Abstract
Arginase 1 (ARG1) metabolizes arginine, thus reducing the availability of arginine as a substrate for nitric oxide synthase (NOS). The decreased production of nitric oxide (NO) by NOS and the production of ornithine by ARG1 affect immune responses and tissue regeneration at sites of infection, respectively. We here demonstrate that ARG1 is synthesized in myelocytes/metamyelocytes and is stored in gelatinase granules. In accordance with this, activated neutrophils coreleased ARG1 and gelatinase to the extracellular environment on stimulation with phorbol-12-myristate 13-acetate (PMA), formyl-methionyl-leucyl-phenylalanine (fMLP), or tumor necrosis factor α (TNF-α). Overall, these findings define ARG1 as a genuine gelatinase granule protein and support a model in which activated neutrophils release ARG1 at sites of infection to modulate immune responses and promote tissue regeneration.
Introduction
Arginase 1 (ARG1) metabolizes arginine to ornithine and thereby reduces extracellular arginine at sites of infection.1 By depleting nitric oxide synthase (NOS) of its substrate, ARG1 leads to reduced synthesis of nitric oxide (NO), which plays a pivotal role in wound healing and immune responses.1-6 Depletion of arginine by ARG1 released from myeloid cells has further been demonstrated to suppress T-cell immune responses.7 Finally, ARG1 generates the amino acid ornithine from arginine. Ornithine can be further metabolized to proline and polyamines, which enhance wound healing by supporting collagen synthesis and cell proliferation, respectively. Hence, the net effect of ARG1 activity at the site of infection is to regulate immune responses and to support tissue regeneration
We have previously argued that the generation of human neutrophil granules, traditionally classified into azurophilic (primary or peroxidase-positive granules), specific granules (secondary granules), and gelatinase granules (tertiary granules), can be viewed as a continuum of partly overlapping subsets, which are formed during the entirety of granulocytic differentiation.8,9 No specific mechanism seems responsible for targeting individual granule proteins to a particular granule subset; therefore, granule proteins formed at the same time will be targeted to the same granule subset.10 Hence, different granule subsets arise as a consequence of differences in the biosynthetic windows of granule proteins. Those who are expressed primarily at the promyelocyte stage will consequently localize to azurophilic granules. Others expressed primarily at the myelocyte and metamyelocyte stages will localize to specific granules, and those expressed at the metamyelocyte and mature neutrophil stages will localize to gelatinase granules.11 This targeting by timing model explains the heterogeneity of human neutrophil granules and has been supported experimentally.9,12-14
We recently performed a comprehensive microarray analysis of human bone marrow populations representing successive stages of granulocytic differentiation, namely promyelocytes (PMs), myelocytes/metamyelocytes (MYs), and bone marrow neutrophils (bm-PMNs). The resulting microarray database enables us to test whether a given protein is expressed at the mRNA level during granulocytic differentiation, and, if so, whether, based on its expression profile, the protein will be targeted primarily to azurophil, specific, or gelatinase granules. So far, this has enabled us to demonstrate that, for example, α1 acid-glycoprotein and haptoglobin are major constituents of human neutrophil specific granules.15,16 Recently, Munder et al17 demonstrated the localization of ARG1 in azurophil granules, indicating that ARG1 is primarily synthesized in PMs.
Our microarray analysis revealed that ARG1 is expressed at the highest levels in MYs and bm-PMNs but only at low levels in PMs, which according to the targeting by timing model implicates that ARG1 is localized in gelatinase granules rather than azurophil granules of neutrophils. Because the findings by Munder et al17 challenge our model of how human neutrophil granules are formed, we decided to reexamine the localization of ARG1.
Materials and methods
Cell isolation
Peripheral blood (PB) and bone marrow (BM) samples were collected from healthy volunteers after informed consent was obtained in accordance with the ethics committee of the cities of Copenhagen and Frederiksberg as well as the Declaration of Helsinki. Neutrophils were isolated from peripheral blood (pb-PMNs) by density centrifugation and hypotonic lysis of erythrocytes.18 Populations highly enriched in PMs, MYs, and bm-PMNs were isolated from samples of human BM by 3-layer density centrifugation and subsequent immunomagnetic depletion of nongranulocytic cells.8,19
Microarray analysis
Microarray analysis was performed as described previously.19
Exocytosis studies
Isolated neutrophils were stimulated by phorbol-12-myristate 13-acetate (PMA; 2.5 μg/mL; Sigma, Poole, United Kingdom), formyl-methionyl-leucyl-phenylalanine (fMLP; 100 nM; Sigma), or tumor necrosis factor α (TNF-α; 50 ng/mL; Sigma). To prevent proteolytic degradation of ARG1, cells were incubated with diisopropyl-fluorophosphate (DFP; Sigma) immediately after stimulation. Granule proteins were analyzed by enzyme-linked immunoabsorbent assay (ELISA) or Western blot as described previously.15
Subcellular fractionation
Western blot analysis
Western blot analysis were performed as described previously using the following primary antibodies: mouse anti–human ARG1 (1:200, HM2163; HyCult Biotechnology, Uden, The Netherlands), rabbit anti–human myeloperoxidase (MPO; 1:2000, A0398; DakoCytomation, Glostrup, Denmark), rabbit anti–human lactoferrin (LF; 1:20 000; DakoCytomation), and rabbit anti–human gelatinase (GEL; 1:100016).20 Recombinant ARG1 was used to test the specificity of the ARG1 antibody at a concentration of 6.6 μg/mL (ALX-201-081; Alexis Biochemicals, San Diego, CA).
Results and discussion
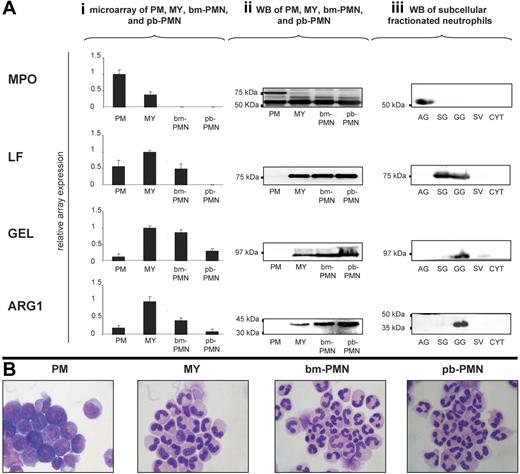
A hallmark of neutrophil granule formation is the sequential emergence of azurophil, specific, and gelatinase granules and their constituent proteins during granulocytic differentiation. To define the time of ARG1 synthesis during granulocytic differentiation, we analyzed the mRNA and protein expression profiles for ARG1 and the marker proteins of azurophil (MPO), specific (LF), and gelatinase (GEL) granules in populations highly enriched in PMs, MYs, bm-PMNs, and pb-PMNs. This analysis demonstrated that ARG1, like the specific/gelatinase granule proteins LF and GEL, is synthesized in MYs and to some extent in bm-PMNs, but not in PMs like the azurophil granule protein MPO (Figure 1Ai–ii,B). Subsequent sequence analysis revealed that ARG1 transcripts expressed in MYs were 100% identical to the ARG1 transcripts expressed in the liver (GenBank locus NM_000045; data not shown). The finding that ARG1 is synthesized in MYs, indicates that ARG1 is stored in specific/gelatinase granules and not in azurophil granules.
ARG1 is synthesized in myelocytes/metamyelozytes and stored in gelatinase granules of mature neutrophils. (Ai) Populations highly enriched in promyelocytes (PMs), myelocytes/metamyelocytes (MYs), bone marrow neutrophils (bm-PMNs), and peripheral blood neutrophils (pb-PMNs) were isolated from bone marrow (BM) and peripheral blood (PB) samples collected from healthy persons. Total mRNA was isolated and subjected to microarray analysis to monitor the mRNA expression profiles for ARG1, myeloperoxidase (MPO; marker for azurophilic granules), lactoferrin (LF; marker for specific granules), and gelatinase (GEL; marker for gelatinase granules) during granulocytic differentiation (mean ± SD, n = 3). (Aii) Western blot analysis of BM and PB populations using antibodies against MPO (proform 89 kDa and heavy chain 64 kDa), LF (78 kDa), GEL (92 kDa), and arginase 1 (ARG1; 35 kDa). Lysates of 3 × 105 cells were loaded in each lane. (Aiii) Subcellular fractions, highly enriched in azurophilic granules (AGs), specific granules (SGs), gelatinase granules (GGs), secretory vesicles (SVs), and cytosol (CYT) were isolated from 108 cells and subjected to Western blot analysis (Aii). (B) Wright Giemsa stained cytospins of BM and PB populations were examined under a BX51 microscope equipped with a DP70 photosystem with analySIS 5.0 software (Olympus, Hamburg, Germany) and a 40×/0.85 numeric aperture oil objective. PowerPoint (Microsoft, Redwood, WA) was used to prepare the images.
ARG1 is synthesized in myelocytes/metamyelozytes and stored in gelatinase granules of mature neutrophils. (Ai) Populations highly enriched in promyelocytes (PMs), myelocytes/metamyelocytes (MYs), bone marrow neutrophils (bm-PMNs), and peripheral blood neutrophils (pb-PMNs) were isolated from bone marrow (BM) and peripheral blood (PB) samples collected from healthy persons. Total mRNA was isolated and subjected to microarray analysis to monitor the mRNA expression profiles for ARG1, myeloperoxidase (MPO; marker for azurophilic granules), lactoferrin (LF; marker for specific granules), and gelatinase (GEL; marker for gelatinase granules) during granulocytic differentiation (mean ± SD, n = 3). (Aii) Western blot analysis of BM and PB populations using antibodies against MPO (proform 89 kDa and heavy chain 64 kDa), LF (78 kDa), GEL (92 kDa), and arginase 1 (ARG1; 35 kDa). Lysates of 3 × 105 cells were loaded in each lane. (Aiii) Subcellular fractions, highly enriched in azurophilic granules (AGs), specific granules (SGs), gelatinase granules (GGs), secretory vesicles (SVs), and cytosol (CYT) were isolated from 108 cells and subjected to Western blot analysis (Aii). (B) Wright Giemsa stained cytospins of BM and PB populations were examined under a BX51 microscope equipped with a DP70 photosystem with analySIS 5.0 software (Olympus, Hamburg, Germany) and a 40×/0.85 numeric aperture oil objective. PowerPoint (Microsoft, Redwood, WA) was used to prepare the images.
To determine the subcellular localization of ARG1 in neutrophils, we isolated subcellular fractions from disrupted neutrophils by 3-layer Percoll density gradient centrifugation. As depicted in Figure 1Aiii, ARG1 protein was found exclusively in the subcellular fraction highly enriched in gelatinase granules, which are defined by a high content of GEL and a low content of LF. Importantly, ARG1 protein was not detectable in subcellular fractions enriched in azurophil granules (high content of MPO) and specific granules (high content of LF, very low content of GEL) (Figure 1Aiii). Despite numerous attempts, immunoelectron microscopy failed to give sufficient specific labeling to allow visualization of ARG1 in neutrophils.
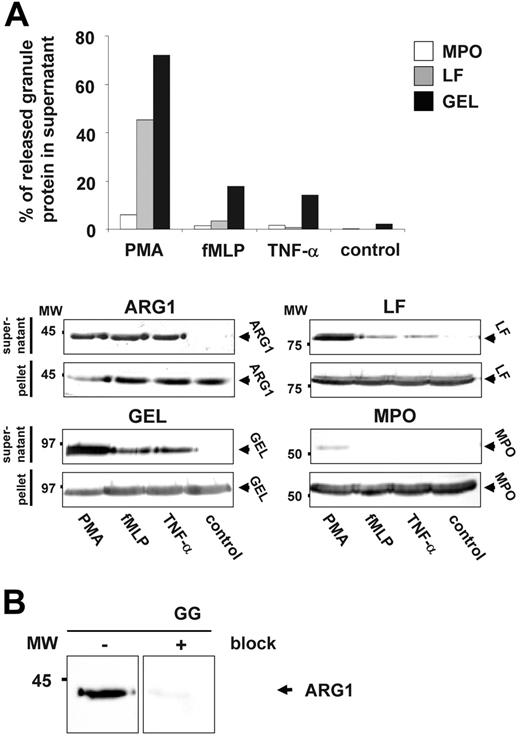
Subsequent exocytosis studies revealed that neutrophils release ARG1 and GEL at higher levels in response to PMA, fMLP, and TNF-α compared with the specific granule protein LF and the azurophilic granule protein MPO (Figure 2A).15,19
ARG1 is exocytosed by activated neutrophils. (A) Purified pb-PMNs were incubated for 15 minutes with or without the addition of 2.5 μg/mL PMA (phorbol myristate acetate), 100 nM fMLP (formyl-methionyl-leucyl-phenylalanine), or 50 ng/mL TNF α (tumor necrosis factor α). Supernatants containing granule proteins and pellets corresponding to equal amounts of cells were subjected to Western blot analysis (ARG1) or Western blot and ELISA analysis (MPO, LF, GEL). For Western blot analysis supernatants obtained from 3.75 × 106 stimulated cells as well as lysates of 3.75 × 106 stimulated cells were used. The percentage of MPO, LF, and GEL released by neutrophils was calculated as the amount of protein detected in the supernatant divided by the total amount of protein detected in the supernatant and pellet multiplied by 100. (B) Highly specific detection of ARG1 in gelatinase granules of neutrophils by Western blot analysis. A subcellular fractionation highly enriched in gelatinase granule (GG) was subjected to Western blot analysis using mouse anti–human ARG1 antibody. Prior blocking of the mouse anti–human ARG1 antibody by recombinant human ARG1 before probing of membranes reveals no cross-reactivity but a highly specific detection of only ARG1.
ARG1 is exocytosed by activated neutrophils. (A) Purified pb-PMNs were incubated for 15 minutes with or without the addition of 2.5 μg/mL PMA (phorbol myristate acetate), 100 nM fMLP (formyl-methionyl-leucyl-phenylalanine), or 50 ng/mL TNF α (tumor necrosis factor α). Supernatants containing granule proteins and pellets corresponding to equal amounts of cells were subjected to Western blot analysis (ARG1) or Western blot and ELISA analysis (MPO, LF, GEL). For Western blot analysis supernatants obtained from 3.75 × 106 stimulated cells as well as lysates of 3.75 × 106 stimulated cells were used. The percentage of MPO, LF, and GEL released by neutrophils was calculated as the amount of protein detected in the supernatant divided by the total amount of protein detected in the supernatant and pellet multiplied by 100. (B) Highly specific detection of ARG1 in gelatinase granules of neutrophils by Western blot analysis. A subcellular fractionation highly enriched in gelatinase granule (GG) was subjected to Western blot analysis using mouse anti–human ARG1 antibody. Prior blocking of the mouse anti–human ARG1 antibody by recombinant human ARG1 before probing of membranes reveals no cross-reactivity but a highly specific detection of only ARG1.
Notably, blocking experiments using recombinant human ARG1 demonstrated a high specificity of the mouse anti–human ARG1 antibody applied in our study for neutrophil ARG1 (Figure 2B).
Collectively, all 3 experimental approaches used in the present study identified ARG1 as a genuine gelatinase granule protein of human neutrophils.
This finding is in direct contrast to the recent study by Munder et al,17 which states that ARG1 is localized exclusively in azurophil granules as demonstrated by subcellular fractionation and immunoelectron microscopy. Although we find that the reported immunoelectron labeling of ARG1 did not allow such conclusion, we cannot explain the data reported by subcellular fractionation. In another study Munder et al7 elegantly demonstrated that neutrophil ARG1 must be released from its intracellular compartment to the extracellular environment to effectively deplete extracellular arginine and thus to suppress T-cell immune responses. Because azurophil granule proteins are primarily released to phagosomes by activated neutrophils, Munder et al7 argued that ARG1 contained in azurophil granules would be liberated by neutrophils at sites of infection as a consequence of necrotic cell lysis. Although necrosis of neutrophils may occur, it is not the major default pathway of neutrophils, because necrotic cell lysis liberates a plethora of toxic compounds that promote tissue destruction and inflammation. Hence, neutrophils that have phagocytosed microorganisms primarily undergo apoptosis and are removed by macrophages to facilitate resolution of inflammation.21,22 In this context, the presence of ARG1 in gelatinase granules rather than azurophilic granules is physiologically meaningful, because activated neutrophils readily mobilize gelatinase granules to the extracellular environment.15,16,23-25
Overall, our study indicates that neutrophils migrate to sites of infection and release ARG1 stored in gelatinase granules. ARG1 generates ornithine from arginine and thereby competes with NOS for arginine as a substrate. As a consequence, ARG1 reduces the synthesis of NO by endothelial cells and activated macrophages locally. Moreover, depletion of arginine by ARG1 might potentially suppress early T-cell immune responses. Finally, ornithine generated by ARG1 can be further metabolized into proline and polyamines, which support wound healing. In this context, our study supports a model in which activated neutrophils regulate early immune responses and support tissue regeneration by release of ARG1.
Authorship
Contribution: L.C.J. designed and performed the experiments and wrote the manuscript; K.T.-M. assisted in designing the experiments and wrote the manuscript; E.I.C. performed the immunoelectron microscopy experiment; and N.B. assisted in designing the experiments and writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Niels Borregaard, Department of Hematology, Rigshospitalet-4042, 9 Blegdamsvej, DK-2100 Copenhagen, Denmark; e-mail: borregaard@rh.dk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Charlotte Horn and Inge Kobbernagel for their expert technical assistance.
This work was supported by the Lundbeck Foundation. L.C.J. received a scholarship from the Lundbeck Foundation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal