Abstract
We compared the gene expression profile of adult acute lymphoblastic leukemia (ALL) to normal hematopoietic and non-ALL samples using oligonucleotide arrays. Connective tissue growth factor (CTGF) was the highest overexpressed gene in B-cell ALL compared with the other groups, and displayed heterogeneous expression, suggesting it might have prognostic relevance. CTGF expression was examined by quantitative reverse transcriptase–polymerase chain reaction (QRT-PCR) on 79 adult ALL specimens. CTGF expression levels were significantly increased in ALL cases with B-lineage (P < .001), unfavorable cytogenetics (P < .001), and blasts expressing CD34 (P < .001). In a multivariate proportional hazards model, higher CTGF expression levels corresponded to worsening of overall survival (OS; hazard ratio 1.36, for each 10-fold increase in expression; P = .019). Further studies are ongoing to confirm the prognostic value of CTGF expression in ALL and to investigate its role in normal and abnormal lymphocyte biology.
Introduction
The prognosis of adults with acute lymphoblastic leukemia (ALL) is poor, especially when contrasted with the impressive progress made in curing pediatric ALL. Even with increasingly intensive chemotherapy regimens, relapse rates remain high, and long-term survival is 40%.1-5 Transplantation regimens can be curative, but it remains challenging to identify high-risk patients suitable for early transplantation. Age, cytogenetic abnormalities, WBC count, and time to achieve complete remission (CR) are risk factors in adult ALL.4-6 New prognostic biomarkers may fine-tune risk assessment in adult ALL
We compared the gene expression profile of adult ALL to control samples, and found that connective tissue growth factor (CTGF) had the highest expression in B-cell ALL, with heterogeneous expression within B-ALL specimens. Quantitative reverse transcriptase–polymerase chain reaction (Q-RT-PCR) in adult patients with ALL showed that high CTGF expression correlated with specific biologic features and poor outcome.
Patients, materials, and methods
Bone marrow (BM) and/or peripheral blood (PB) specimens containing at least 50% blasts from 43 adult ALL cases were included in the microarray experiments, together with 26 acute myeloid leukemia (AML) cases, 10 normal bone marrows (NBMs), 10 normal peripheral bloods (NPBs), 9 CD34-enriched cells from granulocyte colony-stimulating factor (G-CSF) mobilized NPBs, 7 CD34-enriched cells from NBMs, and 2 CD22+ selected B cells from NPBs. Q-RT-PCR experiments were conducted using all 79 patients with available specimens (28 BM and 60 PB) enrolled on SWOG S9400, a study for treating adult non-L3 ALL. Outcomes of the patients included in our study did not differ significantly from those of the remaining patients in S9400.7 Twenty-six patients were included in both microarray and Q-RT-PCR experiments. Approval was obtained from the FHCRC institutional review board for this study. Informed consent was provided in accordance with the Declaration of Helsinki.
Table 1 displays the characteristics and outcomes of the patients analyzed.
Selected demographic, clinical, and outcome variables for S9400 patients included in the Q-RT-PCR analysis, by CTGF expression level tertile
| Variable . | CTGF expression . | Total . | ||
|---|---|---|---|---|
| Low . | Intermediate . | High . | ||
| No. of patients | 27 | 26 | 26 | 79 |
| Median age, y (range) | 31 (18-59) | 32 (17-62) | 41 (18-64) | 35 (17-64) |
| FAB, no. (%) | ||||
| L1 | 9 (33) | 6 (23) | 8 (31) | 23 (29) |
| L2 | 16 (59) | 17 (65) | 18 (69) | 51 (65) |
| NA | 2 (7) | 3 (12) | 0 (0) | 5 (6) |
| Lineage, no. (%) | ||||
| T | 9 (33) | 3 (12) | 1 (4) | 13 (13) |
| B | 11 (41) | 19 (73) | 20 (77) | 50 (63) |
| NA | 7 (26) | 4 (15) | 5 (19) | 16 (20) |
| Performance status, no. (%) | ||||
| 0 | 13 (48) | 14 (54) | 12 (46) | 39 (49) |
| 1 | 11 (41) | 9 (35) | 14 (54) | 34 (43) |
| 2 | 2 (7) | 1 (4) | 0 (0) | 3 (4) |
| NA | 1 (4) | 2 (8) | 0 (0) | 3 (4) |
| Median WBC count, × 109/L (range) | 19.5 (2.1-396.6) | 27.3 (0.6-148.0) | 28.4 (3.3-150.0) | 23.4 (0.6-396.6) |
| Median PB blasts, % (range)* | 49 (1-96) | 60 (0-94) | 48 (0-95) | 56 (0-96) |
| Median BM blasts, % (range)† | 80 (31-99) | 94 (72-99) | 90 (58-99) | 90 (31-99) |
| CD34, % positive cells‡ | 8 (0-96) | 51 (1-95) | 81 (8-98) | 64 (0-98) |
| Cytogenetics, no. (%) | ||||
| UNF | 3 (11) | 12 (46) | 10 (38) | 25 (32) |
| NL | 6 (22) | 3 (12) | 4 (15) | 13 (16) |
| Others | 14 (52) | 7 (27) | 2 (8) | 23 (29) |
| NA | 4 (15) | 4 (15) | 10 (38) | 18 (23) |
| Complete response, no. (%) | 21 (81) | 22 (85) | 21 (81) | 64 (82) |
| Resistant disease, no. (%) | 1 (4) | 2 (8) | 3 (12) | 6 (8) |
| 5-y overall survival, % (95% CI) | 58 (38-78) | 42 (23-61) | 11 (0-24) | 37 (26-48) |
| 5-y disease-free survival,§ % (95% CI) | 54 (32-76) | 41 (20-61) | 5 (0-14) | 33 (21-45) |
| Variable . | CTGF expression . | Total . | ||
|---|---|---|---|---|
| Low . | Intermediate . | High . | ||
| No. of patients | 27 | 26 | 26 | 79 |
| Median age, y (range) | 31 (18-59) | 32 (17-62) | 41 (18-64) | 35 (17-64) |
| FAB, no. (%) | ||||
| L1 | 9 (33) | 6 (23) | 8 (31) | 23 (29) |
| L2 | 16 (59) | 17 (65) | 18 (69) | 51 (65) |
| NA | 2 (7) | 3 (12) | 0 (0) | 5 (6) |
| Lineage, no. (%) | ||||
| T | 9 (33) | 3 (12) | 1 (4) | 13 (13) |
| B | 11 (41) | 19 (73) | 20 (77) | 50 (63) |
| NA | 7 (26) | 4 (15) | 5 (19) | 16 (20) |
| Performance status, no. (%) | ||||
| 0 | 13 (48) | 14 (54) | 12 (46) | 39 (49) |
| 1 | 11 (41) | 9 (35) | 14 (54) | 34 (43) |
| 2 | 2 (7) | 1 (4) | 0 (0) | 3 (4) |
| NA | 1 (4) | 2 (8) | 0 (0) | 3 (4) |
| Median WBC count, × 109/L (range) | 19.5 (2.1-396.6) | 27.3 (0.6-148.0) | 28.4 (3.3-150.0) | 23.4 (0.6-396.6) |
| Median PB blasts, % (range)* | 49 (1-96) | 60 (0-94) | 48 (0-95) | 56 (0-96) |
| Median BM blasts, % (range)† | 80 (31-99) | 94 (72-99) | 90 (58-99) | 90 (31-99) |
| CD34, % positive cells‡ | 8 (0-96) | 51 (1-95) | 81 (8-98) | 64 (0-98) |
| Cytogenetics, no. (%) | ||||
| UNF | 3 (11) | 12 (46) | 10 (38) | 25 (32) |
| NL | 6 (22) | 3 (12) | 4 (15) | 13 (16) |
| Others | 14 (52) | 7 (27) | 2 (8) | 23 (29) |
| NA | 4 (15) | 4 (15) | 10 (38) | 18 (23) |
| Complete response, no. (%) | 21 (81) | 22 (85) | 21 (81) | 64 (82) |
| Resistant disease, no. (%) | 1 (4) | 2 (8) | 3 (12) | 6 (8) |
| 5-y overall survival, % (95% CI) | 58 (38-78) | 42 (23-61) | 11 (0-24) | 37 (26-48) |
| 5-y disease-free survival,§ % (95% CI) | 54 (32-76) | 41 (20-61) | 5 (0-14) | 33 (21-45) |
NA indicates not available; UNF, unfavorable; NL, normal; CI, confidence interval.
N = 74.
N = 64.
N = 67.
Disease-free survival was measured from the date the complete response was established until relapse of leukemia or death from any cause, with observations censored for patients last known to be alive without report of relapse.
For gene expression arrays, 5 μg total RNA was processed and hybridized onto HG-U133A arrays according to Affymetrix's GeneChip expression protocol.8 Gene expression data were normalized and extracted using the RMA method,9 using RMAexpress.10 ArrayMiner (Optimal Design, Brussels, Belgium) was used in posterior analyses.
Sequences of the primers and the fluorescent probe for CTGF Q-RT-PCR were as follows: CTGF-TM-F, 5′-TTGCGAAGCTGACCTGGAA-3′; CTGF-TM-R, 5′-TGCTGGTGCAGCCAGAAA-3′ and 5′-FAM-ACGGATGCACTTTTTGCCCTTCTTAATGTTCT-TAMRA-3′. Beta 2-microglobulin (B2M) was used as an internal control. Samples were run in duplicate and the mean of the ratios of CTGF to B2M was used for analysis.
Comparisons of continuous variables were based on the Kruskal-Wallis test or regression models, and comparisons of dichotomous variable on the χ2 approximation of the Fisher exact test or logistic regression models. Tertile categories for CTGF expression were used for presentation only. Natural log transformation of CTGF expression values was used in parametric models. Distributions of overall survival (OS) and disease-free survival (DFS) were estimated by the Kaplan and Meier,11 method, and compared between groups using the log-rank test. Analyses of prognostic factors and multivariate analysis were based on proportional hazards (PHs) regression models.12 All P values are 2-tailed. Analyses were based on data available as of March 2006.
Results and discussion
Microarray analysis
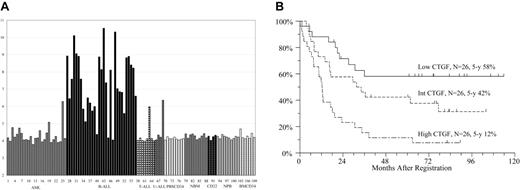
ClassMarker analysis of 43 ALL cases and 66 controls showed that CTGF best discriminated B-ALL for other samples. The median expression of CTGF in B-ALL was approximately 8-fold higher than in other specimens, with levels spanning more than 70-fold across B-ALLs (Figure 1A). Overexpression of CTGF in B-ALL over T-ALL was confirmed in silico by analysis of the microarray data set published by Chiaretti et al.13 This heterogeneous overexpression of CTGF in B-ALL led us to suspect CTGF association with prognosis.
CTGF expression levels in acute leukemias and normal controls, and overall survival of a series of adult acute lymphoblastic leukemia patients by CTGF expression levels. (A) CTGF expression level in different specimens in HG U133A arrays. The intensity of expression is in log2 scale. AML indicates acute myeloid leukemia; B-ALL, B-cell acute lymphoblastic leukemia; T-ALL, T-cell acute lymphoblastic leukemia; U-ALL, acute lymphoblastic leukemia of unknown lineage; PBSCD34, CD34-enriched cells derived from G-CSF–mobilized peripheral blood; NBM, normal bone marrow; CD22, CD22+-selected B cells from normal peripheral blood (2 samples in duplicate); NPB, normal peripheral blood; BMCD34, CD34-enriched cells from normal bone marrow. (B) Overall survival in ALL patients in high, intermediate, and low CTGF expression tertiles, measured by Q-RT-PCR.
CTGF expression levels in acute leukemias and normal controls, and overall survival of a series of adult acute lymphoblastic leukemia patients by CTGF expression levels. (A) CTGF expression level in different specimens in HG U133A arrays. The intensity of expression is in log2 scale. AML indicates acute myeloid leukemia; B-ALL, B-cell acute lymphoblastic leukemia; T-ALL, T-cell acute lymphoblastic leukemia; U-ALL, acute lymphoblastic leukemia of unknown lineage; PBSCD34, CD34-enriched cells derived from G-CSF–mobilized peripheral blood; NBM, normal bone marrow; CD22, CD22+-selected B cells from normal peripheral blood (2 samples in duplicate); NPB, normal peripheral blood; BMCD34, CD34-enriched cells from normal bone marrow. (B) Overall survival in ALL patients in high, intermediate, and low CTGF expression tertiles, measured by Q-RT-PCR.
Q-RT-PCR levels of CTGF
The median expression of CTGF in ALL specimens was 1.2 × 10−3 CTGF/B2M (range, 9.8 × 10−8-2.6 × 10−2), significantly higher than in control samples (median 1.5 × 10−5; range, 1.5 × 10−7-2.3 × 10−4; P < .001). CTGF expression did not differ significantly between BM and PB specimens (P = .73). Comparison of CTGF expression levels between BM and PB in 9 patients with both specimens available showed correlation (correlation coefficient 0.78; P = .013).
CTGF expression and clinical and laboratory variables
Consistent with microarray data, B-cell ALL expressed significantly higher CTGF by Q-RT-PCR compared with T-cell ALL (P < .001). There were no significant differences in CTGF expression among patients when analyzed according to sex, age, French-American-British (FAB) classification, SWOG performance status (PS), WBC counts, and PB or BM blasts.
Cytogenetics were available for 61 of 79 patients (77%). Patients were classified as follows: unfavorable cytogenetics (defined as t(9;22) [n = 18], t(4;11) [n = 2], monosomy 7 [n = 10], and trisomy 8 [n = 3]), normal karyotype (n = 13), and other abnormalities (n = 23).14 Patients with unfavorable cytogenetics expressed significantly higher CTGF levels (median: 2.0 × 10−3; range, 1.3 × 10−5-2.2 × 10−2) than patients without unfavorable cytogenetics (median: 1.7 × 10−4; range, 7.3 × 10−7-8.6 × 10−3) (P < .001).
CD34 status was available in 67 cases. The percentage of blasts expressing CD34 correlated with higher CTGF expression level (P < .001).
CTGF expression and clinical outcome
CTGF expression was not associated with CR rates (P = .72; Table 1). Patients with the lowest CTGF expression levels had a 5-year OS of 58%, whereas patients with intermediate and high levels of expression had 5-year OS rates of 42% and 12%, respectively (Figure 1B). The 5-year DFS rates were 54%, 41%, and 5% for low, intermediate, and high CTGF expression tertiles, respectively. The univariate proportional hazard model showed a statistically significant association of CTGF and OS. On average, each 10-fold increase in CTGF expression corresponded to a 27% increase in mortality hazard rate (HR 1.27; 95% confidence interval [CI]: 1.11-1.61; P < .001). Similarly, the association of CTGF and DFS was statistically significant (HR 1.27; 95% CI: 1.10-1.60; P < .001).
For multivariate analyses, only cases with complete data on included variables were allowed (n = 53). Association of CTGF and OS remained statistically significant (HR 1.36; 95% CI: 1.05-1.77; P = .019) in the proportional hazard model of CTGF adjusted for all significant variables (PS, FAB, cytogenetic group, sex, PB blasts, platelet count, and age). The univariate association between CTGF and OS using this smaller subset of patients remained significant (HR 1.47; 95% CI: 1.21-2.13; P < .001). Association of CTGF and DFS in the multivariate proportional hazard model was consistent with the univariate model for DFS, although it was no longer statistically significant (HR 1.24; 95% CI: 0.98-1.56; P = .076), perhaps due to associations with prognostic factors such as cytogenetics in our model, missing values for other prognostic factors, and/or decrease in the sample size for DFS.
CTGF is located on chromosome band 6q23, and it is not expressed in normal leukocytes.15,16 We show uniformly low CTGF levels in normal PB and BM by microarrays and Q-RT-PCR. Thus, it seems unlikely that other cellular components (ie, other blood or marrow stromal cells) influenced CTGF values in leukemic samples.
In vivo studies suggest a role of CTGF in adhesion,17 cell cycle control,18 and proliferation,19 and it has been linked to the TGF-β20 and Wnt21,22 pathways. CTGF is expressed in solid malignancies, but a role in neoplastic transformation is unclear.23-26 Vorwerk et al16 reported CTGF expression in malignant lymphoblasts in pediatric patients, by qualitative RT-PCR. In contrast to our study, they did not find any difference in the expression of CTGF among patients who relapsed versus patients in continuous CR.27 Differences in these studies may be technical, or due to the different population targeted.
In our study, CTGF expression level was an independent predictor of OS, suggesting it provides additional prognostic stratification in patients with B-ALL. This finding and the role of CTGF in ALL biology will be clarified with validation studies in larger clinical trials and basic biologic studies.
Authorship
Contribution: O.S.-T. designed research, performed experiments, collected and analyzed data, and wrote the paper; H.M.G. collected and analyzed data; D.L.S. designed research; P.A.L. and E.L.P.-A. performed experiments; M.L.S. and D.H.B. contributed reagents and collected data; C.L.W. and S.H. contributed reagents; and J.P.R. designed research, contributed reagents, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Olga Sala-Torra, 1100 Fairview Ave N, Seattle, WA 98119; e-mail: osala@fhcrc.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by NCI grants CA32 102, CA18 029, and CA114 762, and Spanish Health Ministry grant “Beca de Formación en Investigación” (BEFI) 01/9534.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal