Abstract
The aberrant function of transcription factors and/or kinase-based signaling pathways that regulate the ability of hematopoietic cells to proliferate, differentiate, and escape apoptosis accounts for the leukemic transformation of myeloid progenitors. Here, we demonstrate that simultaneous retinoid receptor ligation and blockade of the MEK/ERK signaling module, using the small-molecule inhibitor CI-1040, result in a strikingly synergistic induction of apoptosis in both acute myeloid leukemia (AML) and acute promyelocytic leukemia (APL) cells with constitutive ERK activation. This proapoptotic synergism requires functional RAR and RXR retinoid receptors, as demonstrated using RAR- and RXR-selective ligands and RAR-defective cells. In the presence of MEK inhibitors, however, retinoid-induced chromatin remodeling, target-gene transcription, and granulocytic differentiation are strikingly inhibited and apoptosis induction becomes independent of death-inducing ligand/receptor pairs; this suggests that apoptosis induction by combined retinoids and MEK inhibitors is entirely distinct from the classical “postmaturation” apoptosis induced by retinoids alone. Finally, we identify disruption of Bcl-2–dependent mitochondrial homeostasis as a possible point of convergence for the proapoptotic synergism observed with retinoids and MEK inhibitors. Taken together, these results indicate that combined retinoid treatment and MEK blockade exert powerful antileukemic effects and could be developed into a novel therapeutic strategy for both AML and APL.
Introduction
Acute myeloid leukemia (AML) is a deadly disease, resulting from the clonal expansion and accumulation of hematopoietic stem cells arrested at various stages of development.1 Genetic aberrations that disrupt the function of hematopoietic transcription factors, and/or turn kinase-based signaling pathways constitutively on, play a central role in leukemogenesis and account for the inability of leukemic cells to undergo growth arrest, terminal differentiation, and apoptosis in response to appropriate environmental stimuli.2
Retinoids are natural and synthetic derivatives of vitamin A that, together with their nuclear retinoic acid and retinoid-X receptors (RAR and RXR, respectively), control genetic programs that are essential for embryonic development, organogenesis, organ homeostasis, cell growth, differentiation, and apoptosis.3,4 The pleiotropic actions of retinoids, particularly their ability to force cells into differentiation and death, offer great promise for cancer therapy and prevention.5 From a clinical standpoint, this has been most impressively shown in acute promyelocytic leukemia (APL), a myeloid leukemia (FAB M3/M3v) originating in 98% of cases by the t(15;17)(q22;q21) chromosomal translocation, which generates the PML-RARα fusion protein that is necessary and sufficient to cause the disease.6 Indeed, the introduction of all-trans retinoic acid (ATRA)–based therapeutic strategies has led to our current ability to cure more than 70% of APL patients, thus providing a proof-of-principle that the transcription factor path to leukemia can be successfully targeted with therapeutic intent.7–9 From a molecular standpoint, the presence of a PML-RARα fusion protein results in an enhanced recruitment of histone deacetylase complexes that epigenetically silence cognate gene programs, as well as in a variety of signaling aberrations that affect blast survival and self-renewal of stem cells.5,6,10–13 In this context, supraphysiologic (pharmacologic) ATRA levels induce dissociation of the silencing complex, activate differentiation programs, and trigger maturation-dependent, tumor-selective, death-signaling pathways.5,14,15 Unfortunately, despite their ability to induce a certain degree of growth inhibition, differentiation, Bcl-2 down-regulation, and chemosensitization even in non-M3 AML cells,16–19 retinoids, either alone or combined with standard chemotherapeutic drugs, have had no clinical impact in AML subtypes that do not carry the t(15;17) translocation.20,21
In recent years compelling evidence has accumulated, indicating that, in addition to transcription factor fusion proteins, aberrant activation of the kinase-based signal transduction pathways that normally translate extracellular stimuli into appropriate homeostatic responses can powerfully contribute to leukemogenesis by enabling leukemic cells to grow autonomously and escape programmed cell death.22,23 The mitogen-activated protein kinase (MAPK) pathway that proceeds from Ras, and its downstream effector Raf to MAPK kinase (MEK) and extracellular-signal activated kinase (ERK, p42/44MAPK), is a key integration point along the signal transduction cascades that emanate from receptor- and/or fusion protein–associated tyrosine kinases and links diverse extracellular stimuli to proliferation, differentiation, and survival.24–26 Several groups, including ours, have recently provided substantial evidence that the MEK/ERK signaling module is frequently deregulated in myeloid leukemias, as a result of genetic and epigenetic aberrations involving both receptor-associated and cytoplasmic tyrosine kinases, as well as inhibitory phosphatases.27–30 Not only is MAPK constitutively active in blast cells of the majority of AML patients, but its pharmacologic blockade by selective small-molecule inhibitors profoundly impairs leukemic, but not normal, cell proliferation and clonogenic growth and synergizes with a variety of cytotoxics and novel biologic agents in inducing apoptosis.27,29,31,32 These properties make the MEK/ERK signaling module a prime target for the molecular therapy of AML and support the preclinical/clinical development of MEK inhibitors as therapeutic agents in this disease.29,30,32,33 However, despite their ability to lower the apoptotic threshold, MEK inhibitors exert predominantly cytostatic, rather than cytotoxic, effects in preclinical models of AML.27,31
Mounting evidence indicates that MAPK activity is required to trigger retinoid-induced myeloid differentiation programs,16,17,34 and modulates the phosphorylation and function of retinoid receptors,35 suggesting that these 2 pathways are deeply interconnected; in addition, we have recently shown that MEK blockade sensitizes AML and APL cells to the proapoptotic action of retinoids.27 In the present study, we demonstrate that the proapoptotic interaction between small-molecule MEK inhibitors (CI-1040 and PD98059) and retinoids is strongly synergistic, requires functional RAR and RXR receptors, is not dependent upon retinoid-mediated induction of differentiation, and appears to involve disruption of Bcl-2–mediated maintenance of mitochondrial homeostasis.
Materials and methods
Cell cultures and reagents
AML and APL cell lines (OCI-AML2, OCI-AML3, HL-60, NB4, U937, KG1) were cultured under standard conditions27,36 and harvested in log-phase growth for every experiment. Retinoid-resistant HL-60 cells (HL-60R) and their RARα- and RXRα-overexpressing derivatives have been previously described.37–39 PD184352 (2-[2-chloro-4-iodo-phenilamino]-N-cyclopropylmethoxy-3,4-difluoro-benzamide, also known and hereafter referred to as CI-1040), a highly selective inhibitor of MEK activation,40 was kindly provided by Dr J. S. Sebolt-Leopold (Cancer Molecular Sciences, Pfizer Global Research & Development, Ann Arbor, MI). The novel triterpenoid CDDO (2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid) was synthesized by Dr Takashi Honda et al (Dartmouth Medical College, Hanover, NH41 ) and kindly provided through the Rapid Access to Intervention Development mechanism by Dr Edward Sausville (National Cancer Institute/Cancer Therapy Evaluation Program, Bethesda, MD). The RXR-selective ligand LG10026842 was kindly provided by Dr Reid Bissonnette (Ligand Pharmaceuticals, San Diego, CA). PD98059, ATRA, 9-cis RA, TTNPB (4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphtalenyl)-1-propenyl]benzoic acid), and methoprene acid were purchased from Sigma Chemical (St Louis, MO).
For combination experiments, cell lines were plated at the concentration of 1 × 105/mL and incubated with MEK inhibitors for 30 minutes at 37°C prior to the addition of retinoids, unless otherwise stated. Cell viability was evaluated by triplicate counting of trypan blue dye–excluding cells under a light microscope or using the Vialight Plus assay kit (Cambrex, East Rutherford, NJ) according to the manufacturer's instructions.
Apoptosis analysis
For annexin V binding studies, cells were washed twice with binding buffer (10 mM HEPES, 140 mM NaCl, and 5 mM CaCl2 at pH 7.4; Sigma Chemical) and incubated with a 1:500 solution of FITC-conjugated annexin V (Roche Diagnostic, Indianapolis, IN) for 15 minutes at room temperature. Stained cells were analyzed by flow cytometry using a FACScan flow cytometer (Becton Dickinson, San Jose, CA), while simultaneously assessing membrane integrity by propidium iodide (PI) exclusion. Other measures of apoptosis (mitochondrial transmembrane potential, caspase activation, and decrease of DNA content to sub-G1 levels) were carried out as previously described.27,31
Flow cytometric detection of surface and intracellular protein expression and ROS production
The expression of differentiation-specific cell-surface antigens was measured by flow cytometry, as previously described.43 The PE-conjugated anti-CD11b and FITC-conjugated anti-CD38 monoclonal antibodies (Becton Dickinson) were used at a 1:10 dilution. A matched isotype control was used as a measure of background fluorescence.
For the analysis of intracellular Bcl-2 expression, treated and untreated cells were fixed and permeabilized using the Ortho Permeafix reagent (Ortho Biotech, Milan, Italy). Cell suspensions were then centrifuged and washed once with PBS containing 4% bovine serum albumin (BSA) and then labeled with a FITC-conjugated anti–human Bcl-2 mAb (clone 124; Dako, Glostrup, Denmark) for 30 minutes at 4°C. An irrelevant mouse isotype-matched Ig was used as a negative control to determine background fluorescence.
For the analysis of ROS content, cells were incubated with 4 μM dihydroethidium (DHE; Molecular Probes, Eugene, OR) for 45 minutes at 37°C. After incubation, the cells were analyzed by flow cytometry. ROS production was evaluated at different time points after treatment, as indicated. OCI-AML3 cells were also treated with 15 mM H2O2 for 1 hour, as an internal ROS control. Untreated and H2O2-treated viable cells were then analyzed for ROS production.
Western blot analysis
Quantitative real-time reverse transcriptase–polymerase chain reaction (RT-PCR)
RNA was isolated with TRIzol solution (Life Technologies, Grand Island, NY). Total RNA (1 μg) was reverse transcribed by avian myeloblastosis virus (AMV) reverse transcriptase (Roche Diagnostic) at 42°C for 1 hour. PCR amplification reaction mixtures (25 μL) contained cDNA (RARβ forward primer: 5′-TGCTTCAGTGGATTGACCCA-3′, reverse primer: 5′-CTGGTGCTCTGTGTTTCAATTGA-3′, probe: 5′-CGAATGGCAGCATCGGCACACT-3′) and TaqMan Universal PCR Master Mix (PE Applied Biosystems, Foster City, CA). β2-Microglobulin (β2-m) coamplified with RARβ was included as internal control for normalization of the variable content of cDNA in each sample (forward primer: 5′-AGCTGTGCTCGCGCTACTCT-3′, reverse primer: 5′-TTGACTTTCCATTCTCTGCTGG-3′, probe: 5′-TCTTTCTGGCCTGGAGGGCATCC-3′). Thermal cycle conditions included holding the reactions at 50°C for 2 minutes and at 95°C for 10 minutes, and cycling for 40 cycles between 95°C for 15 seconds and 60°C for 1 minute. Results were collected and analyzed with an ABI Prism 7700 Sequence Detection System (PE Applied Biosystems) as previously described.27
Statistical analysis
Comparisons between different treatment groups were performed using a 2-tailed Student t test for paired samples. Synergism, additive effects, and antagonism were assessed using the Chou-Talalay method44 and Calcusyn software (Biosoft, Ferguson, MO), as previously described.31,45 Briefly, the dose-effect curve for each drug alone was determined based on the experimental observations using the median-effect principle; the combination index (CI) for each experimental combination was then calculated. When CI = 1, the equation used represents the conservation isobologram and indicates additive effects. CI values of less than 0.9 indicate more than expected additive effect (synergism).
Results
MEK inhibitors and retinoids synergistically induce apoptosis in AML cell lines with constitutive MAPK activation
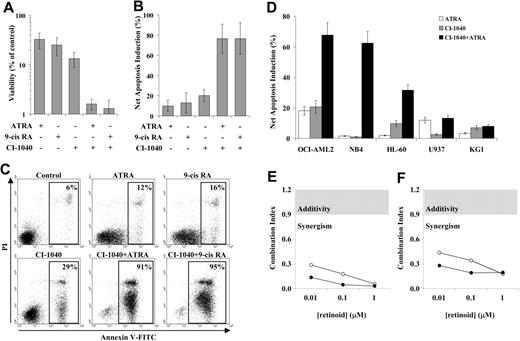
In order to analyze the proapoptotic interaction between MEK inhibitors and retinoids, the AML cell line OCI-AML3, which displays intense constitutive activation of the MEK/ERK MAPK module,27,31 was pre-exposed to the MEK inhibitor CI-1040 (0.5 μM) and subsequently incubated with either ATRA (1 μM) or 9-cis RA (0.1 μM). Under these conditions, 0.5 μM CI-1040 almost completely inhibited both constitutive and ATRA-stimulated ERK phosphorylation (data not shown). After 96 hours, viability in the combined treatment groups was strikingly reduced compared with either CI-1040 (P ≤ .02) or ATRA and 9-cis RA (P = .001 and P < .05, respectively) single treatments, as assessed by trypan blue exclusion counting (data not shown) and ATP measurement (Figure 1A). Consistent with this reduction in viability, combination treatment with CI-1040 and either ATRA or 9-cis RA induced massive apoptosis, as demonstrated by phosphatidylserine exposure on the outer leaflet of the plasma membrane and consequent annexin V binding in up to 96% of the cells (Figure 1B-C). Annexin V binding was accompanied by other markers of apoptosis, such as reduction in DNA content to sub-G1 levels (data not shown), and was significantly higher in the combined treatment groups, compared with either CI-1040 or ATRA/9-cis RA single treatments (P < .001, Figure 1B-C). Virtually identical results were obtained when MEK inhibition was achieved using a different small-molecule inhibitor at appropriate concentrations (25 μM PD98059, data not shown). A similar proapoptotic interaction between CI-1040 and retinoids was observed in other AML (OCI-AML2, HL-60) and APL (NB4) cell lines with constitutive activation of the MEK/ERK MAPK module, but not in AML cell lines (U937, KG1) in which this signaling module is not constitutively activated (Figure 1D).
MEK inhibitors and retinoids synergistically inhibit the growth and induce apoptosis in AML cell lines with constitutive MAPK activation. OCI-AML3 cells were pretreated with the MEK inhibitor CI-1040 (0.5 μM) for 30 minutes and subsequently exposed to either ATRA (1 μM) or 9-cis RA (0.1 μM) for 96 hours. (A) Viability was then assessed by measuring the intracellular ATP content using the Vialight assay. Results are expressed as percentage of viable cells relative to vehicle control–treated cells and represent the average ± SD of 6 independent experiments (for the comparison between CI-1040 and combination treatments, P ≤ .02; for the comparison between ATRA or 9-cis RA and combination treatments, P ≤ .05). (B) Apoptosis was evaluated by flow cytometric analysis of FITC-conjugated annexin V binding, while simultaneously assessing membrane integrity by PI exclusion. Results are expressed as the net apoptosis induction (percentage of apoptosis in treated cells minus percentage of apoptosis in control cells) and represent the average ± SD of 9 independent experiments (for the comparison between CI-1040 and combination treatments, P < .001; for the comparison between ATRA or 9-cis RA and combination treatments, P < .001). (C) Primary data from one representative experiment. Annexin V–positive cells are highlighted in the box, and their percentage is shown in the figure. (D) AML cell lines with (OCI-AML2, NB4, HL-60) or without (U937, KG1) constitutive MAPK activation were pretreated with the MEK inhibitor CI-1040 (0.5 μM) for 30 minutes and subsequently exposed to either ATRA (1 μM) or 9-cis RA (0.1 μM) for 96 hours. Apoptosis was then evaluated as described for panel B. Results are expressed as the net apoptosis induction and represent the average ± SD of at least 3 independent experiments for each cell line. Synergism analysis for (E) viability reduction and (F) apoptosis induction was carried out using a fixed dose of CI-1040 (0.5 μM) and escalating doses of ATRA (0.1-1 μM, ○) and 9-cis RA (0.1-1 μM, •);CI values were then derived using the Chou-Talalay method44 and are plotted against retinoid concentrations. The gray area indicates additive effects (CI = 0.9-1.2), while synergism and antagonism fall below and above the area, respectively.
MEK inhibitors and retinoids synergistically inhibit the growth and induce apoptosis in AML cell lines with constitutive MAPK activation. OCI-AML3 cells were pretreated with the MEK inhibitor CI-1040 (0.5 μM) for 30 minutes and subsequently exposed to either ATRA (1 μM) or 9-cis RA (0.1 μM) for 96 hours. (A) Viability was then assessed by measuring the intracellular ATP content using the Vialight assay. Results are expressed as percentage of viable cells relative to vehicle control–treated cells and represent the average ± SD of 6 independent experiments (for the comparison between CI-1040 and combination treatments, P ≤ .02; for the comparison between ATRA or 9-cis RA and combination treatments, P ≤ .05). (B) Apoptosis was evaluated by flow cytometric analysis of FITC-conjugated annexin V binding, while simultaneously assessing membrane integrity by PI exclusion. Results are expressed as the net apoptosis induction (percentage of apoptosis in treated cells minus percentage of apoptosis in control cells) and represent the average ± SD of 9 independent experiments (for the comparison between CI-1040 and combination treatments, P < .001; for the comparison between ATRA or 9-cis RA and combination treatments, P < .001). (C) Primary data from one representative experiment. Annexin V–positive cells are highlighted in the box, and their percentage is shown in the figure. (D) AML cell lines with (OCI-AML2, NB4, HL-60) or without (U937, KG1) constitutive MAPK activation were pretreated with the MEK inhibitor CI-1040 (0.5 μM) for 30 minutes and subsequently exposed to either ATRA (1 μM) or 9-cis RA (0.1 μM) for 96 hours. Apoptosis was then evaluated as described for panel B. Results are expressed as the net apoptosis induction and represent the average ± SD of at least 3 independent experiments for each cell line. Synergism analysis for (E) viability reduction and (F) apoptosis induction was carried out using a fixed dose of CI-1040 (0.5 μM) and escalating doses of ATRA (0.1-1 μM, ○) and 9-cis RA (0.1-1 μM, •);CI values were then derived using the Chou-Talalay method44 and are plotted against retinoid concentrations. The gray area indicates additive effects (CI = 0.9-1.2), while synergism and antagonism fall below and above the area, respectively.
Pharmacologic interactions between CI-1040 and retinoids were analyzed in further detail by conservative isobologram analysis. For this purpose, OCI-AML3 cells were exposed to a wide range of CI-1040 (0.0625-2 μM) and retinoid (0.01-1 μM for both ATRA and 9-cis RA) concentrations, either alone or in combination, and CI values for both viability reduction and apoptosis induction were then derived using the Chou-Talalay method.44 As shown in Figure 1E-F, isobologram analysis formally demonstrated that the interaction between CI-1040 (0.5 μM) and either ATRA or 9-cis RA is highly synergistic for both viability reduction (CI < 0.3) and apoptosis induction (CI < 0.5) at all retinoid concentrations tested.
Overall, these results indicate that MEK blockade enhances apoptosis induction by retinoids in a highly synergistic fashion in AML cell lines with constitutive activation of the MAPK pathway, regardless of the presence of a PML-RARα fusion protein.
Involvement of RA receptors in the proapoptotic synergism between MEK inhibitors and retinoids
Retinoids exert their pleiotropic effects through interaction with 2 families of nuclear receptors, RAR and RXR. These receptors may homodimerize or heterodimerize and either repress or activate transcription of specific genes, depending on ligand concentration and the consequent recruitment of corepressors/coactivators to the transcriptional complex.5 We therefore analyzed the role of individual retinoid receptors in the described proapoptotic synergism between MEK inhibitors and retinoids.
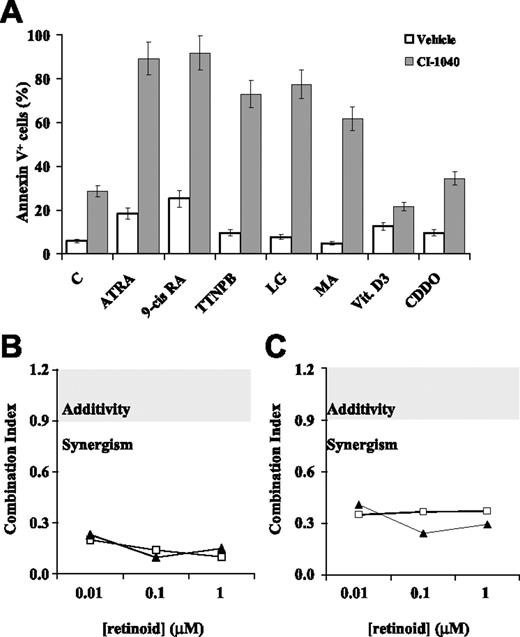
For this purpose, our model AML cell line OCI-AML3 was pretreated with CI-1040 (0.5 μM) and then exposed to panretinoid receptor (ATRA and 9-cis RA), RAR-selective (TTNPB), or RXR-selective (LG100268 and methoprene acid) ligands for 96 hours. As shown in Figure 2A, both RAR-selective and RXR-selective ligands had little, if any, proapoptotic effect when used alone, but induced extensive apoptosis when combined with the MEK inhibitor CI-1040. Superimposable results were obtained when the experiment was conducted in low (0.5%-1%) serum conditions, in an attempt to unmask specific rexinoid-dependent proapoptotic signaling46 (data not shown). Conversely, no proapoptotic cooperation was observed between CI-1040 and either vitamin D3 or the novel triterpenoid CDDO,43,47 which act as ligands for other RXR heterodimerization partners (vitamin D3 receptor and peroxisome proliferator-activated receptor-γ, respectively; Figure 2A). Similar results, in terms of proapoptotic interaction between RAR- or RXR-selective ligands and CI-1040, were also obtained in other AML cell lines with constitutive MAPK activation (OCI-AML2 and HL-60, data not shown), as well as with a different MEK inhibitor (PD98059, data not shown). As previously shown for ATRA and 9-cis RA (Figure 1E-F), isobologram analysis formally demonstrated that, in OCI-AML3 cells, the proapoptotic interaction between RAR- or RXR-selective ligands (TTNPB and LG100268, respectively) and CI-1040 is highly synergistic, with CI values of 0.2 or less and 0.4 or less for viability reduction and apoptosis induction, respectively (Figure 2B-C).
Proapoptotic effects of CI-1040 in combination with RAR- and RXR-selective ligands. (A) OCI-AML3 cells were pretreated with either vehicle (DMSO, □) or the MEK inhibitor CI-1040 (0.5 μM, ⊡) for 30 minutes and subsequently exposed to the following ligands for 96 hours: ATRA (1 μM), 9-cis RA (0.1 μM), TTNPB (1 μM), LG100268 (LG, 1 μM), methoprene acid (MA, 50 μM), 1,25 (OH)2 vitamin D3 (vit D3, 0.1 μM), or CDDO (0.3 μM). Apoptosis was evaluated by flow cytometric analysis of FITC-conjugated annexin V binding, while simultaneously assessing membrane integrity by PI exclusion. Results are expressed as the percentage of annexin V–positive cells and represent the average ± SD of at least 3 independent experiments. Synergism analysis for (B) viability reduction and (C) apoptosis induction was carried out using a fixed dose of CI-1040 (0.5 μM) and escalating doses of TTNPB (0.1-1 μM, □) and LG100268 (0.1-1 μM, ▴); CI values were then derived using the Chou-Talalay method44 and are plotted against ligand concentrations. The gray area indicates additive effects (CI = 0.9-1.2), while synergism and antagonism fall below and above the area, respectively.
Proapoptotic effects of CI-1040 in combination with RAR- and RXR-selective ligands. (A) OCI-AML3 cells were pretreated with either vehicle (DMSO, □) or the MEK inhibitor CI-1040 (0.5 μM, ⊡) for 30 minutes and subsequently exposed to the following ligands for 96 hours: ATRA (1 μM), 9-cis RA (0.1 μM), TTNPB (1 μM), LG100268 (LG, 1 μM), methoprene acid (MA, 50 μM), 1,25 (OH)2 vitamin D3 (vit D3, 0.1 μM), or CDDO (0.3 μM). Apoptosis was evaluated by flow cytometric analysis of FITC-conjugated annexin V binding, while simultaneously assessing membrane integrity by PI exclusion. Results are expressed as the percentage of annexin V–positive cells and represent the average ± SD of at least 3 independent experiments. Synergism analysis for (B) viability reduction and (C) apoptosis induction was carried out using a fixed dose of CI-1040 (0.5 μM) and escalating doses of TTNPB (0.1-1 μM, □) and LG100268 (0.1-1 μM, ▴); CI values were then derived using the Chou-Talalay method44 and are plotted against ligand concentrations. The gray area indicates additive effects (CI = 0.9-1.2), while synergism and antagonism fall below and above the area, respectively.
To further investigate the relative contribution of RAR and RXR receptors to the observed proapoptotic synergism between MEK inhibitors and retinoids, we took advantage of the availability of a retinoid-resistant clone of the HL-60 cell line (HL-60R). HL-60R cells carry a point mutation (C→T) at codon 411 of the RARα ligand-binding domain, which creates a termination signal and results in the production of a truncated, nonfunctional protein that acts in a dominant-negative fashion37,38 ; RA-driven transcriptional programs can be restored in this model by overexpressing functional RARα or RXRα receptors through retrovirally-mediated stable gene transfer.39 As shown in Figure 3A-B, apoptosis induction by the combination of CI-1040 and retinoids (ATRA and 9-cis RA) was completely abrogated in the HL-60R cell line; RXRα overexpression not only restored, but actually further increased, the proapoptotic interaction between CI-1040 and either ATRA or 9-cis RA, compared with the effects observed in the parental HL-60 cell line, resulting in the massive (> 90%) induction of apoptosis with the combined treatment (Figure 3C). Of interest, overexpression of a wild-type RARα receptor rendered transfected cells hypersensitive to the proapoptotic effect of CI-1040, shifting its IC50 for cell growth and ED50 for apoptosis induction from 0.16 μM and 0.33 μM, respectively, in HL-60R to 0.03 μM in RARα-overexpressing HL-60R (Figure 3E-F); this, in turn, prevented an accurate evaluation of the proapoptotic interaction of CI-1040 with either ATRA or 9-cis RA (Figure 3D). Experiments conducted in low-serum (0.5%-1%) conditions or using charcoal-stripped FCS, in order to avoid the possible interference of naturally occurring retinoids, gave virtually identical results (data not shown).
Proapoptotic effects of CI-1040 in combination with retinoids in retinoid-resistant HL-60R cells and their RARα- and RXRα-overexpressing counterparts. (A) Wild-type HL-60 cells, (B) retinoid-resistant HL-60 (HL-60R), (C) RXRα-overexpressing HL-60R, and (D) RARα-overexpressing HL-60R cells were pretreated with either vehicle (DMSO, □) or the MEK inhibitor CI-1040 (0.5 μM, ⊡) for 30 minutes and subsequently exposed to either ATRA (1 μM) or 9-cis RA (0.1 μM) for 96 hours. Apoptosis was then evaluated by flow cytometric analysis of FITC-conjugated annexin V binding, while simultaneously assessing membrane integrity by PI exclusion. Results are expressed as the percentage of annexin V–positive cells and represent the average ± SD of at least 3 independent experiments for each cell line. (E-F) HL-60R (○) and RARα-overexpressing HL-60R cells (•) were exposed to escalating concentrations of CI-1040 (0.03-1 μM) for 96 hours. (E) Viability was then assessed by measuring the intracellular ATP content using the Vialight assay. Results are expressed as percentage of viable cells relative to vehicle control–treated cells and represent the average ± SD of 3 independent experiments. (F) Apoptosis was evaluated by flow cytometric analysis of annexin V binding, as described for panels A-D. Results are expressed as the percentage of annexin V–positive cells and represent the average ± SD of at least 3 independent experiments.
Proapoptotic effects of CI-1040 in combination with retinoids in retinoid-resistant HL-60R cells and their RARα- and RXRα-overexpressing counterparts. (A) Wild-type HL-60 cells, (B) retinoid-resistant HL-60 (HL-60R), (C) RXRα-overexpressing HL-60R, and (D) RARα-overexpressing HL-60R cells were pretreated with either vehicle (DMSO, □) or the MEK inhibitor CI-1040 (0.5 μM, ⊡) for 30 minutes and subsequently exposed to either ATRA (1 μM) or 9-cis RA (0.1 μM) for 96 hours. Apoptosis was then evaluated by flow cytometric analysis of FITC-conjugated annexin V binding, while simultaneously assessing membrane integrity by PI exclusion. Results are expressed as the percentage of annexin V–positive cells and represent the average ± SD of at least 3 independent experiments for each cell line. (E-F) HL-60R (○) and RARα-overexpressing HL-60R cells (•) were exposed to escalating concentrations of CI-1040 (0.03-1 μM) for 96 hours. (E) Viability was then assessed by measuring the intracellular ATP content using the Vialight assay. Results are expressed as percentage of viable cells relative to vehicle control–treated cells and represent the average ± SD of 3 independent experiments. (F) Apoptosis was evaluated by flow cytometric analysis of annexin V binding, as described for panels A-D. Results are expressed as the percentage of annexin V–positive cells and represent the average ± SD of at least 3 independent experiments.
Overall, these data indicate that both RAR and RXR families of retinoid receptors are causally involved in the observed proapoptotic synergism between MEK inhibitors and retinoids.
MEK blockade inhibits ATRA-induced gene expression and differentiation in both AML and APL cells
Retinoid-dependent MAPK activation has been reported to be indispensable for retinoid-induced differentiation of AML cells.16,17 In order to investigate whether induction of differentiation plays a role in the observed proapoptotic synergism, we analyzed induction of differentiation, histone posttranslational modifications, and expression of retinoid-dependent genes, such as RARβ48,49 and CD38,39 in response to combined MEK inhibitor and retinoid treatment. In both OCI-AML3 and NB4 cells, treatment with ATRA induced granulocytic differentiation, as evaluated by increased CD11b membrane expression (Figure 4A-B), morphology, and NBT reduction capacity (data not shown); in both cellular models, MEK inhibition by CI-1040 consistently inhibited retinoid-induced differentiation (Figure 4A-B), in agreement with previous findings in different cellular models.16,17 Similar results were obtained in OCI-AML2 and HL-60 cell lines, and by using a different MEK inhibitor (PD98059) (data not shown). Retinoids have been proposed to affect differentiation through ligand-dependent changes in retinoid receptor–associated transcriptional complexes, which in turn cause chromatin structure modifications and specific differentiation-related gene transcription5 ; consistent with this model, CI-1040–mediated inhibition of ATRA-induced differentiation was accompanied by inhibition of both basal and ATRA-induced histone H3 phosphorylation and acetylation in both OCI-AML3 and NB4 (Figure 4C), resulting in a significant decrease in ATRA-induced expression of retinoid receptor–dependent genes, such as RARβ (as evaluated by real-time RT-PCR assessment of relative mRNA abundance, Figure 4D) and CD38 (as evaluated by flow cytometric analysis of CD38 membrane expression, Figure 4E).
Effects of CI-1040 in combination with ATRA on differentiation, histone posttranslational modifications, and expression of retinoid-responsive genes. (A) OCI-AML3 and (B) NB4 cells were pretreated with CI-1040 (0.5 μM) for 30 minutes and then exposed to ATRA (1 μM). CD11b membrane expression was then assessed by cytofluorimetric analysis after 48 hours. The red histogram refers to a matched IgG control; the black, green, and blue profiles refer to CD11b expression in vehicle control–, ATRA-, and CI-1040 + ATRA–treated cells, respectively. CD11b expression profile in CI-1040–treated cells was superimposable to vehicle control–treated cells and therefore omitted for clarity. One experiment representative of 3 performed with superimposable results is shown. (C) Histone H3 phosphorylation and acetylation status was assessed by Western blot analysis of nuclear extracts prepared after 24 hours of exposure to the indicated treatments, using phosphorylation- or acetylation-specific Abs (p-H3 and Ac-H3, respectively). Coomassie blue staining of the blotting membranes is shown as protein loading control (LC). One experiment representative of 3 performed with superimposable results is shown. (D) Relative RARβ transcript expression was assessed by quantitative real-time PCR, using specific primers, after 24 hours of exposure to the indicated treatments. Results are expressed as RARβ transcript fold induction relative to vehicle-treated control cells and represent the average ± SD of 3 independent experiments. (E) CD38 membrane expression was assessed by cytofluorimetric analysis in OCI-AML3 and NB4 cells, 48 hours after exposure to CI-1040 (0.5 μM) and ATRA (1 μM), either alone or in combination. Results are expressed as mean fluorescence index (MFI) and represent the average ± SD of 3 independent experiments.
Effects of CI-1040 in combination with ATRA on differentiation, histone posttranslational modifications, and expression of retinoid-responsive genes. (A) OCI-AML3 and (B) NB4 cells were pretreated with CI-1040 (0.5 μM) for 30 minutes and then exposed to ATRA (1 μM). CD11b membrane expression was then assessed by cytofluorimetric analysis after 48 hours. The red histogram refers to a matched IgG control; the black, green, and blue profiles refer to CD11b expression in vehicle control–, ATRA-, and CI-1040 + ATRA–treated cells, respectively. CD11b expression profile in CI-1040–treated cells was superimposable to vehicle control–treated cells and therefore omitted for clarity. One experiment representative of 3 performed with superimposable results is shown. (C) Histone H3 phosphorylation and acetylation status was assessed by Western blot analysis of nuclear extracts prepared after 24 hours of exposure to the indicated treatments, using phosphorylation- or acetylation-specific Abs (p-H3 and Ac-H3, respectively). Coomassie blue staining of the blotting membranes is shown as protein loading control (LC). One experiment representative of 3 performed with superimposable results is shown. (D) Relative RARβ transcript expression was assessed by quantitative real-time PCR, using specific primers, after 24 hours of exposure to the indicated treatments. Results are expressed as RARβ transcript fold induction relative to vehicle-treated control cells and represent the average ± SD of 3 independent experiments. (E) CD38 membrane expression was assessed by cytofluorimetric analysis in OCI-AML3 and NB4 cells, 48 hours after exposure to CI-1040 (0.5 μM) and ATRA (1 μM), either alone or in combination. Results are expressed as mean fluorescence index (MFI) and represent the average ± SD of 3 independent experiments.
Overall, these data confirm previous findings indicating that MEK blockade inhibits retinoid-induced differentiation of AML/APL cells and suggest that interference with chromatin remodeling and specific gene transcription may be causally related to the observed inhibition of differentiation. In addition, apoptosis induced by the combination of CI-1040 and ATRA did not appear to involve signaling through death-inducing ligand/receptor pairs, in that it was not blocked by either a TRAIL-R2:Fc chimera or a blocking antibody directed against Fas-L (data not shown). These results strengthen the hypothesis that the proapoptotic synergism between MEK inhibitors and retinoids relies on molecular mechanisms strikingly different from those required for retinoid-induced “postmaturation” apoptosis.
Combined MEK blockade and retinoid treatment results in increased ROS accumulation
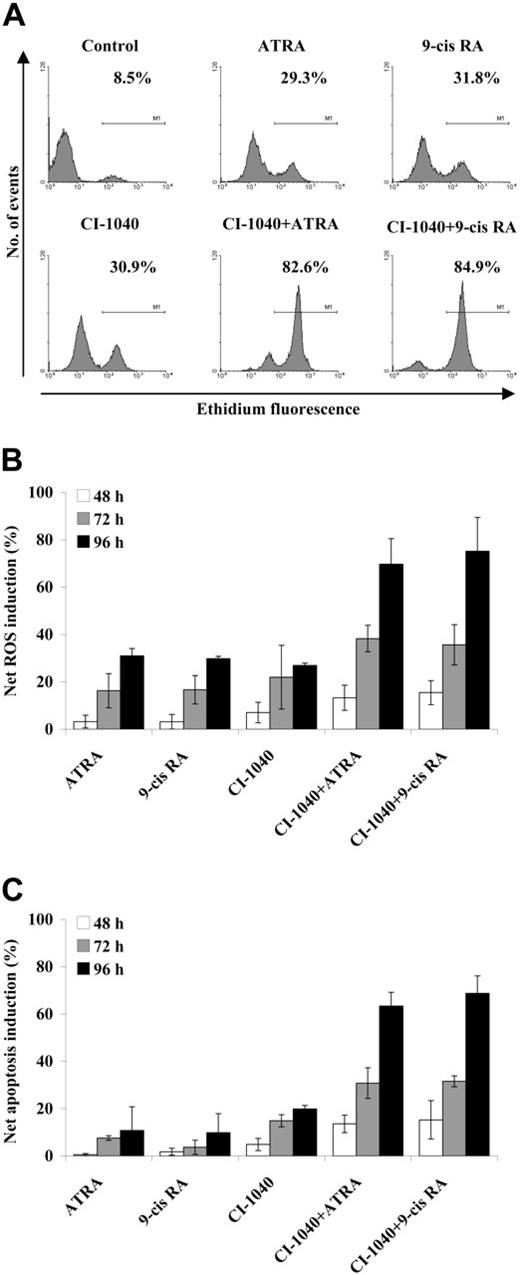
Intracellular accumulation of ROSs may contribute to the proapoptotic activity of retinoids in several cell systems, including leukemic cells.50 Moreover, recent evidence indicates that combined inhibition of kinase-based signaling pathways (such as trastuzumab-mediated inhibition of HER-2 signaling) and retinoid treatment (using either ATRA or fenretinide) results in the additive/synergistic potentiation of ROS accumulation and apoptosis induction in breast cancer models.51 We therefore investigated the ability of combined MEK blockade and ATRA/9-cis RA treatment to modulate ROS accumulation in OCI-AML3 cells. As shown in Figure 5A, CI-1040 (0.5 μM) and ATRA/9-cis RA (1 μM and 0.1 μM, respectively) as individual treatments both induced a modest increase in the proportion of cells oxidizing DHE to the fluorescent product ethidium (a reaction that preferentially detects O2−), while their combination resulted in a strikingly increased accumulation of ROSs in up to 85% of cells (P < .005 and P < .001 for the comparison between combination treatments and CI-1040 or ATRA/9-cis RA single treatments, respectively, at the 96-hour time point). Intracellular ROS accumulation was time dependent, becoming detectable at 48 hours and reaching maximal levels at 96 hours (Figure 5B), and closely paralleled the kinetics of apoptosis induction (Figure 5C).
ROS production in response to combined CI-1040 and retinoid treatment. OCI-AML3 cells were pretreated with the MEK inhibitor CI-1040 (0.5 μM) for 30 minutes and subsequently exposed to either ATRA (1 μM) or 9-cis RA (0.1 μM). At the indicated time points, control and treated cells were then incubated with DHE for 15 minutes, and its oxidation to the fluorescent product ethidium was then monitored by flow cytometric analysis. (A) Primary data from 1 experiment representative of at least 3 performed with superimposable results. The percentage of ROS-positive cells after 96 hours of exposure to the indicated treatments is shown in the figure. (B) Results are expressed as the net ROS induction (percentage of ROS-positive cells in treated samples minus percentage of ROS-positive cells in control samples) and represent the average ± SD of at least 3 independent experiments. (C) Apoptosis was evaluated by flow cytometric analysis of FITC-conjugated annexin V binding, while simultaneously assessing membrane integrity by PI exclusion. Results are expressed as the net apoptosis induction (percentage of apoptosis in treated cells minus percentage of apoptosis in control cells) and represent the average ± SD of at least 3 independent experiments.
ROS production in response to combined CI-1040 and retinoid treatment. OCI-AML3 cells were pretreated with the MEK inhibitor CI-1040 (0.5 μM) for 30 minutes and subsequently exposed to either ATRA (1 μM) or 9-cis RA (0.1 μM). At the indicated time points, control and treated cells were then incubated with DHE for 15 minutes, and its oxidation to the fluorescent product ethidium was then monitored by flow cytometric analysis. (A) Primary data from 1 experiment representative of at least 3 performed with superimposable results. The percentage of ROS-positive cells after 96 hours of exposure to the indicated treatments is shown in the figure. (B) Results are expressed as the net ROS induction (percentage of ROS-positive cells in treated samples minus percentage of ROS-positive cells in control samples) and represent the average ± SD of at least 3 independent experiments. (C) Apoptosis was evaluated by flow cytometric analysis of FITC-conjugated annexin V binding, while simultaneously assessing membrane integrity by PI exclusion. Results are expressed as the net apoptosis induction (percentage of apoptosis in treated cells minus percentage of apoptosis in control cells) and represent the average ± SD of at least 3 independent experiments.
Overall, these results suggest that perturbation of AML cells' redox status may play a role in the observed proapoptotic synergism between MEK inhibitors and retinoids.
Involvement of Bcl-2 modulation in the proapoptotic synergism between MEK inhibitors and retinoids
Since retinoids are known to modulate Bcl-2 expression in leukemic cells,18 and we have recently demonstrated that simultaneous MEK blockade and interference with Bcl-2 expression and/or function result in a highly synergistic induction of apoptosis in AML cells,31 we investigated whether Bcl-2 modulation could play a role in the observed proapoptotic synergism between MEK inhibitors and retinoids. For this purpose, Bcl-2 expression was analyzed by flow cytometry in OCI-AML3 cells treated with CI-1040 and either ATRA or 9-cis RA, alone or in combination; both ATRA and 9-cis RA decreased Bcl-2 expression by approximately 50%, while CI-1040 did not affect Bcl-2 expression per se (as we previously reported27 ) nor did it increase retinoid-mediated Bcl-2 down-regulation (Figure 6A). From a kinetic standpoint, Bcl-2 down-regulation by retinoids, alone or combined with CI-1040, was already maximal after 48 hours (Figure 6A), when apoptosis started to be detectable by annexin V binding (Figure 5C), and did not increase further up to 96 hours, when apoptosis reached maximal levels. Conversely, intracellular Bax expression levels did not change significantly in response to either CI-1040 or retinoids, as assessed by flow cytometry (data not shown). Virtually identical results in terms of Bcl-2/Bax expression levels were obtained using the MEK inhibitor PD98059 (data not shown).
Retinoid-mediated down-regulation of Bcl-2 expression and effects of Bcl-2 overexpression on apoptosis induced by combined CI-1040 and retinoids. OCI-AML3 cells were pretreated with CI-1040 (0.5 μM) for 30 minutes and then exposed to ATRA (1 μM) or 9-cis RA (0.1 μM). (A) Bcl-2 cytoplasmic expression was then assessed by cytofluorimetric analysis. Results are expressed as mean fluorescence index (MFI) after 48 hours (□) and 96 hours (⊡) of treatment and represent the average ± SD of 3 independent experiments. (B) Control vector–transfected (Neo, □) and Bcl-2–transfected (Bcl-2, ⊡) OCI-AML3 cells were treated as described for panel A. Apoptosis was then evaluated by flow cytometric analysis of annexin V binding after 48 hours (top panel) and 96 hours (bottom panel) of incubation. Results are expressed as the net apoptosis induction (percentage of apoptosis in treated cells minus percentage of apoptosis in control cells) and represent the average ± SD of 3 independent experiments.
Retinoid-mediated down-regulation of Bcl-2 expression and effects of Bcl-2 overexpression on apoptosis induced by combined CI-1040 and retinoids. OCI-AML3 cells were pretreated with CI-1040 (0.5 μM) for 30 minutes and then exposed to ATRA (1 μM) or 9-cis RA (0.1 μM). (A) Bcl-2 cytoplasmic expression was then assessed by cytofluorimetric analysis. Results are expressed as mean fluorescence index (MFI) after 48 hours (□) and 96 hours (⊡) of treatment and represent the average ± SD of 3 independent experiments. (B) Control vector–transfected (Neo, □) and Bcl-2–transfected (Bcl-2, ⊡) OCI-AML3 cells were treated as described for panel A. Apoptosis was then evaluated by flow cytometric analysis of annexin V binding after 48 hours (top panel) and 96 hours (bottom panel) of incubation. Results are expressed as the net apoptosis induction (percentage of apoptosis in treated cells minus percentage of apoptosis in control cells) and represent the average ± SD of 3 independent experiments.
To further explore the role of retinoid-induced Bcl-2 modulation in the observed proapoptotic effects, we next evaluated the effects of CI-1040/retinoid combinations in OCI-AML3 cells engineered to constitutively overexpress murine Bcl-2, through retrovirally-mediated stable gene transfer. As shown in Figure 6B, apoptosis induction by combined CI-1040 and retinoid treatment was completely abrogated at the 48-hour time point (top panel, P < .05 compared with control vector–transfected cells) and significantly reduced at the 96-hour time point (bottom panel, P < .05 compared with control vector–transfected cells) in Bcl-2–overexpressing OCI-AML3 cells; conversely, apoptosis induction by CI-1040 alone was relatively unaffected by Bcl-2 overexpression.
Overall these results indicate that retinoid-mediated Bcl-2 down-regulation plays an important, though not exclusive, role in the synergistic induction of apoptosis by combined MEK inhibitors and retinoids in AML cells with constitutive activation of the MEK/ERK signaling module.
Discussion
Here, we demonstrate that combined MEK inhibition and retinoid treatment result in the strikingly synergistic induction of apoptosis in cell line models of both AML and APL with constitutive activation of the MEK/ERK signaling module.
Recent evidence produced from our group as well as others indicates that constitutive activation of the MEK/ERK signaling module is frequently observed and could constitute a therapeutically relevant molecular target in AML23,28–30,33,52 ; in most instances, however, MEK blockade per se has predominantly cytostatic, rather than cytotoxic, effects.32 Retinoids, on the other hand, have not fulfilled their therapeutic promise in this disease, with the relevant exception of the PML-RARα fusion protein–driven APL subgroup, despite their differentiating and Bcl-2–modulating activity.20,21 Our results suggest that the frequently observed deregulation of the MAPK pathway may account for the relative resistance of non-APL AML cells to the growth-inhibitory and proapoptotic action of retinoids; of most importance from a clinical standpoint, these data indicate that the rational combination of 2 therapeutic modalities endowed with modest proapoptotic activity when used alone elicits a massive apoptotic response in AML cells and results in a strikingly synergistic antileukemic effect that may have important therapeutic implications. This apoptosis-sensitizing activity is one of the most intriguing features of MEK inhibitors as potential anticancer agents. MEK blockade, indeed, lowers leukemic cells' apoptotic threshold, setting the stage for increased sensitivity to the proapoptotic action of classical cytotoxic drugs, ionizing radiation, and other biologic agents that modulate apoptosis; together with their amenability to pharmacodynamic evaluation and negligible systemic toxicity, these actions make MEK inhibitors an ideal starting point to build pharmacologic combinations with synergistic antileukemic effects,32 which, based on the results presented here, may usefully include RA derivatives.
Results presented in Figures 2–3 clearly indicate that the presence of functional RAR and RXR receptors is instrumental to the synergistic induction of apoptosis by combined MEK inhibition and retinoid treatment. RA nuclear receptors and their associated transcriptional coregulators could likely constitute a point of convergence between MAPK and retinoid actions: indeed, both RXR and RAR family members, as well as the corepressor SMRT, could be regulated through MEK/ERK-dependent phosphorylation.35,53,54 While MAPK-dependent phosphorylation of nuclear RA receptors appears to influence the ability of retinoids and vitamin D3 to induce receptor-dependent transcriptional activation, cell growth arrest, and differentiation,16,17,34,35 its involvement in the proapoptotic cross talk described here is presently unclear and will require further investigation.
In the classical NB4 model of APL, ATRA-induced cell death occurs late (7 days) after exposure to retinoids, has features of postmaturation apoptosis, and appears to involve TRAIL expression and paracrine interaction with its cognate receptors.14,15 On the contrary, apoptosis induced by the combination of MEK inhibitors and retinoids in our model systems of both AML and APL has strikingly different features: first, it occurs earlier (48-72 hours) in the time course of exposure to combined treatment and reaches massive levels (> 80% of apoptotic cells in both OCI-AML3 and NB4) within 96 hours; second, it is not preceded by any appreciable sign of cell maturation; third, it is entirely independent of signaling through death-inducing ligand/receptor pairs. These features closely resemble those of rexinoid-induced apoptosis: in fact, under conditions of low serum, rexinoid agonists powerfully induce an apoptotic program that is entirely distinct from ATRA-induced, TRAIL-mediated apoptosis of APL cells, in that it occurs rapidly (72 hours) and it is not preceded by differentiation.46 Although experiments conducted in low-serum conditions failed to unmask specific rexinoid-dependent proapoptotic signaling in our cellular models (data not shown), one could reasonably speculate that the constitutive MAPK activation observed in OCI-AML3 and NB4 cells27,31 may substitute for exogenous growth/survival factors, thereby counteracting rexinoid-dependent apoptosis that becomes apparent only when the MEK/ERK module is pharmacologically blocked. In addition to blocking a MAPK-driven survival program, which may counteract rexinoid-dependent default death pathways, MEK inhibitors also interfere with chromatin remodeling and RAR-dependent transcriptional activation (as demonstrated in Figure 4), thereby potentially inhibiting an RARα-induced terminal maturation program, which acts dominantly over the rexinoid-induced program that triggers apoptosis of immature blasts.46 Although data generated using selective RXR ligands and RXR-overexpressing HL-60R cells support the hypothesized importance of RXR signaling, several lines of evidence suggest that in our model system signaling through RARs (particularly RARα) is equally, if not more, important in the proapoptotic synergism between MEK inhibitors and retinoids: first, RXR-selective ligands fail to synergize with MEK inhibitors in cells that lack a functional RARα, such as the NB4 cell line, which carries the PML-RARα fusion protein and the RXR-overexpressing HL-60R cell line (M.M., unpublished observation, August 2005); second, ligands for RXR heterodimerization partners other than RAR (ie, vitamin D3 and CDDO) fail to synergize with MEK inhibitors in inducing apoptosis (Figure 2A); third, MEK blockade partly inhibits ATRA-induced CD38 expression (Figure 4E), an event previously shown to be directly dependent on the expression of functional RARα receptors39 ; fourth, wild-type RARα overexpression renders the cells hypersensitive to MEK blockade, even in the absence of receptor ligation (Figure 3E-F).
Although the precise definition of the molecular mechanisms underlying the observed proapoptotic synergism between MEK inhibitors and retinoids will require further studies, the evidence presently available suggests that Bcl-2–dependent maintenance of mitochondrial homeostasis may play a prominent role. The data presented here confirm previous reports that retinoid treatment,18 but not MEK blockade,27 rapidly down-regulates Bcl-2 expression in AML cells, while providing evidence that abrogation of retinoid-mediated Bcl-2 modulation by stable gene transfer substantially reduces and delays (Figure 6B) apoptosis induced by the combination of CI-1040 and retinoids. Since we have previously demonstrated31 that interference with Bcl-2 expression and function strikingly synergizes with MEK blockade in inducing apoptosis in AML cells, a reasonable explanation for these findings is that, in our model system, retinoids act at least in part by interfering with Bcl-2 function and lowering the apoptotic threshold in a manner similar to that achieved by Bcl-2 antisense oligonucleotides or small-molecule inhibitors. MEK inhibitors, in turn, may either interfere with the expression of alternative/complementary survival mediators, such as Mcl-1 and BCL2A1 (Milella et al27 ; and M.M., unpublished observation, August 2005), which are up-regulated in response to retinoids in leukemic cells,14,55 or prevent/abrogate ERK-dependent phosphorylation of the residual Bcl-2 protein on specific serine residues, such as S28 and S70, which results in enhanced antiapoptotic activity.56–58 The failure of Bcl-2 overexpression to completely abrogate apoptosis induced by the combination of MEK inhibitors and retinoids at late time points (96 hours), however, leaves open the possibility that other, yet-undiscovered, Bcl-2–independent proapoptotic mechanisms may be operational in leukemic cells in response to the combined treatment. The finding that combined treatment substantially enhances cellular accumulation of ROSs (Figure 5A-B) also supports the hypothesis that disruption of mitochondrial homeostasis may be a convergence point for the proapoptotic action of retinoids and MEK inhibitors; however, since cell-permeable antioxidants, such as N-acetyl cysteine and glutathione ethyl ester, do not affect apoptosis induction by the combination of CI-1040 and ATRA in OCI-AML3 cells (data not shown), the question of whether ROS accumulation plays a causal role in or is a secondary byproduct of apoptosis induction remains open and is currently under investigation. Finally, preliminary evidence indicates that apoptosis induced by the combination of retinoids and MEK inhibitors is only marginally affected by pan-caspase inhibitors, such as IDN1965,59 again pointing to mitochondrial dysfunction as a major determinant of the observed proapoptotic synergism (M.M., unpublished observation, August 2005).
Overall, these findings support a model in which retinoid treatment of AML cells induces RA receptor– and MAPK-dependent transcription of differentiation-related genes; in order to successfully complete the differentiation process, however, retinoid-treated cells need to temporarily increase their resistance to apoptosis, possibly by up-regulating antiapoptotic Bcl-2 family members or yet-undiscovered mediators, thereby counteracting Bcl-2 down-regulation. In this context, blockade of MEK to ERK signaling efficiently prevents this burst of antiapoptotic signaling, shifting the balance away from differentiation toward apoptosis. As a consequence, combined treatment with MEK inhibitors and retinoids results in massive induction of apoptosis and strikingly synergistic antileukemic effects. Although further mechanistic studies are needed to elucidate the molecular details of this proapoptotic interaction, especially with regard to the role of mitochondrial dysfunction and ROS generation, the data presented here warrant the continued preclinical evaluation of antileukemic strategies based on combined MEK inhibition and retinoid treatment in both AML and APL models. Since up to 70% of non-M3 AML blasts do retain a certain degree of responsiveness to retinoids,19 thereby indicating the presence of functional RA receptors, and display constitutive ERK phosphorylation,28,60 such a therapeutic strategy could be usefully explored in the clinical setting in a substantial proportion of AML and APL patients, eventually opening new, mechanism-based avenues for the treatment of myeloid leukemias.
Authorship
Contribution: M.M., M.K., and M.A. designed and performed research, analyzed data, and wrote the paper; C.M.P., Y.T., M.R.R., C.G., B.Z.C., and C.D'A. performed research and analyzed data; S.J.C. contributed vital new reagents; and M.T.P., R.F., F.C., and A.T. analyzed data and reviewed the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michele Milella, Division of Medical Oncology A, Regina Elena National Cancer Institute, Via Elio Chianesi, n. 53, 00144 Rome, Italy; e-mail: milella@ifo.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by Associazione Italiana per la Ricerca sul Cancro (AIRC) and the Italian Ministry of Health (M.M.), Leukemia and Lymphoma Society (M.K.), and the National Institutes of Health (R01 CA089346 and P01 CA55164 to M.A.).
The authors wish to thank Dr Judith S. Sebolt-Leopold (Cancer Molecular Sciences, Pfizer Global Research & Development, Ann Arbor, MI) for kindly providing CI-1040; Dr Edward Sausville (National Cancer Institute/Cancer Therapy Evaluation Program, Bethesda, MD) for providing access to CDDO; and Dr Reid Bissonnette (Ligand Pharmaceuticals, San Diego, CA) for kindly providing LG100268. We also would like to greatly thank Dr Lucia Altucci (Dipartimento di Patologia Generale, Seconda Università degli Studi di Napoli, Naples, Italy) for helpful discussions and scientific advice.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal