Abstract
We have previously shown that engagement of the integrins VLA-4 and VLA-5 to the fibronectin fragment CH-296 in combination with cytokines sustained the capacity of cultured human CD34+ cells to undergo hematopoiesis in immunodeficient mice for 7 to 12 months, whereas this capacity was rapidly lost in cells cultured in suspension with the same cytokines. In the current study, we assessed the molecular pathways that might explain the loss of long-term engraftment capacity in cells cultured in suspension. Although the cell cycle profile was similar between cells cultured in suspension versus on fibronectin, levels of cell death were higher in the suspended cultures. While the CDK inhibitors p27Kip1 and p57Kip2 were present at equal levels in cells from both cultures, low levels of p21Cip1 were detectable only in the cytoplasmic compartment of cells cultured in suspension. Cytoplasmic location of p21Cip1 has been linked to monocytic differentiation. The levels of c-myb and GATA-2, transcription factors associated with stem cell maintenance, were higher in cells cultured on fibronectin as compared with suspension. In contrast, the levels of PU.1, which is induced during myeloid differentiation, were higher in cells cultured in suspension. There were no significant differences in surface expression of CD34 on the cells after culture, but total CD34 protein, assessed by immunoblotting, was significantly higher in cells cultured on fibronectin. Our data suggest that, in the presence of cytokines, the engagement of VLA-4 and VLA-5 integrins to the fibronectin fragment CH-296 preserves the expression of specific transcription factors associated with primitive stem cell maintenance. In contrast, a lack of integrin engagement leads to the induction of cellular markers associated with myeloid differentiation.
Introduction
Human bone marrow–derived hematopoietic stem cells (HSCs) are currently one of the best characterized stem cells in the field of biomedical research. Phenotypically, most HSCs express CD34 and lack expression of CD38.1 As the cells differentiate, CD34 is down-regulated and CD38 is up-regulated in most cases. At the cellular level, HSCs can be induced to self-renew as well as to give rise to both myeloid and lymphoid lineages under optimal cytokine stimulation.2 At the molecular level, the multipotentiality and self-renewal potential of HSCs are under the control of transcription factors such as c-myb, GATA-2, and homeobox proteins.3–6 In vivo studies have demonstrated that a single murine HSC can reconstitute the complete hematopoietic system of a lethally irradiated recipient, highlighting the potency of this pluripotent stem cell.7
Transplantation of hematopoietic stem cells is a common method for assessing the primitive stem cell characteristics of HSCs.8 By definition, primitive HSCs possess the capacity for long-term engraftment, whereas their differentiation-committed progeny can only sustain short-term engraftment.9 Our laboratory has previously reported that direct continuous contact between HSCs and stromal cells was critical in maintaining the long-term reconstitution ability of cultured human hematopoietic stem/progenitor cells.10 We demonstrated that HSCs maintained in suspension during a 72-hour ex vivo culture with IL-3, IL-6, and stem-cell factor (SCF) (36S) could engraft immunodeficient mice for a short-term period of 4 to 6 months; however, these cells were not detected at the long-term engraftment period of 7 to 12 months. In contrast, human HSCs maintained on human stromal cells retained the ability to engraft the mice for 1 year, reconstituting the mice with both lymphoid and myeloid cells.10 We next showed that CH-296, the purified fibronectin (FN) fragment that contains the binding sites for VLA-4 and VLA-5, could be used as a substitute for stromal cells in maintaining the long-term reconstitution ability of HSCs.11 We concluded that integrin engagement is important in maintaining the pool of primitive HSCs during ex vivo culture.
As an extension of our in vivo observations, in the current studies we investigated the molecular mechanisms by which integrin engagement regulates the primitive state of CD34+ progenitors in vitro. One of the features of HSCs is their deeply quiescent state.12 We have shown that HSCs can be recruited out of quiescence by transiently reducing the levels of 27Kip1, a G0 cell cycle gatekeeper; transient reduction of p27Kip1 allowed cell division without a loss of engraftment potential.13 In murine HSCs, p21Cip1, a closely related member of p27Kip1, was reported to be important in maintaining the hematopoietic stem cell pool.14 Of note, p21Cip1 has a role in the myeloid differentiation of human and murine hematopoietic cells.15,16 These studies highlight a correlation between cell cycle regulation and the characteristics of primitive stem cells. Once recruited out of quiescence, a primitive HSC can either self-renew or commit to differentiation. In murine studies, a differentiation-committed hematopoietic progenitor is reported to increase its cell cycle kinetics until it reaches its terminally differentiated state.17 Based on these studies, we hypothesized that integrin engagement may regulate the primitive state of HSCs through alteration of p27Kip1 or p21Cip1.
CD34 has long been used as a surface marker for isolation and identification of primitive hematopoietic cells.1 However, there is evidence that surface expression depends on the activation state of the progenitor cell, making surface detection of this molecule less reliable after culture.18–20 For this reason, we chose to assess the levels of c-myb, a transcription factor that binds to the promoter of the CD34 gene.6 C-myb is also a transcription factor for c-kit and flt3, receptor tyrosine kinases found on primitive hematopoietic cells. In terms of hematopoietic development, c-myb has been shown to be expressed at high levels in primitive stem cells and to decrease as the cells differentiate, making this transcription factor attractive for our current studies.3 Another transcription factor with similar expression patterns as c-myb is GATA-2, found to be high in primitive cells and low in differentiated cells. GATA-2–enforced expression has been shown to preserve the primitive state of HSCs by blocking the differentiation process.4,21–23 Based upon these excellent studies, we hypothesized that down-regulation of c-myb and/or GATA-2 expression could be induced through loss of integrin engagement during suspension culture. This loss could then cause the differentiation process to begin following a reduction in expression of CD34 and other proteins controlled by c-myb. In the current studies we documented that the inability to engage integrins during culture with cytokines in human stem/progenitor cells correlated with a loss of expression of c-myb, GATA-2, and CD34.
In the current studies, we show that although the cell-cycle kinetics are not significantly different between HSCs cultured on FN versus in suspension in the presence of growth factors, there was a higher degree of cell death when cells were maintained in suspension. At the molecular level, hematopoietic progenitors expanded in suspension expressed p21Cip1 protein, a CDK inhibitor (CDKI) previously shown to be absent in freshly isolated CD34+ cells15 but present in abundance in monocytes and megakaryocytes.24 In addition, hematopoietic progenitors cultured in suspension expressed lower levels of c-myb, GATA-2, and CD34 proteins but higher levels of PU.1 protein as compared with hematopoietic progenitors cultured on FN. Collectively, our results suggest that during short-term ex vivo culture with cytokines, at least 2 molecularly distinct pools of hematopoietic cells can be generated—one expressing high levels of c-Myb, GATA-2, and CD34 and the other expressing lower levels of these 3 components—and that integrin engagement plays an important role in this process.
Materials and methods
Isolation of CD34+ cells and culture
Human bone marrow samples were used as the sources of CD34+ progenitors in the current study. All samples obtained were approved by the Childrens Hospital Los Angeles institutional review board (IRB) and the Washington University Human Subjects Committee. Informed consent was obtained in accordance with the Declaration of Helsinki. Mononuclear cells were isolated by ficoll gradient and washed, red-cell lysis buffer was then added, and cells were incubated at 37°C for 10 minutes. Cells were washed twice in phosphate-buffered saline (PBS) and once in CD34+ isolation buffer (2% BSA, 0.05 M EDTA in PBS). Using the MiniMacs Isolation Kit (Miltenyi Biotec, Auburn, CA), CD34+ cells were selected by incubation with antibody to CD34 and then with magnetic beads. Cells were passed through a positive selection column, which retains the cells bound to CD34. Using a syringe plunger, CD34+ cells retained in the column were ejected and transferred to a second column to repeat the process. The purity of CD34+ cells, after 2 columns, was consistently more than 97%. Each experiment was done with CD34+ cells isolated from an individual bone marrow sample, except in some cases in which the number of CD34+ cells recovered per bone marrow sample was too low and CD34+ cells were pooled from 2 bone marrow samples. We have not observed significant differences between experiments with single or pooled CD34+ samples.
Six-well plates were coated with either BSA or 50 mg/mL Retronectin CH-296 (Takara Shuzo, Otsu, Japan) overnight at 4°C. Plates were then rinsed once with cold PBS, and the FN-coated plates were blocked with 2% BSA in PBS for 1 hour at room temperature. After rinsing twice with PBS, plates were used for culture without drying. A total of 106 CD34+ cells were cultured in 5% fetal calf serum–containing Iscove modified Dulbecco medium containing 10 ng/mL IL-3, 50 ng/mL IL-6, and 50 ng/mL SCF for 48 hours to assess cell cycle changes or for 96 hours to assess cell differentiation and survival.
Immunoprecipitation and in vitro kinase assay
After culture, CD34+ cells were collected and normalized by cell counts. In general, 106 cells were lysed in 50 μL of 1% NP-40 lysis buffer containing proteinase inhibitors for 30 minutes on ice with vortexing every 5 minutes. Preclearing of the cell lysates was performed by preincubation for half an hour at 4°C with A/G agarose beads. One tenth of each lysate was transferred to a new tube, equal volume of 2× Laemmli buffer was added, and samples were frozen at −80°C for total protein analyses later. Kinase assays were done as described by Matsushime et al25 using histone H1 as the substrate for cyclin A-, E-, and B-associated kinases immunoprecipitated as described above. Briefly, the remaining portion of each lysate was then diluted with 700 μL lysis buffer and subjected to immunoprecipitation with 10 μL cyclin A, cyclin E, or cyclin B antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 hours followed by incubation with protein A or G agarose beads (Roche Diagnostics, Basel, Switzerland) for 1 hour at 4°C. After 4 washes in lysis buffer, immunoprecipitated samples were washed once in kinase buffer (20 mM HEPES [pH 7 7.2], 25 mM β-glycerophosphate, 1 mM EGTA, 1 mM sodium orthovanadate, 1 mM DTT, 1 mM NaF). In vitro kinase assay was performed in 12.5 μL volume with final concentration of 200 μM cold ATP plus 1 μCi/μL (0.037 MBq/μL) γ-32P ATP plus 1 μg histone H1 at 32°C for 15 minutes. To terminate the reactions, 12.5 μL of 2× Laemmli buffer was added to each tube. Samples were loaded on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels, and gels were dried and exposed to film. The results shown here were obtained after overnight film exposure.
For Western blotting of total lysates, membranes were blocked in 5% nonfat milk/1× PBS/0.05% Tween for 1 hour at room temperature. Incubation with the primary antibody was carried out at room temperature for 2 hours or overnight at 4°C, followed by 4 10-minute washes with 1× PBS/0.05% Tween. Primary antibodies, anti–GATA-2 (H-116), anti–c-myb (C-19), anti-pu.1 (H-135), anti-CD34 (ICO-115), anti-actin (I-19), anti-tubulin (B-7), anti-p21 (C-19), anti-p27 (C-19), anti-p57, anti–cyclin D3 (C-16), anti–cyclin E (M-20), anti-CDK2 (M-2), anti-CDK6 (C-21), anti–cyclin A (C-20), and anti–cyclin B1(H-20), were purchased from Santa Cruz Biotechnology and used at 1:500 dilution. Incubation with the appropriate secondary antibody was then performed using anti–rabbit HRP at a 1:20 000 dilution and anti–mouse HRP at a 1:10 000 dilution. Detection of protein was done using the ECL chemiluminescent assay (Amersham Pharmacia Biotech, Piscataway, NJ).
Subcellular fractionation
Cell samples recovered after culture were normalized for 106 to 2 × 106 cells per sample, for equal loading, by trypan blue exclusion cell count. Initial lysates were made using nuclei extraction buffer (NEB) (10 mM Tris HCl, 10 mM NaCl, 1.5 mM MgCl, 1.0 mM CaCl, 1.0 mM NaF, 5.0 mM β-glycerophosphate, 0.1% NP-40, and protease inhibitors). On ice, using a dounce homogenizer with the “loose” pestle, 20 strokes were performed, followed by 20 strokes with the “tight” pestle. Lysates were then spun at 13 000 rpm for 15 minutes at 4°C, and the supernatant containing the cytoplasmic fraction was transferred to a new tube. The pellets containing nuclei were resuspended in hypertonic NEB containing 420 mM NaCl, sonicated for 10 seconds on ice, and pelleted at 356 g for 15 minutes at 4°C. Cytoplasmic and nuclear fractions were loaded onto 10%, 12%, or 15% SDS-PAGE gels and run, transferred, and immunoblotted as described above.
Cell cycle assessment by Ki67/7-AAD staining
For Ki67/7-AAD staining, to assess the percentage of cells in G0, G1, and S/G2M after 48 hours of culture on FN- versus BSA-coated plates, 106 cells from each culture were fixed in 2% paraformaldehyde for 20 minutes, followed by permeabilization with 0.5% saponin for 15 minutes. Cells were then washed twice in PBS/0.05% saponin and then split equally into 2 tubes for staining with IgG-FITC versus Ki67-FITC (BD Biosciences/PharMingen, San Jose, CA) for 15 minutes, followed by staining with 7-AAD (Sigma, St Louis, MO). Cells were acquired and analyzed on FACSCalibur on FL-1 (for IgG-FITC and Ki67-FITC) and FL-3 (for 7-AAD).
Cell death assessment by annexin V/7-AAD staining
A total of 500 000 cells from 96-hour cultures on FN- versus BSA-coated plates were washed and fixed in 1× binding buffer (0.01 M HEPES [pH 7.4], 0.14 M NaCl, and 2.5 mM CaCl2) prior to addition of 2 μL annexin V antibody (BD Biosciences/PharMingen) and 2 μL 7-AAD antibody (Sigma). Acquisitions and analyses were performed on FACSCalibur on FL-1 and FL-3 channels.
Results
Assessment of cell-cycle profile and subcellular levels of p27Kip1 and p21Cip1
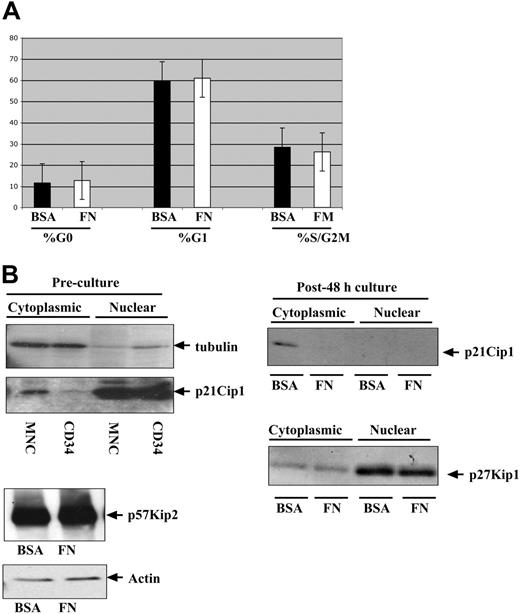
Although hematopoietic cells are often classified as adherence-independent cells, they do respond to integrin engagement to various extracellular matrix proteins. To determine whether integrin engagement in the presence of IL-3, IL-6, and SCF regulates cell-cycle entry in quiescent bone marrow CD34+ progenitors, we performed Ki67 and 7-AAD intracellular staining on CD34+ progenitors after 48 hours of culture on BSA- versus FN-coated plates. As shown in Figure 1A, there were no significant differences in the percentages of cells in the G0, G1, or S/G2M phases between the 2 cultures.
Comparison of cell cycle kinetics and regulators between CD34+ progenitors cultured on FN- versus BSA-coated plates. Human bone marrow CD34+ progenitors were cultured in the presence of IL-3, IL-6, and SCF for 48 hours on either FN- or BSA-coated plates prior to analysis. (A) Ki67/7-AAD staining was performed to identify the percentage of G0, G1, and S/G2M cells after 48 hours on FN- (□) and BSA- (▪) coated plates. (B) Subcellular fractionations were performed on 106 cells recovered from FN- and BSA-coated plates and immunoblotted with an antibody against p21Cip1 and p27Kip1. Freshly isolated CD34+ progenitors and mononuclear cells were subfractionated and immunoblotted with an antibody against p21Cip1 (left) and compared with cells harvested after 48-hour culture on FN- versus BSA-coated plates (right). Total protein lysates were immunoblotted to assess for p57Kip2 (lower left). MNC indicates mononuclear cells. Error bars indicate standard deviation.
Comparison of cell cycle kinetics and regulators between CD34+ progenitors cultured on FN- versus BSA-coated plates. Human bone marrow CD34+ progenitors were cultured in the presence of IL-3, IL-6, and SCF for 48 hours on either FN- or BSA-coated plates prior to analysis. (A) Ki67/7-AAD staining was performed to identify the percentage of G0, G1, and S/G2M cells after 48 hours on FN- (□) and BSA- (▪) coated plates. (B) Subcellular fractionations were performed on 106 cells recovered from FN- and BSA-coated plates and immunoblotted with an antibody against p21Cip1 and p27Kip1. Freshly isolated CD34+ progenitors and mononuclear cells were subfractionated and immunoblotted with an antibody against p21Cip1 (left) and compared with cells harvested after 48-hour culture on FN- versus BSA-coated plates (right). Total protein lysates were immunoblotted to assess for p57Kip2 (lower left). MNC indicates mononuclear cells. Error bars indicate standard deviation.
At the molecular level, we assessed the common gatekeepers of cell-cycle entry—namely, the Cip/Kip family proteins. Previous reports from Jiang et al showed that p27Kip1 is elevated upon integrin engagement but that this effect is counteracted by cytokines.26 In agreement with those studies, as shown in Figure 1B, there were no differences in the levels of p27Kip1 protein in cells cultured on BSA- or FN-coated plates in the presence of IL-3, IL-6, and SCF, making p27Kip1 an excellent internal control for assessing other cell-cycle inhibitors, p21Cip1 and p57Kip2, which to date, have not been studied in the context of integrin regulation of CD34+ progenitors. Similar to p27Kip1, there were no differences in the protein levels of p57Kip1 detected in cultures on BSA- versus FN-coated plates (Figure 1B). In contrast, p21Cip1 was detectable at low levels in the cytoplasmic compartment of progenitors cultured in suspension. Our data suggest that integrin engagement in the presence of cytokines exerts differential effects on the Cip/Kip proteins.
Comparison of the expression and activity of various cyclins
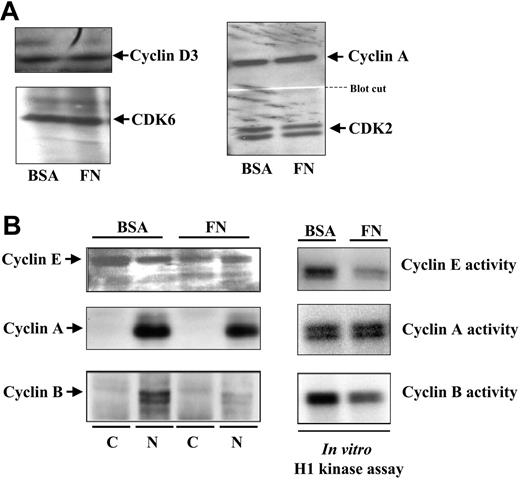
The cytoplasmic detection of p21Cip1 led us to investigate the kinase activity of various cyclin/CDK complexes known to be targeted by p21Cip1. Cyclin D is the mitogenic cyclin that associates with CDK6 to initiate the hypophosphorylation of the retinoblastoma protein in G1. Comparing cultures on BSA- versus FN-coated plates, we observed no differences in the levels of cyclin D or CDK6 Figure 2A). Cyclin E is the regulatory subunit of CDK2 in early S phase.27 We detected higher levels of cyclin E protein in hematopoietic progenitors cultured on BSA-coated plates, and in vitro kinase assays using immunoprecipitates of cyclin E showed higher activity in these cells (Figure 2B). Cyclin A is the regulatory subunit of CDK2 throughout S phase.28 There were no significant differences in cyclin A protein levels and, accordingly, there were no significant differences in cyclin A–associated CDK2 kinase activity, as assessed by in vitro kinase assays (Figure 2B).
Assessment of the levels of cyclin E, A, and B and their CDK-associated kinase activity in CD34+ cells cultured on FN- versus BSA-coated plates. After 48 hours of culture in cytokines on FN- versus BSA-coated plates, cell numbers were equalized and half of the cells were used for subcellular fractionation, immunoblotting for cyclin D3 and CDK6 (A, left), cyclin A and CDK2 (A, right), cyclin E (B, left), cyclin A (B, left), and cyclin B (B, left). The other half of the cells were used for immunoprecipitation with antibodies against specific cyclins (as indicated) followed by in vitro histone H1 kinase assay to assess activity of CDKs associated with cyclin E (A, right), cyclin A (B, right), and cyclin B (B, right).
Assessment of the levels of cyclin E, A, and B and their CDK-associated kinase activity in CD34+ cells cultured on FN- versus BSA-coated plates. After 48 hours of culture in cytokines on FN- versus BSA-coated plates, cell numbers were equalized and half of the cells were used for subcellular fractionation, immunoblotting for cyclin D3 and CDK6 (A, left), cyclin A and CDK2 (A, right), cyclin E (B, left), cyclin A (B, left), and cyclin B (B, left). The other half of the cells were used for immunoprecipitation with antibodies against specific cyclins (as indicated) followed by in vitro histone H1 kinase assay to assess activity of CDKs associated with cyclin E (A, right), cyclin A (B, right), and cyclin B (B, right).
Finally, we assessed cyclin B, the regulatory subunit of CDC2 in G2/M phase.29 Similar to cyclin E, there was a higher detection of cyclin B protein in hematopoietic cells cultured on BSA-coated plates as compared with cells on FN-coated plates. Accordingly, there was also more kinase activity in cyclin B immunoprecipitates from hematopoietic cells cultured on BSA-coated plates (Figure 2B). These observations suggest that integrin engagement differentially affects the expression of specific cyclins and their associated kinases.
Assessment of CD34 surface expression and total cellular protein expression
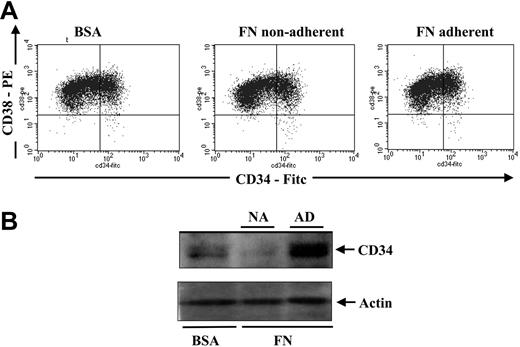
During myeloid differentiation of human hematopoietic progenitors, p21Cip1 has been shown to be up-regulated.15 To determine if the induction of p21Cip1 in hematopoietic progenitors cultured on BSA-coated plates might be suggestive of differentiation, we assessed the expression of CD34, a marker found to be highly expressed on the surface of stem and progenitor cells and low on actively differentiating cells. Cell-surface measurements of CD34 showed no significant differences between cells cultured in suspension versus on fibronectin (Figure 3A). However, when we assessed total cellular CD34 levels in protein lysates of hematopoietic cells after culture, we noted a significantly higher level of total CD34 protein in hematopoietic cells cultured on FN-coated plates as compared with cells cultured on BSA-coated plates (Figure 3B). These observations suggest a possible role for integrin engagement in regulating CD34 expression.
Comparison of CD34 surface expression versus total CD34 protein expression in CD34+ progenitors after culturing on FN- versus BSA-coated plates. After a 48-hour culture, 200 000 cells were stained with anti-CD34–FITC and anti-CD38–PE and analyzed by flow cytometry to assess CD34 cell-surface expression (A). An additional 2 × 106 cells from the same cultures (n = 4) were lysed in protein lysis buffer for CD34 total protein expression by immunoblotting (B). The top half of the blot was immunoblotted for CD34, and the bottom half was immunoblotted for actin as a loading control.
Comparison of CD34 surface expression versus total CD34 protein expression in CD34+ progenitors after culturing on FN- versus BSA-coated plates. After a 48-hour culture, 200 000 cells were stained with anti-CD34–FITC and anti-CD38–PE and analyzed by flow cytometry to assess CD34 cell-surface expression (A). An additional 2 × 106 cells from the same cultures (n = 4) were lysed in protein lysis buffer for CD34 total protein expression by immunoblotting (B). The top half of the blot was immunoblotted for CD34, and the bottom half was immunoblotted for actin as a loading control.
Expression of c-myb, GATA-2, and PU.1
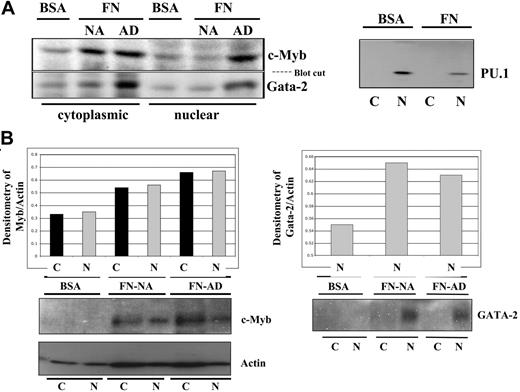
The induction of p21Cip1 and the lower levels of CD34 protein in the progenitors cultured in suspension led us to hypothesize that the lack of integrin engagement during ex vivo culture might induce the differentiation process. In contrast, the lack of p21Cip1 and the higher levels of CD34 protein in the progenitors cultured on FN led us to hypothesize that integrin engagement could preserve some primitive characteristics of CD34+ progenitors. In support of this hypothesis, we demonstrated that c-myb and GATA-2, both of which are expressed at high levels in primitive hematopoietic cells, are higher in hematopoietic cells cultured on FN-coated plates than in cells cultured on BSA-coated plates (Figure 4). In contrast, we showed that PU.1, a transcription factor that is expressed at high levels in differentiating monocytes,30,31 is more abundant in hematopoietic cells cultured on BSA-coated plates as compared with cells cultured on FN-coated plates (Figure 4B). These results suggest that 2 different pools of hematopoietic cells can be generated during ex vivo culture with cytokines—one pool of cells with high GATA-2 and c-myb and the other with low GATA-2 and c-Myb levels—depending on the presence or lack of integrin engagement.
Detection of c-Myb, GATA-2, and PU.1 protein after ex vivo culture on FN- versus BSA-coated plates. To determine whether integrin engagement preserves the primitiveness of CD34+ cells, the cells were cultured on FN- versus BSA-coated plates in the presence of IL-3, IL-6, and SCF for 96 hours. A total of 2 × 106 cells were normalized at the end of each culture and subjected to subcellular fractionation and protein analysis for c-Myb and GATA-2, 2 transcription factors reported to be abundant in primitive cells versus PU.1, a transcription factor highly expressed in committed monocytes (A). A quantitative representation of 1 of the 4 bone marrow experiments assessing c-Myb and GATA-2 is shown by normalizing the densitometry value of the transcription factor with that of the corresponding actin densitometry value (B). NA indicates nonadherent; AD, adherent.
Detection of c-Myb, GATA-2, and PU.1 protein after ex vivo culture on FN- versus BSA-coated plates. To determine whether integrin engagement preserves the primitiveness of CD34+ cells, the cells were cultured on FN- versus BSA-coated plates in the presence of IL-3, IL-6, and SCF for 96 hours. A total of 2 × 106 cells were normalized at the end of each culture and subjected to subcellular fractionation and protein analysis for c-Myb and GATA-2, 2 transcription factors reported to be abundant in primitive cells versus PU.1, a transcription factor highly expressed in committed monocytes (A). A quantitative representation of 1 of the 4 bone marrow experiments assessing c-Myb and GATA-2 is shown by normalizing the densitometry value of the transcription factor with that of the corresponding actin densitometry value (B). NA indicates nonadherent; AD, adherent.
Assessment of the levels of cell death
Overexpression of cytoplasmic p21Cip1 has been reported to render differentiating monocytes resistant to apoptosis-inducing factors such as TNF-α or hydrogen peroxide.32 We therefore asked whether p21Cip1 induction suggests that hematopoietic progenitors cultured in suspension are under stress which, in turn, might affect their viability. To assess cell death, we compared the annexin V/7-AAD staining profile of hematopoietic cells cultured on BSA-coated plates versus FN-coated plates (Figure 5A). We observed a significantly higher percentage of necrotic and apoptotic cells in BSA-coated plates. Within the cells cultured on FN-coated plates, we compared the staining profile between the nonadherent floating cells versus the adherent cells and detected higher levels of cell death in the floating cell fractions. This pattern of decreased cell survival in cells cultured on BSA-coated plates as well as in floating cells from FN-coated plates was noted in 3 independent experiments with individual bone marrow samples (Figure 5B). These results highlight the role of integrin engagement in regulating cell survival during ex vivo culture.
Assessment of cell death in cultures of CD34+ progenitors maintained on FN- versus BSA-coated plates. (A) A total of 500 000 cells from each of the culture conditions were subjected to annexin V (FITC) and 7-AAD staining. FACS acquisition and analysis were done on FACSCalibur. (B) Percentage of live cells from 3 independent experiments using marrow samples from 3 donors.
Assessment of cell death in cultures of CD34+ progenitors maintained on FN- versus BSA-coated plates. (A) A total of 500 000 cells from each of the culture conditions were subjected to annexin V (FITC) and 7-AAD staining. FACS acquisition and analysis were done on FACSCalibur. (B) Percentage of live cells from 3 independent experiments using marrow samples from 3 donors.
Discussion
In vivo, primitive hematopoietic progenitors are lodged in the bone marrow within a specialized niche shared with osteoblasts.33 Osteoblasts express VCAM-1, the counterpart of VLA-4 integrin found on primitive hematopoietic progenitors.34 In addition, hematopoietic progenitors express VLA-5 integrin, which binds to the RGD domain of FN, an extracellular matrix protein found in the bone marrow compartment. Verfaillie et al reported that integrin engagement through the VLA-4 and/or VLA-5 integrins reduced the proliferation rate of CD34+ cells.35 We have reported that integrin engagement preserves the long-term engraftment potential of CD34+ cells during ex vivo gene transfer on CH-296.11 Because, in hematopoietic cells, differentiation is coupled with increased cell cycle and loss of long-term engraftment potential,17,36 we hypothesized that integrin engagement during ex vivo culture could preserve the primitive characteristics of CD34+ progenitors. First, we demonstrated that p21Cip1, a CDKI protein that is up-regulated during monocyte differentiation, is detectable at low levels in hematopoietic cells cultured in suspension and not in cells on FN. Second, we demonstrated that the cells cultured in suspension have lower expression of CD34 protein, a common marker of primitive progenitors. Third, in cells cultured in suspension, we observed lower levels of c-myb and GATA-2, two transcription factors known to be most abundant in primitive cells, and a higher level of PU.1, a transcription factor up-regulated during myeloid differentiation. These observations suggest that the presence of the cytokines IL-3, IL-6, and SCF coupled with a lack of integrin engagement during ex vivo culture of CD34+ progenitors leads to changes in intracellular proteins indicative of differentiation and/or loss of primitiveness.
The role of p21Cip1 in hematopoietic progenitors has been controversial. In p21Cip1-deficient mice, there is strong evidence that p21 has a role in maintaining the quiescent state of primitive hematopoietic progenitors.14 In human hematopoietic progenitors, p21 mRNA and protein are induced during differentiation along the myeloid/monocytic lineage,15 and p21 protein is found in abundance in monocytes and megakaryocytes.24 Interestingly, in monocytes, p21 protein is retained in the cytoplasmic compartment through its binding with Brap2, a protein partner of BRCA1.37 The cytoplasmic p21 has a special role as an antiapoptotic regulator in monocytes.32 In our cultures, we observed a higher percentage of cell death when cells were expanded in suspension. Cytoplasmic p21 expression corresponded to the live cells present in the suspended culture. Our results also suggest that ex vivo culture of hematopoietic progenitors in the absence of integrin engagement may be a stress-inducing condition even in the presence of cytokines.
The cytoplasmic localization of p21Cip1 observed in the cells cultured in suspension reduces its role as a cell-cycle inhibitor. Accordingly, we noted no decrease in the activity of cyclin/CDK kinases in the cells cultured in suspension despite the higher levels of cytoplasmic p21Cip1. Although we consistently observed a direct correlation between cyclin levels and the activity of their catalytic subunits, it was interesting that only cyclin E and cyclin B are higher in cells cultured in suspension. In contrast, cyclin D and cyclin A did not show significant differences. It is possible that at the time of harvest, the cells were in a phase that is dependent on p27Kip1 and cyclin A. Because we noted no difference in p27Kip1 or p57Kip2 levels, cyclin A activity may be under the regulation of p27Kip1, which would account for no differences in its activity when comparing cells in suspension versus on FN. Collectively, although we saw some minor differences in the percentages of G0, G1, and G2/M when comparing cultures in suspension versus on FN, the differences were not significant, again in support of a previous report that cytokines override the cell-cycle –inhibitory impact of integrin engagement.38 It is known that specific cyclins are involved in differentiation. For instance, cyclin D3 is critical for megakaryocyte differentiation.39 Overexpression of cyclin D2 and D3 impedes granulocyte differentiation.40 Whether the higher levels and activity of cyclin E and cyclin B correlate with differentiation remains to be determined.
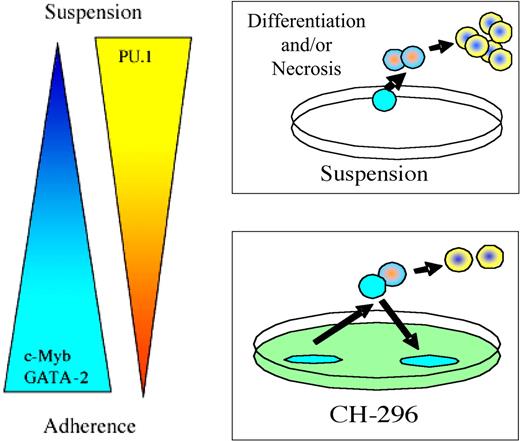
In conclusion, we previously reported that human bone marrow CD34+ progenitors lose their long-term engraftment potential when cultured in suspension; in contrast, CD34+ progenitors cultured on fibronectin retain the potential to durably engraft and reconstitute beige/nude/xid mice with a complete human multilineage hematopoietic compartment.11 We present evidence here that the lack of integrin engagement during ex vivo culture leads to intracellular changes suggestive of increased differentiation and reduced cell survival of hematopoietic progenitors (Figure 6).
Hypothesis model. We hypothesize that maintenance of CD34+ progenitors in suspension culture with cytokines induces differentiation and sensitivity to cell death. In contrast, CD34+ progenitors maintained in culture on CH-296–coated plates generate 2 pools of cells: the less primitive nonadherent cell pool and the more primitive adherent cell pool.
Hypothesis model. We hypothesize that maintenance of CD34+ progenitors in suspension culture with cytokines induces differentiation and sensitivity to cell death. In contrast, CD34+ progenitors maintained in culture on CH-296–coated plates generate 2 pools of cells: the less primitive nonadherent cell pool and the more primitive adherent cell pool.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jan A. Nolta, Washington University School of Medicine, Department of Internal Medicine, Division of Oncology, Hematopoietic Development & Malignancy Program, Southwest Tower, 6th Floor, Rm 643, 4940 Parkview Place, St Louis, MO 63110; e-mail: jnolta@im.wustl.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health's (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (2R01DK53041 and 2RO1DK61848) and National Heart, Lung, and Blood Institute (NHLBI) (SCOR P50-HL54850).
We thank the physicians in the Division of Research Immunology/Bone Marrow Transplantation (BMT) at Childrens Hospital Los Angeles and in the Division of Oncology at Washington University for drawing bone marrow samples for these studies.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal