Abstract
Flk-1+ endothelial progenitors contribute critically to the definitive onset of hematopoiesis during embryogenesis. Recent studies have suggested that adult sources of endothelial cells also possess hematopoietic activity. In this study, we sought to determine whether transplantation of primary vascular endothelial cells (ECs) could enhance the hematopoietic recovery and survival of irradiated mice. C57Bl6 mice were exposed to sublethal and lethal doses of irradiation and were subsequently given transplants of either primary murine brain–derived ECs (MBECs) or fetal blood-derived ECs (FBECs). Mice that received a transplant with MBECs alone demonstrated accelerated BM cellular recovery, radioprotection of BM c-kit+sca-1−lin− progenitors and enhanced regeneration of c-kit+sca-1+lin− (KSL) stem/progenitor cells following irradiation compared with controls. MBEC transplantation also facilitated the recovery of circulating white blood cell and platelet counts following radiation exposure. Remarkably, 57% of mice that received a transplant with MBECs alone survived long term following 1050 cGy exposure, which was 100% lethal in control mice. FBEC transplantation was also associated with increased survival compared with controls, although these mice did not survive in the long term. These data suggest that reestablishment of endothelial cell activity can improve the hematopoietic recovery and survival of irradiated mice.
Introduction
Studies have shown that hematopoietic stem cells (HSCs) reside in close association with osteoblasts within the BM niche, and this association contributes to the maintenance of the HSC pool in vivo.1,2 HSCs also reside in intimate anatomic proximity to vascular endothelial cells (ECs) from the earliest stages of embryonic development,3 through their migration to the fetal liver,4 and ultimately throughout their adult residence within the BM compartment.5 During embryogenesis, mice lacking flk-1+ endothelial precursor cells fail to initiate normal hematopoiesis,3 and gene marking studies suggest that hematopoietic and vascular endothelial cells derive from a common precursor cell, the hemangioblast.6 Similarly, bone marrow–, umbilical vein–, and yolk sac–derived endothelial cells elaborate growth factors that support the proliferation of myeloid, erythroid, and megakaryocytic progenitors in vitro.7–9 Our laboratory has shown that primary human brain-derived endothelial cells produce a soluble hematopoietic activity that supports a 1 to 2 log expansion of the most primitive assayable human hematopoietic cell, which is capable of long-term repopulation in nonobese diabetic/severe combined immunodeficient mice.10–12 Li et al13 have also confirmed that murine brain- and heart-derived endothelial cells can support the maintenance of colony-forming unit-spleen day-8 colonies in vitro. Taken together, these data implicate vascular endothelial cells as a source of proliferative and regenerative signals for hematopoietic stem and progenitor cells.
Although these studies have demonstrated the capacity of endothelial cells to support hematopoiesis in vitro,7–13 evidence of the in vivo contribution of endothelial cells to adult hematopoiesis has begun to emerge. Blockade of N-cadherin on vascular endothelial cells in the BM has been associated with delayed megakaryocyte recovery in 5-FU–treated mice,5 and accelerated repair of this BM vascular niche has been associated with early megakaryocytopoiesis after chemotherapy. More recently, adenoviral delivery of angiopoietin, which can signal through the Tie-2 receptor on vascular endothelial cells, has been associated with improved hematologic recovery following 5-FU treatment in mice.14 Although the effects of angiopoietin-1 delivery may have been accounted for by direct effects on BM HSCs, which also express Tie-2,15 these data suggest the possibility that vascular endothelial cells in the BM contribute to hematopoietic regeneration in vivo.5,14 On the basis of our own observations that primary ECs support the amplification of HSCs in vitro, we initiated studies to determine whether systemic transplantation of primary ECs alone, in the absence of hematopoietic stem cell transplantation, could ameliorate the myeloablative effects of high-dose radiation therapy. Our results indicate that systemic administration of primary ECs alone accelerates hematopoietic recovery and enhances the survival of lethally irradiated mice. Moreover, this effect is mediated directly through the enhanced regeneration of BM stem and progenitor cells.
Materials and methods
Isolation and passage of primary MBECs
To establish murine brain–derived EC (MBEC) monolayers, 6-well culture plates (Corning, Corning, NY) were coated with 0.2% gelatin (Sigma, St Louis, MO) in DPBS, for 1 hour at room temperature. Plates were then washed twice with DPBS, and 5 mL/well of endothelial cell growth media, containing M199 (Invitrogen, Carlsbad, CA), 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 2 mM L-glutamine (Invitrogen), 100 U/mL penicillin, 100 μg/mL streptomycin (1% pcn/strp; Invitrogen), 4 U/mL heparin (Sigma), and 60 μg/mL endothelial cell growth supplement (Sigma) were added. MBECs were seeded at 1 × 105/well and brought to greater than 80% confluence by incubating for 48 hours at 37°C in 5% CO2. GFP+ MBECs were generated from cortical brain vessel explants from C57BL/Ka-Thy1.1 GFP-transgenic mice that were generated in the laboratory of Dr Irving Weissman (Stanford University, Palo Alto, CA) and provided courtesy of Dr Jos Domen (Duke University, Durham, NC).
Reverse transcriptase–polymerase chain reaction (RT-PCR) analysis of MBECs and Matrigel assay
Total RNA was extracted from 5 × 106 MBECs using a RNeasy Mini spin column (Qiagen, Valencia, CA), according to the manufacturer's protocol. RNA was quantified using a SmartSpec 3000 spectrophotometer (Bio-Rad, Hercules, CA), and total RNA was reverse transcribed using the SuperScript III First-Strand Synthesis System (Invitrogen), according the manufacturer's instructions. PCR amplification of cDNA was conducted using Platinum Taq polymerase (Invitrogen) to detect the presence of mRNA for the following endothelial associated genes: vascular endothelial-cadherin (CD144), tyrosine kinase with immunoglobulin and epidermal growth factor homology domains-2 (Tie 2), von Willebrand factor (VWF), Flk-1, and CD31 (PECAM) using sequence-specific primers as previously described.16
Capillary-like tube formation assay was conducted using Matrigel basement membrane matrix (Becton Dickinson, San Jose, CA), according to the manufacturer's recommended procedure. Briefly, 300 μL/well Matrigel was placed into a precooled 24-well plate (Corning) and incubated at 37°C for 30 minutes to solidify. Then, 4 × 104 MBECs/well were placed onto the Matrigel matrix and incubated at 37°C in a 5% CO2 humidified environment for 24 to 48 hours. Capillary tube formation was assessed via phase-contrast microscopy as previously described.16 A Nikon Diaphot 200 inverted microscope was used with Nikon ACT-1 imaging software (version 2.63) and a DXM 1200 digital camera (Nikon Instruments, Melville, NY). Photographs were taken using a 10×/0.25 NA lens.
Transplantation of MBECs into irradiated C57Bl6 mice
Eight- to 10-week-old C57Bl6 mice (Jackson Laboratory, Bar Harbor, ME) were irradiated with 700 cGy total body irradiation at a rate of 100 cGy/minute using the X-rad 320 irradiation system (AGFA NDT, Lewistown, PA). Two hours following irradiation, mice were given either intravenous or intraperitoneal transplants of MBECs (passage > 5) that had been washed twice and resuspended in PBS prior to tail vein injection. Mice were injected with MBECs daily from day 0 through day +4 (5 total doses) and then observed for survival. At days +5, +10, +15, +20, +25, and +30, subsets of mice that received a transplant and controls were killed, and femurs were harvested. A subset of mice had fresh BM cells collected from the femurs, Ficoll-Hypaque collection of mononuclear cells (MNCs), and viable MNC counts were obtained. Another set of mice had femurs collected and fixed in 4% paraformaldehyde (Sigma) and dissolved in DPBS for 24 hours at room temperature. After fixation, one femur was processed for histology using hematoxylin and eosin (H&E) staining, and one femur was used for GFP visualization.
To detect native GFP, femurs were decalcified using daily changes of 14% EDTA (EMD Chemicals, Gibbstown, NJ) (pH 7.1) for 7 to 10 days at 4°C. The femurs were washed thoroughly with DPBS and soaked in 20% sucrose (Fisher Biotech, Fair Lawn, NJ) dissolved in DPBS, overnight at 4°C. Decalcified bones were then placed into Tissue-Tek cryomolds (Sakura Finetek, Torrance, CA), embedded in Tissue-Tek OCT compound (Sakura Finetek), and frozen in a dry ice-ethanol bath. Cryosectioning was performed on a Leica CM1850 cryotome (Meyer Instruments, Houston, TX), and 10-μm serial sections were adhered to Poly-prep poly-L-lysine coated slides (Sigma). Slides were visualized on an Olympus FluoView FV500 confocal microscope (Olympus, Melville, NY). Wild-type and GFP+ BL6 mouse femur sections were used to distinguish and subtract background fluorescence.
Measurement of BM stem and progenitor cell content, hematologic parameters, and survival studies
Groups of mice were also irradiated with 700 cGy; given transplants of MBECs for 5 days; and then killed at dasy +10, +15, and +20 to measure BM stem and progenitor cell content. Untreated control mice, irradiated mice that were treated intravenously with MBECs, and mice that received intraperitoneal MBEC injections were compared. Flow cytometric analysis was performed to measure the percentages of c-kit+sca-1−lin− progenitor cells and c-kit+sca-1+lin− (KSL) stem cell–enriched populations within the BM in all mice. Briefly, bilateral femurs were harvested, and BM cells were collected, following red blood cell lysis, as previously described.17 BM cells were incubated with a fluorscein isothiocyanate–conjugated anti–stem cell antigen-1 (sca-1), a phycoerythrin–conjugated anti-CD117 (c-kit), and an allophycocyanin–conjugated lineage antibody cocktail (Becton Dickinson), containing anti-CD3e, CD11b, B220, Ly-76, and Gr-1. The stained cells were washed with DPBS, resuspended in 100 μL/sample DPBS + 10% FBS + 1% pcn/strp, and incubated with 5 μL 7AAD (Becton Dickinson) for 10 minutes on ice. Samples were analyzed using a FACScalibur cytometer (Becton Dickinson).
Groups of mice were analyzed under the identical treatment conditions described above for measurement of recovery of white blood cells (WBCs) and platelet counts in the peripheral blood. For these studies, peripheral blood samples (100 μL) were collected via the orbital vein beginning at day +5 and then every 5 days through day +30. Complete blood counts and differentials were measured within each sample using an Abbott CELL-DYN 3700 Hematology Analyzer (Abbott Laboratories, Abbott Park, IL). Equal numbers of mice that received a transplant with MBECs and control mice that did not receive a transplant were evaluated to compare hematologic recovery in each group. Statistical comparisons were performed using a t test.
Additional groups of mice were irradiated with a lethal dose of irradiation and subsequently systemically given transplants of either MBECs, murine fetal blood ECs (FBECs), or murine mesenchymal stem cells (MSCs; courtesy of Dr Victor Dzau, Duke University), and their survival was compared with untreated control mice. Briefly, 8- to 10-week-old C57Bl6 mice were irradiated with 1050 cGy irradiation (500 cGy and 550 cGy, split dose by 4 hours, dose rate 100 cGy/minute) and given transplants, beginning 2 hours after irradiation, with 1 × 106 MBECs intravenously × 1 and then 1 × 106 MBECs daily for 4 days via intraperitoneal administration. Additional groups of mice were irradiated identically with 1050 cGy total body irradiation and subsequently given transplants of 1 × 106 FBECs or MSCs via identical route of administration, and their survival was compared with control, irradiated mice. Survival analyses were conducted using the log-rank test.
Cytokine assays and cytokine administration in lethally irradiated mice
The concentrations of known hematopoietic cytokines were measured in MBEC- and FBEC-derived conditioned medium (CM) using enzyme-linked immunoabsorbent assay (ELISA). Conditioned media from MBECs and FBECs were produced as follows: MBECs and FBECs were seeded at 2 × 106/flask in gelatin-coated 175-cm2 tissue culture flasks (Becton Dickinson) containing 25 mL/flask of endothelial cell growth media and allowed to come to greater than 95% confluence during 10 days at 37°C in a 5% CO2 environment. After 10 days, media was removed, the cells were washed twice with excess PBS to remove residual media, and the media were replaced with 35 mL/flask of Iscove Modified Dulbecco Medium containing 1% pcn/strp. The cultures were then incubated for another 10 days at 37°C in 5% CO2, and the media was collected, centrifuged to remove cell debris, and filter sterilized through a 0.2-μm filter unit (Nalgene Nunc, Rochester, NY). CM was concentrated to 50 × in a stirred cell concentrator (Millipore, Bedford, MA) over a 3-kDa cellulose ultrafiltration membrane (Millipore) using nitrogen gas, in a 4°C cold room. Following concentration, 50 × CM was filter sterilized using a 0.2-μm Acrodisc syringe filter (Pall, Ann Arbor, MI) and stored at −80°C in 0.5-mL aliquots. For ELISA, CM was diluted to 1 × concentration using sterile water.
ELISA analyses of CM were conducted using Quantikine ELISA kits (R&D Systems, Minneapolis, MN), according to the manufacturer's suggested protocol. The following murine cytokines were measured: SCF, platelet-derived growth factor AA (PDGF-AA), interleukin 1-α (IL-1α), IL-1β, IL-6, TPO, granulocyte macrophage colony-stimulating factor (GM-CSF), Flt-3 ligand, vascular endothelial growth factor (VEGF), IL-11, IL-3, tumor necrosis factor α, stromal-derived factor 1 (SDF-1), leukemia inhibitory factor, granulocyte colony-stimulating factor (G-CSF), and angiopoietin 1. ELISAs were also performed on serum collected via retroorbital bleed from isofluorane-anesthetized C57BL6 mice at day +4 following 1050 cGy irradiation and 2 hours following the final dose of MBECs. Serum was also collected from 1050 cGy-irradiated mice that did not receive MBECs and from nonirradiated control mice. Blood was centrifuged in a tabletop microfuge at 2300 g for 10 minutes to pellet the cellular components, and serum was collected and stored at −20°C prior to ELISA analyses. Analyses were conducted in triplicate on serum samples diluted 1:8 with PBS.
Survival studies were also performed on additional sets of mice that were lethally irradiated with 1050 cGy total body irradiation and subsequently received 5 days of VEGF, PDGF, SDF-1, IL-6, or the combination of these 4 cytokines, beginning at 2 hours after irradiation. C57BL6 mice irradiated with 1050cGy were given either 100 ng murine VEGF, 100 ng rat PDGF-AA, 2 μg IL-6, 1.5 μg SDF-1 (R&D Systems) daily for 5 days via intraperitoneal administration, or the combination of these cytokines at the same doses. Doses of each growth factor were determined based on prior studies demonstrating the systemic activity of each cytokine following administration in rodents.18–21 Log-rank analyses were performed to compare the survival of the cytokine-treated animals versus control, irradiated mice. All animal studies were performed under protocols approved by the Duke University Animal Use Committee.
Results
Characterization of primary murine brain endothelial cells (MBECs) and systemic administration in irradiated mice
Because our previous studies demonstrated the hematopoietic capacity of primary human brain-derived vascular endothelial cells as compared with ECs from other tissues,10 we generated primary MBEC cultures from mouse brain cortical vessel explants (anterior cerebral artery, middle cerebral artery) harvested from 6- to 8-week-old C57Bl6 mice, as previously described.10 To facilitate in vivo tracking of transplanted ECs, primary MBEC cultures were also generated from brain vessels obtained from C57Bl6 mice that were transgenic for the expression of green fluorescent protein (GFP+).22 Transcriptional analysis demonstrated that these primary cells expressed VE-cadherin, von Willebrand factor, vascular endothelial growth factor receptor 2 (VEGFR-2), and PECAM (CD31) and readily formed vascular tubes when plated in Matrigel, verifying their endothelial origin (Figure 1A-B).
We next sought to determine whether transplanted MBECs could engraft in the BM of recipient mice. As a preliminary experiment, we irradiated C57Bl6 mice (n = 5 per condition) with 700 cGy and gave the mice transplants of 5 × 105 GFP+ MBECs via tail vein injections daily for 5 days, beginning 2 hours after radiation exposure on day 0. Beginning at day +5 and continuing every 5 days through day +30, mice were killed, bilateral femurs were harvested, and BM was analyzed for evidence of MBEC engraftment. Spleens and lung tissue were also harvested from mice to assess engraftment in other tissues. GFP+ cells were not detected in the BM in any mice analyzed, whereas GFP+ cells were identified consistently at low levels (range, 0.5%-1.0% of cells per high power field) in the lung tissue in all treated animals. For comparison, additional mice also received GFP+ MBECs via intraperitoneal injection, and these animals also demonstrated no detectable GFP+ cells in the BM at any time point, indicating a lack of engraftment of MBECs in the BM in these mice (data not shown).
MBECs express endothelial-specific genes and display endothelial functional characteristics. (A) Primarily passaged MBECs were collected, and total RNA was isolated and prepared for reverse-transcriptase PCR analysis for expression of multiple endothelial cell genes. VE-cadherin, VWF, VEGFR-2, and PECAM (CD31) were all expressed by MBECs, confirming their endothelial cell lineage. (B) Primary MBECs (5 × 104) were plated in 24-well tissue culture plates precoated with Matrigel to analyze for evidence of capillary tube forming capability. As shown, MBECs readily formed tubelike structures, consistent with an endothelial cell lineage.
MBECs express endothelial-specific genes and display endothelial functional characteristics. (A) Primarily passaged MBECs were collected, and total RNA was isolated and prepared for reverse-transcriptase PCR analysis for expression of multiple endothelial cell genes. VE-cadherin, VWF, VEGFR-2, and PECAM (CD31) were all expressed by MBECs, confirming their endothelial cell lineage. (B) Primary MBECs (5 × 104) were plated in 24-well tissue culture plates precoated with Matrigel to analyze for evidence of capillary tube forming capability. As shown, MBECs readily formed tubelike structures, consistent with an endothelial cell lineage.
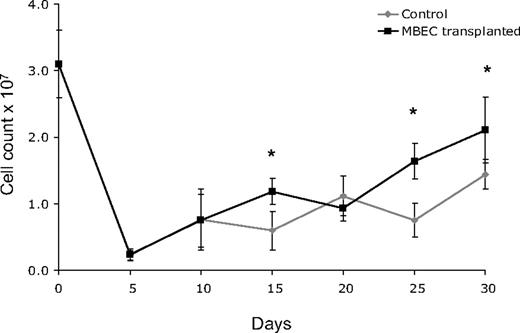
Because 700 cGy total body irradiation can be expected to acutely induce hypoplasia within the BM, we next examined the recovery of BM cellularity in mice given transplants of MBECs versus irradiated, control mice. For these measurements, bilateral femurs were collected from groups of mice in each condition (n = 5 per condition), and total viable cell counts were performed every 5 days from day 0 (before irradiation) through day +30. Both control mice and mice that received transplants of MBECs displayed a marked reduction in BM cell counts through day +10, but mice that received transplants of MBECs demonstrated significantly increased BM cell counts at day +15, +25, and +30 compared with untreated controls (P = .004, P = .001, P = .019; Figure 2). These quantitative results corresponded with microscopic analysis of BM cellularity in tissue sections as well (data not shown). In addition, the MBEC-treated mice showed earlier recovery of BM vascular architecture by day +20 compared with the irradiated, control mice which demonstrated persistent disruption of the vascular architecture through day +30.
Mice that received transplants of MBECs demonstrate accelerated BM cellular recovery following total body irradiation. C57Bl6 mice were irradiated with 700 cGy total body irradiation and then observed without treatment or given transplants of 5 × 105 MBECs daily for 5 days. BM cells were collected from bilateral femurs from mice in each condition at day 0 and every 5 days through day +30, and mean viable mononuclear cell (MNC) counts were performed. Standard errors for each mean are shown as error bars. Mice that received transplants of MBECs demonstrated significantly increased numbers of viable MNCs at day +15, +25, and +30 compared with irradiated control mice. The * indicates a significant difference at each time point; P = .004, P = .001, P = .019, respectively.
Mice that received transplants of MBECs demonstrate accelerated BM cellular recovery following total body irradiation. C57Bl6 mice were irradiated with 700 cGy total body irradiation and then observed without treatment or given transplants of 5 × 105 MBECs daily for 5 days. BM cells were collected from bilateral femurs from mice in each condition at day 0 and every 5 days through day +30, and mean viable mononuclear cell (MNC) counts were performed. Standard errors for each mean are shown as error bars. Mice that received transplants of MBECs demonstrated significantly increased numbers of viable MNCs at day +15, +25, and +30 compared with irradiated control mice. The * indicates a significant difference at each time point; P = .004, P = .001, P = .019, respectively.
MBEC transplantation radioprotects BM progenitors and enhances the regeneration of BM stem/progenitor cells
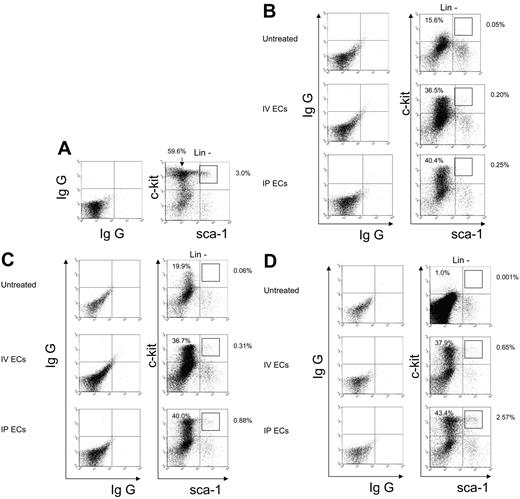
To determine whether systemic administration of MBECs could mediate a radioprotective or regenerative effect on BM stem and progenitor cells following radiation injury, additional mice (n = 2-4 per condition) were irradiated with 700 cGy and subsequently given transplants of 5 × 105 MBECs per day for 5 days via either intravenous or intraperitoneal injection. At day +10 following 700 cGy irradiation, untreated control mice demonstrated a 5.1-fold decline in c-kit+sca-1−lin− progenitor cells in the marrow compared with normal mice (mean, 14.0% ± 2.0% versus 60.7% ± 4.4% of the lin− population, P = .002; Figure 3A-B). Conversely, mice treated intravenously or intraperitoneally with MBECs maintained significantly higher numbers of BM c-kit+sca-1−lin− progenitor cells at day +10 compared with untreated control mice (mean, 31.5% ± 7.1% and 34.1% ± 9.0% of lin− population versus 14.0% ± 2.0%, respectively; P = .04 and P = .04), suggesting that administration of MBECs protected BM progenitor cells from early radiation-induced toxicity. We next examined the recovery of BM c-kit+sca-1+lin− (KSL) cells which are enriched for hematopoietic stem cell content.23,24 At day +10 following 700 cGy exposure, untreated control mice demonstrated a 90-fold reduction in the percentage of KSL cells compared with normal mice (mean, 0.001% ± 0.001% versus 0.09% ± 0.01% of the total BM population, P = .002; Figure 3A-B), reflecting radiation-induced loss of BM stem/progenitor cells at this early time point. Mice treated with MBECs via either intravenous or intraperitoneal administration also demonstrated significant reduction in KSL populations at day +10 compared with normal mice (mean, 0.007% ± 0.002% and 0.005% ± 0.002% versus 0.09% of the total BM population, respectively; P = .002 and P = .002, respectively; Figure 3B). However, BM analysis between day +15 and day +20 following irradiation revealed accelerated recovery of the KSL population within the MBEC-treated animals as compared with the irradiated control animals (Figure 3C-D). At day +20, mice that were treated with MBECs via either intravenous or intraperitoneal administration demonstrated significantly increased percentages of KSL cells in the BM compared with untreated control mice (mean, 0.04% ± 0.01% and 0.07% ± 0.02% versus 0.0003% ± 0.0001% of the total BM population; P = .01 and P = .03, respectively). Taken together, these data suggested that systemic administration of MBECs mediated the radioprotection of BM progenitor cells and promoted early regeneration of hematopoietic stem/progenitor cells following radiation injury. Of note, to exclude the possibility that transplanted MBECs contained contaminating cells with hematopoietic capacity that could account for the observed results, we examined the peripheral blood and BM of mice that received a transplant of MBECs between day +10 and +30 after irradiation and found no donor-derived GFP+CD45+ cells (data not shown). These results indicated a very low probability that transplanted MBEC grafts contained contaminating hematopoietic progenitor cells or underwent dedifferentiation in vivo to account for the hematopoietic recovery we observed.
MBEC transplantation provides radioprotection of BM progenitors and augments the regeneration of stem cell–enriched populations. BM mononuclear cells were collected from mice on days +10, +15, and +20 following 700 cGy of total body irradiation. The percentages of c-Kit+sca-1−lin− progenitors and the more primitive KSL cells were measured by flow cytometry in the BM at each time point in normal mice (A), in 700 cGy-irradiated control mice, and in mice treated with MBECs by intravenous or via intraperitoneal administration for 5 days. As shown in panel B, control mice demonstrated marked reduction in viable c-kit+sca-1−lin− progenitors at day +10, whereas all animals treated with MBECs maintained significantly higher numbers of this population following radiation exposure. At days +15 (C) and day +20 (D), both groups of MBEC-treated animals demonstrated recovery of KSL cells compared with untreated controls. Percentages shown in the upper quadrants of each figure represent the percentage of that population within the lin− subset.
MBEC transplantation provides radioprotection of BM progenitors and augments the regeneration of stem cell–enriched populations. BM mononuclear cells were collected from mice on days +10, +15, and +20 following 700 cGy of total body irradiation. The percentages of c-Kit+sca-1−lin− progenitors and the more primitive KSL cells were measured by flow cytometry in the BM at each time point in normal mice (A), in 700 cGy-irradiated control mice, and in mice treated with MBECs by intravenous or via intraperitoneal administration for 5 days. As shown in panel B, control mice demonstrated marked reduction in viable c-kit+sca-1−lin− progenitors at day +10, whereas all animals treated with MBECs maintained significantly higher numbers of this population following radiation exposure. At days +15 (C) and day +20 (D), both groups of MBEC-treated animals demonstrated recovery of KSL cells compared with untreated controls. Percentages shown in the upper quadrants of each figure represent the percentage of that population within the lin− subset.
MBEC transplantation accelerates WBC and platelet recovery and improves survival of irradiated mice
To determine whether the accelerated recovery of the BM stem and progenitor cell pool in the animals that received a transplant of MBECs affected hematologic recovery, we collected peripheral blood (PB) from mice irradiated with 700 cGy beginning at day +7 and every 3 to 4 days through day +20 (n = 10 mice per condition). Total WBCs and platelet (Plt) count were measured in each animal over time. From day +7 through day +14 following irradiation, all MBEC-treated and untreated control mice demonstrated severe leukopenia and thrombocytopenia (Figure 4A-B). However, at day +20, mice treated with MBECs via either intraperitoneal or intravenous administration demonstrated increased WBC counts in the PB compared with untreated controls (mean WBC count, 0.7 and 0.9 versus 0.1, respectively; P = .01 and P = .05; Figure 4A). Similarly, mice treated intravenously with MBECs demonstrated significantly increased platelet counts at day +17 versus untreated controls (mean Plt count, 176 × 109/L versus 68 × 109/L, P = .04; Figure 4B), and both groups of MBEC-treated mice had higher platelet counts at day +20 compared with untreated controls (mean Plt counts, 377 and 232 versus 78, respectively; P = .03 and P = .06; Figure 4B). These data suggested that systemic administration of MBECs accelerated hematologic recovery in irradiated animals.
MBEC transplantation is associated with accelerated recovery of mature blood counts. Peripheral blood was collected every 3 to 4 days following the administration of 700 cGy total body irradiation to C57Bl6 mice. Groups of mice were given intravenous (n = 10, blue line) or intraperitoneal (n = 10, gray line) transplants of MBECs, and hematologic recovery was compared with that of irradiated control mice (n = 10, black line). The mean total WBCs and Plt counts were markedly reduced in all mice through day +14. By day +20, mice that received either intravenous or intraperitoneal MBECs demonstrated increased total WBC counts compared with control mice (A). (B) Mice that received intravenously administered MBECs displayed significantly increased platelet counts at day +17 compared with untreated controls, and mice that received either intravenous or intraperitoneal MBECs had higher platelet counts at day +20. *Significant difference in the levels between the group that received intraperitoneally administered MBECs and control mice; ∧significant difference between the mice that received intravenously administered MBECs versus untreated control animals. Standard errors about the mean are shown as error bars.
MBEC transplantation is associated with accelerated recovery of mature blood counts. Peripheral blood was collected every 3 to 4 days following the administration of 700 cGy total body irradiation to C57Bl6 mice. Groups of mice were given intravenous (n = 10, blue line) or intraperitoneal (n = 10, gray line) transplants of MBECs, and hematologic recovery was compared with that of irradiated control mice (n = 10, black line). The mean total WBCs and Plt counts were markedly reduced in all mice through day +14. By day +20, mice that received either intravenous or intraperitoneal MBECs demonstrated increased total WBC counts compared with control mice (A). (B) Mice that received intravenously administered MBECs displayed significantly increased platelet counts at day +17 compared with untreated controls, and mice that received either intravenous or intraperitoneal MBECs had higher platelet counts at day +20. *Significant difference in the levels between the group that received intraperitoneally administered MBECs and control mice; ∧significant difference between the mice that received intravenously administered MBECs versus untreated control animals. Standard errors about the mean are shown as error bars.
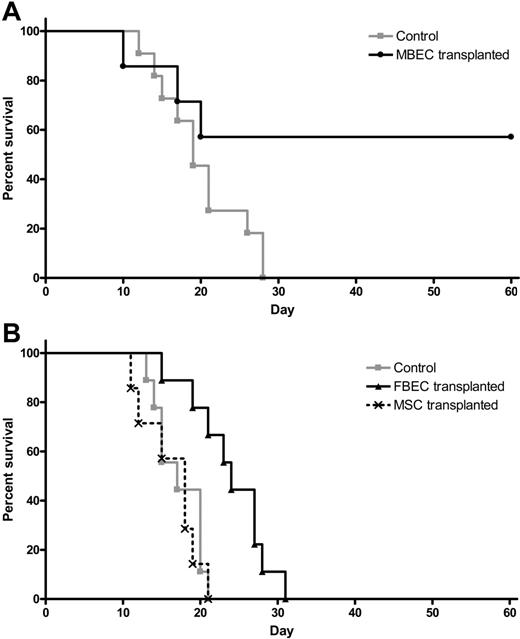
Because systemic administration of MBECs was strongly associated with enhancement of hematopoietic recovery in irradiated mice, we sought to determine whether such therapy could affect the survival of animals following lethal dose irradiation. We irradiated a group of C57Bl6 mice with 1050 cGy (split dose), which we have previously shown to be 100% lethal in this strain of mice by day +30 (LD100/30).17 We then examined whether systemic administration of MBECs alone, in the absence of transplanted HSCs, beginning 2 hours after exposure, could improve the survival of these animals compared with irradiated controls. One hundred percent of irradiated control mice died prior to day +30 (Figure 5A). In contrast, 57% of mice that were irradiated with 1050 cGy and subsequently transplanted with MBECs survived through day +60 with no signs of morbidity (Figure 5A). These data demonstrated that systemic administration of MBECs alone, in the absence of HSCs, significantly increased the survival of animals after exposure to lethal dose irradiation (P = .04, log-rank test).
Transplantation of MBECs is associated with improved survival in mice following 1050 cGy total body irradiation. (A) Control C57Bl6 mice were irradiated with 1050 cGy total body irradiation, and their survival was compared with mice that were irradiated with 1050 cGy and then given transplants of MBECs for 5 days, as described in “Materials and methods.” Whereas 100% of control, irradiated mice (n = 11) died prior to day +30 (gray line), 57% of mice that received a transplant of MBECs (n = 7) survived through day +60 without evidence of morbidity (black line, P = .04). (B) The survival of control C57Bl6 mice that were irradiated with 1050 cGy (n = 9, gray line) was compared with the survival of C57Bl6 mice that were irradiated with 1050 cGy and given transplants of FBECs (n = 9, solid black line) or MSCs (n = 7, dashed black line). FBEC transplantation was associated with a significant increase in percent survival compared with controls or mice that received a transplant of MSCs (P = .002 and P = .002, respectively), although these mice failed to survive in the long term.
Transplantation of MBECs is associated with improved survival in mice following 1050 cGy total body irradiation. (A) Control C57Bl6 mice were irradiated with 1050 cGy total body irradiation, and their survival was compared with mice that were irradiated with 1050 cGy and then given transplants of MBECs for 5 days, as described in “Materials and methods.” Whereas 100% of control, irradiated mice (n = 11) died prior to day +30 (gray line), 57% of mice that received a transplant of MBECs (n = 7) survived through day +60 without evidence of morbidity (black line, P = .04). (B) The survival of control C57Bl6 mice that were irradiated with 1050 cGy (n = 9, gray line) was compared with the survival of C57Bl6 mice that were irradiated with 1050 cGy and given transplants of FBECs (n = 9, solid black line) or MSCs (n = 7, dashed black line). FBEC transplantation was associated with a significant increase in percent survival compared with controls or mice that received a transplant of MSCs (P = .002 and P = .002, respectively), although these mice failed to survive in the long term.
We next sought to determine whether the effect of MBEC transplantation on the survival of lethally irradiated mice was specifically due to an effect of MBECs or could be mediated by other sources of ECs or nonendothelial cells. For this purpose, we compared the survival of groups of mice irradiated with 1050 cGy without treatment (controls) versus 1050 cGy-irradiated mice that were given transplants of equal doses of FBECs or MSCs. One hundred percent of the control mice (n = 9) that received 1050 cGy without treatment died by day +21, as did 100% of irradiated mice given transplants of MSCs alone (n = 7, Figure 5B). Interestingly, mice that were irradiated and given transplants of FBECs (n = 9) demonstrated a significant increase in survival compared with both control mice and mice that received a transplant of MSCs, although these mice failed to survive long term (P = .002 and P = .002, respectively; Figure 5B).
MBEC-derived cytokines fail to rescue lethally irradiated mice
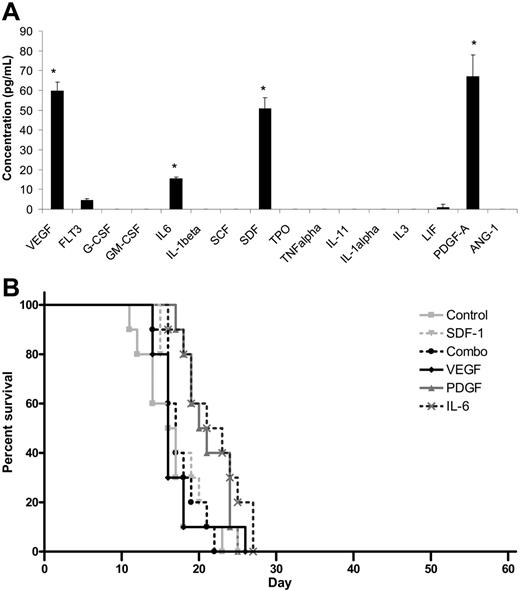
Because transplantation of MBECs supported the survival of mice following lethal dose irradiation, we sought to determine whether MBECs produced soluble hematopoietic growth factors that could account for the results we had observed. ELISA analysis of a broad panel of candidate hematopoietic cytokines revealed that MBEC-CM (1 ×) contained significantly increased levels of VEGF, PDGF-AA, SDF-1, and IL-6 compared with all other cytokines tested (P < .03 for all comparisons, Tukey-Kramer multiple comparison test; Figure 6A). MBECs notably produced no significant levels of TPO, SCF, G-CSF, GM-CSF, or IL-3. For comparison, ELISA analysis of FBEC-CM was also performed, which revealed increased levels of SCF and IL-11 but not VEGF, PDGF, SDF-1, or IL-6 (data not shown). These results indicated that MBECs and FBECs were distinct in their production of known hematopoietic cytokines.
Cytokine production does not account for the in vivo effects of MBECs. (A) ELISAs performed on MBEC-CM (1 ×) revealed significantly increased mean concentrations of VEGF, PDGF-AA, SDF-1, and IL-6 compared with all other cytokines that were measured (*P < .03 for multiple comparison test). Standard errors about the mean are represented by error bars. (B) C57Bl6 mice were irradiated with 1050 cGy and subsequently treated with 5 days of intraperitoneal administration of VEGF, PDGF-AA, SDF-1, or IL-6 or the combination of VEGF, PDGF-AA, SDF-1, and IL-6, beginning at 2 hours after irradiation. The percent survival in each treatment group is shown as compared with control, irradiated mice. No animals in any group survived beyond day +28.
Cytokine production does not account for the in vivo effects of MBECs. (A) ELISAs performed on MBEC-CM (1 ×) revealed significantly increased mean concentrations of VEGF, PDGF-AA, SDF-1, and IL-6 compared with all other cytokines that were measured (*P < .03 for multiple comparison test). Standard errors about the mean are represented by error bars. (B) C57Bl6 mice were irradiated with 1050 cGy and subsequently treated with 5 days of intraperitoneal administration of VEGF, PDGF-AA, SDF-1, or IL-6 or the combination of VEGF, PDGF-AA, SDF-1, and IL-6, beginning at 2 hours after irradiation. The percent survival in each treatment group is shown as compared with control, irradiated mice. No animals in any group survived beyond day +28.
We next tested whether the in vivo administration of the most overexpressed MBEC-derived cytokines (VEGF, PDGF, SDF-1, IL-6) or their combination could reproduce the effect of MBEC transplantation toward promoting the survival of lethally irradiated mice. For these studies, the survival of control C57Bl6 mice that were irradiated with 1050 cGy was compared with 1050 cGy-irradiated mice that were treated, beginning 2 hours after irradiation, with 100 ng VEGF, 100 ng PDGF-AA, 2 μg IL-6, or 1.5 μg SDF-1 or the combination of all 4 cytokines given intraperitoneally for 5 days. All control mice (n = 10) died by day +23, and no mice treated with VEGF alone (n = 10), PDGF-AA (n = 10), IL-6 (n = 10), or SDF-1 (n = 10) or with the combination of all 4 factors (n = 10) survived past day +28 (Figure 6B). Taken together, these data suggested that these growth factors alone did not account for the in vivo survival benefit that was observed in mice given transplants of MBECs.
As a final analysis to determine whether MBEC transplantation might have indirectly promoted the survival of irradiated animals via the systemic induction of antiapoptotic or hematopoietic growth factors in vivo, we compared the levels of cytokines in the serum of mice that were irradiated and given transplants of MBECs versus irradiated controls and nonirradiated controls. Interestingly, 1050 cGy irradiation was associated with a significant increase in the serum concentrations of GCSF and Flt-3 ligand compared with nonirradiated control mice (P = .002 and P < .001, respectively), and mice that were irradiated and given transplants of MBECs displayed a further increase in G-CSF levels and a significant decrease in PDGF-AA levels in the serum compared with irradiated, untreated mice (P = .02 and P = .03, respectively).
Discussion
Administration of high-dose chemotherapy or radiotherapy in the treatment of cancer is limited by the potentially prolonged BM aplasia and pancytopenia that can occur as a result of toxicity to proliferating stem and progenitor cells.20 The only established treatment for the resulting marrow aplasia is transplantation of autologous or allogeneic hematopoietic stem cells coupled with intensive supportive care.25 Experimental studies have demonstrated that supportive cells within the BM niche (stromal cells, endothelial cells) are also severely damaged by exposure to therapeutic doses of ionizing radiation.26,27 We postulated that cellular therapy directed at replenishing normal endothelial cell activity could potentially accelerate hematopoietic recovery following radiation-induced BM aplasia. Our results suggest that systemic administration of vascular endothelial cells provides radioprotection to BM progenitor cells, induces the regeneration of primitive stem/progenitor cell populations, accelerates the recovery of mature peripheral blood counts, and facilitates the improved survival of mice following radiation injury. These results indicate that the targeted restoration of endothelial cell activity is a potentially viable strategy to accelerate hematologic recovery following myelosuppressive chemotherapy or radiotherapy.
Although the contribution of vascular endothelial precursors to hematopoiesis has been well demonstrated during embryogenesis,3,4 the contribution of ECs to adult hematopoiesis in vivo has been less well characterized. Two recent investigations have suggested that signals provided by the bone marrow vascular niche may be important in hematopoietic regeneration following chemotherapy-induced myelosuppression.5,14 Montfort et al28 demonstrated that transplantation of segments of thoracic aorta or inferior vena cava under the kidney capsule was associated with radioprotection in lethally irradiated mice, and Li et al29 have preliminarily reported that transplantation of PECAM/CD31+ cells was radioprotective in mice as well. Our results significantly extend these observations by demonstrating that transplanted ECs exert a prosurvival effect on susceptible BM progenitor cells in vivo during the early period after irradiation (day 0-10) and a regenerative effect on the more primitive BM stem cell population which occurs later (day 10-20). The combination of these effects coincided temporally with the earlier recovery of mature WBCs and platelets at day +20 after irradiation. Furthermore, 100% of lethally irradiated, mice that received a transplant of ECs which were alive at day +20 ultimately survived long term, suggesting a causal relationship between the recovery of hematopoiesis and survival. These data indicate that restoration of the hematopoietic activity normally provided by vascular endothelial cells can enhance survival following myelosuppressive radiotherapy.
Additional studies performed here suggest the possibility that transplantation of other sources of ECs, particularly fetal blood ECs, has the potential to ameliorate radiation-induced toxicity. In a preliminary study, fetal blood EC transplantation was significantly less potent than transplantation of brain-derived ECs, perhaps reflecting the distinct pattern of cytokine production between the 2 sources of ECs. Nonetheless, we plan to further study the utility of fetal blood ECs to augment hematopoiesis and survival following myelosuppressive therapy in mice because this could be a model for the use of umbilical cord blood ECs to augment human cord blood transplantation. Interestingly, transplantation of MSCs had no impact on the survival of irradiated mice, suggesting that the beneficial effects of MBEC transplantation reflect an endothelial cell-specific activity, rather than a nonspecific effect inducible by different cell types.
We also sought to determine whether the beneficial effects of MBEC transplantation could be ascribed to the production of known hematopoietic cytokines. Not surprisingly, MBEC-CM contained increased levels of VEGF, PDGF-AA, and SDF-1, along with IL-6. However, when we administered pharmacologic doses of these 4 growth factors alone or in combination to lethally irradiated mice, we did not observe a significant improvement in the survival of any mice compared with controls. Although additional dose and scheduling studies will be necessary to confirm these results, the current data suggest that additional, perhaps novel, MBEC-derived soluble factors account for the effects we have observed. It is also possible that MBEC transplantation indirectly mitigates radiation damage in vivo via the induction of other systemic growth factors. However, preliminary studies we have performed indicate that noncontact culture with MBECs directly reduces apoptosis and increases the recovery of viable BM progenitor cells harvested from irradiated mice (J.P.C., manuscript in preparation).
To advance the observations presented here for therapeutic purposes, it will be important to delineate the precise mechanism(s) through which ECs mediate hematopoietic regeneration following radiation damage. Our results suggest that transplanted ECs promote hematopoietic repair and regeneration via a soluble or endocrine effect. First, we found no evidence that intravenously administered MBECs engrafted in the recipient BM within the first 30 days after transplantation, yet these recipient animals had earlier recovery of BM cellularity, stem and progenitor cell content, and circulating WBCs and platelets. Second, mice treated with intraperitoneal administration of MBECs also did not demonstrate detectable GFP+ MBECs in their BM, and these animals demonstrated nearly identical protection of the BM progenitors, regeneration of more primitive KSL cells, and recovery of mature blood counts compared with mice that received intravenous MBECs. Taken together, these results suggest that transplanted MBECs mediate their in vivo activity via soluble effects on the BM stem and progenitor cell compartment. We plan to confirm the mechanism of action in 2 ways: first, we will examine the efficacy of systemically administered MBEC-derived conditioned media alone in the treatment of irradiated animals, and, second, we will test whether direct intrafemoral injection of MBECs, which will eliminate homing inefficiencies,30 can further augment the hematoepoietic recovery and survival in mice following high-dose irradiation.
The most surprising result from this study was the demonstration that transplantation of primary MBECs alone, in the absence of HSCs, promoted the survival of mice following lethal dose total body irradiation. It has been established in experimental models and clinical practice that survival following lethal dose irradiation depends on either the transplantation of sufficient numbers of HSCs to repopulate the ablated host marrow31,32 or transplantation of myeloid-erythroid progenitor cells33 which can serve as a “bridge” providing short-term (2-4 weeks) hematopoietic production until endogenous hematopoietic stem/progenitor cell activity occurs. Our study suggests that a third strategy can be applied to facilitate survival following exposure to lethal-dose irradiation: replenishment of endothelial cell–derived hematopoietic activity. Because the BM vascular niche is significantly damaged following high-dose radiation exposure, it is plausible that diminished vascular niche activity contributes to the delayed recovery of hematopoiesis that invariably ensues. This study provides proof of principle that provision of vascular endothelial cell activity can accelerate hematopoietic recovery following radiation injury. Pharmacologic or cellular therapies aimed at restoring endothelial cell–mediated activity represent a novel approach to enhance hematopoietic recovery in vivo following radiation-induced myelosuppression.
Authorship
J.P.C. designed the research, analyzed the data, and wrote the paper; G.G.M., A.B.S., S.K.M., B.C., and H.A.H. performed the research; D.R. performed the research and analyzed data; N.J.C. helped design research and contributed to the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John P. Chute, Division of Cellular Therapy, Duke University Medical Center, 2400 Pratt St, Durham, NC 27710; e-mail: john.chute@duke.edu.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported in part by the National Institute of Allergy and Infectious Diseases (grant AI067798-01) (J.P.C.).



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal