Abstract
We investigated the clinical effects of low-power laser therapy (LPLT) on prevention and reduction of severity of conditioning-induced oral mucositis (OM) for hematopoietic stem cell transplantation (HSCT). We randomized 38 patients who underwent autologous (AT) or allogeneic (AL) HSCT. A diode InGaAlP was used, emitting light at 660 nm, 50 mW, and 4 J/cm2, measured at the fiberoptic end with 0.196 cm2 of section area. The evaluation of OM was done using the Oral Mucositis Assessment Scale (OMAS) and the World Health Organization (WHO) scale. In the LPLT group, 94.7% of patients had an OM grade (WHO) lower than or equal to grade 2, including 63.2% with grade 0 and 1, whereas in the controls group, 31.5% of patients had an OM grade lower than or equal to grade 2 (P < .001). Remarkably, the hazard ratio (HR) for grades 2, 3, and 4 OM was 0.41 (range, 0.22-0.75; P = .002) and for grades 3 and 4 it was 0.07 (range, 0.11-0.53; P < .001). Using OMAS by the calculation of ulcerous area, 5.3% of the laser group presented with ulcers of 9.1 cm2 to 18 cm2, whereas 73.6% of the control group presented with ulcers from 9.1 cm2 to 18 cm2 (P = .003). Our results indicate that the use of upfront LPLT in patients who have undergone HSCT is a powerful instrument in reducing the incidence of OM and is now standard in our center.
Introduction
High-dose chemotherapy administered as part of the preparative regimen prior to hematopoietic stem cell transplantation (HSCT) has a direct cytotoxic effect on the oral epithelium, connective tissue, and extracellular matrix, leading to injury or disruption of the mucosal barrier.1 Oral and gastrointestinal mucositis may occur in up to 100% of the patients undergoing high-dose chemotherapy with HSCT. Oral mucositis (OM) is associated with an increase in the incidence of systemic infections because of disruption of the natural mucosal barrier, and impacts both the length of hospital stay and the complications associated with HSCT.1–3
Clinical presentation of OM consists of mucosal burning, erythema, and edema, and progresses to ulceration with or without pseudomembrane formation that develops most commonly on the nonkeratinized mucosa of the floor of the mouth, tongue, buccal mucosa, and soft palate.4 The initial stage of tissue injury occurs rapidly following the administration of radiation or chemotherapy, which trigger both DNA and non-DNA damage. DNA strand breaks result in direct cellular injury that targets cells in the basal epithelium and within the submucosa. Although the mucosa seems to be absolutely normal at this stage, a cascade of events is ongoing in the submucosa that ultimately results in mucosal destruction.5 The current management of such OM is directed at prevention, palliation, infection prevention, and treatment.6,7
Low-power laser therapy (LPLT) has been used in an attempt to reduce the incidence of OM and its associated pain in patients who are receiving high-dose chemotherapy or chemoradiotherapy prior to HSCT.8–12 Over the last several years, appropriate laboratory and clinical evidence have been accumulating steadily to also support the use of LPLT to promote biomodulation.13–15 It has been reported that LPLT promotes wound healing and reduces pain and inflammation. Different effects appear to be related to laser characteristics and the particular type of tissue being treated.16 Helium-neon (He-Ne) laser (632.8 nm) treatment has been the most frequently studied form of LPLT for the prevention or reduction of OM and oral pain associated with cancer therapy (including HSCT). Research is currently underway on the use of diode lasers with wavelengths ranging from 650 nm to 905 nm. It appears that laser therapy produces no toxicity and is not traumatic to patients.5
In the current trial we investigated the clinical effects of the InGaAlP (660 nm) laser on the prevention and reduction of the severity of conditioning-induced OM for HSCT patients.
Patients, materials, and methods
Patients
This was a randomized, placebo-controlled, quantity, and prospective clinical trial. Between January 4, 2004, and May 20, 2005, 38 patients were evaluated and underwent HSCT at Centro de Transplante de Medula Óssea (CEMO). The research was performed in compliance with resolution no. 196/96 of the National Health Counsel of Brazil, and was submitted to both the Ethics Committee of the Instituto Nacional de Câncer (INCA) and of the Universidade do Vale do Paraíba (UNIVAP). Informed consent was obtained in accordance with the Declaration of Helsinki. Patients were randomized on the day of admission for the transplantation, between receiving laser therapy (laser or experimental group), or not receiving laser therapy (placebo or control group).
Inclusion criteria
The criteria for inclusion in the study were age 18 years old or older, hematologic disease–nominated HSCT, oral mucous intact on the first day of the conditioning (D-7), absence of visible plaque on the teeth upon inspection, and capacity to cooperate with the treatment. Patients must also have signed the consenting text giving free confirmation, after the information and instruction section.
Exclusion criteria
Patients who did not meet the inclusion criteria (eg, allogeneic or autologous not myeloablative transplantation), patients who were receiving drugs for the treatment and/or prevention of mucositis, and patients who were not previously evaluated and released by the author were excluded from this study.
Oral care
Dental care was performed by a dentist before admission for HSCT. Dental care included educating patients about oral hygiene; panoramic radiograph; oral examination with attention to soft tissues and bones; tooth and periodontal exam; removal of sub and supragingival calculus; elimination of sources of trauma caused by orthodontic bands and brackets, teeth, or prosthesis; extraction of teeth with signs or symptoms indicative of potentially bad prognosis (active periodontal disease, teeth requiring endodontic treatment or with extended caries and coronary destruction).17–18 All the patients had carried out oral hygiene with extra-soft toothbrushes, dental paste with a peroxidase system after every meal, and mouth rinses with an ethanol-free 0.12% chlorhexidine solution19–21 containing xylitol from D-7 until neutrophil recovery (granulocytes > 500/mm3), 3 times a day (morning, afternoon, and night).
Conditioning regimens
The characteristics of the conditioning regimens are summarized in Table 1. Patients received 200 mg fluconazole intravenously every 12 hours from D-2 and 500 mg/m2 acyclovir intravenously every 8 hours from D-2 until neutrophil recovery.
Conditioning regimens
| Regimen . | Dose, period . | Laser group, n . | Control group, n . |
|---|---|---|---|
| Regimen 1 | Cyclophosphamide 1800 mg/m2/d, d −6 and d −3; carmustine 450 mg/m2/d, d −2; etoposide 2400 mg/m2, d −7 (34 h) | 5 | 5 |
| Regimen 2 | Cyclophosphamide 60 mg/kg/d, d −3 and d −2; TBI 22 Gy every 12 h, d −7 to d −5; antithymocyte globulin 15 mg/kg/d, d −5 to d −4 | 3 | 5 |
| Regimen 3 | Cyclophosphamide 60 mg/kg/d, d −3 and d −2; busulfan 4 mg/kg/d, d −7 to d −4 | 11 | 9 |
| Regimen . | Dose, period . | Laser group, n . | Control group, n . |
|---|---|---|---|
| Regimen 1 | Cyclophosphamide 1800 mg/m2/d, d −6 and d −3; carmustine 450 mg/m2/d, d −2; etoposide 2400 mg/m2, d −7 (34 h) | 5 | 5 |
| Regimen 2 | Cyclophosphamide 60 mg/kg/d, d −3 and d −2; TBI 22 Gy every 12 h, d −7 to d −5; antithymocyte globulin 15 mg/kg/d, d −5 to d −4 | 3 | 5 |
| Regimen 3 | Cyclophosphamide 60 mg/kg/d, d −3 and d −2; busulfan 4 mg/kg/d, d −7 to d −4 | 11 | 9 |
TBI indicates total body irradiation.
Samples for blood cultures were collected by central catheter and peripheral veins in case of febrile episodes, followed by empiric therapy with broad-spectrum antibiotics.
Laser application
LPLT was started on the first day of the conditioning (D-7) and stopped on the day of neutrophil recovery. The dentists were the only members of the team who knew which group the patient was randomized to. The same equipment was used in all applications. The irradiation used was a 50-mW InGaAlP diode laser, emitting continuous light at 660 nm, with a real power output of 46.7 mW and energy density (ED) of 4 J/cm2, measured at the fiberoptic end with 0.196 cm2 of section area during the experiment. It was applied in a punctual form, side by side, touching the material, for 16.7 seconds per point, totaling 15 points per region. The oral cavity regions previously treated were the upper lip, lower lip (redness and lip mucosa), buccal mucosa, dorsum, ventral and lateral tongue, the floor of the mouth, and the hard and soft palates. Before the application of the laser the tip was wrapped with a PVC film and after this procedure it was disinfected with 70% alcoholic solution. For protection all patients received eyeglasses to totally block the light, to be used during the application of the laser.
A crossover was allowed for patients from the control group who presented with a grade 4 oral mucositis index of the World Health Organization (WHO)22 and/or an ulcer area more than or equal to 12 cm according to the Oral Mucositis Assessment Scale (OMAS).23 A therapeutic laser with 8 J/cm2 per point was applied to these patients. The patients in the laser group who had presented with erythema or ulcers continued to receive the preventive laser with 4 J/cm2.
Laser therapy evaluation
With the purpose of minimizing interobserver variation and familiarizing the team with the measurement scales for mucositis, a CD-ROM containing the research protocol as well as photographic examples of normal and damaged oral mucosa (mucositis) was given to all the professionals involved in the application of LPLT and in the evaluation of patients. One dentist and 3 nurses (blinded for the study) performed daily oral evaluation of the patients from D-7 until neutrophil recovery. The results were catalogued and analyzed according to the WHO scale, the OMAS, and the Visual Analogue Scale (VAS).23
Statistical analysis
For the WHO scale and the OMAS the chi-square test (χ2 ) was applied. Correlation between the scores in the WHO scale and the OMAS was assessed by the F-test in analysis of variance (ANOVA), and the Bonferroni test. Mucositis-free survival was calculated from the first day of conditioning through neutrophil recovery by the Kaplan-Meier method. The concordance index (CI) between the evaluators was measured (CI = 81.7% ± 1.96 vs
Results
All 38 patients completed the study and none were lost to follow-up or excluded for failure to complete the laser application protocol. The treatment was well tolerated and no toxicity from LPLT was recorded. Patient characteristics are summarized in Table 2.
Patient characteristics
| Patient characteristic . | Laser group . | Control group . |
|---|---|---|
| Age, y | ||
| Average | 36.5 | 36.8 |
| Median | 38.0 | 36.0 |
| Gender, n | ||
| Male | 12 | 11 |
| Female | 7 | 8 |
| HSCT, n | ||
| Related allogeneic | 11 | 9 |
| Related allogeneic with TBI | 0 | 2 |
| Unrelated allogeneic with TBI | 1 | 3 |
| Unrelated allogeneic umbilical cord blood cells with TBI | 2 | 0 |
| Autologous | 5 | 5 |
| Diagnostic, n | ||
| Chronic myeloblastic leukemia | 8 | 8 |
| Acute myeloblastic leukemia | 3 | 3 |
| Hodgkin lymphoma | 6 | 2 |
| Non-Hodgkin lymphoma | 1 | 3 |
| Acute lymphoblastic leukemia | 1 | 0 |
| Myelodysplastic syndrome | 0 | 3 |
| Patient characteristic . | Laser group . | Control group . |
|---|---|---|
| Age, y | ||
| Average | 36.5 | 36.8 |
| Median | 38.0 | 36.0 |
| Gender, n | ||
| Male | 12 | 11 |
| Female | 7 | 8 |
| HSCT, n | ||
| Related allogeneic | 11 | 9 |
| Related allogeneic with TBI | 0 | 2 |
| Unrelated allogeneic with TBI | 1 | 3 |
| Unrelated allogeneic umbilical cord blood cells with TBI | 2 | 0 |
| Autologous | 5 | 5 |
| Diagnostic, n | ||
| Chronic myeloblastic leukemia | 8 | 8 |
| Acute myeloblastic leukemia | 3 | 3 |
| Hodgkin lymphoma | 6 | 2 |
| Non-Hodgkin lymphoma | 1 | 3 |
| Acute lymphoblastic leukemia | 1 | 0 |
| Myelodysplastic syndrome | 0 | 3 |
TBI indicates total body irradiation.
Mucositis evaluation by WHO criteria
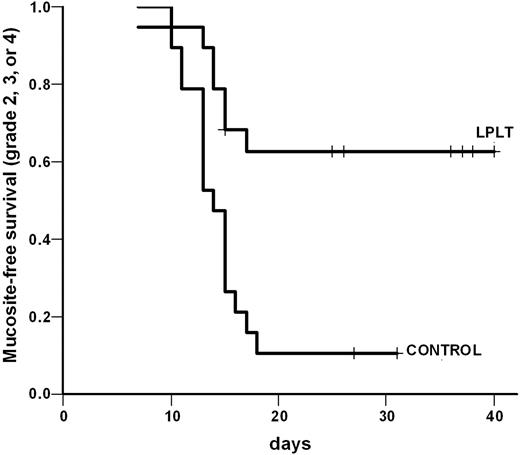
Using the WHO scale, it was observed that the laser group patients presented with less intense OM (WHO grades 0-1; Figure 1). The proportion of patients in the LPLT and placebo groups who developed grade 0 or 1 mucositis (without ulcers) was 63.2% (12 of 19), including 3 patients submitted to total body irradiation (TBI) and 10.5% (2 of 19), respectively (P < .001). Six patients in the LPLT group (31.5%) had small ulcers (WHO grade 2), totaling 94.7% of the patients in this group with a WHO grade between 0 and 2. The control group behaved in the opposite way (P < .001). In order to better estimate the impact of LPLT, the mucositis-free survival was analyzed separately in the strata of patients with grades 2, 3, and 4 and grades 3 and 4. The hazard ratio for grades 2, 3, and 4 mucositis was 0.41 (range, 0.22-0.757; P = .002), whereas for grades 3 and 4 only it was 0.07 (range, 0.11-0.53; Figures 2 and 3).
Oral mucositis evaluation by OMAS criteria
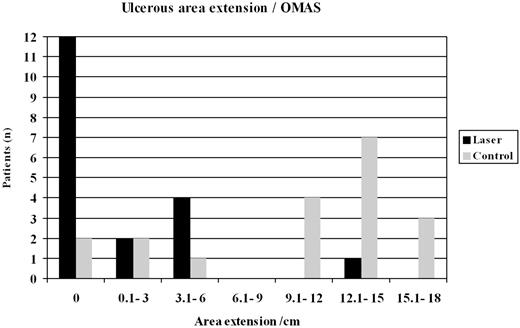
The evaluation by OMAS criteria was done by using both the calculation of the weighted average of the ulcerous area plus the erythema's intensity, and by ulcerous area only. Sixteen patients from the laser group (84.2%) stayed on the weighted average zone of 0 to 2.9, while only 5 patients from the control group (26.3%) stayed in the same zone (P = .007; Figure 4). It was observed that the laser group patients presented with a small extension of the ulcerous area (P = .003; Figure 5). In addition, the control group patients showed mucositis earlier (D+5) than the laser group (D+6; U* [Mann-Whitney test] = 0.42 with P = .67), had a longer duration of mucositis (average of 6 days for laser group versus average of 9 days for control group; U* = 1.52 with P = .13), and therefore needed more time for the mucositis to heal, compared with the laser group (U* = 1.45 with P = 0.15). The average time for the laser application in the control group was 6 days. In total, 24 patients presented with an ulcer in the oral cavity and the most affected areas were buccal mucosa (20.5 patients, 85.4%), lateral tongue (19 patients, 79.1%), and ventral tongue (17 patients, 70.8%).
Mucositis incidence, OMAS scale. P = .007. (WA = 2.5 × [(Σui : 3 ×Nu) + (Σei : 2 × Ne)], in which Σui is the sum of the ulcerous area, Nu equals the number of ulcerous areas, Σei is the sum of the erythema's intensity, and Ne is the number of areas with erythemas.
Mucositis incidence, OMAS scale. P = .007. (WA = 2.5 × [(Σui : 3 ×Nu) + (Σei : 2 × Ne)], in which Σui is the sum of the ulcerous area, Nu equals the number of ulcerous areas, Σei is the sum of the erythema's intensity, and Ne is the number of areas with erythemas.
Correlation between the WHO scale and OMAS
In this analysis arithmetic averages and standard deflection were used for comparison of the WHO scale with the OMAS weighted average (WA). A significant difference was detected between grade 1 (WA = 1.25) and grade 2 (WA = 2.07); grade 1 (WA = 1.25) and grade 3 (WA = 3.72); grade 1 (WA = 1.25) and grade 4 (WA = 3.5); grade 2 (WA = 2.07) and grade 3 (WA = 3.72); and grade 2 (WA = 2.07) and grade 4 (WA = 3.5), with °°F” (ANOVA) = 149.98 (P < .001). However, a significant difference was not observed between grade 3 (WA = 3.72) and grade 4 (WA = 3.5).
Level of agreement among evaluators and pain evaluation
A significant agreement among the evaluators occurred, with an agreement index of 81.7%. Regarding the presence and intensity of pain, a significant difference was not noticed: 14 (73.7%) patients from the laser group (VAS 7) and 16 (84.2%) patients from the control group (VAS 8) reported pain (P = .13).
Impact of LPLT on clinical outcomes
Concerning the results of the blood cultures, 28 (73.8%) patients overall presented with negative blood cultures and 10 (26.3%) presented with positive blood cultures. No differences between the LPLT and control groups were observed. Among the positive blood cultures, no Streptococcus was identified and no tooth and gingival complications detected. Although not planned, an analysis of the impact of LPLT on survival and treatment-related mortality (TRM) was performed. Marginal differences in survival (P = .04), but not in TRM, favoring the LPLT group were detected (data not shown).
Discussion
The potential positive effects from LPLT as a preventive treatment method for patients with high probability to develop OM, such as those submitted to HSCT, has been postulated.8,10,12 However, confirmatory randomized trials with appropriate design are lacking. The study presented here differs from 2 previous randomized trials in 3 ways: the population examined, the LPLT, and the criteria (scores) used for analysis.
The majority of our patients underwent allogeneic transplantation (Table 2), whereas both the Cowen et al10 and Barasch et al8 studies enrolled patients who underwent autologous HSCT, except for one patient. Since allogeneic transplantation leads to a more severe OM than does autologous HSCT, our population may be considered more vulnerable and the results more remarkable. However, the fact that TBI was applied to 100% of the patients in Cowen et al's study, but to only 10% of our patients, may counterbalance the previous difference, making comparisons to the population described here and elsewhere8,10 difficult.
Despite the use of preventive LPLT, a high incidence of ulcers was still observed in previous randomized studies.8,10 One study applied continuous laser illumination with a 632.8-nm wavelength, 25 mW of power, and 1 J/cm2 of ED, from day −1 to +3.8 It should be noted that in that study, 20 patients served as their own control since the randomization was done between the right and left of the buccal mucosa midline. Cowen et al10 applied continuous laser illumination with a 632.8-nm wavelength, 25 mW of power, and 1.5 J/cm2 of energy density, from day −5 to −1, to 30 patients. In our trial both a higher energy density (4 J/cm2) and a longer length of administration (from day −7 until neutrophil recovery) were applied. In marked contrast to previous data, this strategy proved to be highly effective: 63.2% of the patients did not present with OM, whereas 100% of the patients presented with OM after HSCT in the studies by Barasch et al8 and Cowen et al.10 Further, Migliorati12 applied continuous laser illumination with a 780-nm wavelength, 60 mW of power, and 2 J/cm2 of energy density, from day −5 to day +5, to 11 patients and reported that 63.7% of the patients presented with OM grades 3 and 4 (WHO) and 9% of the patients presented with OM grade 2 after HSCT and high-dose chemotherapy (QT). Our data suggest that both high laser energy density and length of the application may be pivotal for the outcome of LPLT preventive treatment. In line with that, recent data from a randomized clinical trial in which 60 children received LPLT (780 nm wavelength, 60 mW of power, and 4 J/cm2 of energy density) for a short period of time (days 1 to 5) showed no difference between LPLT and control groups.24 It should be noted that the groups treated in that study were heterogeneous, including patients treated with both conditioning and conventional chemotherapy regimens.24
Following the same rationale, a higher ED (8 J/cm2) was applied with therapeutic intention for patients of the control group who received only LPLT when they achieved grade 4 OM or 12 cm of ulcerous area. This unprecedented strategy was successful, since patients recovered in 6 days from the start of the laser application, whereas in a previous report8 this recovery took 16 days. Of note, the fast recovery from OM shown in our study cannot be attributed to neutrophil recovery, since the medium score of neutrophils in the group was 94 mm3.
In contrast to the scores selected in previous randomized trials, both the WHO scale and OMAS were used here. The WHO scale was selected for its easy handling, straightforward applicability, and previous validation, whereas the OMAS was picked because it measures and quantifies both ulcers and erythema. This is an important characteristic because some patients mentioned difficulty in swallowing because of pain in the oropharyngeal area, even without lesions on the oral cavity, which may skew evaluation by the WHO scale or any other scale that mixes signs and symptoms. The fact that both the WHO scale and the OMAS showed a strong difference between LPLT and control groups in favor of the experimental arm, coupled to the correlation between the 2 scales, strengthen our data. Previous studies8,10 did not evaluate the quantity and extension of the areas attacked by ulcers, but in the current study 7 patients from the laser group who presented with ulcers had them in smaller proportion than the 17 patients from the control group. This fact confirms the methodologic option for the use of OMAS. These results support OMAS as an instrument capable of portraying the clinical condition of the patient and suggest that the WHO scale and the OMAS may be complementary in the evaluation of OM. It is worthwhile to point out that none of the previous randomized studies used OMAS. Barasch et al8 used the modified Oral Mucositis Index Scale (OMI) and the Eastern Cooperative Oncology Group Oral Toxicity Scale (ECOG) and Cowen et al10 used the scale published by Walsh et al.25
Although a delay in the start of OM was expected in patients from the laser group, a significant statistical difference between the laser and control groups was not observed, and this result deserves further investigation. Further, no significant statistical difference was seen between the laser and control groups in the total time with OM. It should be noted that control group patients who reached grade 4 OM or a total area of OM of 12 cm, started to receive the therapeutic laser with 8 J/cm2 on the ulcerous areas. This crossover may have masked a true difference between the groups. In contrast to our trial, Cowen et al10 reported an increase in the time of mucositis grade 0 and grade 1 in the patients who received laser (17 days) compared with those in the control group (14 days). Here the comparison of the whole time of OM between the groups did not show a significant statistical difference, probably due to the crossover.
Previous studies using VAS for pain report both a decrease in pain and in the use of morphine in patients receiving preventive laser.8,10 In contrast, no difference between the 2 arms was noted in our series. It is important to emphasize that the initial idea was to use this scale to evaluate pain in the oral cavity. However, it was observed that the pain started and was predominant in the oropharyngeal area, triggering the start of narcotics. Since these events preceded the onset of ulcers in the oral cavity, patients were already taking morphine when the oral lesions appeared and this may have been an important bias in the analysis of the VAS. Further, it was observed that morphine provides better pain control for the oral cavity than for oropharyngeal lesions. These observations are unprecedented and should be taken into account and confirmed in future studies. Moreover, no statistical difference was seen between grade 3 and grade 4, which probably reflects both the difficulty in the evaluation of the symptoms (subjective) and the administration of narcotics that mask the pain. This observation is in agreement with the observation mentioned by Sonis et al.23
The presence of OM associated with infection increases the hospital confinement time of the patient undergoing HSCT to a total of 5 days, increasing hospital costs.26 The negative results of the blood cultures for Streptococcus in 38 patients indicate that the mouth environment adaptation method was effective. The method objectively supplied an eventual alteration in the quality and quantity of saliva, using a toothpaste with the lactoperoxidase enzymes lysozyme and glucoseoxidase and the lactoferrin protein bactericides, which in normal conditions are produced by the salivary glands, in association with a mouth solution containing chlorhexidine 0.12%, without ethanol. This approach is in harmony with Karthaus et al,7 who reported positivity for Streptococcus viridans in the blood cultures of 70% of the patients with severe OM, and with Barker17 and Meurman et al,27 who referred to the possibility of a reduction of the quantity and quality of the saliva. Chlorhexidine (0.12%) has been regarded as a powerful antibacterial.19–21
It should not be overlooked that our study was not blinded to one of the evaluators. This may have been balanced by the 3 additional evaluators who were not aware which treatment arm the patients had been allocated to, and mainly by the fact that the method adopted for the evaluators' calibration proved to be efficient, with a concordance of 81.7% among them. Another caveat of the present study is the fact that it does not address the question of whether the high efficacy of the strategy adopted was due to the higher energy density, the length of its application, or the combination of both. In addition, this study did not assess a potential impact of LPLT on graft-versus-host disease (GVHD), the number of days on antibiotics, use of total parenteral nutrition, and hospital confinement time. Additional studies with these endpoints as well as quality-of-life and cost-effectiveness analyses are warranted and should be pursued in future studies with preventive LPLT. Another endpoint that deserves further study is survival. Although a marginal difference was observed in our study favoring the LPLT group, these data should be analyzed with caution because our trial was not designed to detect these differences and confounding factors may hamper the analyses.
In conclusion, our results indicate that the upfront use of LPLT in HSCT patients is a powerful instrument in reducing the incidence of OM, and this practice is now standard in our center.
Authorship
Contribution: H.S.A. designed and performed research, analyzed data, wrote the paper, and gave final approval of the manuscript; A.M.d.A. designed research and gave final approval of the manuscript; L.F.d.S.B. designed research, provided administrative support, and gave final approval of the manuscript; C.A.E.A., C.T.P., R.M., L.H.P., R.A., and V.D'A.d.M. performed research; P.C.R. and I.A.S. analyzed data; R.A.Z. and C.G.F. designed research, analyzed data, wrote the paper, and gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Héliton Spíndola Antunes, Seção de Odontologia, Serviço de Pesquisa Clínica, Instituto Nacional de Câncer (INCA), Rua André Cavalcante 37, 2° andar, Rio de Janeiro, Brazil 20231-050; e-mail: hspindola@inca.gov.br.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



![Figure 4. Mucositis incidence, OMAS scale. P = .007. (WA = 2.5 × [(Σui : 3 ×Nu) + (Σei : 2 × Ne)], in which Σui is the sum of the ulcerous area, Nu equals the number of ulcerous areas, Σei is the sum of the erythema's intensity, and Ne is the number of areas with erythemas.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/5/10.1182_blood-2006-07-035022/4/m_zh80050708850004.jpeg?Expires=1765917574&Signature=qEYtA26DveDXIiFVqhYN93rXkjemdi2enKGvI0Xy5tSMorOHWusiUYB5PS~oBLQbTyBymqnuUfNpSEQIdEi3W3JnCLKIiaQTwUyFROXRPT1q5tYHfsaDqDLTvo-MP6nzNgiWKCHMaEfxqK-4n1Ut7xUroh70M90Gaspof3Af94Vt~vs5OoVN96kkASgwnYZEsfhIOwmavxjnbi~iyjZaGvN8UDVGSLQysSX5f0R0~H7kAmmPlRr2boljy49nbhv4EDP-QSjS31RUzEXsyJbVgXrdLTq8L7TrrtVAcZspfdbdIn9d~69XNTbcVYkng3U7Bg-xdQ2vCX9UJ51YthJwWA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal