The CD28 T-cell costimulatory molecule was shown to regulate in vivo migration of memory T cells independent of TCR stimulation, suggesting that CD28 can have additional functions beyond costimulation. However, the mechanism involved, the physiological relevance, and the therapeutic implications await further investigation.
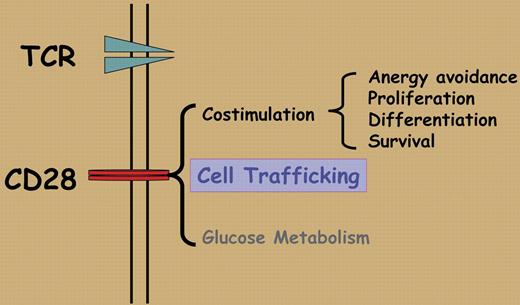
To position the right T cells at the right place is essential in T-cell homeostasis and in productive T-cell immune responses in vivo. CD28 is a key T-cell costimulatory molecule that is required for the initial phase of T-cell activation including prevention of anergy, differentiation, and promotion of cell survival.1 Infiltration of inflamed tissues by activated T cells and the subsequent migration of memory T cells after the primary T-cell response are thought to be guided by homing receptors, adhesion molecules, and chemokine/chemokine receptors. In this issue of Blood, Mirenda and colleagues show that CD28 has a surprising new role beyond its costimulatory function. In a set of carefully designed experiments, the authors discovered that ligation of CD28 on the surface of resting T cells did not interfere with T-cell homing behaviors, and T cells traffic within the secondary lymphoid organs, which is entirely expected given what is already known about the role of CD28. What is surprising is that ligation of CD28 on memory T cells profoundly disrupted their migration properties and caused CD28-stimulated memory T cells to preferentially accumulate in the kidney, liver, and the heart rather than in the lymphoid organs. Similar to studies in other models, the effect of CD28 can be diametrically opposed by that of the inhibitory receptor CTLA-4.2 The authors further demonstrate, in a different set of experiments, that T cells with a mutated CD28 that is defective in intracellular signaling can be activated normally, but such activated T cells fail to accumulate at inflammatory sites. Clearly, besides being a key costimulatory molecule, CD28 can also direct the homing and migration of effector/memory T cells.
The unexpected function of CD28 in memory T-cell migration appears to be dependent on CD28 signaling, especially CD28-triggered PI3 kinase activation and integrin clustering, but is totally independent of TCR stimulation and chemokine CXCL-12–mediated chemotaxis. These features put CD28 into the category of adhesion receptors, in addition to being a T-cell costimulatory molecule. These new findings clearly expand the role of CD28 in the ever-changing T-cell response including bioenergetics and glucose metabolism3 (Figure 1), and may offer an additional explanation for the observed effects associated with the disruption of CD28/CTLA-4:B7 pathway.
Many questions have yet to be answered. First, CD28 is constitutively expressed by all naive and memory T cells in the mouse; it is not clear why CD28 ligation selectively affects the trafficking of memory T cells. It is possible that CD28 may have distinct signaling capabilities in naive and memory T cells, but such distinctions are likely modified by the early phase of TCR-mediated T-cell activation. The new finding also suggests an interesting dichotomy in which CD28 acts as a costimulatory molecule to naive T cells but may function as a homing receptor for memory T cells. Clearly, a detailed understanding of this dichotomy is going to be significant and clinically relevant. Those are important issues and need to be explored further. Second, it remains to be defined why CD28-stimulated memory T cells selectively accumulate in some organs but not in others, and what the physiologic significance is of such trafficking in normal and disease states. A related issue is the effect of CD28 blockade on such trafficking in different disease states, as therapies to block CD28-B7 costimulation are in clinical development.4,5 This may have major implications on the outcome of therapies on the disease versus response to infectious agents. Second, whether CD28 stimulation disrupts the migration of all memory T cells or whether the effect is restricted to a specific memory subset remains unknown. Third, T cells with potent regulatory properties (Tregs) also express CD28 on the cell surface, and phenotypically, Tregs and memory T cells share certain common features; thus, it would be important to know whether stimulation of CD28 also disrupts their migration and homing behaviors. This is highly clinically relevant as the outcome of T-cell–mediated diseases and effects of immunomodulatory therapies are likely determined by the balance between effector/memory T cells on one hand and Tregs on the other. Fourth, very little is known about the relationships and potential interactions between the classical homing receptors and CD28 in regulating the migration of memory T cells, and the therapeutic implications of blocking CD28-B7 interactions in different disease states. Finally, unlike memory T cells in mice, a significant fraction of memory T cells in humans does not express CD28. Thus, caution needs to be taken to extrapolate mouse studies into humans. Nonetheless, the data presented by Mirenda and colleagues certainly open an exciting new avenue of research on the ever-expanding role of CD28 in T-cell immunobiology.
The author declares no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal