Abstract
Productive T-cell immunity requires both the activation and the migration of specific T cells to the antigenic tissue. The costimulatory molecule CD28 plays an essential role in the initiation of T-cell–mediated immunity. We investigated the possibility that CD28 may also regulate migration of primed T cells to target tissue. In vitro, CD28-mediated signals enhanced T-cell transendothelial migration, integrin clustering, and integrin-mediated migration. In vivo, T cells bearing a mutation in the CD28 cytoplasmic domain, which abrogates PI3K activation, displayed normal clonal expansion but defective localization to antigenic sites following antigenic rechallenge. Importantly, antibody-mediated CD28 stimulation led to unregulated memory T-cell migration to extra-lymphoid tissue, which occurred independently of T-cell receptor (TCR)–derived signals and homing-receptor expression. Finally, we provide evidence that CD28- and CTLA-4–mediated signals exert opposite effects on T-cell trafficking in vivo. These findings highlight a novel physiologic function of CD28 that has crucial implications for the therapeutic manipulation of this and other costimulatory molecules.
Introduction
The end point of productive T-cell immunity requires the localization of primed T cells to the antigenic site. Following priming in the lymph nodes, T cells must re-enter the blood stream and finally reach and infiltrate their target tissue. To this aim, primed T cells up-regulate and redistribute adhesion molecules and homing receptors that allow extravasation into nonlymphoid tissues.1 It has recently emerged that T-cell receptor (TCR)–derived signals can also regulate T-cell localization to target tissue,2,3 possibly by inducing surface integrin clustering and enhancing their adhesive function.4-7
In vivo models investigating the role of CD28-mediated costimulation in the generation of adaptive immunity have clearly shown that not only the initiation but also the development of the effector phase of T-cell responses require the delivery of CD28-mediated signals.8,9 However, primed T cells display reduced requirements for CD28-mediated costimulation for cell expansion and cytokine production.10,11 Although primed T cells display reduced requirements for CD28-mediated costimulation for proliferation and cytokine production,10,11 a prominent but poorly understood feature of CD28-deficient immune responses is the inefficient localization of primed T cells to the antigenic site.8,9,12 While vigorous T-cell responses to myelin-derived antigens could be measured ex vivo, CD28-deficient mice had significantly reduced delayed-type hypersensitivity responses to myelin antigen and failed to develop experimental autoimmune encephalomyelitis (EAE).8,9 Moreover, in the absence of CD28 ligands, adoptively transferred encephalitogenic T cells localized to meningeal but not parenchymal sites,8 suggesting that CD28 signaling can influence the trafficking of effector T cells to target tissue.12 CD28 signaling has previously been associated with the regulation of integrin-mediated T-cell adhesion13,14 as well as cytoskeletal reorganization15 and can activate signaling pathways, which are known to regulate these events.15,16 For example, CD28 can recruit and activate PI3K, which binds to a YMNM motif in the cytoplasmic domain via the SH2 domains of the p85 regulatory subunit.16 PI3K has been shown to increase integrin adhesion,17 raising the possibility that CD28 may be involved in the regulation of T-cell adhesion and/or trafficking.
Materials and methods
Mice
Mice aged 4 to 8 weeks were purchased from Olac Harlan (Bicester, United Kingdom). CD28Y170F and OT-II mice were previously described.18,19 OT-II mice were backcrossed to the CD28Y170F backgrounds for 6 to 8 generations. The level and frequency of transgenic TCR expression was indistinguishable between OT-II and OT-II/Y170F mice. Experiments with animals were performed under the Home Office authority (PPL 70/5872).
Cells
Human T-cell blasts were generated by treating peripheral-blood mononuclear cells with PHA (2 μg/mL) for 2 hours at 37°C. Following washing, T cells were further expanded with recombinant IL-2 (rIL-2; 10 U/mL; Roche, Welwyn Garden City, United Kingdom) for 2 weeks. At this stage 70% to 80% of the T cells were CD4+ and 20% to 30% CD8+, and at least 95% expressed the CD45RO isoform (data not shown). Memory CD8+ T cells specific for the male-specific minor transplantation antigen HY in the context of H2-Db molecules20 were obtained by 2 fortnightly intraperitoneal immunizations of female mice with male splenocytes, as previously described,21 and further expanded by stimulation with male C57BL/6 splenocytes and rIL-2 (20 U/mL; Roche). T-cell specificity was determined by major histocompatibility complex (MHC):peptide tetramer binding and IFNγ production (data not shown). For adoptive transfer, T cells were used 2 weeks after stimulation. The phenotype of T cells following antigen stimulation in vitro and at the time of injection is shown in Figure S3 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Murine naive T cells were isolated by immunomagnetic negative selection (Miltenyi Biotech, Bergisch Gladbach, Germany). Human umbilical vein endothelial cells (HUVECs) were purchased from Biowhittaker (Wokingham, United Kingdom). For experiments, cells were used between 2 and 4 passages. The cell lines DAP.3 and derivatives expressing human ICAM-1 (DAP.3–ICAM-1) as well as the human fibroblast cell lines M1 (M1) and derivatives expressing human CD80 (M1-CD80) or CD86 (M1-CD86) have been described previously.22,23 T-cell hybridomas wild-type (CD28WT) and M194 mutants (M194CD28C, carrying a mutation in the PYMNM motif, a docking site for PI3K) were a kind gift of C. Rudd (Cambridge University, United Kingdom) and have been described previously.24
Reagents, mAbs, and antibody-mediated stimulation
The OVA323-339 peptide was purchased from Pepscan Systems (Lelystad, The Netherlands). Murine IFNγ was obtained from Pepro Tech (Peterborough, United Kingdom). Allophycocyanin (APC)–labeled Db tetramers bearing the immunodominant HY/Uty epitope were generated as previously described.21 A detailed description of the tetramer generation is provided in Supplemental Methods. Anti–mouse CD80, anti–mouse CD86, anti–human CD80, anti–human CD86, hamster IgG2, rat IgG2a, APC–conjugated rat IgG2a, anti–human CD28, and fluorescein isothiocyanate (FITC)–conjugated mouse IgG1 were purchased from BD Biosciences (Oxford, United Kingdom); APC-conjugated anti–mouse CD4 was from Caltag Laboratories (Burlingame, CA); FITC-conjugated anti–human CD11a and rabbit anti–mouse IgG were from DAKO (Glostrup, Denmark); and FITC-conjugated anti–human CD49d and mouse IgG1 were from Serotec (Oxford, United Kingdom). Anti–mouse CD28 (37.51), anti–mouse CTLA-4 (UC10-4F10-11), hamster Ig, and rabbit anti–hamster Ig were purchased from Pharmingen (Cowley, United Kingdom). The doses of anti–human and mouse anti–CD28 antibodies to be used in the cross-linking experiments were chosen based on their ability to induce optimal T-cell proliferation in conjunction with anti-CD3 monoclonal antibodies (mAbs; data not shown). The dose of anti–mouse CTLA-4 mAb was chosen based on its ability to augment proliferation by the C6 T-cell clone in response to cognate peptide.
To induce CD28 signaling, human T cells were incubated for 30 minutes at 4°C with a mouse anti–human CD28 antibody (5 μg/106 cells) or with an isotype-matched control mAb (5 μg/106 cells) and then with a rabbit anti–mouse Ig antibody (2.5 μg/106 cells) for 60 minutes at 37°C.
HY-specific T cells were treated with a mixture of hamster anti–mouse CD28 (5 μg/5 × 106 cells) and rabbit anti–hamster Ig (2.5 μg/5 × 106 cells) for 30 minutes at 37°C and labeled with the cell-linker PKH26 (5 μM; Sigma, St Louis, MO). As a control, T cells were treated with hamster Ig (5 μg/5 × 106 cells) and rabbit anti–hamster Ig (2.5 μg/5 × 106 cells) labeled with CFSE (5 μM; Sigma).
Lymphocyte migration assays
The transmigration experiments were carried out using HUVECs, M1, M1-CD80, M1-CD86, DAP.3, and DAP.3–ICAM-1 cell monolayers (2 × 104/well) grown on Costar Transwell tissue-culture well inserts (Costar, High Wycombe, United Kingdom; diameter 6.5 mm) mounted with polycarbonate membranes with a 5-μm pore size. In the experiments with HUVECs, cells were seeded onto fibronectin-coated (10 μg/mL; Sigma) polycarbonate membranes. T cells (5 × 105/well) were added into each insert and left to migrate through the monolayers. The number of migrated T cells was determined by hemocytometric counting of the cells present in the well media at the indicated time points over the next 24 hours. Results are expressed as percentage of transmigrated cells.
In assays evaluating integrin-induced migration, T cells (5 × 105/well) were seeded onto fibronectin-coated (10 μg/mL; Sigma) 5-μm pore polycarbonate membranes, and T-lymphocyte migration was evaluated as described in the previous paragraph.
For chemotaxis assays with CXCL-12, T cells were seeded (5 × 105 to 10 × 105/well) in the upper chamber of a 5-μm pore polycarbonate Transwell (Costar). A 0.5-mL volume of the chemotaxis medium (RPMI 2% FCS) containing CXCL-12 (50 ng/mL; Pepro Tech) was added to the bottom chamber of the transwell, while 0.2 mL of cell suspension was added to the top chamber. As a control, spontaneous T-cell migration through uncoated transwells and in the absence of CXCL-12 was monitored. Transwells were incubated for 6 to 24 hours at 37°C with 5% CO2. The number of migrated cells was evaluated as described in the previous paragraph.
LFA-1 and VLA-4 clustering
Human T-cell blasts were incubated for 30 minutes at 4°C with 5 μg/mL of mouse anti–human CD28 or isotype-matched control Abs, as previously described.5 Cells were then washed 3 times and primary Abs cross-linked by incubating with 2.5 μg/mL rabbit anti–mouse Abs at 37°C for 1 hour. As a positive control, T cells were incubated with phorbole myristate acetate (PMA; 10 ng/mL; Sigma) and ionomycin 0.5 μg/mL (Sigma). Following 3 washes, cells were transferred onto poly-L-lysine–coated coverslips (BDH, Poole, United Kingdom). Cells were then fixed in 4% paraformaldehyde for 20 minutes at room temperature, washed 3 times, and in some assays stained with anti-LFA1–FITC, anti–VLA-4–FITC, or isotype control–FITC. Coverslips were extensively washed and mounted in fluorescent mounting medium (DAKO) on glass slides.
Integrin clustering was visualized by a Leica TCS SP2 confocal laser-scanning microscope using a × 100 PL APO 1.40 oil PH3 objective (Leica, Heidelberg, Germany) FITC and Green Argon (488 nm) laser.
Recruitment of circulating T cells into nonlymphoid tissues
Tissue samples were embedded in optimal cutting temperature (OCT) compound (Agar Scientific, Stansted, United Kingdom), snap-frozen, and stored until analysis, as previously described.25 To obtain an accurate representation of the number of infiltrating labeled T cells, sections from a different cutting level per sample without any field selection were made. The number of infiltrating cells obtained in 10 randomly selected × 10-magnified fields/sample from at least 3 animals were then averaged and assessed statistically.
Criostat sections were laid onto Polysine Microscope slides (VWR International, Lutterworth, United Kingdom), left to dry overnight, and then mounted in Vectorshield mounting medium for fluorescence with DAPI (Vector Laboratories, Peterborough, United Kingdom). Slides were visualized with a Coolview 12-cooled CCD camera (Photonic Science, Newbury, United Kingdom) mounted over a Zeiss Axiovert S100 microscope equipped with Metamorph software (Zeiss, Welwyn Garden City, United Kingdom). A 10×/0.6 NA objective and standard epi-illuminating fluorescence filter cubes were used and 12-bit image data sets were generated.
Quantification of T-cell infiltrates observed by wide-field fluorescence microscopy was performed using a specifically designed software to run in the LabView (V7.1; National Instruments, Austin, TX) environment. This automatic cell-counting algorithm is based on a combination of background subtraction, multiple thresholding, and morphologic processing approaches (Figure S1), which allows identification of single fluorescent cells within the tissue.
Enrichment of labeled T cells in the peritoneal lavage was assessed by flow cytometry using a FACSCalibur (Becton Dickinson, Oxford, United Kingdom). To control for autofluorescence, the peritoneal lavage of mice injected with PBS without T cells was analyzed.
Statistical analysis
In the in vitro experiments, comparisons between groups were made using the Student t test. In the in vivo experiments, the Mann-Whitney test was used. All reported P values are 2 sided.
Results
CD28-triggering enhances human T-cell transendothelial migration, integrin-mediated migration, and integrin clustering
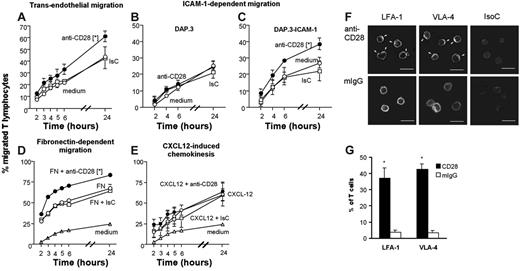
To examine the effect of CD28 signaling on T-cell motility, we used an in vitro model of lymphocyte transmigration. To exclude effects due to TCR signaling, human T-cell blasts were generated by PHA stimulation, followed by 2-week culture in medium plus IL-2. Following CD28 ligation, recently activated T cells were seeded onto human endothelial cells (ECs) or DAP.3 (a mouse fibroblastoid cell line) or DAP.3–ICAM-1 cell monolayers grown on transwells. Antibody triggering of CD28 led to increased T-cell migration through ECs (Figure 1A) and enhanced T-cell migration through human ICAM-1–transfected (Figure 1C) but not control (Figure 1B) DAP.3 cells and through fibronectin-coated transwells (Figure 1D). However, CD28 triggering did not enhance CXCL12-mediated chemotaxis (Figure 1E). Parallel experiments conducted with ICAM-1–expressing fibroblasts transfected with either CD80 or CD86 molecules showed that both CD28 ligands can enhance T-cell migration (Figure S2).
CD28 triggering enhances integrin-mediated T-cell migration. (A-C) Percentage of T-cell blasts (5 × 105/well) migrated at the indicated time points through endothelium (A), DAP.3 (B), or DAP.3–ICAM-1 (C) monolayers, fibronectin (D), or to a gradient of CXCL-12 (E) following CD28 triggering. The average percentage (±SE) of migrated T cells at the specified time points in 3 experiments with similar design is shown. (F) LFA-1 and VLA-4 clustering on the T-cell surface following CD28 triggering (indicated by arrows). Scale bar: 20 μm. (G) Mean percentage of T cells displaying polarized LFA-1 and VLA-4 aggregates in 300 cells from each sample in 3 independent experiments ± SD. (A) *P < .05 at all time points except 5 hours; (C) *P < .006; (D) *P < .002; (G) *P < .001.
CD28 triggering enhances integrin-mediated T-cell migration. (A-C) Percentage of T-cell blasts (5 × 105/well) migrated at the indicated time points through endothelium (A), DAP.3 (B), or DAP.3–ICAM-1 (C) monolayers, fibronectin (D), or to a gradient of CXCL-12 (E) following CD28 triggering. The average percentage (±SE) of migrated T cells at the specified time points in 3 experiments with similar design is shown. (F) LFA-1 and VLA-4 clustering on the T-cell surface following CD28 triggering (indicated by arrows). Scale bar: 20 μm. (G) Mean percentage of T cells displaying polarized LFA-1 and VLA-4 aggregates in 300 cells from each sample in 3 independent experiments ± SD. (A) *P < .05 at all time points except 5 hours; (C) *P < .006; (D) *P < .002; (G) *P < .001.
Integrin-mediated adhesion is enhanced by signals that induce integrin clustering on the cell surface.26 To test the possibility that increased T-cell migration induced by CD28 ligation was associated with integrin clustering, the distribution of LFA-1 and VLA-4 on the cell surface of CD28-activated T cells was analyzed by confocal microscopy. As shown in Figure 1F–G, LFA-1 and VLA-4 clustering was induced in T cells by CD28 stimulation in the absence of TCR triggering.
CD28-dependent regulation of T-cell migration requires PI3K activation
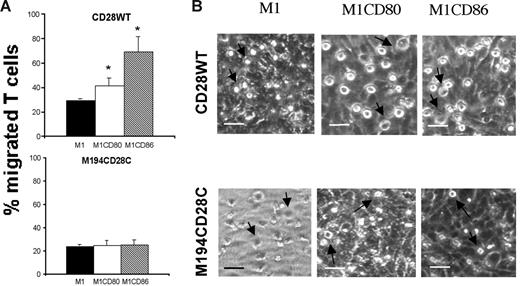
To characterize the signaling pathway(s) activated in the CD28-mediated regulation of T-cell migration, mouse T-cell hybridomas transfected with either wild-type human CD28 molecules (CD28WT) or molecules carrying a mutation that disrupts PI3K binding (M194CD28C)24 were seeded onto monolayers of ICAM-1–expressing human M1, M1-CD80, and M1-CD86 fibroblasts grown on transwells. Both T-cell hybridomas showed a similar migration through matrix (data not shown) or untransfected M1 cells (Figure 2A). CD28WT T cells migrated more efficiently through the CD80- or CD86-expressing fibroblasts than through the parental cell line. In contrast, migration of M194 T cells was unaffected by expression of CD80 or CD86 by the fibroblasts. Figure 2B shows that CD28 wild-type cells adhered inefficiently to M1 cells, as judged by their smaller size and less-evident nuclei, whereas in the presence of CD80 and CD86 molecules the T cells became spread (ie, firmly adherent) on the monolayers. In contrast, mutant M194CD28C cells interacted very inefficiently with the fibroblast monolayer irrespective of CD28 engagement. These data suggest that PI3K is required in the CD28 signaling pathways promoting T-cell transmigration.
PI3-kinase recruitment is required for CD28-mediated enhancement of T-cell adhesion and motility. (A) Murine T-cell hybridomas (5 × 105/transwell) expressing either human CD28 wild-type (top row) or M194CD28C were seeded onto M1, M1-CD80, and M1-CD86 monolayers grown on transwells. The average percentage of migrated cells after 24 hours in 3 independent experiments is shown. Standard error bars are shown. M1-CD80 versus M1, *P = .023; M1-CD86 versus M1, *P = .013. (B) CD28WT (top row) or M194CD28C (bottom row) T-cell hybridomas (indicated by arrows; 5 × 105/dish) were coincubated for 4 hours at 37°C in 25-mm Petri dishes with confluent M1, M1-CD80, or M1-CD86 cells. Images were analyzed by bright-field phase-contrast microscopy (scale bar: 20 μm).
PI3-kinase recruitment is required for CD28-mediated enhancement of T-cell adhesion and motility. (A) Murine T-cell hybridomas (5 × 105/transwell) expressing either human CD28 wild-type (top row) or M194CD28C were seeded onto M1, M1-CD80, and M1-CD86 monolayers grown on transwells. The average percentage of migrated cells after 24 hours in 3 independent experiments is shown. Standard error bars are shown. M1-CD80 versus M1, *P = .023; M1-CD86 versus M1, *P = .013. (B) CD28WT (top row) or M194CD28C (bottom row) T-cell hybridomas (indicated by arrows; 5 × 105/dish) were coincubated for 4 hours at 37°C in 25-mm Petri dishes with confluent M1, M1-CD80, or M1-CD86 cells. Images were analyzed by bright-field phase-contrast microscopy (scale bar: 20 μm).
Intact CD28 signaling is required for efficient localization of primed T cells to antigenic sites in vivo
Having established the effect that antibody stimulation of CD28 molecules has on memory T-cell migration in vitro, we sought to investigate the relevance of physiologic CD28 engagement on memory T-cell trafficking. Since our in vitro experiments suggested that PI3K is essential for the effect of CD28 on T-cell migration, and CD28Y170F T cells (in which CD28 is uncoupled from PI3K recruitment19 ) can be activated normally,19 OT-II/Y170F double-transgenic mice were generated to allow discrimination of conventional costimulation-driven clonal expansion from their ability to infiltrate antigenic tissue. CFSE-labeled OT-II and OT-II/Y170F naive T cells (107/mouse) were injected intravenously in C57BL/6 mice. Some mice were immunized intraperitoneally with OVA323-339 peptide (100 μg/mouse) in LPS (10 μg/mouse; + Ag) or LPS alone (no Ag) 24 hours later. This induced similar levels of OT-II and OT-II/Y170F T-cell division in the lymph nodes (Figure 3A–B) and spleen (data not shown) of recipient mice. A detailed analysis is summarized in Table 1 To up-regulate MHC class II molecule expression and induce a nonlymphoid antigenic site in the peritoneum, all recipients were also injected with 600U IFNγ intraperitoneally 24 hours after T-cell transfer and finally received a small dose of OVA323-339 peptide (10 μg/mouse) intraperitoneally 48 hours after the injection of IFNγ. We have previously established that this procedure induces optimal conditions for local MHC molecule up-regulation, antigen presentation, and recruitment of antigen-specific memory T cells.3 Peritoneal localization of labeled T cells was assessed 24 hours after the intraperitoneal administration of antigenic peptide by wide-field fluorescence microscopy and quantified as described in “Recruitment of circulating T cells into nonlymphoid tissues.” Control mice (No Ag) received T cells intravenously and LPS and IFNγ intraperitoneally but were not challenged with OVA323-339 peptide. The localization of labeled T cells in these mice was assessed at the same time point as that used for Ag-challenged mice.
OT-II/CD28Y170F T cells display normal antigen-driven expansion but defective localization to antigenic sites following priming in vivo. (A-B) CFSE-labeled OT-II and OT-II/Y170F CD4+ T cells detected in draining (mesenteric) lymph nodes after immunization with OVA323-339 peptide and LPS (cells are gated based on CD4 expression). (C-D) Localization of CFSE-labeled T cells 96 hours after immunization in the peritoneal membrane of mice that received an intraperitoneal injection of LPS and IFNγ only (No Ag) or that had been also injected with OVA323-339 peptide 48 hours later (+ Ag; scale bar: 40 μm). Panel D represents data averaged from at least 3 animals, and standard error bars are shown. *P < .03.
OT-II/CD28Y170F T cells display normal antigen-driven expansion but defective localization to antigenic sites following priming in vivo. (A-B) CFSE-labeled OT-II and OT-II/Y170F CD4+ T cells detected in draining (mesenteric) lymph nodes after immunization with OVA323-339 peptide and LPS (cells are gated based on CD4 expression). (C-D) Localization of CFSE-labeled T cells 96 hours after immunization in the peritoneal membrane of mice that received an intraperitoneal injection of LPS and IFNγ only (No Ag) or that had been also injected with OVA323-339 peptide 48 hours later (+ Ag; scale bar: 40 μm). Panel D represents data averaged from at least 3 animals, and standard error bars are shown. *P < .03.
T-cell division following immunization with OVA323-339 peptide in OT-II and OT-II/CD28 Y170F mice
| . | Total CD4+ T cells, % . | No. of divisions, % of total CD4+ T cells . | % divided . | % undivided . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | ||||
| OT-2 C | 36.8 ± 3.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.62 ± 0.62 | 1.42 ± 0.87 |
| OT-2 + Ag | 33.33 ± 1.06 | 0.04 ± 0.01 | 0.12 ± 0.03 | 0.36 ± 0.1 | 0.82 ± 0.3 | 1.27 ± 0.3 | 1.47 ± 0.2 | 1.17 ± 0.12 | 4.90 ± 1.2 | 0.06 ± 0.01 |
| OT-2 Y170F C | 32.1 ± 2.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.44 ± 0.3 | 0.96 ± 0.7 |
| OT-2 Y170F + Ag | 32.73 ± 0.3 | 0.12 ± 0.03 | 0.18 ± 0.06 | 0.47 ± 0.2 | 0.67 ± 0.3 | 0.96 ± 0.3 | 1.17 ± 0.3 | 1.48 ± 0.2 | 5.08 ± 1.3 | 0.19 ± 0.06 |
| . | Total CD4+ T cells, % . | No. of divisions, % of total CD4+ T cells . | % divided . | % undivided . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | ||||
| OT-2 C | 36.8 ± 3.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.62 ± 0.62 | 1.42 ± 0.87 |
| OT-2 + Ag | 33.33 ± 1.06 | 0.04 ± 0.01 | 0.12 ± 0.03 | 0.36 ± 0.1 | 0.82 ± 0.3 | 1.27 ± 0.3 | 1.47 ± 0.2 | 1.17 ± 0.12 | 4.90 ± 1.2 | 0.06 ± 0.01 |
| OT-2 Y170F C | 32.1 ± 2.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.44 ± 0.3 | 0.96 ± 0.7 |
| OT-2 Y170F + Ag | 32.73 ± 0.3 | 0.12 ± 0.03 | 0.18 ± 0.06 | 0.47 ± 0.2 | 0.67 ± 0.3 | 0.96 ± 0.3 | 1.17 ± 0.3 | 1.48 ± 0.2 | 5.08 ± 1.3 | 0.19 ± 0.06 |
Evaluation of CFSE-labeled OT-II and OT-II/Y170F CD4+ T-cell division in draining (mesenteric) lymph nodes 96 hours after immunization with OVA323-339 peptide and LPS intraperitoneally. Cells are gated based on CD4 expression (CD4-APC, FL-2). The total percentage of CD4+ T cells, the percentage of CD4+ CFSE-labeled cells that underwent the indicated number of divisions, and the total number of CD4+ T cells that underwent division or did not divide are indicated in the relevant columns.
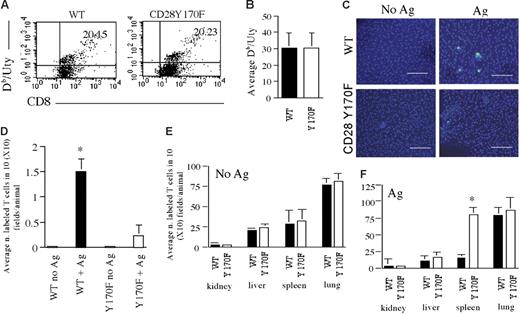
As shown in Figure 3C–D, only OT-II T cells efficiently localized in the peritoneal membrane of Ag-challenged mice, suggesting that CD28 signaling is required for efficient localization of primed T cells to nonlymphoid antigenic sites. To rule out that defective T-cell localization to the antigenic site was due to increased susceptibility of Y170F T cells to apoptosis-inducing signals,19 we analyzed the migration of HY-specific WT and CD28Y170F T cells in response to antigen challenge. WT and CD28Y170F female mice were immunized twice intraperitoneally with male-derived splenocytes (5 × 106/mouse).21 Expansion of CD8+ T cells specific for the immunodominant Uty epitope in the context of Db molecules was equivalent in immunized WT and CD28 Y170F mice (as assessed by Db/Uty tetramer-binding (Figure 4A–B), confirming that T-cell division is not impaired in CD28Y170F mice.19 Following in vitro expansion, HY-specific memory T cells lost the ability to undergo further proliferation following antigenic challenge in vivo (data not shown), possibly due to terminal differentiation (Figure S3). This allowed us to quantify the tissue distribution of T cells in the absence of proliferation. T cells were labeled with CFSE and injected intravenously in WT female mice (5 × 106/mouse). To provide CD28 stimulation in the context of antigen rechallenge, mice (Ag-challenged) received an intraperitoneal injection of 15 × 106 male-derived splenocytes concomitant to the intravenously infused labeled T cells. Recipients were also injected with 600U IFNγ intraperitoneally 24 hours after T-cell transfer and finally received Uty peptide (10 ng/mouse) intraperitoneally 48 hours after the injection of IFNγ to induce localized HY antigen presentation in the peritoneum.3 Tissue distribution of labeled T cells was assessed 24 hours after the intraperitoneal administration of antigenic peptide by wide-field fluorescence microscopy and quantified as described in “Materials and methods.” Control mice (No Ag) received T cells intravenously and IFNγ intraperitoneally but were not challenged with antigen (either in the form of male-derived splenocytes or as Uty peptide).
HY-specific memory CD28Y170F T cells display defective localization to antigenic sites in vivo. (A) Percentage of Uty-specific CD8+ T cells (as determined by Db/Uty tetramer binding) detected in WT and CD28Y170F female mice after 2 immunizations with male splenocytes (cells are gated based on CD8 expression). (B) Average percentage of Uty-specific T cells in 3 samples. Standard error bars are shown. (C-D) Localization of CFSE-labeled T cells obtained from immunized WT and CD28Y170F female mice and injected in a female recipient that had either received an intraperitoneal injection of IFNγ only (No Ag) or that had also been injected with male splenocytes on the same day and Uty peptide 48 hours later (+ Ag). Cells were visualized in the peritoneal membrane (C; scale bar: 40 μm) and in the organs indicated in panels E-F 24 hours later by wide-field fluorescence microscopy and quantified as described in “Materials and methods.” Panels D-F represent data averaged from at least 3 animals, and standard error bars are shown. (D) *P < .03; (F) *P < .04.
HY-specific memory CD28Y170F T cells display defective localization to antigenic sites in vivo. (A) Percentage of Uty-specific CD8+ T cells (as determined by Db/Uty tetramer binding) detected in WT and CD28Y170F female mice after 2 immunizations with male splenocytes (cells are gated based on CD8 expression). (B) Average percentage of Uty-specific T cells in 3 samples. Standard error bars are shown. (C-D) Localization of CFSE-labeled T cells obtained from immunized WT and CD28Y170F female mice and injected in a female recipient that had either received an intraperitoneal injection of IFNγ only (No Ag) or that had also been injected with male splenocytes on the same day and Uty peptide 48 hours later (+ Ag). Cells were visualized in the peritoneal membrane (C; scale bar: 40 μm) and in the organs indicated in panels E-F 24 hours later by wide-field fluorescence microscopy and quantified as described in “Materials and methods.” Panels D-F represent data averaged from at least 3 animals, and standard error bars are shown. (D) *P < .03; (F) *P < .04.
As shown in Figure 4C–D, only WT T cells efficiently localized in the peritoneal membrane of Ag-challenged mice. In contrast, labeled CD28Y170F T cells did not gain access to the peritoneal membrane but accumulated in the spleen of antigen-challenged mice (Figure 4E–F), suggesting that their failure to localize to the antigenic site was not due to cell death.
CD28 stimulation by antibody triggering induces aberrant infiltration of nonlymphoid tissue by memory T cells in vivo
Antibody-mediated manipulation of costimulatory signals, including CD28 antibody triggering,27 has been proposed as a potential strategy for the therapy of a variety of human diseases including autoimmunity and cancer.28-30 In order to assess the effect of antibody-induced, CD28-mediated signals on memory T-cell trafficking in vivo, memory HY-specific H2-Db–restricted CD8+ T cells were generated by immunization of female C57BL/6 WT and CD28 Y170F mice with syngeneic male splenocytes and further in vitro expansion (Figure S3). T cells were pretreated with a mixture of hamster anti–mouse CD28 and rabbit anti–hamster Ig and labeled with PKH26 prior to intravenous injection into female C57BL/6 mice. As a control, T cells treated with hamster Ig and rabbit anti–hamster Ig labeled with CFSE were coinjected.
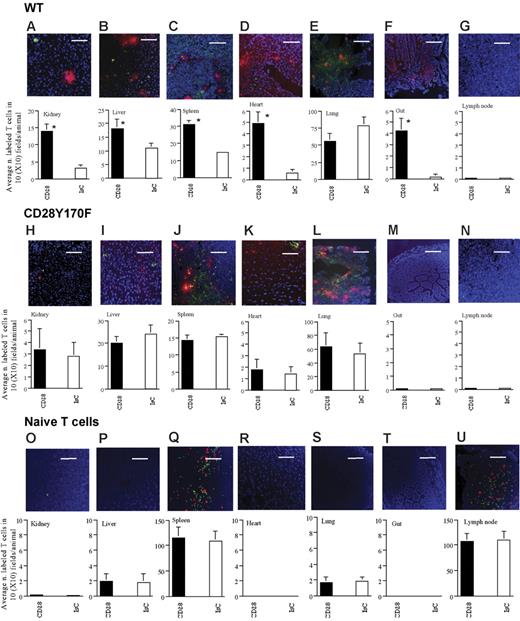
Tissue infiltration by labeled T cells was assessed 24 hours later. Both stimulated and unstimulated T cells recirculated normally and could be detected in the spleen, lung, liver, and kidney. CD28 ligation led to enhanced infiltration of kidney, liver, spleen, and heart (Figure 5A–D). Interestingly, no significant difference was detected in the lung (the first site reached by intravenously injected T cells), suggesting that increased adhesion and trapping could not explain the increased infiltration of other tissues by CD28-activated T cells (Figure 5E). Localization of HY-specific T cells in the gut and lymph nodes was also analyzed: access to these sites requires the expression of homing receptors (such as L-selectin and α4β7 integrins1 ), which were not present on the surface of the T cells used in this study even following CD28 ligation (data not shown). Surprisingly, a considerable proportion of T cells that underwent CD28 triggering were found in the gut wall (Figure 5F). In contrast, CD28 triggering did not permit T-cell access to the lymph nodes (Figure 5G). These data suggest that CD28 cross-linking promotes memory T-cell homing to nonlymphoid organs independently of the expression of homing receptors.
CD28-triggering enhances T-cell trafficking into nonlymphoid organs and promotes promiscuous T-cell recruitment to homing-“privileged” sites. Infiltration of the indicated tissues by HY-specific WT (A-G) and Y170F T cells (H-N) following CD28 ligation (PKH26 labeled) or treatment with a control antibody (CFSE labeled) is shown. Each panel shows a representative tissue image (scale bar: 30 μm). The mean T-cell infiltration ± SD observed in samples from at least 3 animals is shown (A, *P < .007; B, *P < .05; C, *P < .03; D, *P < .004; F *P < .003).
CD28-triggering enhances T-cell trafficking into nonlymphoid organs and promotes promiscuous T-cell recruitment to homing-“privileged” sites. Infiltration of the indicated tissues by HY-specific WT (A-G) and Y170F T cells (H-N) following CD28 ligation (PKH26 labeled) or treatment with a control antibody (CFSE labeled) is shown. Each panel shows a representative tissue image (scale bar: 30 μm). The mean T-cell infiltration ± SD observed in samples from at least 3 animals is shown (A, *P < .007; B, *P < .05; C, *P < .03; D, *P < .004; F *P < .003).
In parallel experiments, we compared the effect of CD28 triggering of HY-specific H2-Db–restricted CD8+ CD28Y170F T-cell trafficking. Although HY-specific CD28Y170F T cells displayed a similar phenotype as the WT counterpart (data not shown) and normal constitutive trafficking ability as shown by the comparable levels of tissue infiltration in the absence of CD28 triggering (see columns indicated with IsC-treated T cells in Figure 5A–N) and as suggested by experiments in which unstimulated WT and CD28Y170F T cells were coinjected (data not shown), as shown in Figure 5H–N, cross-linking of CD28 did not affect tissue infiltration by CD28Y170F T cells.
Finally, as CD28 is expressed by naive T lymphocytes, we assessed the effect of CD28 triggering on their trafficking ability. CD28 ligation did not affect the recruitment of syngeneic female-derived naive T cells (Figure 5O–U), which selectively localized in secondary lymphoid organs in equal amounts.
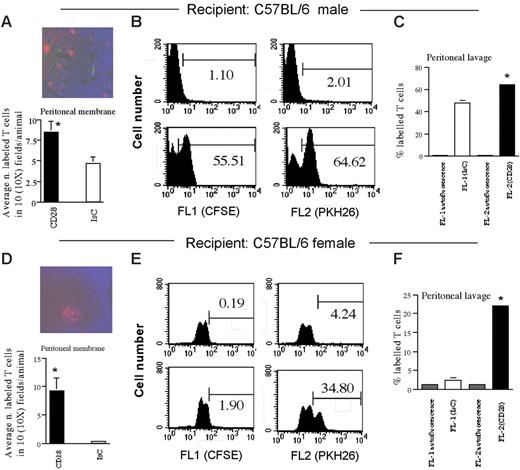
Antibody-induced, CD28-mediated regulation of T-cell trafficking can act independently of TCR-mediated signals
The previously described observations suggest that CD28-induced enhancement of memory T-cell trafficking following antibody triggering does not require coengagement of the TCR. To clarify whether TCR- and CD28-mediated signals can act independently on T-cell migration, we compared the recruitment of CD28-activated and nonactivated HY-specific T cells from the circulation into the peritoneum of male and female mice. In this model, HY-specific T cells exclusively localize in the peritoneal cavity and membrane of male but not female mice in which local MHC molecule up-regulation (and subsequently antigen presentation) is achieved by an intraperitoneal injection of IFNγ.3 H2-Db–restricted CD8+ T cells (107/mouse), which had either undergone CD28 ligation (PKH26 labeled) or had been pretreated with an isotype control antibody (CFSE labeled), were coinjected intravenously into male or female C57BL/6 mice that had received an intraperitoneal injection of IFNγ (600 U) 48 hours earlier. In male mice, T-cell recruitment to the peritoneal membrane and cavity was significantly increased by CD28 stimulation, suggesting a cooperative role of CD28- and TCR-mediated signals in T-cell recruitment (Figure 6A–C). As expected, CFSE-labeled (unstimulated) T cells did not localize in the peritoneal lavage (Figure 6F) or membrane (Figure 6D) of IFNγ-treated female mice. However, following CD28 ligation, significant amounts of T cells (PKH26 labeled) were detected in the peritoneal membrane and cavity of female mice (Figure 6E–F), suggesting that TCR- and CD28-derived signals can independently support T-cell infiltration of inflammatory sites. CD28 triggering failed to enhance HY-specific CD28Y170F T-cell migration to the peritoneal membrane and lavage of male mice or to induce access of these T cells to the peritoneal membrane and cavity of female mice (data not shown).
CD28- and TCR-mediated signals exert independent effects on T-cell migration. Male (A-C) and female (D-F) C57BL/6 mice were coinjected intravenously with HY-specific CD28-triggered (PKH26 labeled) or control (CFSE labeled) T cells 48 hours after receiving 600U IFNγ. A representative image (scale bar: 30 μm) and the mean T-cell infiltration of the peritoneal membrane, assessed as specified in “Materials and methods,” are shown. (C,F) Representative histograms and average number (+SE) of labeled T cells in the peritoneal lavage of at least 3 mice that received either PBS (top histograms and autofluorescence bars) or T cells 24 hours earlier are shown. (A, *P < .01; C, *P < .05; D, *P < .003; F, *P < .005).
CD28- and TCR-mediated signals exert independent effects on T-cell migration. Male (A-C) and female (D-F) C57BL/6 mice were coinjected intravenously with HY-specific CD28-triggered (PKH26 labeled) or control (CFSE labeled) T cells 48 hours after receiving 600U IFNγ. A representative image (scale bar: 30 μm) and the mean T-cell infiltration of the peritoneal membrane, assessed as specified in “Materials and methods,” are shown. (C,F) Representative histograms and average number (+SE) of labeled T cells in the peritoneal lavage of at least 3 mice that received either PBS (top histograms and autofluorescence bars) or T cells 24 hours earlier are shown. (A, *P < .01; C, *P < .05; D, *P < .003; F, *P < .005).
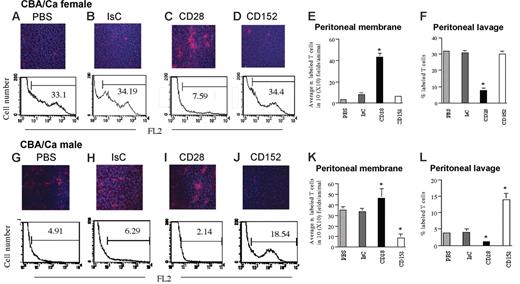
CD28- and CTLA-4–mediated signals elicit opposite effects on tissue infiltration by T cells
It has recently been reported that CTLA-4/CD152-mediated signals can induce integrin clustering and enhanced adhesion.31 To compare the relative effect of CD28- and CD152-mediated signals in the regulation of T-cell motility we used a model of peritoneal infiltration by intraperitoneally injected CD8+ HY-specific Kk-restricted C6 T cells. In this model, T-cell access into the peritoneal membrane is strictly dependent on cognate recognition of male antigen on the mesothelium and endothelium.25 C6 T cells do not infiltrate the peritoneal membrane of female mice and remain localized in the peritoneal cavity. The C6 T-cell clone used in this model was chosen based on its constitutive expression of low levels of CD152 (data not shown) and its susceptibility to CD152-mediated signals,32 which avoid the need for T-cell manipulation to induce CD152 expression (ie, activation in vitro followed by antibody-mediated positive selection) prior to their use. In addition, C6 T cells do not display regulatory properties unless they are rendered anergic (F.M.M.-B., unpublished observations, December 1999). In these experiments, intraperitoneal injection of T cells was used, as we have previously established that while this clone promptly infiltrates tissue in static conditions, it does not recirculate efficiently following intravenous injection (ie, it localizes exclusively in the lung possibly due to a deficient expression of selectin ligands and chemokine receptors; data not shown). Male and female CBA/Ca mice were treated intraperitoneally with 600 U IFNγ. After 48 hours, 2 × 106 to 3 × 106 PKH26-labeled HY-specific CD8+ C6 T cells were injected intraperitoneally. The presence of labeled T cells in the peritoneal membrane and lavage was analyzed after 24 hours by wide-field fluorescence microscopy and flow cytometry, respectively. T cells did not localize in the peritoneal membrane of female mice and remained in the peritoneal cavity (Figure 7A) while they progressively infiltrated the peritoneal membrane of male mice, becoming undetectable in the peritoneal lavage by 24 hours after injection (Figure 7G). Following tissue infiltration, T cells remained localized in the peritoneal membrane and were not detected in either draining lymph nodes or in the spleen (data not shown).
CD28- and CTLA-4–mediated signals elicit opposite effects on tissue infiltration by T cells. Female and male CBA/Ca mice were treated intraperitoneally with 600 U IFNγ. After 48 hours, 5 × 106 PKH26-labeled HY-specific CD8+ C6 T cells were injected intraperitoneally in PBS (A-B). In some mice, T cells were coinjected with a mixture of hamster anti–mouse CD28 (5 μg/5 × 106 cells) and rabbit anti–hamster Ig (2.5 μg/5 × 106 cells; C,I) or hamster anti–mouse CD152 (2.5 μg/5 × 106 cells) and rabbit anti–hamster Ig (1.25 μg/5 × 106 cells; D,J) together with the PKH26-labeled C6 cells. As a control, mice were injected with a mixture of hamster Ig (5 μg/5 × 106 cells, the highest Ig dose used for cross-linking) and rabbit anti–hamster Ig (2.5 μg/5 × 106 cells) together with labeled T cells (B,H). The presence of labeled T cells in the peritoneal membrane and lavage was analyzed after 24 hours by wide-field fluorescence microscopy and flow cytometry, respectively. To minimize the effect of arbitrary choice of field, × 10 magnifications are shown. Tissue infiltration was quantified by randomly selecting ten × 10-magnified fields and assessing the number of fluorescent cells in each field. The mean T-cell infiltration ± SD observed in samples from at least 3 animals are summarized in panels E,K (infiltration of the peritoneal membrane) and F,L (cells retrieved in the peritoneal lavage). E, *P < .004; F, *P < .007; K, *P < .05 for CD28 and *P < .001 for CD152; L, *P < .02 for CD28 and *P < .001 for CD152.
CD28- and CTLA-4–mediated signals elicit opposite effects on tissue infiltration by T cells. Female and male CBA/Ca mice were treated intraperitoneally with 600 U IFNγ. After 48 hours, 5 × 106 PKH26-labeled HY-specific CD8+ C6 T cells were injected intraperitoneally in PBS (A-B). In some mice, T cells were coinjected with a mixture of hamster anti–mouse CD28 (5 μg/5 × 106 cells) and rabbit anti–hamster Ig (2.5 μg/5 × 106 cells; C,I) or hamster anti–mouse CD152 (2.5 μg/5 × 106 cells) and rabbit anti–hamster Ig (1.25 μg/5 × 106 cells; D,J) together with the PKH26-labeled C6 cells. As a control, mice were injected with a mixture of hamster Ig (5 μg/5 × 106 cells, the highest Ig dose used for cross-linking) and rabbit anti–hamster Ig (2.5 μg/5 × 106 cells) together with labeled T cells (B,H). The presence of labeled T cells in the peritoneal membrane and lavage was analyzed after 24 hours by wide-field fluorescence microscopy and flow cytometry, respectively. To minimize the effect of arbitrary choice of field, × 10 magnifications are shown. Tissue infiltration was quantified by randomly selecting ten × 10-magnified fields and assessing the number of fluorescent cells in each field. The mean T-cell infiltration ± SD observed in samples from at least 3 animals are summarized in panels E,K (infiltration of the peritoneal membrane) and F,L (cells retrieved in the peritoneal lavage). E, *P < .004; F, *P < .007; K, *P < .05 for CD28 and *P < .001 for CD152; L, *P < .02 for CD28 and *P < .001 for CD152.
In female mice, ligation of CD28 induced C6 T cells to promptly infiltrate the peritoneal membrane and disappear from the peritoneal lavage (Figure 7C). IsC or anti-CD152 treatment did not induce tissue infiltration by T cells, which were still detectable in the peritoneal lavage (Figure 7B and E, and D and F, respectively).
In male mice, anti-CD28 ligation enhanced peritoneal membrane infiltration by HY-specific T cells and their depletion from the peritoneal cavity (Figure 7I,K-L). In contrast, CD152 ligation prevented T cells from migrating from the peritoneal cavity into the peritoneal membrane (Figure 7J–L). T cells pretreated with control hamster Ig and rabbit anti–hamster Ig promptly infiltrate the peritoneal membrane (Figure 7H,K-L). Similarly to what we observed in the experiments assessing peritoneal membrane infiltration in female mice, no T cells were detected in draining lymph nodes or in the spleen (data not shown).
These data show that while CD28 signals promote tissue infiltration by antigen-specific T cells, CD152-mediated signals prevent TCR-dependent tissue infiltration by T cells.
Discussion
The key role of CD28-mediated signals in supporting T-cell activation in response to antigen is well established both in vitro and in vivo.12,33,34 Besides its costimulatory function, it remains unclear whether CD28 signaling independently of the TCR servesa physiologic purpose. In this study, we have analyzed the effects of CD28 engagement on T-cell motility in vitro and trafficking in vivo.
CD28 triggering induced integrin clustering and increased T-cell motility and migration in vitro via PI3K-dependent mechanisms. In this context, CD28 has previously been reported to enhance integrin-mediated adhesion13,14,17,35 and mediate cytoskeleton rearrangements via the Rho family GTPase Rac136 and Cdc42.15
In vivo, CD28-mediated activation was required for efficient localization of primed T cells to nonlymphoid antigenic sites. This is in line with previous studies in models of tissue-specific autoimmune disease in CD28- and B7-deficient mice, which have highlighted a key role for B7:CD28 interactions in the regulation of effector T-cell trafficking.8,9,12
Interestingly, antibody stimulation of CD28 endowed T cells with the ability to infiltrate “homing”-privileged sites, such as the gut, despite lacking the expression of gut-specific homing receptors, and lost the requirement for TCR triggering in antigen-dependent T-cell trafficking. A prominent feature of the pathologic events following CD28 superagonists in humans is a prolonged lymphocyte depletion,37 which, in view of our observations, could be related to unregulated T-cell extravasation to nonlymphoid tissue. In addition, the aberrant homing ability of CD28-activated memory T cells that we describe here may well explain the pathologic events observed in CTLA-4–deficient mice.38,39 It is possible that dominant CD28 signaling in CTLA-4 knockout mice may lead to the extensive T-cell infiltration of nonlymphoid organs observed in these mice. Consistent with this notion, we provide evidence that CTLA-4 exerts potent inhibition of TCR-dependent tissue infiltration by T cells.
Our studies with mutant T cells indicate that CD28-mediated regulation of T-cell motility and trafficking requires intact CD28 signaling and, in particular, PI3K activity. The essential role of PI3K in regulating CD28-dependent adhesion events and migration of memory T cells contrasts with the nonessential role of PI3K in transmitting signals that lead to proliferation and interleukin-2 production downstream of CD28 in naive T lymphocytes.19,40 In addition, naive T-cell migration was not affected by CD28 triggering, suggesting that CD28-initiated signaling pathways may regulate different functions in naive and memory T cells. In this context, it will be interesting to establish the relative contribution of different PI3K subunits to the effects described there.
In summary, the regulation of memory T-cell trafficking by CD28 as described in this study provides a novel function for this molecule in the regulation of effector T-cell responses. In addition, we describe an alternative mechanism to account for the interplay of CD28- and CTLA-4–mediated signals in the regulation of immunity and tolerance. The clinical relevance of the data presented here lays in the growing interest on antibody-mediated manipulation of CD28- and CTLA-4–mediated signals in the therapy of human diseases, which is often burdened by side effects associated with T-cell–mediated inflammation of nonlymphoid tissue as well as lymphocyte depletion from the blood.28-30,37
Although most studies have focused of the effect of triggering/blockade of these molecules on the activation and/or regulation of T-cell clonal expansion, our data highlight the fact that CD28 engagement can have profound antigen-independent effects on memory T-cell trafficking to nonlymphoid organs. In humans, 50% of T cells have a memory phenotype. In contrast, animal models such as the mouse and primates41 have relatively few memory T cells and hence the profound effect that CD28 has on memory T-cell trafficking may have been overlooked.42
Authorship
Contribution: V.M. performed research and wrote the paper; S.J.J. performed research; R.D., J.D., and D.S. performed research work and contributed reagents; Y.G. contributed analytical tools; R.I.L. analyzed data; K.O. analyzed data and wrote the paper; and F.M.M.-B. designed research, analyzed data, and wrote the paper.
V.M. and S.J.J. contributed equally to the study.
Correspondence: Federica Marelli-Berg, Department of Immunology, Division of Medicine, Imperial College London, Hammersmith Campus, London W12 ONN, United Kingdom; e-mail: f.marelli@imperial.ac.uk.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by the British Heart Foundation (PG/2001014 and PG/03/104/15944) and by the Juvenile Diabetes Research Foundation (1-2002-742).
We are grateful to J. Emery and C. Wilson for assistance with immunizations. We are also grateful to E. Simpson, C. Rudd, and H. Schneider for discussion; to A. Ridley and J. Millan for their suggestions in the integrin clustering studies; and to F. Hashim for her help with confocal microscopy.
The current address for V.M. and R.I.L. is as follows: Department of Diabetes, Endocrinology, and Internal Medicine (V.M.) or Immunoregulation (R.I.L.), King's College London, 5th Floor, Thomas Guy House, Guy's Hospital, London SE1 9RT United Kingdom.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal