Abstract
Autologous hematopoietic stem cell transplantation (ASCT) is an increasingly accepted treatment for refractory autoimmune diseases. Refractory celiac disease with aberrant T cells (RCD type II) is unresponsive to available therapies and carries a high risk of transition into enteropathy associated T-cell lymphoma (EATL). This study reports on the feasibility, safety, and efficacy of ASCT in patients with RCD type II. Thirteen patients with RCD type II were evaluated. Seven patients (4 men, 3 women, mean age 61.5 years [range, 51-69 years]) underwent transplantation. After conditioning with fludarabine and melphalan, ASCT was performed. Patients were monitored for response, adverse effects, and hematopoietic reconstitution. All 7 patients completed the mobilization and leukapheresis procedures successfully and subsequently underwent conditioning and transplantation. Engraftment occurred in all patients. No major nonhematologic toxicity or transplantation-related mortality was observed. There was a significant reduction in the aberrant T cells in duodenal biopsies associated with improvement in clinical well-being and normalization of hematologic and biochemical markers (mean follow-up, 15.5 months; range, 7-30 months). One patient died 8 months after transplantation from progressive neuroceliac disease. These preliminary results showed that high-dose chemotherapy followed by ASCT seems feasible and safe and might result in long-term improvement of patients with RCD type II whose condition did not respond promptly to available drugs.
Introduction
Autologous hematopoietic stem cell transplantation (ASCT) is an increasingly accepted effective treatment option for patients with severe autoimmune diseases refractory to conventional treatment1 and has been used successfully in patients with multiple sclerosis,2 rheumatoid arthritis,3 systemic sclerosis,4 systemic lupus erythematosus,5 and Crohn disease.6 The rationale for this strategy is based on the concept of immunoablation by intense immunosuppression using high-dose chemotherapy, with subsequent regeneration of naïve T lymphocytes derived from reinfused hematopoietic progenitor cells.7
In celiac disease (CD), HLA-DQ molecules bind and present gluten peptides to antigen-specific T cells. These HLA-DQ–peptide complexes induce inflammatory responses in the small intestine consisting of lymphocytic infiltration of the lamina propria, expansion of the intraepithelial lymphocyte population, hyperplasia of the crypts, and atrophy of the villi.8 In a small percentage (2%-5%) of adult patients with CD diagnosed as adults, a refractory state develops despite strict adherence to a gluten-free diet (GFD).9 In refractory celiac disease (RCD) the number of intraepithelial lymphocytes (IELs) is markedly raised and it is from these IELs that enteropathy associated T-cell lymphoma (EATL) may arise.9,10 Immunophenotyping of the IELs identifies 2 groups of RCD patients: those with normal IELs (RCD I) and those with aberrant IELs, lacking surface expression of CD3 and CD8 (RCD II).10,11 RCD II can be regarded as a “cryptic” lymphoma.9 Strong molecular and immunophenotypic evidence now shows that a monoclonal neoplastic T-cell population may emerge from IELs in RCD. Clonal expansion of this monoclonal T-cell population eventually leads to frank EATL. The genesis and expansion of these monoclonal T cells involve both inappropriate immune responses to gluten and acquisition of genetic abnormalities. Although the monoclonal IELs in patients with RCD are neoplastic, they are not cytologically abnormal and do not form tumor masses, which differentiate these patients from those with EATL, in addition to the absence of radiologic and bone marrow evidence of lymphoma.10,12–14
RCD II is usually resistant to any known therapy, including azathioprine/prednisone, cyclosporine, and IL-10 therapy15–18 and has a high risk of developing EATL (60%-80% within 5 years).10,19 This specific type of peripheral T-cell lymphomas has a very poor outcome with 1- and 5-year survival rates in the range of 31% to 39% and 11% to 20%, respectively.19–21 In a prospective multicenter study of 35 patients with EATL treated with 6 cycles of cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP), the cumulative 2-year survival was only 28%.11 Therefore, new treatment strategies for patients with “premalignant” CD (RCD II) are urgently needed to improve their clinical condition with the ultimate goal of resetting the immune response, which might prevent or delay development of overt EATL.
This study reports on the feasibility, safety, and efficacy of high-dose chemotherapy followed by ASCT in patients with RCD II.
Patients, materials, and methods
Patients
Between March 2004 and March 2006, 13 patients were evaluated for ASCT. The 4 men and 3 women (mean age, 61.5 years; range, 51-69 years) with RCD II underwent ASCT. Six other patients were excluded because of the presence of coexistent coronary artery disease and heart failure (New York Heart Association classification III in 2 patients), EATL found on evaluation before transplantation (3 patients), and low performance status (1 patient). One patient could not be treated due to unsuccessful leukapheresis; she developed EATL and died subsequently despite chemotherapy and immunotherapy with anti-CD52 (alemtuzumab).22 The 2 patients with congestive heart failure died from progressive disease and cachexia (first patient) and bronchiectasis (second patient). The 3 patients with EATL all died within few months, whereas the patient with low performance status died from cachexia.
The baseline characteristics of the patients are shown in Table 1. All patients received therapy with prednisone and cladribine (2-CDA) several months before undergoing ASCT (not within 6 months of transplantation). The first 3 patients (patients A, B, and C) were diagnosed with CD at relatively advanced age, had persistent diarrhea and weight loss and failed to respond to GFD, steroids, and immunosuppressives. Because of the presence of active disease and high percentage of aberrant T cells in the small bowel mucosa, they were included in this study protocol. At the age of 48 years, patient D was diagnosed with CD in association with dermatitis herpetiformis. Furthermore, he had a clinical picture of neuroceliac disease with ataxia. After exclusion of structural brain and infectious disorders, he underwent ASCT at the age of 63.5 years. Patient E has, in addition to CD with ulcerative jejunitis, Hashimoto thyroiditis, and patient F has CD with ulcerative jejunitis. One patient (patient G) was included because of the presence of very extensive ulcerative jejunitis with multiple small bowel strictures necessitating repeated resections although initially biopsies showed a low percentage of aberrant T cells. He had clinically short bowel syndrome (remaining small bowel approximately 100-150 cm) requiring total parenteral nutrition (TPN).
Baseline characteristics of the patients
| Characteristic . | Patient A . | Patient B . | Patient C . | Patient D . | Patient E . | Patient F . | Patient G . |
|---|---|---|---|---|---|---|---|
| Age, y/sex | 62/M | 70/M | 65/F | 63/M | 64/F | 59/F | 51/M |
| Age at CD diagnosis, y | 56 | 62 | 61 | 48 | 44 | 47 | 50 |
| Age at RCD II diagnosis, y | 59 | 64 | 63 | 63 | 56 | 58 | 51 |
| Age at ASCT, y | 60 | 68 | 64 | 63 | 64 | 59 | 51 |
| Date of ASCT | Mar 2004 | Aug 2004 | May 2005 | Aug 2005 | Nov 2005 | Dec 2005 | Mar 2006 |
| HLA-DQ2 | Homozygous | Homozygous | Heterozygous | Homozygous | Heterozygous | Heterozygous | Homozygous |
| Marsh at RCD diagnosis | III-A | III-B | III-A | III-A | III-C | III-C | III-A |
| BMI, kg/m2 | 19.4 | 18.9 | 17.1 | 24.1 | 20.1 | 21.3 | 20.5 |
| Performance | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| Symptoms/associations | Diarrhea, pain, weight loss | Pain, diarrhea | Diarrhea, weight loss, hypocalcemia | Diarrhea, weight loss, dermatitis, herpetiformis, neurologic symptoms (ataxia) | Weight loss, skin rash, Hashimoto thyroiditis | Weight loss, diarrhea | Diarrhea, hypocalcemia, weight loss, extensive small bowel resection |
| Serology at CD diagnosis | EMA+, anti-tTG+ | EMA+, anti-tTG+ | EMA+, anti-tTG+ | EMA+, anti-tTG+ | EMA+, anti-tTG+ | EMA+, anti-tTG+ | EMA+, anti-tTG+ |
| Serology at RCD diagnosis | EMA−, anti-TTG− | EMA−, anti-tTG− | EMA−, anti-tTG− | EMA−, anti-tTG− | EMA−, anti-tTG− | EMA−, anti-tTG− | EMA−, anti-tTG− |
| Endoscopy* | Nodular mucosa | Mosaic mucosa, erosions, and ulcerations | Mosaic mucosa, visible vessels, no ulcerations | Nodular mucosa, disappearance of folds, erosions | Ulcerative jejunitis | Ulcerative jejunitis | Ulcerative jejunitis with multiple stenoses |
| CT scan | Splenic atrophy, thickened SI wall | Thickened SI loops | Splenic atrophy, dilated SI loops | Splenic atrophy | No abnormality | No abnormality | SI ileus |
| PET scan | Increased uptake in SI | Increased uptake in SI | Increased uptake in SI | No abnormality | No abnormality | No abnormality | No abnormality |
| Characteristic . | Patient A . | Patient B . | Patient C . | Patient D . | Patient E . | Patient F . | Patient G . |
|---|---|---|---|---|---|---|---|
| Age, y/sex | 62/M | 70/M | 65/F | 63/M | 64/F | 59/F | 51/M |
| Age at CD diagnosis, y | 56 | 62 | 61 | 48 | 44 | 47 | 50 |
| Age at RCD II diagnosis, y | 59 | 64 | 63 | 63 | 56 | 58 | 51 |
| Age at ASCT, y | 60 | 68 | 64 | 63 | 64 | 59 | 51 |
| Date of ASCT | Mar 2004 | Aug 2004 | May 2005 | Aug 2005 | Nov 2005 | Dec 2005 | Mar 2006 |
| HLA-DQ2 | Homozygous | Homozygous | Heterozygous | Homozygous | Heterozygous | Heterozygous | Homozygous |
| Marsh at RCD diagnosis | III-A | III-B | III-A | III-A | III-C | III-C | III-A |
| BMI, kg/m2 | 19.4 | 18.9 | 17.1 | 24.1 | 20.1 | 21.3 | 20.5 |
| Performance | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| Symptoms/associations | Diarrhea, pain, weight loss | Pain, diarrhea | Diarrhea, weight loss, hypocalcemia | Diarrhea, weight loss, dermatitis, herpetiformis, neurologic symptoms (ataxia) | Weight loss, skin rash, Hashimoto thyroiditis | Weight loss, diarrhea | Diarrhea, hypocalcemia, weight loss, extensive small bowel resection |
| Serology at CD diagnosis | EMA+, anti-tTG+ | EMA+, anti-tTG+ | EMA+, anti-tTG+ | EMA+, anti-tTG+ | EMA+, anti-tTG+ | EMA+, anti-tTG+ | EMA+, anti-tTG+ |
| Serology at RCD diagnosis | EMA−, anti-TTG− | EMA−, anti-tTG− | EMA−, anti-tTG− | EMA−, anti-tTG− | EMA−, anti-tTG− | EMA−, anti-tTG− | EMA−, anti-tTG− |
| Endoscopy* | Nodular mucosa | Mosaic mucosa, erosions, and ulcerations | Mosaic mucosa, visible vessels, no ulcerations | Nodular mucosa, disappearance of folds, erosions | Ulcerative jejunitis | Ulcerative jejunitis | Ulcerative jejunitis with multiple stenoses |
| CT scan | Splenic atrophy, thickened SI wall | Thickened SI loops | Splenic atrophy, dilated SI loops | Splenic atrophy | No abnormality | No abnormality | SI ileus |
| PET scan | Increased uptake in SI | Increased uptake in SI | Increased uptake in SI | No abnormality | No abnormality | No abnormality | No abnormality |
All patients were treated with prednisone and 2-CDA (cladribine).
SI indicates small intestine; tTG, tissue transglutaminase; and EMA, endomysial antibody.
Gastroduodenoscopy (GDS), video capsule enteroscopy (VCE), or double balloon enteroscopy (DBE).
Criteria for diagnosis of RCD
Patients with CD were considered to be refractory when symptoms of malabsorption due to villous atrophy persisted or recurred after a former good response despite strict adherence to a GFD for at least 1 year. Furthermore, possible underlying diseases such as autoimmune enteritis, bacterial overgrowth, giardiasis, amyloidosis, intestinal lymphangiectasia, Whipple disease, hypogammaglobulinemia, eosinophilic enteritis, EATL, and inflammatory bowel disease were excluded.11 The diagnosis of RCD was established as type II when 20% or more aberrant T cells were present.10,11,15
Inclusion criteria
Patients were included only when the diagnosis of true RCD with aberrant T cells was confirmed (except for patient G who was included based on the extensive ulcerative jejunitis with short bowel syndrome despite having only 10% aberrant T cells), after verifying their strict adherence to a GFD. Performance status according to the World Health Organization (WHO) criteria had to be 0 to 2, and no severe concomitant cardiac, pulmonary, renal or hepatic disease could be present. EATL was excluded by endoscopic examination with multiple biopsies, computed tomography (CT) scan, positron emission tomography (PET), and a trephine bone marrow biopsy. Furthermore, neither active uncontrolled infection nor HIV positivity was permitted.
Evaluation
Before proceeding to ASCT, the patients were extensively evaluated as to their performance status, the presence of concomitant diseases, and extraintestinal disease or EATL. This evaluation included clinical assessment noting particularly signs and symptoms of malabsorption, body mass index (BMI), and performance according to the WHO score23 ; evaluation of adherence to a GFD including frequent consultation with dietitian (advice and follow-up) in addition to checking serology (antiendomysial [EMA] and anti–tissue transglutaminase antibody [anti-tTG], both of which usually revert to negative after strict adherence to the GFD); and evaluation by upper gastrointestinal endoscopy (UGIE), video capsule endoscopy (VCE), and double balloon enteroscopy (DBE). Duodenal biopsies (4 biopsies) were classified according to the modified Marsh criteria.24,25 T-cell receptor (TCR) gene rearrangement study,12–14 T-cell flow cytometry, and IEL phenotyping were performed.15,26,27 Laboratory evaluation included whole blood cell counts and serum levels of creatinine, bilirubin, liver enzymes, lactate dehydrogenase, albumin, electrolytes, iron, ferritin, folic acid, and vitamin B12 were determined. EMA and anti-tTG assays, HLA-DQ typing, thyroid function tests, stool examination for Giardia and other parasites, and HIV serology were also performed.28 For radiologic evaluation, the patients underwent whole-body CT scanning and whole-body PET to exclude intestinal and extraintestinal localization of EATL.29,30
Immunophenotyping of IELs
IELs were isolated from 3 duodenal biopsies by passing them through nylon filters (1 × 100 μm, 1 × 40 μm, BD Biosciences, Discovery Labware, Bedford, MA). Cells were stained with fluorescent-labeled monoclonal antibodies to CD3, CD7, CD8, CD45, CD103, and TCRγδ, as well as with relevant isotype controls.
All monoclonal antibodies were from BD (BD Biosciences, San Jose, CA), except for CD103, which was from IQ Products (Groningen, The Netherlands) and analyzed by 4-color flow cytometry (FACSCalibur, BD Biosciences, San Jose, CA). Leukocyte common antigen (CD45) was always included to identify the lymphocyte population. In some tubes cell surface CD3 staining (anti–CD3-APC) was followed by permeabilization (Cytofix/Cytoperm, BD Biosciences PharMingen, San Diego, CA) and subsequent cytoplasmic staining with anti–CD3-FITC or isotype control. Aberrant T cells were defined either as CD7+ surface CD3− cells (expressed as percent of CD103+ lymphocytes) or as cytoplasmic CD3+, surface CD3− cells (expressed as percent of CD103+ lymphocytes).12,26
All flow cytometry analyses were performed by an analyst and interpreted by the same medical immunologist; histopathology was performed by the same pathologist to ensure uniformity, reproducibility, and consistency of results.
Assessment of TCR gene rearrangement by PCR
TCRγ gene rearrangements studies were performed in separate 3 to 4 duodenal specimens that were preserved on Histocon (Polysciences Europe, Eppelheim, Germany) and frozen at −20°C. DNA was extracted from cryosections of duodenal specimens by a standard procedure using proteinase-K digestion and ethanol precipitation of the gDNA. TCR-γ gene rearrangements were analyzed by multiplex polymerase chain reaction (PCR) amplification under standardized conditions. A monoclonal and polyclonal control was included in each experiment. Clonality assessment for TCR-γ gene rearrangements was done according to the Biomed-2 concerted action BM H4-CT98-3936 on PCR-based clonality studies for early diagnosis of lymphoproliferative disorders.12–14
Peripheral blood stem cells mobilization and collection
Mobilization of hematopoietic progenitor cells from the bone marrow into the peripheral blood was achieved using granulocyte colony-stimulating factor (G-CSF) 2 × 5 μg/kg by subcutaneous injection for at least 4 days. Hematopoietic stem cells were harvested from the peripheral blood by leukapheresis and kept frozen until ASCT. The target CD34+ count was more than 2 × 106/kg.
Conditioning and ASCT
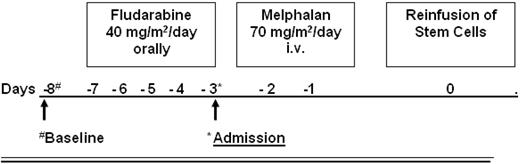
The conditioning regimen consisted of fludarabine given orally for 5 days (40 mg/m2/d) and melphalan (given intravenously, 2 days, 70 mg/m2/d) as shown in Figure 1. At day 0, the frozen stem cell suspension was thawed and reinfused. The rationale for this conditioning regimen was based on T-cell depletion by a purine analog combined with a modified dose of melphalan (total dose 140 mg/m2) for myeloablation.
Supportive care
Patients A, C, and D were supported with parenteral feeding during the 2-week period of oral mucositis after ASCT; patient G was receiving parenteral nutritional support before receiving the transplant. After discharge, all patients except patient G were able to be fed enterally. Patient G was supported to gain weight for several months with a duodenal feeding tube and limited TPN (twice a week). During admission, all patients received standard antibacterial and antifungal prophylaxis. Pneumocystis jiroveci pneumonia prophylaxis was initiated (trimethoprim-sulfamethoxazole gluten-free syrup 480-960 mg daily) until 6 months after transplantation. No patient received antidiarrheal or narcotic medications in the peritransplantation period. Blood and platelet transfusions were given as indicated.
Follow-up and criteria of response
During follow-up, WHO performance status, nutritional status, and changes in weight and stool frequency were noted, as well as relevant biochemical markers. An endoscopic and histologic examination of the small intestine was performed (3, 12, and 24 months after ASCT). From the second part of the duodenum, 4 biopsies were taken for histologic assessment and 4 to 6 specimens for T-cell flow cytometry study. Hematologic data (hemoglobin, white blood cell [WBC] count, differential, and platelets) were registered before inclusion, after preconditioning, and after transplantation until recovery. The nadir WBC count, duration of neutropenia, infectious complications, bleeding tendency, and need for supportive therapies such as blood and platelet transfusions were documented.
Ethics approval and informed consent
Approval of the medical ethics committee was obtained, and all treated patients signed an informed consent in accordance with the Declaration of Helsinki.
Results
Table 1 summarizes the demographic and clinical characteristics of the patients before ASCT. The mean age at diagnosis of CD was 52.5 years (range, 47-62 years) and for RCD II 59 years (range, 51-64 years). Four patients were DQ2 homozygous and 3 were heterozygous.31 The mean follow-up was 15.5 months (range, 7-30 months). All patients had a WHO performance status of 1 except patient G, whose performance status was 2. Patients B, E, F, and G had ulcerative jejunitis. Patients A, C, and D had splenic atrophy on CT scan. PET scan showed an increased uptake in the small intestine in patients A, B, and C. At the time of diagnosis of CD, all patients were positive for anti-tTG and EMA, but all reverted to negative after GFD. Before and after ASCT all patients remained negative for anti-tTG and EMA. There was no transplantation-related mortality. The conditioning regimen seems feasible in this group of patients. The mean duration of hospitalization was 19.5 days (range, 18-22 days). ASCT-related toxicity was relatively mild. Patient B had transient diarrhea and fever of undetermined origin, which was treated with intravenous antibiotics. Three weeks after discharge from the hospital, he suffered from a transient visual disturbance caused by minor retinal bleeding, which was not related to thrombocytopenia. Patient D experienced fever of undetermined origin and recovered after administration of intravenous antibiotics. One month after ASCT, patient E developed self-limiting erythematous plaque skin lesions with central necrosis. Detailed histopathologic tests excluded EATL and showed aberrant T-lymphocyte infiltration (CD8−CD7+CD30+).
The mean time from the day of transplantation to neutrophil recovery was 17.8 days (range, 10-21 days). Only one patient (patient B) had a transient 5-day period of severe thrombocytopenia of 5 × 109/L; all other patients had nadir platelet counts between 17 and 32 × 109/L without need for platelet transfusions.
Clinical and laboratory tests before and after ASCT are shown in Table 2. Patients A, C, and D were supported with parenteral feeding during the period of oral mucositis. No patient received antidiarrheal or long-term narcotic medications. Within 3 months after ASCT, all patients showed impressive clinical improvement with normalization of stool frequency, disappearance of abdominal pain, and improvement of biochemical markers. In addition, improvement of BMI was documented (from mean 20.2 at baseline to 24.1 after ASCT). Mean serum albumin level increased from 29 g/L to 40.7 g/L. Patient G showed a remarkable clinical improvement 3 to 4 months after ASCT and was able to be fed partly enterally with parenteral nutritional support twice a week.
Clinical and laboratory tests before and at the last follow-up after ASCT
| Characteristic . | Patient A . | Patient B . | Patient C . | Patient D . | Patient E . | Patient F . | Patient G . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before . | After . | Before . | After . | Before . | After . | Before . | After . | Before . | After . | Before . | After . | Before . | After . | |
| BMI, kg/m2 | 19.4 | 25.6 | 18.9 | 26.1 | 22 | 24.1 | 24 | 24.1 | 20.1 | 22 | 22.1 | 23 | 20.5 | 25.8 |
| Diarrhea | Yes | No | Yes | No | Yes | Yes | Yes | No | Yes | No | Yes | No | Yes | No |
| Performance status | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 |
| Albumin, g/L | 32 | 43 | 33 | 41 | 30 | 46 | 32 | 41 | 20 | 41 | 30 | 44 | 26 | 46 |
| Serum iron, μM | 18 | 26 | 11 | 18 | 14 | 10 | 14 | 15 | 17 | 13 | 7 | 17 | 13 | 18 |
| Serum calcium, mM | 2.20 | 2.36 | 2.33 | 2.45 | 2.26 | 2.26 | 2.26 | 2.32 | 2.33 | 2.41 | 2.02 | 2.35 | 2.00 | 2.29 |
| Serum folic acid, nM | 10 | 44 | 10.4 | 24 | 14 | 91 | 14 | 15 | 14 | 78 | 4.4 | 35 | 29 | 18 |
| Serum B12, pM | 470 | 560 | 169 | 307 | 440 | 290 | 440 | 790 | 206 | 666 | 107 | 317 | 269 | 221 |
| Characteristic . | Patient A . | Patient B . | Patient C . | Patient D . | Patient E . | Patient F . | Patient G . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before . | After . | Before . | After . | Before . | After . | Before . | After . | Before . | After . | Before . | After . | Before . | After . | |
| BMI, kg/m2 | 19.4 | 25.6 | 18.9 | 26.1 | 22 | 24.1 | 24 | 24.1 | 20.1 | 22 | 22.1 | 23 | 20.5 | 25.8 |
| Diarrhea | Yes | No | Yes | No | Yes | Yes | Yes | No | Yes | No | Yes | No | Yes | No |
| Performance status | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 |
| Albumin, g/L | 32 | 43 | 33 | 41 | 30 | 46 | 32 | 41 | 20 | 41 | 30 | 44 | 26 | 46 |
| Serum iron, μM | 18 | 26 | 11 | 18 | 14 | 10 | 14 | 15 | 17 | 13 | 7 | 17 | 13 | 18 |
| Serum calcium, mM | 2.20 | 2.36 | 2.33 | 2.45 | 2.26 | 2.26 | 2.26 | 2.32 | 2.33 | 2.41 | 2.02 | 2.35 | 2.00 | 2.29 |
| Serum folic acid, nM | 10 | 44 | 10.4 | 24 | 14 | 91 | 14 | 15 | 14 | 78 | 4.4 | 35 | 29 | 18 |
| Serum B12, pM | 470 | 560 | 169 | 307 | 440 | 290 | 440 | 790 | 206 | 666 | 107 | 317 | 269 | 221 |
Normal ranges: albumin, 34-50 g/L; iron, 10-32 μM; calcium, 2.20-2.60 mM; folic acid, > 5.9 nM; B12, 156-672 pM. Duration of follow-up for each patient: A, 30 mo; B, 27 mo; C, 16 mo; D, 8 mo; E, 11 mo, F, 10 mo; and G, 7 mo.
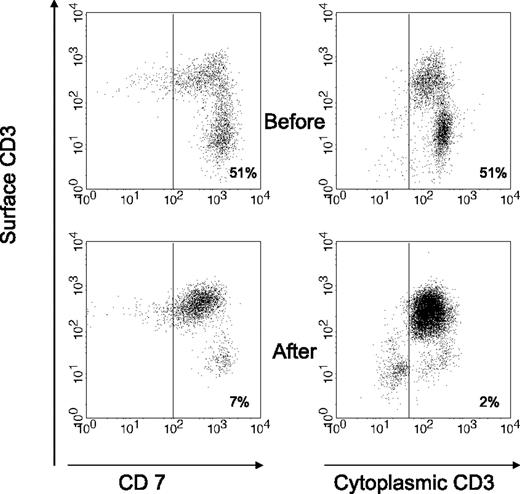
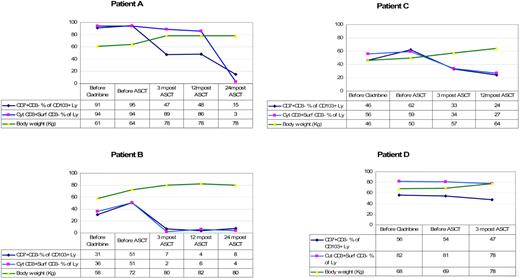
Table 3 shows the endoscopic and immunologic results. All patients were monoclonal for the TCR-γ. Endoscopically there was disappearance of erosions and ulcerations in the jejunum in all patients (patients B, E, F, and G) who had ulcerative jejunitis before ASCT, and histology of the small intestine showed significant regeneration as documented by down-staging of the Marsh class (patients A, B, C, E, F, and G). Overall, the aberrant (CD7+CD3−) T-cell percentage of CD103+ lymphocytes decreased from a mean of 63% (range, 11%-95%) at baseline to 38% (range, 7%-68%) 3 to 4 months after transplantation. Aberrant cytoplasmic CD3+ surface CD3− T-cell percentage of CD103+ lymphocytes decreased from a mean of 61% (range, 10%-94%) to 42% (range, 2%-89%). Furthermore, the mean percentage of CD8+ cells increased from 23% to 30% after ASCT. This was particularly noticeable in the first 3 patients. Patient D did not show a significant increase in CD8+ cells and the last 3 patients have not yet shown a significant change. Individual responses to ASCT differed from each patient as shown in Table 3. Patient B showed the most impressive response with a virtual complete disappearance of aberrant T cells. The fluorescent-activated cell sorting (FACS) data from patient B are shown in Figure 2. The trend of aberrant T cells and body weight for the first 4 patients who have a follow-up period of at least 1 year is shown in Figure 3. Follow-up of patients E, F, and G is as yet limited. Two years after transplantation, our first patient (patient A) is showing further improvement in his immunopathology status as demonstrated in further decline in the percentage of aberrant T cells to 3% and histologically improved from Marsh III-A to Marsh I and the second patient (patient B) is still showing persistent complete clinical and histologic response. Patient D had no significant change in the percentage of aberrant T cells and showed no histologic improvement and no significant improvement in CD8+ percentage; he died 8 months after transplantation. After ruling out structural and infectious (bacterial and viral) causes, we assumed that progressive disease of RCD II with oligoclonal T lymphocytes infiltrating the brain was the cause of death in this particular patient. EATL could not be detected. Autopsy confirmed the presence of chronic encephalitis of the right temporal lobe with T-lymphocyte infiltration. Immunohistochemistry showed that the lymphocyte infiltrate was CD3+ and the majority of cells expressed CD8 positivity. TCR gene analysis showed that the T cells were oligoclonal.
Flow cytometric analysis of duodenal cells obtained from patient B, showing the change in the percentage of aberrant T-cell population before and after ASCT. Aberrant population is shown as CD7+CD3− within CD103+ lymphocytes (left) or as cytoplasmic (cyt) CD3+ surface (surf) CD3− within lymphocyte gate (right). Normal range for cyt CD3+ surf CD3− % of CD103+ lymphocytes is 10% or less.
Flow cytometric analysis of duodenal cells obtained from patient B, showing the change in the percentage of aberrant T-cell population before and after ASCT. Aberrant population is shown as CD7+CD3− within CD103+ lymphocytes (left) or as cytoplasmic (cyt) CD3+ surface (surf) CD3− within lymphocyte gate (right). Normal range for cyt CD3+ surf CD3− % of CD103+ lymphocytes is 10% or less.
The trend of aberrant T cells and body weight per patient. Ly indicates lymphocytes; before, 1 to 3 months. Normal range for cyt CD3+ surf CD3− % of lymphocytes is 10% or less.
The trend of aberrant T cells and body weight per patient. Ly indicates lymphocytes; before, 1 to 3 months. Normal range for cyt CD3+ surf CD3− % of lymphocytes is 10% or less.
Histological and phenotypical flow cytometric analysis of IELs in duodenal biopsies before (1-6 mo) and after ASCT
| Patient . | Marsh category . | CD7+CD3−, % of CD103+ ly . | Cyt CD3+ surf CD3−, % of CD103+ ly . | CD8+, % of CD103+ ly . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before . | After . | Before . | After . | Before . | After . | Before . | After . | |||||||||
| 3 mo . | 12 mo . | 24 mo . | 3 mo . | 12 mo . | 24 mo . | 3 mo . | 12 mo . | 24 mo . | 3 mo . | 12 mo . | 24 mo . | |||||
| Patient A | III-A | III-A | III-A | I | 95 | 47 | 48 | 15 | 94 | 89 | 86 | 3 | 1 | 20 | 7 | 52 |
| Patient B | III-B | I | I | I | 51 | 7 | 4 | 8 | 51 | 2 | 6 | 4 | 28 | 68 | 62 | 67 |
| Patient C | III-C | III-A | III-A | — | 62 | 33 | 24 | — | 59 | 34 | 27 | — | 15 | 41 | 36 | — |
| Patient D | III-A | III-A | — | — | 54 | 47 | — | — | 81 | 78 | — | — | 7 | 13 | — | — |
| Patient E | III-B | I | — | — | 44 | 68 | — | — | 30 | 36 | — | — | 22 | 2 | — | — |
| Patient F | III-B | III-A | — | — | 71 | 40 | — | — | 50 | 31 | — | — | 23 | 11 | — | — |
| Patient G | III-C | III-A | — | — | 11 | 30 | — | — | 10 | 27 | — | — | 63 | 52 | — | — |
| Mean | — | — | — | — | 63 | 38 | — | — | 61 | 42 | — | — | 23 | 30 | — | — |
| Patient . | Marsh category . | CD7+CD3−, % of CD103+ ly . | Cyt CD3+ surf CD3−, % of CD103+ ly . | CD8+, % of CD103+ ly . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before . | After . | Before . | After . | Before . | After . | Before . | After . | |||||||||
| 3 mo . | 12 mo . | 24 mo . | 3 mo . | 12 mo . | 24 mo . | 3 mo . | 12 mo . | 24 mo . | 3 mo . | 12 mo . | 24 mo . | |||||
| Patient A | III-A | III-A | III-A | I | 95 | 47 | 48 | 15 | 94 | 89 | 86 | 3 | 1 | 20 | 7 | 52 |
| Patient B | III-B | I | I | I | 51 | 7 | 4 | 8 | 51 | 2 | 6 | 4 | 28 | 68 | 62 | 67 |
| Patient C | III-C | III-A | III-A | — | 62 | 33 | 24 | — | 59 | 34 | 27 | — | 15 | 41 | 36 | — |
| Patient D | III-A | III-A | — | — | 54 | 47 | — | — | 81 | 78 | — | — | 7 | 13 | — | — |
| Patient E | III-B | I | — | — | 44 | 68 | — | — | 30 | 36 | — | — | 22 | 2 | — | — |
| Patient F | III-B | III-A | — | — | 71 | 40 | — | — | 50 | 31 | — | — | 23 | 11 | — | — |
| Patient G | III-C | III-A | — | — | 11 | 30 | — | — | 10 | 27 | — | — | 63 | 52 | — | — |
| Mean | — | — | — | — | 63 | 38 | — | — | 61 | 42 | — | — | 23 | 30 | — | — |
Mean was calculated for values at 3 mo after ASCT. Normal range for Cyt CD3+ surf CD3−% of lymphocytes is 10% or less. TCRγ-PCR analysis showed monoclonality in all patients.
Ly indicates lymphocytes; —, not applicable; TCRγ-PCR analysis, T-cell receptor γ–polymerase chain reaction rearrangement.
Discussion
In this pilot study, ASCT in patients with RCD II was shown to be feasible. The conditioning regimen was well tolerated in all patients and there was a substantial clinical improvement. The rapid initial response (within 3 months) and the duration (2 years in patient A and B and 14 months in patient C) of the remission up to now are promising. Complications included the occurrence of neutropenic fever in 2 patients and retinal bleeding not related to thrombocytopenia in one patient, all with full recovery. The nadir leukocyte and platelet counts and the duration of leukopenia and thrombocytopenia were comparable to our experience in patients with non-Hodgkin lymphomas and multiple myeloma undergoing ASCT after a combination of carmustine, etoposide, cytarabine, and melphalan (BEAM) or high-dose melphalan (HDM; 200 mg/m2).32 Because there is no standard conditioning regimen for ASCT used in autoimmune disease,33 a standard regimen from our institution was used. Fludarabine induces T-cell depletion and the alkylating agent melphalan was used to achieve myeloablation.
One patient was excluded due to unsuccessful leukapheresis. Although we could achieve successful leukapheresis in all patients despite earlier 2-CDA therapy, it is possible that the reason for failure of stem cell mobilization in one particular patient might be related to the use of 2-CDA.34 T cells play an essential role in the pathogenesis of CD and RCD II/EATL.8,10,15 Through the activity of the enzyme tissue transglutaminase (tTG) glutamine residues in gluten are converted into glutamic acid.35,36 Subsequently a multitude of gluten-derived peptides is generated that, when bound to either HLA-DQ2 or -DQ8 can induce T-cell responses in patients with CD.8,24 A particular glutamine- and proline-rich 33-mer α-gliadin peptide that contains 6 different T-cell stimulatory sequences and is resistant to gastric and duodenal proteolysis might be the primary initiator of the inflammatory response to gluten. In the large majority of patients, even in children with CD, inflammatory T-cell responses to other gluten peptides are also observed, implicating multiple gluten peptides in the disease process.26,27
The definition of RCD I/II has undergone refinement in recent years. It seems that the most reliable available method to differentiate between RCD I and RCD II is flow cytometry of intestinal biopsies revealing the presence of aberrant T cells. Detection of a clonal T-cell population by testing for TCR rearrangement was thought to be highly predictive of EATL development. However, oligoclonal or monoclonal IEL populations can be detected in the large majority of both RCD I and RCD II patients and also in patients who do not develop an EATL. Clonality is therefore of limited use in establishing the diagnosis of RCD and to predict the development of EATL.14,37,38
RCD II is usually resistant to any known immunosuppressive therapy, including azathioprine/prednisone,15 cyclosporine,16 and IL-10 therapy.17 Recently, we treated 17 patients with 2-CDA on intention to induce remission. Within a mean follow-up period of 22 months (range, 7-67 months) 47% had a significant decrease in aberrant T-cell percentages with or without clinical response.39 However, another 41% did not respond clinically, histologically, nor immunopathologically and subsequently died from EATL.
Remissions of autoimmune diseases have been described in adults after both allogenic and autologous ASCT1–7 most probably due to the extreme immunosuppressive effects of these strategies,1 resulting in immunoablation with subsequent regeneration of naïve T lymphocytes derived from reinfused hematopoietic progenitor cells.7 Furthermore, recently, interesting insights into possible unsuspected mechanisms by which stem cell transplantation could affect the gut have emerged. In both animal and patient studies, sex-mismatched allogeneic stem cell transplantations have shown in both mice and women that a population of myofibroblasts derived from the donor populates the intestinal mucosa. Given the importance of myofibroblasts in orchestrating the function of epithelial cells, these data suggest a mechanism other than one targeted at immunosuppression that could beneficially reset patient functions, for example, enhancing barrier function following stem cell transplantation.40
These positive results, the high risk of transforming into EATL, and the absence of effective therapy for RCD with aberrant T cells led us to introduce this new strategy with the ultimate goal of resetting the immune response that might prevent or delay development of overt EATL. On follow-up, our patients showed improvement in the small intestinal histology, together with impressive clinical improvement as demonstrated by disappearance of diarrhea and abdominal pain; normalization of serum albumin, electrolytes, and hemoglobin; increase in BMI; and improvement of the performance status. Two years after transplantation, our first patient is showing further improvement in his immunopathology status as demonstrated by further decline in the percentage of aberrant T cells to 3% and histologic improvement from Marsh III-A to Marsh I. We propose that enhanced apoptosis of activated but aberrant T cells has led to this late but remarkable decline.41 One patient died 8 months after ASCT from progressive neurologic manifestations in association with CD. Autopsy excluded any structural or infectious cause. One patient developed self-limiting erythematous plaque skin lesions with central necrosis 2 months after ASCT. Detailed analysis excluded the presence of EATL. Our most recent patient with clinically short bowel syndrome is showing remarkable clinical, endoscopic, and immunologic improvement. All our patients had negative serology before inclusion, confirming their strict adherence to GFD, and after ASCT all patients remained negative for anti-tTG and EMA. Furthermore, the first 3 patients showed a significant increase in the percentage of CD8+ lymphocytes, which is seen as a marker of lymphocyte regeneration after ASCT.42 Patient D did not show a significant increase in CD8+ cells and the last 3 patients have not yet shown a significant change. Absence of a demonstrable improvement in the surface expression of CD8 on the IEL might be regarded as a poor prognostic indicator of response; this is only to be proved or disproved on longer-term follow-up.
Although the short-term results in these patients are promising, follow-up at present is too short to permit firm conclusions as to efficacy. The selection of patients for this treatment should be restricted to those patients with a substantial population of aberrant T cells, even after therapy with 2-CDA, who have a greater tendency to progress to highly lethal EATL. High-dose chemotherapy followed by ASCT seems feasible and safe and might result in long-term improvement of disease activity in RCD patients with aberrant T cells whose condition previously did not respond to available treatments. Longer-term follow-up and additional pilot studies with larger groups of patients are needed to confirm the efficacy of this therapy.
Authorship
Contribution: A.A.-t., O.J.V., and W.H.M.V. drafted the manuscript and provided patient care; H.M.v.R. collected and analyzed data; B.M.E.v.B. and P.E.T.S. performed T-cell flow cytometry; G.J.O., P.C.H., and C.J.J.M. revised and gave final approval of the manuscript; and C.J.J.M. supervised the management of patients before and after transplantation.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: Chris J. J. Mulder, VU University Medical Center, Department of Gastroenterology, PO Box 7057, 1005 MB Amsterdam, The Netherlands; e-mail: cjmulder@vumc.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal