBMC culture, IDO induction by HIV does not require secretion of these cytokines. However, the binding of viral gp120 to cellular CD4, which is also required for inducing IFN production, is necessary for the activation of IDO expression and the enzymatic activity induced by HIV.
Discussion
Infection with HIV is characterized by both quantitative and functional immune deficits.2-5 Although the causes for this qualitative dysfunction are unclear, the fact that both CD4+ and CD8+ T-cell responses are affected suggests that these changes are the consequence of aberrant immune activity, rather than being directly dependent on infection of CD4+ cells. We recently reported that IDO is overexpressed in lymphoid tissues during HIV infection,25 and the increased rate of tryptophan catabolism, measured as increased kyn/Trp ratio, has been previously shown in HIV-infected patients.18,25,43,44 However, the mechanism of IDO induction by HIV and the functional consequences of this IDO expression have been only partially investigated. In this report, we show direct induction of IDO by HIV in the absence of productive infection, and identify pDCs as the major producers of IDO in response to HIV. The interaction of gp120 with CD4, but not production of type I and type II IFNs, was required for induction of IDO. In addition, we uniquely demonstrated that HIV-induced IDO contributes to the loss of CD4+ T-cell function even in patients with high CD4 count, and that this defect can be corrected by 1-mT.
Similar to our previous results, we found that IDO activity appears to have different effects on CD4+ and CD8+ T cells.37 Here, we show that although both T-cell subsets are impaired in their proliferative activity during HIV infection, only the CD4+ T-cell defect can be explained by an IDO-mediated mechanism. Several functional alterations have been suggested to contribute to CD8+ T-cell dysfunction during HIV infection, including down-regulation of CD3ζ and CD28, with subsequent deficient T-cell receptor stimulation and costimulation45,46 ; and up-regulation of the inhibitory receptor PD-1.47-49 Although IDO-mediated tryptophan catabolism was shown to affect CD8+ T-cell activity in other settings,11,39,50,51 it is probable that non–IDO-mediated mechanisms affect this T-cell subset during HIV infection, which may prevent their functional recovery by inhibition of IDO.
Using AT-2–treated virions, others and we have shown that HIV can initiate a cascade of immunologic effects, even in the absence of productive infection of CD4+ cells.40,41,52-56 In particular, pDCs produce a large amount of IFN-α upon exposure to infectious or noninfectious HIV.40 Here, we showed that IFN-β and IFN-γ gene expression is also increased in PBMCs in presence of HIV particles and that gp120-CD4 interaction, but not productive infection, is required for IFN gene activation. We demonstrated that addition of type I or type II IFN to PBMC cultures directly induced IDO expression in pDCs. Moreover, type I or type II IFN was shown to mediate CTLA-4–induced IDO expression in DCs.35-37 Remarkably, our model showed that blockade of either type I or type II IFN was ineffective in reducing HIV-induced IDO, suggesting that mechanisms not dependent on IFN production may be exploited by HIV to achieve the activation of this immune-suppressive enzyme. In addition, HIV was strikingly more potent (approximately 10-fold) than CTLA-4-Fc in inducing IDO expression in PBMCs, suggesting that even if CTLA-4 and/or IFN contribute to HIV-induced IDO expression, their contribution is probably marginal. When the gp120-CD4 interaction was blocked, the effect of HIV on IDO, as well as on IFN-β and IFN-γ, was abolished. This finding raises the possibility that pathways other than IFN signaling may be responsible for HIV-induced IDO. An alternate hypothesis is that IFN signaling may occur within the intracellular compartment of pDCs, in a privileged site not accessible to the blocking antibodies used for in vitro experiments. Our discovery that efficient induction of IDO was obtained with noninfectious virions suggests that the trigger for IDO activation occurs during the phases of viral binding and/or entry, or is mediated by viral protein contained in the native virion that does not need to be synthesized de novo by productively infected cells. In addition, R5- and X4-tropic viruses were equally potent in inducing IDO, suggesting that HIV-mediated IDO activation is not restricted by coreceptor usage.
We previously reported increased IDO expression associated with increased expression of regulatory T-cell (Treg) markers, such as FoxP3 and CTLA-4, in tonsils of HIV-infected patients.25,26 In addition, we recently demonstrated that blockade of CTLA-4 can synergize with ART in reducing IDO and viral RNA in lymphoid tissues of SIV-infected macaques.57 The findings reported in the current study appear to contrast with other reports,25,26,57 in that IDO induction through CTLA-4-B7 signaling requires either type I or type II IFN signaling,36,58 which do not appear to be involved in our in vitro model of HIV-induced IDO in pDCs. However, our in vitro experiments using PBMCs may not mimic all aspects of the cell-cell interactions occurring in vivo in lymphoid tissues. Thus, 2 different mechanisms may result in increased IDO expression in vivo: (1) activation and migration of Tregs into lymphoid tissues and CTLA-4–mediated induction of IDO; and (2) direct stimulation of pDCs by HIV to express IDO. An alternate hypothesis takes into account the recent findings by Fallarino et al showing that IDO-mediated tryptophan catabolism can induce a Treg phenotype in T-helper cells.51 From this perspective, direct induction of IDO in pDCs by HIV may be the driving force for the accumulation of Tregs in lymphoid tissues. This hypothesis is also consistent with the decreased number of circulating pDCs during HIV infection, potentially a consequence of their activation and migration into lymphoid tissues.59 Thus, HIV may activate pDCs first, which migrate to lymphoid tissues and overexpress IDO, resulting in the suppression of CD4+ T-helper cells and their conversion into Tregs.
The importance of gp120-CD4 interactions in HIV/AIDS disease is not limited to its requirement for infection of CD4+ T cells. Numerous reports demonstrated that engagement of gp120 with CD4 results in immunologic events that have deleterious consequences for the patient.60,61 We reported that gp120-CD4 interactions may contribute to the depletion of CD4+ T cells through an IFN-α/β–induced, TRAIL-mediated apoptotic mechanism.40,41,54 Our finding in the present study that anti-CD4 Abs prevent HIV-induced IDO expression suggests that gp120-CD4 interactions may also be involved in HIV-related immune dysfunction.
Another link between HIV-induced TRAIL-mediated CD4+ T-cell depletion and IDO-mediated immune suppression is the central role played by pDCs.40 We reported that production of type I IFN by HIV-exposed pDCs is pivotal in the TRAIL-mediated apoptosis model.40 Here, we propose a new role for pDCs in HIV disease, which does not appear to be associated with IFN secretion. Thus, activation of pDCs by HIV may result in IDO-mediated T-cell inhibition and TRAIL-mediated CD4+ T-cell death. Of interest, the interference with gp120-CD4 interactions can effectively prevent both pathogenic mechanisms. Thus, therapeutic approaches based on blockade of gp120-CD4 engagement may have a triple beneficial effect: (1) interfering with the first step of productive infection of CD4+ T cells; (2) preventing production of type I IFN by pDCs, and the subsequent induction of TRAIL-mediated apoptosis of CD4+ T cells; and (3) as we showed here, inhibiting HIV-mediated expression of IDO by pDCs and subsequent immune suppression.
Inhibition of IDO with the competitive molecule 1-mT has been used in vivo in murine models to induce antitumor immunity and allograft rejection.11,62 Potential applications have been proposed for 1-mT in immunotherapeutic approaches against tumors in humans.63 Our findings suggest that 1-mT may be a promising candidate for enhancing immune responses, including anti–HIV immunity, in HIV-infected patients. Consistent with this hypothesis, we recently found that diminished IDO expression correlated with reduced viral RNA in lymph nodes of SIV-infected macaques treated with anti–CTLA-4 Ab in combination with antiretroviral therapy.57 Of interest, the present study demonstrates that the reversal of IDO-mediated immune suppression by 1-mT is more efficient in patients with high CD4 count. A corollary to this finding is that restoration of immune function was not achieved with 1-mT when extensive depletion of CD4+ T cells was detected in peripheral blood. Therefore, a therapeutic approach involving direct inhibition of IDO or IDO-inducing pathways should be considered in the early stages of the asymptomatic phase of HIV disease and/or in combination with ART.
Immunotherapeutic approaches to HIV infection are inevitably biased by the compromised status of the immune system.64 The goal of therapeutic immunization is to provide the immune system with the tools to clear HIV infection.64 CD4+ T-helper cells are key players in immune responses against pathogens, and their function may be compromised by HIV through an IDO-dependent mechanism. A better understanding of the consequences of HIV-induced IDO on T cells and studies aimed at clarifying the pathways involved in the up-regulation of IDO by HIV should give new insights into the intricate pathology of HIV/AIDS and provide new potential targets for therapy.
Authorship
Contribution: A.B. and G.S. designed research and wrote the paper; A.B., J.P.H., A.W.H., and D.F. performed research; S.A.A. and M.J.D. contributed vital reagents. J.P.H. and A.W.H. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adriano Boasso, National Institutes of Health, National Cancer Institute, Experimental Immunology Branch, 10 Center Dr, Bldg 10, Rm 4B36, Bethesda, MD 20815; e-mail: boassoa@mail.nih.gov.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by the Intramural Program of the Centers for Cancer Research, NCI, NIH, by the Intramural AIDS Targeted Antiviral Program (IATAP), and by the government of the State of the Austrian Tyrol.
The authors thank Dr J. D. Lifson (AIDS Vaccine Program, SAIC Frederick, NCI-Frederick, Frederick, MD) for providing the noninfectious and infectious HIV viruses, for reviewing the paper, and for helpful suggestions.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal