Abstract
How diverse environmental cues are integrated to regulate B-cell activation and development remains poorly understood. Here we show that Notch activity synergizes with B-cell receptor (BCR) and/or CD40 signaling to enhance several aspects of B-cell activation and function. We find that costimulation of follicular B cells with the Notch ligand Delta-like-1 leads to significant increases in BCR- and CD40-mediated proliferation and enhances production of IgG1+ cells in vitro and in vivo. We further find that coengagement of Notch and the BCR results in increased activation of the MAPK pathway, and MAPK and Notch inhibitors prevent B-cell activation events mediated by coengagement of Notch and the BCR. These data suggest that the BCR and CD40 signaling pathways collaborate with the Notch pathway to optimize B-cell activation.
Introduction
In adult mice most mature B cells can be divided into follicular (FO) or marginal zone (MZ) B cells. FO B cells constitute the bulk of the recirculating peripheral B-cell pool and generate both plasmablasts and memory B cells in response to a wide array of potential pathogens. In contrast, MZ B cells are actively retained in the marginal sinus of the spleen where they rapidly generate plasmablasts in response to bloodborne pathogens.1,2 B-cell receptor (BCR)–mediated activation of naive cells within either population can be modified through coengagement of several cell surface receptors including the costimulatory receptor CD40, the CD19/CD21 complement receptor complex, and Toll-like receptor-9 (TLR-9).3-5 BCR and CD19 signaling also influence whether immature B cells colonize the FO or MZ B-cell pools, but how signals derived from these and additional cell surface receptors are integrated to control B-cell development and activation is unclear.
Notch receptors regulate cell fate decisions in a wide variety of biologic systems including lymphocyte development and function. The mouse genome contains 4 Notch receptors (Notch1 to Notch4). Activation of Notch receptors on the cell surface requires cell-cell contact with neighboring cells expressing Notch ligands (reviewed by Maillard et al6 ). Effective Notch-Notch ligand interactions result in proteolytic cleavage of Notch, thus allowing the intracellular portion of Notch (ICN) to translocate to the nucleus to form a transcriptional activation complex consisting of ICN, the DNA-binding transcription factor CSL (CBF1, Serrate, Lag1), and coactivators of the Mastermind-like (MAML) family.6 Recent experiments illustrate that Notch2, CSL, MAMLs, and the Notch ligand Delta-like-1 (DL1) play critical and nonredundant roles in B-cell development. Notch2 is the primary Notch gene expressed by peripheral B cells,7 and genetic deletion of the genes encoding Notch2, CSL, or DL1 and expression of a dominant negative form of MAML1 (DNMAML1) all result in a selective failure to generate MZ B cells.7-10
Here we show that BCR- and CD40-mediated proliferation and production of IgG1+ cells are greatly enhanced by costimulating FO B cells with DL1. Further, we demonstrate that CSL/MAML1-dependent Notch signaling contributes to the generation of IgG1+ plasma cells during a T-dependent (TD) immune response. These observations suggest that Notch signaling may influence both the development and antigen-driven differentiation of B cells by amplifying BCR- and CD40-mediated signaling events.
Materials and methods
Mice and cell purification
C57BL/6 mice, Mx1-Cre mice, and CD19-Cre were purchased from Jackson ImmunoResearch Labs (West Grove, PA). DNMAML mice have been previously described.11 All animal procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
To purify CD23+ cells, splenocytes were resuspended in 2 mL FACS buffer (PBS, 0.5% BSA, 1 mM EDTA) with 5 μL anti-CD23–biotin (B3B4) (eBioscience, San Diego, CA) for 10 minutes at 4°C. Cells were washed, resuspended in 900 μL fluorescence-activated cell sorting (FACS) buffer, and incubated with 90 μL Streptavidin Microbeads (Miltenyi Biotec, Auburn, CA) for 15 minutes at 4°C, followed by another wash. The cells were then positively selected using magnetic-activated cell separation (MACS) LS columns, resulting in purity above 95%.
For induction of DNMAML1, Mx1-Cre+ DNMAML1f/+ and control Mx1-Cre− DNMAML1f/+ mice were given intraperitoneal injections of poly(I:C) (Sigma, St Louis, MO) (500 μg every 2 days for 10 days, repeated once after 1 week of rest). For the generation of bone marrow (BM) chimeras, C57BL/6 recipient mice received lethal irradiation (9 Gy [900 rad]), followed by intravenous injection of 5 × 105 total BM cells from poly(I:C)-induced Mx-Cre+ DNMAML1f/+ or control Mx-Cre– DNMAML1f/+ mice. Mice receiving transplants were kept on antibiotics for 2 weeks and used at least 8 weeks after reconstitution. Induction of DNMAML1 was monitored at the time of killing, and more than 95% of BM Lin−Sca-1hic-Kithi (LSK) progenitors were GFP+.
In vitro B-cell cultures
OP9-DL1 and OP9 control cells were generated as previously described.12 One day prior to initiation of B-cell cultures, 1.5 × 104 OP9 cells per well were cultured in OP9 medium (αMEM with 10% FBS [HyClone, Logan, UT] and 2.2 g/L sodium bicarbonate) in 96-well plates and incubated at 37°C in a humidified chamber with 5% CO2. A total of 2 × 104 CD23+ B cells per well were cultured on the resulting OP9 monolayers in RPMI 1640 with 10% FBS, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 1 × OPI, 55 μM β-ME, and 10 mM HEPES. B cells were cultured with either goat anti–mouse IgM (F(ab′)2), 10 μg/mL anti-CD40 (clone HM40-3; BD PharMingen, San Diego, CA), 10 ng/mL IL-4 (R&D Systems, Minneapolis, MN), or 1 μg/mL LPS (Sigma). γ-Secretase Inhibitor XVIII (GSI) was used at 1 μM (Calbiochem, San Diego, CA). The small molecule inhibitors U0126, PD98059, Ly294002, and SB203580 were purchased from Cell Signaling Technology (Beverly, MA) and dissolved in DMSO. For [3H]-thymidine experiments, OP9 cultures were irradiated (30 Gy [3000 rad]) at least 6 hours prior to addition of B cells. For [3H]-thymidine analysis, 1 μCi (0.037 MBq) [3H]-thymidine was added to each well 8 hours before harvesting. Cells were collected onto Filtermat A filters (PerkinElmer Life Sciences, Shelton, CT) and analyzed on a MicroBeta Trilux scintillation counter (PerkinElmer Life Sciences).
Flow cytometry
Antibodies were obtained from the indicated vendor or made in our laboratory. Alexa 488–conjugated antibodies included anti–phospho-p44/42 MAPK (E10), anti–phospho-Akt (193H12), anti–phospho-p38 MAPK (28B10), and rabbit IgG–Alexa 488 isotype control (Cell Signaling Technologies). Mouse IgG1–Alexa 488 isotype control, anti-CD3–PE-Cy7 (145-2C11), and anti–Gr-1–PE-Cy7 (RB6-8C5) were obtained from eBioscience. Anti-IgG1–PE (A85-1) and anti-CD138 (281-2) were obtained from BD PharMingen. Anti-B220 (6B2) was conjugated to APC or APC-Cy5.5, anti-IgD (11-26) was conjugated to biotin and revealed with streptavidin–PE–Texas red, and NP-conjugated APC was kindly provided by Dr Michael Cancro (University of Pennsylvania).
For division tracking, CD23+ B cells were labeled for 2 minutes with 5 μM CFSE (Invitrogen, Carlsbad, CA) in PBS. Cultures were harvested after 3 days, surface stained for B220, and analyzed using either DAPI, Topro-3, or 7-AAD to exclude dead cells.
For phospho staining, cultured cells were resuspended in a final concentration of 1% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). Cells were incubated at 37°C for 10 minutes, transferred to 12 × 75 mm FACS tubes, and incubated on ice for 1 minutes. Following centrifugation cells were resuspended in 250 μL ice-cold 90% methanol while vortexing and incubated for 30 minutes on ice. Samples were washed with 500 μL FACS buffer, centrifuged, and resuspended in 90 μL FACS buffer followed by 10 minutes of incubation at room temperature (RT). A total of 10 μL antiphosphoantibody or isotype control (matched by concentration) was then added and the samples incubated for 60 minutes at RT in the dark. The previous wash was repeated and the samples analyzed. A forward scatter/side scatter (FSC/SSC) gate was used to distinguish lymphocytes from OP9 stromal cells.
For analysis of secreted immunoglobulin (Ig), 50 μL of day 3 culture supernatants was assessed with a CBA Mouse Immunoglobulin Isotyping Kit (BD PharMingen) as per the manufacturer's instructions.
For flow cytometry analysis compensation was performed after data collection using FlowJo (Tree Star, Ashland, OR) software. A viability dye was included in all samples to exclude dead cells. With the exception of histograms, flow cytometry plots are presented using logicle displays to demonstrate proper compensation.13
Immunization
Nitrophenyl–chicken γ-globulin (NP-CGG) was purchased from Biosearch Technologies (Novato, CA) and precipitated in alum. Mice received intraperitoneal injections of 200 μL containing alum alone or alum plus 50 μg NP-CGG and were killed for analysis on day 7.
Results
DL1 signals enhance B-cell proliferation
To assess the impact of Notch signaling on B-cell proliferation, FO B cells were cultured on either Delta-like-1–expressing OP9 stromal cells (OP9-DL1 cells) or control OP9 stromal cells.12 This system has been used extensively to study Notch signaling in T-cell development and provides an ideal approach to examine B-cell proliferation in the presence or absence of Notch signaling mediated by DL1.
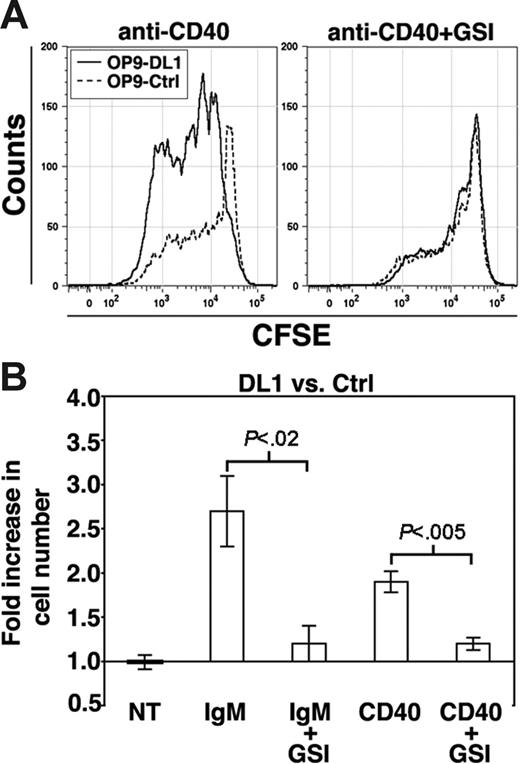
We first tested the effect of Notch signaling on B cells stimulated via BCR cross-linking. Purified CD23+ splenic B cells were stimulated with increasing concentrations of anti-IgM antibodies in cocultures with either OP9 stromal cells expressing GFP (OP9-Ctrl cells) or OP9-DL1 cells. Proliferation was measured by uptake of 3H-thymidine. As shown, proliferation was enhanced in FO B cells cultured on OP9-DL1 cells compared with controls in a manner that was anti-IgM dose dependent (Figure 1A). We next analyzed B-cell proliferation on OP9-DL1 cells at the single-cell level using the cell division tracking dye CFSE. We found that anti-IgM stimulation of FO B cells cultured on OP9-DL1 cells resulted in increased B-cell recovery (2.6-fold higher for OP9-DL1 versus OP9-Ctrl after 12.5 μg/mL anti-IgM, P < .001) (Figures 1B and 2B). The enhanced proliferation did not reflect altered survival, as there was no difference in the frequency of dead cells as determined by uptake of the DNA dyes DAPI or Topro-3 (using a FSC gate the frequency of dead cells for OP9-DL1 versus OP9-Ctrl was 2.8 ± 0.4 and 3.4 ± 0.5; without a FSC gate, 20.2 ± 2 and 23.2 ± 1.4, respectively).
DL1 signaling enhances B-cell proliferation after BCR stimulation. (A) Histogram presents 3[H]-thymidine uptake by CD23+ splenic B cells (FO B cells) stimulated with anti-IgM for 72 hours. Error bars represent standard error of the mean (SEM) (n = 9). (B) FO B cells were labeled with CFSE and cultured for 72 hours on OP9-DL1 (solid lines) or OP9-Ctrl (dashed lines) cultures with the indicated amounts of anti-IgM and without or with GSI (γ-secretase inhibitor). Results are representative of 5 independent experiments.
DL1 signaling enhances B-cell proliferation after BCR stimulation. (A) Histogram presents 3[H]-thymidine uptake by CD23+ splenic B cells (FO B cells) stimulated with anti-IgM for 72 hours. Error bars represent standard error of the mean (SEM) (n = 9). (B) FO B cells were labeled with CFSE and cultured for 72 hours on OP9-DL1 (solid lines) or OP9-Ctrl (dashed lines) cultures with the indicated amounts of anti-IgM and without or with GSI (γ-secretase inhibitor). Results are representative of 5 independent experiments.
To determine if enhanced DL1-mediated B-cell proliferation is unique to BCR stimulation, we asked whether Notch signaling influenced B-cell proliferation in response to anti-CD40 or LPS. Similar to BCR cross-linking, DL1 stimulation enhanced B-cell proliferation in response to CD40 engagement (1.8-fold higher for OP9-DL1 versus OP9-Ctrl, P < .001) (Figure 2A), again without altering survival (not shown). However, B-cell proliferation in response to LPS was unaffected by coculture with OP9-DL1 cells (not shown).
DL1 signaling enhances B-cell proliferation after CD40 or BCR stimulation. (A) CFSE-labeled FO B cells were stimulated with anti-CD40 antibodies for 72 hours on OP9-DL1 (solid lines) or OP9-Ctrl (dashed lines) cultures without or with GSI. (B) Histogram presents the fold increase in the number of B cells recovered from OP9-DL1 versus OP9-Ctrl cultures after 72 hours of the indicated treatment. Error bars represent SEM. For no treatment (NT), n = 9; IgM and IgM+GSI, n = 5 (12.5 μg/mL anti-IgM); CD40 and CD40+GSI, n = 6.
DL1 signaling enhances B-cell proliferation after CD40 or BCR stimulation. (A) CFSE-labeled FO B cells were stimulated with anti-CD40 antibodies for 72 hours on OP9-DL1 (solid lines) or OP9-Ctrl (dashed lines) cultures without or with GSI. (B) Histogram presents the fold increase in the number of B cells recovered from OP9-DL1 versus OP9-Ctrl cultures after 72 hours of the indicated treatment. Error bars represent SEM. For no treatment (NT), n = 9; IgM and IgM+GSI, n = 5 (12.5 μg/mL anti-IgM); CD40 and CD40+GSI, n = 6.
Notch activation by DL1 is mediated by a multiprotein complex with γ-secretase activity and is inhibited by γ-secretase inhibitors (GSIs).6 To determine if the DL1-enhanced B-cell activation is γ-secretase dependent, we stimulated FO B cells with anti-IgM or anti-CD40 in the presence of GSI. As shown, GSI abrogated the increased B-cell recovery from OP9-DL1 cultures (Figures 1B–2). However, the presence of GSI in anti-IgM–stimulated cultures decreased survival under all conditions (the fold increase in the frequency of dead cells for OP9-DL1+GSI versus OP9-DL1 was 2.7 and for OP9-Ctrl+GSI versus OP9-Ctrl was 2.4). GSI did not alter survival of anti-CD40–stimulated cultures (not shown). Further, for anti-IgM–stimulated cultures there was no difference in the frequency of dead cells for OP9-DL1+GSI versus OP9-Ctrl+GSI, suggesting that inhibition of Notch signaling by GSI was responsible for abrogating the increased recovery of B cells from OP9-DL1 cultures. We conclude that DL1 stimulation synergizes with BCR and CD40 signaling to enhance proliferation.
DL1 signaling enhances MAPK phosphorylation
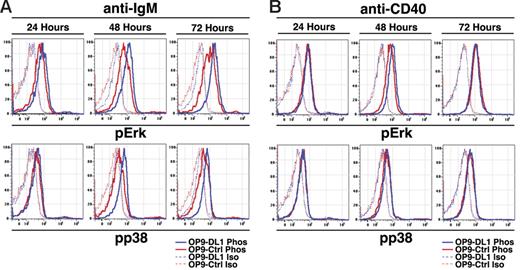
We next asked if DL1-mediated enhanced BCR- and CD40-induced proliferation influenced signaling pathways associated with proliferation. To address this issue we employed flow cytometry to assess phosphorylation of Erk/MAPK, Akt, and p38 MAPK and validated our staining technique using anti-IgM stimulation of freshly isolated CD23+ B cells (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). We cultured FO B cells on OP9-Ctrl or OP9-DL1 cells for 24, 48, or 72 hours with no treatment, anti-IgM, or anti-CD40. Analysis of FO B cells cultured with no treatment on OP9-Ctrl or OP9-DL1 cells demonstrated similarly low levels of phosphorylated Erk/MAPK compared with isotype controls at all time points, while phosphorylated Akt and p38 MAPK were indistinguishable from isotype controls (Figure S1B). However, anti-IgM stimulation of FO B cells cultured on OP9-DL1 cells demonstrated enhanced Erk/MAPK phosphorylation compared with OP9-Ctrl cultures (Figure 3). Enhanced Erk/MAPK phosphorylation was evident as early as 24 hours after initiation of cultures, well before the first cell division has occurred, and was further enhanced after 48 and 72 hours. Similarly, we observed enhanced p38 phosphorylation after 48 and 72 hours (Figure 3). Akt phosphorylation was enhanced after 48 hours but not at 72 hours (Figure S1C). Anti-CD40 stimulation of FO B cells cultured on OP9-DL1 cells also demonstrated enhanced Erk/MAPK phosphorylation after 48 hours but no differences after 24 or 72 hours and no differences in p38 MAPK phosphorylation at any time points (Figure 3). Akt phosphorylation was also unaffected at any time points (Figure S1D). These results suggest that DL1 stimulation enhances proliferation in response to BCR signaling and, to a lesser degree, CD40 signaling by altering signaling thresholds associated with MAPK activation.
DL1 signals enhance MAPK activation during BCR- and CD40-mediated proliferation. (A) FO B cells were stimulated with anti-IgM (12.5 μg/mL) for the indicated times and then analyzed for expression of phosphorylated Erk (pErk) or p38 (pp38). Dashed lines indicate isotype controls for phosphostaining, while solid lines indicate phosphostaining in cells cultured on OP9-Ctrl (red) or OP9-DL1 (blue) cells. Results are representative of 3 independent experiments. (B) As in panel A, except that cells were stimulated with anti-CD40.
DL1 signals enhance MAPK activation during BCR- and CD40-mediated proliferation. (A) FO B cells were stimulated with anti-IgM (12.5 μg/mL) for the indicated times and then analyzed for expression of phosphorylated Erk (pErk) or p38 (pp38). Dashed lines indicate isotype controls for phosphostaining, while solid lines indicate phosphostaining in cells cultured on OP9-Ctrl (red) or OP9-DL1 (blue) cells. Results are representative of 3 independent experiments. (B) As in panel A, except that cells were stimulated with anti-CD40.
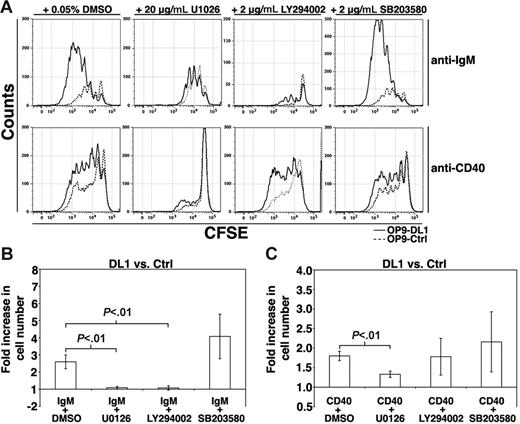
To evaluate whether DL1-mediated increases in B-cell proliferation require activation of the MAPK pathway, we cocultured anti-IgM–stimulated FO B cells on OP9-Ctrl or OP9-DL1 cells in the presence of the MEK1/2 inhibitor U0126.14 U0126 abrogated the enhanced proliferation in response to BCR plus DL1 stimulation (Figure 4). Significantly, inclusion of U0126 reduced the proliferative response to that observed with BCR stimulation of FO B cells in cocultures with OP9-Ctrl cells (Figure 4A), suggesting that MEK1/2 plays a critical role in the synergistic proliferative response observed following costimulation of the BCR and Notch. However, in contrast to inhibition of MEK1/2, inclusion of the p38 MAPK inhibitor SB203580 15 had insignificant effects on the BCR-mediated enhanced proliferation on OP9-DL1 cells. The PI3K inhibitor LY294002 16 also abrogated the enhanced proliferation on OP9-DL1 cells, although few cells were recovered from these cultures, likely due to the essential role of PI3K in BCR signaling. In the presence of anti-CD40, U0126 abrogated the synergy observed by coengagement of DL1 and CD40, while neither LY294002 nor SB203580 affected proliferation induced by DL1 and CD40 costimulation (Figure 4). Together, these data suggest that DL1-mediated enhancement of the proliferative response to BCR or CD40 engagement requires MEK1/2 activity.
Inhibition of DL1-mediated BCR- and CD40-mediated proliferation. (A) FO B cells were labeled with CFSE and stimulated with either anti-IgM (12.5 μg/mL) or anti-CD40 in the presence of DMSO, U0126, LY294002, or SB203580 for 72 hours on OP9-DL1 (solid lines) or OP9-Ctrl (dashed lines) cultures. Results are representative of at least 3 independent experiments. (B) Histogram presents the fold increase in the number of B cells recovered from OP9-DL1 versus OP9-Ctrl cultures after 72 hours of the indicated treatment. Error bars represent SEM. For IgM+DMSO, n = 5; IgM+U0126, n = 4; IgM+LY294002, n = 3; IgM+SB203580, n = 5; CD40+DMSO, n = 4; CD40+U0126, n = 4; CD40+LY294002, n = 4; CD40+SB203580, n = 4.
Inhibition of DL1-mediated BCR- and CD40-mediated proliferation. (A) FO B cells were labeled with CFSE and stimulated with either anti-IgM (12.5 μg/mL) or anti-CD40 in the presence of DMSO, U0126, LY294002, or SB203580 for 72 hours on OP9-DL1 (solid lines) or OP9-Ctrl (dashed lines) cultures. Results are representative of at least 3 independent experiments. (B) Histogram presents the fold increase in the number of B cells recovered from OP9-DL1 versus OP9-Ctrl cultures after 72 hours of the indicated treatment. Error bars represent SEM. For IgM+DMSO, n = 5; IgM+U0126, n = 4; IgM+LY294002, n = 3; IgM+SB203580, n = 5; CD40+DMSO, n = 4; CD40+U0126, n = 4; CD40+LY294002, n = 4; CD40+SB203580, n = 4.
DL1 stimulation enhances production of IgG1+ cells
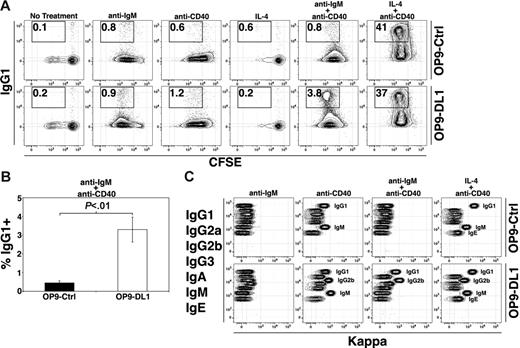
We next asked whether the capacity of DL1 stimulation to enhance B-cell activation extends to certain aspects of B-cell function. Previous experiments have demonstrated that induction of Ig class switching to IgG1 via stimulation of B cells with CD40 ligand and IL-4 correlates with proliferation.17 We therefore reasoned that DL1 stimulation in concert with additional B-cell mitogens might increase Ig class switching. Therefore, we analyzed proliferation and surface IgG1 expression after stimulation with anti-IgM, anti-CD40, IL-4, anti-IgM + anti-CD40, or IL-4 + anti-CD40. Similar to stimulation with anti-IgM alone or anti-CD40 alone, FO B cells stimulated with anti-IgM + anti-CD40 exhibited enhanced proliferation on DL1-expressing cells compared with controls (2-fold higher for OP9-DL1 versus OP9-Ctrl, P < .05, not shown). However, proliferation of FO B cells was not enhanced in the presence of DL1 signals after stimulation with IL-4 alone or IL-4 + anti-CD40 (not shown). Analysis of surface IgG1 expression demonstrated that DL1 signals enhanced production of IgG1+ cells when FO B cells were stimulated with anti-IgM + anti-CD40 by about 6-fold (Figure 5A–B). However, after treatment with IL-4 + anti-CD40, which strongly induces Ig class switching to IgG1, FO B cells cultured on OP9-DL1 and OP9-Ctrl cells produced IgG1+ cells equally well.
DL1 signaling alters Ig switching and secretion. (A) FO B cells were labeled with CFSE and stimulated as indicated (anti-IgM at 12.5 μg/mL) for 72 hours. Results are representative of at least 3 independent experiments. (B) Histogram presents the frequency of IgG1+ B cells after treatment with anti-IgM + anti-CD40 for 72 hours. Error bars represent SEM (n = 6). (C) Flow cytometric analysis of supernatants from cultures stimulated as in Figure 3A using a CBA assay that identifies all 7 mouse Ig isotypes in the order indicated. Presence of a particular isotype is indicated by kappa fluorescence (with labels provided to indicate positive cultures) (ie, anti-IgM–treated cultures are negative for all isotypes, while the OP9-Ctrl anti-CD40 culture is positive for secreted IgG1 and IgM).
DL1 signaling alters Ig switching and secretion. (A) FO B cells were labeled with CFSE and stimulated as indicated (anti-IgM at 12.5 μg/mL) for 72 hours. Results are representative of at least 3 independent experiments. (B) Histogram presents the frequency of IgG1+ B cells after treatment with anti-IgM + anti-CD40 for 72 hours. Error bars represent SEM (n = 6). (C) Flow cytometric analysis of supernatants from cultures stimulated as in Figure 3A using a CBA assay that identifies all 7 mouse Ig isotypes in the order indicated. Presence of a particular isotype is indicated by kappa fluorescence (with labels provided to indicate positive cultures) (ie, anti-IgM–treated cultures are negative for all isotypes, while the OP9-Ctrl anti-CD40 culture is positive for secreted IgG1 and IgM).
To address whether DL1 stimulation of FO B cells influenced secretion of each Ig isotype, cells were stimulated as described in Figure 5A and culture supernatants harvested after 3 days and analyzed using a cytometric bead array (CBA) assay for the 7 mouse Ig isotypes.18 We found no significant differences in Ig secretion in untreated cultures (not shown) or anti-IgM–stimulated cultures with or without DL1 signals (Figure 5C). However, supernatants from OP9-DL1+IL-4 cultures contained IgM, while OP9-Ctrl cultures did not (not shown). Further, supernatants from OP9-Ctrl cultures treated with anti-CD40 contained IgG1 and IgM, while OP9-DL1 culture supernatants contained IgG1, IgM, and IgG2b. In addition, treatment with anti-IgM + anti-CD40 resulted in secretion of IgG1 from B cells in OP9-DL1 cultures, in agreement with surface staining, but also IgG2b, while neither isotype was present in OP9-Ctrl culture supernatants. Finally, treatment with IL-4 + anti-CD40 resulted in secretion of high amounts of IgG1 from B cells in OP9-Ctrl and OP9-DL1 cultures, in agreement with the high frequency of surface IgG1+ B cells seen after such treatment, as well as IgM and low amounts of IgE. However, supernatants from OP9-DL1 cultures treated with IL-4 + anti-CD40 also contained IgG2b. Thus, DL1 stimulation enhances IgG1 and IgG2b secretion from FO B cells.
DL1-mediated B-cell activation is CSL/MAML1 dependent
A large body of work indicates that Notch signaling occurs when Notch ligands (such as DL1) initiate cleavage events that release ICN, which then translocates to the nucleus to form a multimeric protein complex with the transcription factor CSL and transcriptional coactivators of the MAML family.6 However, other studies show that Notch receptors can also signal in a CSL/MAML-independent manner.19 Although our data showing that GSI inhibits enhanced DL1-mediated proliferation of B cells suggest the effects of DL1 stimulation on B cells are Notch dependent, GSI is known to inhibit a diverse array of signaling events.20 Thus, it was uncertain whether DL1-mediated enhanced proliferation and production of IgG1+ cells are CSL dependent.
To address whether enhanced B-cell activation mediated by DL1 stimulation requires formation of a CSL/MAML1 coactivator complex, we used conditional DNMAML1f/+ mice. In DNMAML1f/+ mice a dominant negative form of MAML1 fused to GFP (DNMAML1-GFP) was targeted to the ROSA26 locus and preceded by a loxP-flanked PGK-neo-tpA cassette that prevents transcriptional read-through. Upon Cre expression, the PGK-neo-tpA cassette is excised and CSL-dependent signaling through all Notch receptors, including Notch2, is inhibited by DNMAML1-GFP.10,11
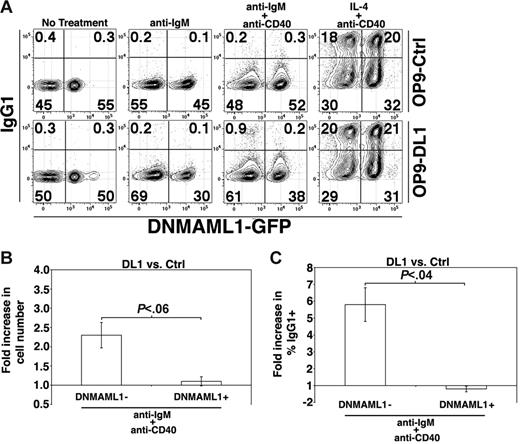
To generate mice that express DNMAML1-GFP in FO B cells we used Mx1-Cre to induce Cre expression (see “Materials and methods”).21 FO B cells were harvested from mice that express DNMAML1-GFP and were uniformly GFP+, indicating DNMAML1-GFP expression (not shown). We subsequently harvested FO B cells from Mx1-Cre+ DNMAML1f/+ and control DNMAML1f/+ mice, mixed the cells at a 1:1 ratio, cultured the B cells on OP9-Ctrl or OP9-DL1 cells in the presence of different stimuli, and then analyzed the cultures for expression of GFP and IgG1. In OP9-Ctrl cultures the ratio of control (GFP−) to DNMAML1-GFP+ B cells remained at about 1:1 regardless of treatment with anti-IgM, anti-IgM + anti-CD40, or IL-4 + anti-CD40 (Figure 6A), indicating that CSL-dependent Notch signaling was unimportant in these cultures. Expression of surface IgG1 was also similar on control and DNMAML1-GFP+ cells. In contrast, the ratio of control to DNMAML1-GFP+ B cells from OP9-DL1 cultures treated with anti-IgM was about 2:1, indicating that the ability to signal through Notch to CSL provided an advantage in these cultures. This was also evident in the number of recovered control cells on OP9-DL1 cells versus OP9-Ctrl cells after treatment with anti-IgM + anti-CD40 (Figure 6B). In agreement with our experiments demonstrating that proliferation induced by IL-4 + anti-CD40 is not enhanced on OP9-DL1 cultures, following such treatment the ratio of control to DNMAML1-GFP+ B cells was still about 1:1. Further, analysis of IgG1 surface expression after treatment with anti-IgM + anti-CD40 demonstrated that there was an approximate 6-fold increase in the frequency of IgG1+ control B cells from OP9-DL1 versus OP9-Ctrl cultures, identical to that seen in Figure 5A–B, while DNMAML1-GFP+ B cells showed no increase in frequencies of IgG1+ cells (Figure 6A,C). Importantly, DNMAML1-GFP+ B cells were capable of producing IgG1+ cells, as there was no difference in the frequency of IgG1+ cells after treatment with IL-4 + anti-CD40 on OP9-DL1 cells (Figure 6A). We conclude that enhanced DL1-mediated proliferation and production of IgG1+ cells is CSL/MAML1 dependent.
CSL is required for enhanced B-cell proliferation and production of IgG1+ cells in the presence of DL1 signals. (A) DNMAML1− and DNMAML1+ FO B cells were cultured together at a 1:1 ratio and stimulated as indicated for 72 hours. Results are representative of 3 independent experiments. (B) Histogram presents the fold increase in the number of DNMAML1− or DNMAML1+ B cells recovered from OP9-DL1 versus OP9-Ctrl cultures after 72 hours of anti-IgM + anti-CD40 treatment. Error bars represent SEM (n = 3). (C) Histogram presents the fold increase in the frequency of DNMAML1− or DNMAML1+ B cells recovered from OP9-DL1 versus OP9-Ctrl cultures after 72 hours of anti-IgM + anti-CD40 treatment. Error bars represent SEM (n = 3).
CSL is required for enhanced B-cell proliferation and production of IgG1+ cells in the presence of DL1 signals. (A) DNMAML1− and DNMAML1+ FO B cells were cultured together at a 1:1 ratio and stimulated as indicated for 72 hours. Results are representative of 3 independent experiments. (B) Histogram presents the fold increase in the number of DNMAML1− or DNMAML1+ B cells recovered from OP9-DL1 versus OP9-Ctrl cultures after 72 hours of anti-IgM + anti-CD40 treatment. Error bars represent SEM (n = 3). (C) Histogram presents the fold increase in the frequency of DNMAML1− or DNMAML1+ B cells recovered from OP9-DL1 versus OP9-Ctrl cultures after 72 hours of anti-IgM + anti-CD40 treatment. Error bars represent SEM (n = 3).
CSL/MAML1 enhances in vivo production of IgG1+ plasma cells
The observation that CSL/MAML1-dependent Notch signaling combined with BCR and CD40 signaling enhances B-cell proliferation and production of IgG1+ cells suggests that CSL/MAML1 might be important in vivo during a TD immune response. To test this possibility we generated mice that express DNMAML1 exclusively in B cells using CD19-Cre (CD19-Cre-DNMAML1 mice, see “Materials and methods”). Compared with littermate controls, CD19-Cre-DNMAML1 mice had a slight increase in FO B-cell numbers, while the number of MZ precursor B cells22 appeared slightly reduced (Figure S2; Table S1). In addition, CD19-Cre-DNMAML1 mice had a severe decrease in the number of MZ B cells compared with littermate controls. Further, analysis of DNMAML1-GFP expression in FO, MZ precursor, and MZ B cells demonstrated that B cells expressing DNMAML1-GFP were selected against in the formation of MZ B cells. Thus, CD19-Cre-DNMAML1 mice are very similar in phenotype to both the CSL8 and Notch2 conditional knockouts.7 Further, FO B cells isolated from CD19-Cre-DNMAML1 mice did not respond synergistically to IgM, CD40, or CD40+IgM on OP9-DL1 versus OP9-GFP cultures (not shown). Thus, CD19-Cre-DNMAML1 mice are a suitable model for testing the role of CSL/MAML1 in the formation of IgG1+ cells during a TD immune response.
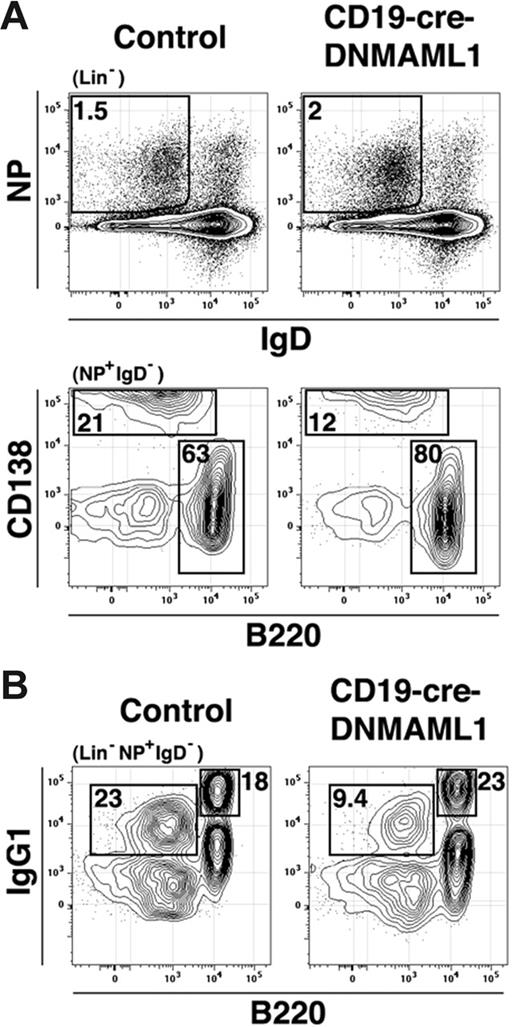
CD19-Cre-DNMAML1 mice, littermate controls, and B6 controls were immunized with NP-CGG and analyzed 7 days later for the frequency of responding NP-specific plasma cells and germinal center B cells by flow cytometry similar to the method of McHeyzer-Williams and colleagues.23 Compared with littermate controls, immunized CD19-Cre-DNMAML1 mice had similar numbers of NP+ B cells (Figures 7 and S3; Table 1). However, in immunized CD19-Cre-DNMAML1 mice we observed a decreased ratio of NP-specific plasma cells (CD138+) versus germinal center cells (CD138−). Further, CD19-Cre-DNMAML1 mice had decreased frequencies and absolute numbers of IgG1+B220− cells. Moreover, frequencies of GFP+ plasma cells and IgG1+B220− cells were significantly lower than frequencies of GFP+ germinal center and IgG1+B220+ cells (Table 1). Together these results suggest that CSL/MAML1-dependent Notch signaling enhances the in vivo production of IgG1+ plasma cells.
CSL enhances the production IgG1+ plasma cells. (A) CD19-Cre-DNMAML1 mice or littermate controls were immunized with NP-CGG and splenocytes analyzed 7 days later. Numbers are the percentage of cells in the gate indicated in parentheses above the plot. Lin− indicates CD3− and Gr-1−. Gates were drawn using B6 mice immunized with alum alone or alum+NP-CGG (Figure S3) (B). Numbers represent the percentage of cells in the gate indicated in parentheses above the plot but from a sample stained separately from that presented in panel A.
CSL enhances the production IgG1+ plasma cells. (A) CD19-Cre-DNMAML1 mice or littermate controls were immunized with NP-CGG and splenocytes analyzed 7 days later. Numbers are the percentage of cells in the gate indicated in parentheses above the plot. Lin− indicates CD3− and Gr-1−. Gates were drawn using B6 mice immunized with alum alone or alum+NP-CGG (Figure S3) (B). Numbers represent the percentage of cells in the gate indicated in parentheses above the plot but from a sample stained separately from that presented in panel A.
T-dependent immune responses to NP-CGG in control and CD19-Cre-DNMAML1 mice
| . | No. of mice . | NP+ cells: % live / Abs no. . | Germinal center cells: % NP+ / Abs no. / % GFP+ . | Plasma cells: % NP+ / Abs no. / % GFP+ . | IgG1+B220+ cells: % NP+ / Abs no. / % GFP+ . | IgG1+B220− cells: % NP+ / Abs no. / % GFP+ . |
|---|---|---|---|---|---|---|
| Control | 7 | 0.94 ± 0.2 / 96 ± 17 | 50 ± 5 / 51 ± 12 / NA | 24 ± 3 / 23 ± 5 / NA | 19 ± 2 / 20 ± 5 / NA | 23 ± 1 / 22 ± 4 / NA |
| CD19-Cre-DNMAML1 | 9 | 0.79 ± 0.1 / 73 ± 13 | 81 ± 5* / 57 ± 10 / 86 ± 2 | 14 ± 2† / 11 ± 3 / 68 ± 4 | 22 ± 1 / 16 ± 3 / 88 ± 2 | 10 ± 1* / 8 ± 2†/ 69 ± 5 |
| . | No. of mice . | NP+ cells: % live / Abs no. . | Germinal center cells: % NP+ / Abs no. / % GFP+ . | Plasma cells: % NP+ / Abs no. / % GFP+ . | IgG1+B220+ cells: % NP+ / Abs no. / % GFP+ . | IgG1+B220− cells: % NP+ / Abs no. / % GFP+ . |
|---|---|---|---|---|---|---|
| Control | 7 | 0.94 ± 0.2 / 96 ± 17 | 50 ± 5 / 51 ± 12 / NA | 24 ± 3 / 23 ± 5 / NA | 19 ± 2 / 20 ± 5 / NA | 23 ± 1 / 22 ± 4 / NA |
| CD19-Cre-DNMAML1 | 9 | 0.79 ± 0.1 / 73 ± 13 | 81 ± 5* / 57 ± 10 / 86 ± 2 | 14 ± 2† / 11 ± 3 / 68 ± 4 | 22 ± 1 / 16 ± 3 / 88 ± 2 | 10 ± 1* / 8 ± 2†/ 69 ± 5 |
B-cell populations defined by flow cytometry as shown in Figures 7 and S3. Frequencies indicate percentage of live cells or percentage of NP+ cells; absolute numbers (Abs no.), × 105. Results represent combined data from 4 independent experiments.
P < .001.
P < .02.
Discussion
Our findings illustrate that Notch activity amplifies antigen receptor signaling in B cells. Previous experiments examining the impact of loss-of-function Notch mutations on B-cell function focused primarily on serum antibody concentrations or plasma cell frequencies in nonimmunized or immunized mice.7,8 Because MZ B cells fail to develop in Notch2, CSL, and DL1-deficent mice, interpretation of these experiments can be clouded by the possibility that MZ B cells may contribute to but not dominate responses to certain antigens. Indeed, CSL-deficient mice exhibit marginal changes in the antibody response to T-independent haptens but exhibit a significant inability to respond to the bloodborne bacterium Staphylococcus aureus.24 By assessing the impact of DL1 stimulation on purified FO B cells in vitro and the impact of inhibiting Notch/CSL activity on frequencies of antigen-specific B cells in vivo, we have uncovered the capacity of Notch activity to enhance B-cell activation and differentiation into isotype-switched plasma cells. These findings should lead to studies into how manipulation of the Notch pathway might increase the efficacy of the humoral response to a variety of antigens and also lead to further study of the impact of loss of Notch2 on the function of discrete B-cell subpopulations.
Early T-dependent humoral responses are characterized by antigen-driven clonal expansion and differentiation of naive antigen-specific cells into plasma cells and germinal center B cells.25,26 Previous analyses of antigen-specific antibody response in Notch loss-of-function mutants focused on serum antibody titers rather than frequencies of responding B cells.7,24 While informative, measurements of serum antibody titers are influenced by several factors including Ig serum half-lives as well as numbers of antigen-specific plasmablasts. In contrast, our assessment of frequencies of antigen-binding germinal center and plasma cells in immunized DNMAML1+ mice revealed a somewhat subtle yet significant decrease specifically in CD138+ and IgG1+B220− plasma cells (Figure 7; Table 1). Given that much of the long-term antibody response may derive from long-lived plasma cells in the BM,27 it is tempting to speculate that induced inhibition of Notch activity in marrow plasma cells long after immunization would result in decreased antibody titers. In addition, plasma cell differentiation requires the activity of the transcription factor Blimp-1.28 Thus, one possibility is that Notch activity optimizes plasma cell differentiation by increasing Blimp-1 expression and/or activity. However, this model does not address the impact of DL1 stimulation on isotype switching observed here. An alternative possibility is that Notch activity increases the proliferative capacity of antigen-responding B cells, with alterations in plasma cell frequencies and isotype switching constituting secondary effects.
The decreased numbers of plasma cells upon inhibition of Notch activity in immunized mice also raises questions about the identity and source of Notch ligands during the course of a humoral immune response. Given that Notch loss-of-function mutations appear to selectively affect MZ B-cell development7-10 and MZ B cells are restricted to the spleen, one possibility is that cell-cell interactions required for immune responses that initiate largely in the spleen are uniquely regulated by poorly described splenocytes characterized by Notch ligand expression. Given that differentiation of MZ B cells into plasma cells involves cell-cell interactions between MZ B cells and novel blood dendritic cells characterized by rapid migration to the spleen and production of antiapoptotic cytokines of the BLyS/BAFF family,29 it is tempting to speculate that B-cell–dendritic-cell interactions enhance plasma cell differentiation by increasing Notch signaling as well as BLyS/BAFF receptor signaling on antigen-responsive B cells. This model gains support from data showing that increased BLyS/BAFF concentrations increase MZ B-cell numbers30-32 as well as recent data showing that antigen-responsive B cells in the lymph node also form cell-cell contacts with dendritic cells.33 Indeed, given that DL1 stimulation also enhances CD40-mediated B-cell activation, it is conceivable that Notch activity enhances B-cell responsiveness regardless of whether these responses initiate in the spleen or lymph node. Further study will be required to address these issues.
Previous work on the impact of Notch activation on T-lineage differentiation and selection showed that constitutive Notch1 activation dampened responses to T-cell receptor (TCR) stimulation in immature CD4+ CD8+ thymocytes.34 In contrast, studies with mature T cells suggest Notch activity plays a positive role in T-cell activation, as TCR signaling induces Notch1 activity and inhibition of Notch activity decreases activation-induced γ-IFN production.35 These observations, together with the capacity of Notch activity to enhance mature B-cell activation, suggest the outcome of Notch activity on antigen receptor signaling may depend in part on the developmental context of the cells in question. This possibility is supported by several studies showing that antigen receptor crosslinking on immature B and T cells promotes apoptosis rather than proliferation.36,37 Alternatively, Notch1 and Notch2 may play distinct roles in B- versus T-cell activation. Additional experiments will be needed to distinguish between these possibilities and completely elucidate the mechanism underpinning Notch-mediated regulation of antigen receptor signaling.
Our data show that activation of the Notch pathway transcription factor CSL together with BCR signaling leads to increased activation of the MAPK/Erk pathway and that the synergy achieved through costimulation of these pathways requires activation of the MAPK/Erk pathway component MEK1/2. These data suggest that Notch signaling in peripheral B cells may enhance BCR signaling and consequently influence cell fate decisions controlled by the BCR. To what extent Notch activation affects other signaling pathways typically activated by BCR signaling remains unclear. In this regard, it was surprising that an inhibitor of p38 failed to inhibit the synergy achieved through coengagement of Notch and the BCR. In addition, we emphasize that the ability of DL1 stimulation to enhance B-cell activation was CSL dependent and thus likely reflects the regulated expression of key mediators of BCR and CD40 signaling. Interestingly, the Ikaros family transcription factor Aiolos appears to play an opposing role in B-cell activation, as BCR-mediated proliferation is increased in Aiolos null B cells.38 Clearly, additional work is warranted to address how transcription factors such as CSL and Aiolos influence BCR signaling.
Two main pathways promoting MZ B-cell differentiation are BCR and Notch signaling.7,8,39 At first glance, our data suggest that Notch-DL1 interactions may promote MZ B-cell selection and differentiation by amplifying BCR-mediated positive selection events. However, whether MZ B-cell differentiation is favored or limited by increasing BCR signaling is currently controversial, as several different genetic models can be cited to support either viewpoint.40-42 Notably, most studies correlating BCR signal strength with MZ B-cell differentiation infer BCR signaling intensity by quantitating the proliferative response to BCR crosslinking. Although this approach and the resulting data are informative, recent data suggest that progression of transitional B cells into the MZ B-cell pool is not associated with measurable proliferation.22 Therefore, how certain mutations affect BCR-mediated proliferation in vitro may not be directly relevant to why these mutations also influence MZ B-cell differentiation in vivo. Furthermore, given that the capacity of the BCR to transmit a proliferative signal increases substantially as immature B cells mature, it is also conceivable that positive and negative regulators of BCR signaling each regulate the FO versus MZ cell fate decision at distinct stages of peripheral B-cell development. In this regard, developing B cells may only gain access to DL1 at one or more discrete stages of development. Further studies will be needed to determine how developing B cells access DL1 and whether Notch signaling promotes MZ B-cell differentiation by modifying BCR signaling events.
Authorship
Contribution: M.T. designed and performed research, analyzed data, and wrote the paper; M.C., B.S., and I.M. performed research; W.S.P. created a unique research tool; and D.A. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Allman, University of Pennsylvania School of Medicine, 230 John Morgan Bldg, 36th and Hamilton Walk, Philadelphia, PA 19104-6082; e-mail: dallman@mail.med.upenn.edu.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by National Institutes of Health (NIH) grants AI52861, AI58066 (D.A.), and AI47833 (W.S.P.); Career Development Awards (M.T., D.A.) and a Specialized Center of Research (SCOR) grant (W.S.P.) from the Leukemia and Lymphoma Society; NIH training grant 5T32HL007971 (M.T.); and the Damon Runyon Cancer Research Foundation (DRG-102-05) (I.M.).
We thank Drs Michael Cancro, Avinash Bhandoola, and Michael McHeyzer-Williams for helpful discussions.

![Figure 1. DL1 signaling enhances B-cell proliferation after BCR stimulation. (A) Histogram presents 3[H]-thymidine uptake by CD23+ splenic B cells (FO B cells) stimulated with anti-IgM for 72 hours. Error bars represent standard error of the mean (SEM) (n = 9). (B) FO B cells were labeled with CFSE and cultured for 72 hours on OP9-DL1 (solid lines) or OP9-Ctrl (dashed lines) cultures with the indicated amounts of anti-IgM and without or with GSI (γ-secretase inhibitor). Results are representative of 5 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/8/10.1182_blood-2006-09-046698/4/m_zh80080711350001.jpeg?Expires=1765894987&Signature=MDHid39UTtgApZsFj86LdlZ64~kCRAfajMZTiPgrMPLL3Rw81I~AE56axhHNz27pvrBIJj7c460Pjvf9wquYD3pjHOSmox7oP8P0RpaKbzCjN~47F8lIajU3jnsRuOqejTwCV6CoHtinXyrIZUgUNHv6ajaejSTuT5Qe3l~4vi5S-dN7L22YU6gWbO0bqzTfOzHJbY9mBG4IwSGaMwGU6hnL4WMNB6~pDEc7hAlyeNR8wpPsz5K2zniKkXiPiG031yim643t-D5jQg5BqiCn8xbF2h9yoeskqCOICmw3b92xee8RyBoAX8NfGdXCbQUmvNjqaGdmr~AatmqgyoWx9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal