Abstract
Expression of ZAP-70 is an important negative prognostic factor in chronic lymphocytic leukemia (CLL). This protein tyrosine kinase is a key mediator of T-cell receptor (TCR) signaling and is structurally homologous to Syk, which plays an analogous role in B-cell receptor (BCR) signaling. Recent studies indicate that ZAP-70 may participate in BCR signaling as well, but the mechanism of action is not completely understood. We have now compared antigen receptor-induced activation of ZAP-70 in B cells and T cells by analyzing phosphorylation of critical regulatory tyrosine residues. We show that BCR-mediated activation of ZAP-70 is very inefficient in CLL and lymphoma B cells and is negligible when compared to activation of Syk. Despite the inefficient catalytic activation, the ability of ZAP-70 to recruit downstream signaling molecules in response to antigen receptor stimulation appeared relatively preserved. Moreover, ectopic expression of ZAP-70 enhanced and prolonged activation of several key mediators of BCR signaling, such as the Syk, ERK, and Akt kinases, and decreased the rate of ligand-mediated BCR internalization. We conclude that the role of ZAP-70 in BCR signaling is quite distinct from its role in TCR signaling and is likely mediated by inhibition of events that terminate the signaling response.
Introduction
B-cell chronic lymphocytic leukemia (CLL) is a malignancy of mature monoclonal CD5+ B cells that typically express low levels of surface IgM. The clinical course is highly variable, characterized by progressive disease and unfavorable prognosis in approximately half the cases and a relatively indolent disease and normal life span in the remaining patients.1
Significant associations have recently been observed between the clinical course and certain features of the B-cell receptor (BCR), indicating that antigen stimulation and signaling may play an important role in the pathogenesis of this disease.2,3 In particular, the leukemic cells of patients with progressive disease typically display BCRs encoded by unmutated immunoglobulin variable heavy-chain genes (IgVH) and express the protein tyrosine kinase (PTK) ZAP-70, which plays an essential role in antigen receptor signaling in T cells. In contrast, CLL B cells from most patients with stable disease express mutated IgVH genes and lack ZAP-70.4–8 The 2 subsets also appear to differ in their capacity to transmit BCR-derived stimuli because IgM ligation induced stronger signaling responses in CLL B cells from patients with a poor prognosis.9–11
ZAP-70 is a key signaling molecule in T cells, where it couples the antigen-activated T-cell receptor (TCR) to downstream signaling pathways.12 It is structurally homologous to Syk, a PTK that is involved in proximal BCR signaling. In normal B cells, stimulation of the BCR by antigen leads to phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) within the cytoplasmic tails of Igα and Igβ. Syk is subsequently recruited to these motifs and, following its activation by tyrosine phosphorylation, propagates the signal by activation of downstream signaling molecules, such as phosphatidylinositol 3-kinase (PI3K) and phospholipase Cγ2 (PLCγ2). PI3K generates the key second messenger phosphatidylinositol-3,4,5-triphosphate, which recruits other BCR-signaling molecules to the membrane and activates the kinase Akt. Activation of PLCγ2 leads to the release of intracellular Ca2+ and activation of protein kinase C (PKC). The signaling cascade proceeds with activation of mitogen-activated protein kinases (MAPKs), such as extracellular signal-regulated kinase (ERK), c-JUN NH2-terminal kinase (JNK) and p38 MAPK, and transcription factors, including nuclear factor-κB (NF-κB) and nuclear factor of activated T cells (NFAT).13 The balance among the activation of these signaling molecules determines the B-cell response, which can include proliferation, survival, differentiation, or cell death.
The functional significance of ZAP-70 expression in CLL B cells is still unclear. Experiments with avian lymphoma B cells have shown that ZAP-70 can restore some BCR-signaling events when Syk is not expressed, indicating a certain degree of functional homology between the 2 PTKs.14 More recently, ZAP-70 was shown to undergo tyrosine phosphorylation and to associate with the BCR complex in antigen-stimulated CLL B cells, which suggested that ZAP-70 may be involved in BCR signal transduction even when Syk is present.11 In addition, introduction of ZAP-70 in CLL B cells by adenoviral gene transfer resulted in stronger activation of certain BCR-signaling molecules, such as Syk, BLNK, and PLCγ.15 These findings indicate that the function of ZAP-70 in CLL B cells may be to enhance BCR signaling, which in turn could promote the growth and survival of the malignant clone.16,17 However, the mechanism through which ZAP-70 can enhance BCR signaling in CLL B cells is still unclear, considering that ZAP-70 has been shown to have an approximately 100 times lower intrinsic enzymatic activity than Syk in in vitro immune complex kinase assays.18 Moreover, we have recently shown that ZAP-70 is expressed at lower levels than Syk in the majority of ZAP-70+ CLL cases.19
ZAP-70 and Syk are activated by a multistep process that involves phosphorylation of several shared tyrosine residues by Src family PTKs or by autophosphorylation. The first event involves phosphorylation of Tyr319 and Tyr352 in the interdomain B of ZAP-70 and Syk, respectively, which in turn releases the kinase domain from an autoinhibitory configuration.20 Subsequently, Tyr493 in the activation loop of ZAP-70 and Tyr526 in the activation loop of Syk become phosphorylated, leading to full enzymatic activation of the 2 kinases. Several other tyrosine residues in ZAP-70 and Syk are involved in the negative regulation of these PTKs or are required for interactions with downstream signaling molecules or adaptor proteins. In particular, Tyr292 in ZAP-70 and Tyr323 in Syk are considered to have a negative regulatory function, possibly by allowing interaction with the c-Cbl ubiquitin ligase.21,22 However, these Tyr residues were recently shown to function also as binding sites for the p85 regulatory subunit of PI3K, indicating that they may also play a positive role in antigen receptor signal transduction.23 The tyrosine at position 474 of ZAP-70 is required for association with the Shc adaptor protein and coupling of the activated TCR to the Ras/Raf/Erk signaling pathway, whereas Tyr315 is the binding site for Vav, a guanine nucleotide exchange factor for the Rac/Rho/Cdc42 family of small GTP-binding proteins.24,25 Additional sites of tyrosine phosphorylation in ZAP-70 include Tyr69, Tyr126, and Tyr178, but their role in signal transduction is unclear at present.26
To further define the role of ZAP-70 in BCR signaling, we compared phosphorylation of the activating tyrosine residues in ZAP-70 and Syk following antigen receptor cross-linking of CLL B lymphocytes and transfected lymphoma cell lines. Unexpectedly, we observed that in B cells phosphorylation of the activating tyrosines in ZAP-70 is highly inefficient and negligible when compared to phosphorylation of Syk. Despite this inefficient activation, we show that expression of ZAP-70 can prolong and enhance BCR signaling and can decrease the rate of receptor internalization.
Patients, materials, and methods
Patients and CLL B-cell samples
Blood samples were collected from 19 patients who satisfied standard morphologic and immunophenotypic criteria for B-cell CLL.27 Informed consent was obtained from all patients according to the Declaration of Helsinki and approval for the study was obtained from the Institutional Human Research Committee at the Catholic University Medical School in Rome.
Peripheral blood mononuclear cells were separated by Ficoll gradient centrifugation (Amersham Biosciences, Uppsala, Sweden). CLL B cells were isolated by negative selection using anti-CD3, anti-CD14, and anti-CD16 mouse monoclonal antibodies (kindly provided by F. Malavasi, University of Turin, Italy) and Dynabeads conjugated with a pan anti–mouse IgG antibody (Dynal Biotech, Oslo, Norway). The purity of the selected CLL B cells was evaluated by staining with anti-CD5 R-phycoerythrin (R-PE)–conjugated and anti-CD19 fluorescein (FITC)–conjugated antibodies (BD Biosciences, Franklin Lakes, NJ), followed by flow cytometry analysis on a FACSCalibur flow cytometer (Becton Dickinson, Milan, Italy). Expression of ZAP-70 was evaluated by immunoblotting and reverse transcription–polymerase chain reaction (RT-PCR) analysis, as described previously.19
Cell cultures and stimulations
The human T-cell line Jurkat (clone E6.1), Burkitt lymphoma B-cell line BJAB (kindly provided by E. A. Clark, University of Washington, Seattle, WA), and CLL B cells were maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, 0.1 mg/mL streptomycin, 2 mM l-glutamine, and 1 mM sodium pyruvate (Invitrogen, Carlsbad, CA) and cultured at 37°C in a humidified atmosphere containing 5% CO2. CLL and BJAB B cells were stimulated with 5 μg/mL goat F(ab′)2 anti–human IgM (Southern Biotechnology, Birmingham, AL) at a density of 1 × 107 cells/mL. Jurkat T cells were stimulated at a density of 5 × 107 cells/mL with 10 μg/mL anti–human CD3-ϵ mouse monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Stimulations were stopped by adding ice-cold phosphate-buffered saline (PBS), followed by centrifugation at 4°C and cell lysis.
Constructs and transfections
The ZAP-70 expression vector (pcDNA3-ZAP-70-Myc) was created by excising a DNA fragment from the pSXSRα-ZAP-Myc plasmid (kindly provided by R. L. Wange, National Institutes of Health, Baltimore, MD) that encodes for the full-length human ZAP-70 in frame with the Myc-tag epitope, and subcloning it into the polylinker BamHI site of pcDNA3. An expression vector without the Myc-tag was generated by PCR of the same construct. The integrity of ZAP-70 was confirmed by nucleotide sequencing. The pcDNA3-ZAP-70-Myc construct was introduced by electroporation into BJAB cells using the GenPulser II Electroporator System (Bio-Rad Laboratories, Hercules, CA). Clonal selection with geneticin/G418 was performed by limiting dilution. Expression of ZAP-70 in the selected stable transfectant clones was verified by RT-PCR and immunoblotting.
Transient transfection of the pcDNA3-ZAP-70-Myc or pcDNA3-ZAP-70 vector into purified CLL, BJAB, and Jurkat cells was performed using the Nucleofector System (Amaxa Biosystems, Cologne, Germany). Typically, 1.2 × 107 CLL B cells were resuspended in 100 μL Cell Line Nucleofector Solution V and mixed with 3 μg ZAP-70 expression vector or the control vectors pCDNA3 and pmaxGFP (Amaxa Biosystems). The GFP expression vector was used to evaluate transfection efficiency. Nucleofections were done using the Amaxa Nucleofector II device and the U-13 program. Cell viability and transfection efficiency were evaluated on 5 × 105 GFP-nucleofected cells after 16 to 20 hours by propidium iodide (PI) staining and flow cytometry analysis. The transfection efficiency was on average 60%, based on the number of PI−/GFP+ cells. Transfection of BJAB and Jurkat cells was performed as described, except for the use of different nucleofection programs (T-16 and S-18, respectively). Twenty hours after nucleofection the cells were stimulated and processed for immunoblotting or immunoprecipitation.
Immunoblotting analysis
Cells were lysed in ice-cold RIPA lysis buffer (10 mM Tris-HCl, pH 7.4, 5 mM EDTA, 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 0.1% sodium deoxycholate, containing 1:40 dilution of protease and 1:75 dilution of phosphatase inhibitor cocktail for mammalian cells [Sigma-Aldrich, St Louis, MO]). The protein concentration of each cell lysate was determined with the RC DC Protein Assay (Bio-Rad Laboratories). Proteins were separated by 8% or 10% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred on Immobilon-P polyvinylidene difluoride membranes (Millipore, Bedford, MA). Membranes were incubated at 4°C with the following primary antibodies: phospho-tyrosine (P-Tyr-100), phospho-ZAP-70Tyr319/SykTyr352, phospho-ZAP-70Tyr493, phospho-ERK1/2Thr202/Tyr204, phospho-JNKThr183/Tyr185, phospho-AKTSer493, phospho-PLCγ2Tyr1217, ZAP-70 (clone L1E5), Syk, ERK1/2, IκB-α, Shc (Cell Signaling Technology, Danvers, MA), c-Cbl, Cbl-b (Santa Cruz Biotechnology), PI3K p85α (Upstate Technology, Lake Placid, NY), β-actin, and tubulin (Sigma-Aldrich). Immunodetection was done with anti–rabbit or anti–mouse IgG HRP-linked antibodies (Cell Signaling Technology) and the ECL Plus enhanced chemiluminescence detection system (Amersham Biosciences, Buckinghamshire, United Kingdom) with BioMax MR films (Eastman Kodak, Rochester, NY).
Immunoprecipitation assays
Cells were lysed for 30 minutes in ice-cold buffer containing 1% NP-40, 20 mM Tris-HCl, pH 7.4, 2 mM EGTA, 150 mM NaCl, 50 mM sodium fluoride, and a 1:40 dilution of protease and 1:75 dilution of phosphatase inhibitor cocktails. Nuclei were removed by centrifugation at 16 000 g (4°C) and protein concentration was determined as described. Cell lysates were precleared for 1 hour at 4°C with protein G–Sepharose-4B Fast Flow beads (Sigma-Aldrich) and subjected to immunoprecipitation for 2 hours at 4°C with 20 μL protein G-Sepharose beads preadsorbed with primary antibody. After 3 washes with ice-cold lysis buffer, the bound proteins were eluted in SDS sample buffer, boiled, and subjected to electrophoresis and immunoblotting analysis.
Analysis of BCR internalization
BCR internalization was measured as described in detail elsewhere.28,29 Briefly, 2 × 106 cells were incubated for each investigated time point in 200 μL complete RPMI medium with 20 μL R-PE–coupled goat F(ab′)2 anti–human IgM (Caltag Laboratories, Burlingame, CA) in microcentrifuge tubes on ice for 30 minutes. Cells were washed, resuspended in 200 μL medium, and split in equal amounts into 3 new tubes. Two tubes were kept at 4°C, whereas the third tube was incubated at 37°C for the indicated time. Cells were washed again to eliminate unbound F(ab′)2, and then the sample that was incubated at 37°C together with one of the samples kept at 4°C were resuspended in 0.4 mL 150 mM NaCl, 150 mM acetic acid, pH 2.7, and incubated on ice for 3 minutes. Acid treatment was stopped by adding 0.4 mL FBS. Cells were pelleted by centrifugation, subjected to one more acid treatment, resuspended in PBS 0.1% BSA, and analyzed by flow cytometry. The percentage of BCR internalization was calculated using the formula: (F−Fmin)/(Fmax−Fmin) × 100. F is the mean fluorescent intensity (MFI) of the sample incubated at 37°C and is a measure of internalized F(ab′)2-IgM complexes at the indicated time point; Fmin is the MFI of the acid-treated sample that was incubated at 4°C and serves to control that R-PE F(ab′)2 was eliminated from the cell surface by acid treatment; Fmax is the MFI of the sample incubated at 4°C without acid treatment and represents the starting amount of R-PE F(ab′)2 bound to the cells.
Results
Tyrosine residues required for ZAP-70 kinase activation are not phosphorylated in CLL B cells following BCR stimulation
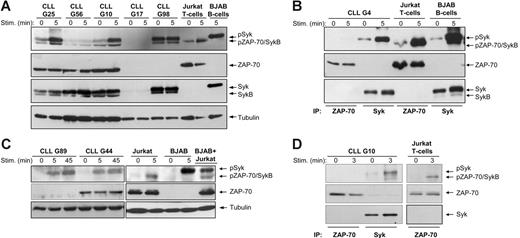
To investigate whether ZAP-70 becomes activated following BCR stimulation, we first performed immunoblotting analysis with an antibody that recognizes the phosphorylated regulatory tyrosine residue in interdomain B (Tyr319). This antibody also reacts with Syk when phosphorylated at the corresponding Tyr352, thus providing a convenient means to simultaneously follow activation of both kinases. Experiments were performed with purified CLL B cells from 19 patients, of which 13 were ZAP-70+ and 6 were ZAP-70−. In 12 samples BCR ligation with anti-IgM induced activation of Syk, as evidenced by increased phosphorylation of a 72-kDa protein (Figure 1A and Figure S1, which is available on the Blood website; see the Supplemental Figures link at the top of the online article). In 3 cases Syk was constitutively phosphorylated at Tyr352 and did not show an increase in signal intensity following BCR stimulation, whereas in 4 cases no band of the expected size was visible, indicating that Syk was not activated.
ZAP-70 is not phosphorylated on positive regulatory tyrosine residues following BCR stimulation in CLL B cells. (A) Immunoblotting analysis of total cellular extracts from 3 ZAP-70+ (G25, G56, and G10) and 2 ZAP-70− CLL samples (G17 and G98) using the phospho-ZAP-70Tyr319/SykTyr352 antibody. Cells were stimulated with anti-IgM, as indicated. Jurkat T cells stimulated for 5 minutes with anti-CD3 antibody were used as a positive control for ZAP-70 activation. BJAB B cells stimulated for 5 minutes with anti-IgM were used as a positive control for Syk activation. The same membrane was reprobed with anti–ZAP-70, anti-Syk, and antitubulin antibodies, as indicated in the bottom panels. Arrows indicate the expected position of the ZAP-70, Syk, and SykB proteins. (B) Immunoblotting analysis with phospho-ZAP-70Tyr319/SykTyr352 antibody of ZAP-70 and Syk proteins immunoprecipitated from unstimulated or anti-IgM–stimulated ZAP-70+ CLL B cells (sample G4) is shown in the top panel. Jurkat T cells unstimulated or stimulated with anti-CD3 were used as a positive control for ZAP-70 phosphorylation, whereas unstimulated or anti-IgM–stimulated BJAB B cells were used as a positive control for Syk phosphorylation. The total amount of immunoprecipitated ZAP-70 and Syk protein is shown in the bottom panels. (C) Immunoblotting analysis of total cellular extracts from ZAP-70+ and ZAP-70− CLL B cells, BJAB B cells, and Jurkat T cells using the phospho-ZAP-70Tyr493 antibody, which also cross-reacts with phospho-SykTyr526. Stimulation with anti-IgM or anti-CD3 was performed for the indicated times. (D) Immunoblotting analysis of immunoprecipitated ZAP-70 and Syk from unstimulated or anti-IgM–stimulated ZAP-70+ CLL B cells. Jurkat T cells stimulated with anti-CD3 are shown as a positive control for ZAP-70 activation. Membranes were probed with the same phospho-ZAP-70/Syk antibody as in panel C (top panels) and reprobed with anti–ZAP-70 (middle panels) and anti-Syk (bottom panel).
ZAP-70 is not phosphorylated on positive regulatory tyrosine residues following BCR stimulation in CLL B cells. (A) Immunoblotting analysis of total cellular extracts from 3 ZAP-70+ (G25, G56, and G10) and 2 ZAP-70− CLL samples (G17 and G98) using the phospho-ZAP-70Tyr319/SykTyr352 antibody. Cells were stimulated with anti-IgM, as indicated. Jurkat T cells stimulated for 5 minutes with anti-CD3 antibody were used as a positive control for ZAP-70 activation. BJAB B cells stimulated for 5 minutes with anti-IgM were used as a positive control for Syk activation. The same membrane was reprobed with anti–ZAP-70, anti-Syk, and antitubulin antibodies, as indicated in the bottom panels. Arrows indicate the expected position of the ZAP-70, Syk, and SykB proteins. (B) Immunoblotting analysis with phospho-ZAP-70Tyr319/SykTyr352 antibody of ZAP-70 and Syk proteins immunoprecipitated from unstimulated or anti-IgM–stimulated ZAP-70+ CLL B cells (sample G4) is shown in the top panel. Jurkat T cells unstimulated or stimulated with anti-CD3 were used as a positive control for ZAP-70 phosphorylation, whereas unstimulated or anti-IgM–stimulated BJAB B cells were used as a positive control for Syk phosphorylation. The total amount of immunoprecipitated ZAP-70 and Syk protein is shown in the bottom panels. (C) Immunoblotting analysis of total cellular extracts from ZAP-70+ and ZAP-70− CLL B cells, BJAB B cells, and Jurkat T cells using the phospho-ZAP-70Tyr493 antibody, which also cross-reacts with phospho-SykTyr526. Stimulation with anti-IgM or anti-CD3 was performed for the indicated times. (D) Immunoblotting analysis of immunoprecipitated ZAP-70 and Syk from unstimulated or anti-IgM–stimulated ZAP-70+ CLL B cells. Jurkat T cells stimulated with anti-CD3 are shown as a positive control for ZAP-70 activation. Membranes were probed with the same phospho-ZAP-70/Syk antibody as in panel C (top panels) and reprobed with anti–ZAP-70 (middle panels) and anti-Syk (bottom panel).
A weak band of approximately 70 kDa was observed in 12 cases. This protein appeared to migrate slightly faster than ZAP-70 from Jurkat T cells and was also present in 2 CLL samples that were clearly ZAP-70− (G98 in Figure 1A and G140 in Figure S1). Moreover, a band of the same size was detected with an antibody that reacts specifically with total Syk, indicating that this phosphoprotein corresponds to a variant of Syk rather than to ZAP-70 (Figure 1A and data not shown).
A variant of Syk that lacks part of the interdomain B region has been previously reported.18,30 This isoform, known as SykB, is only 0.8 kDa smaller than ZAP-70, which precludes size discrimination between these 2 proteins. Therefore, to determine the identity of this 70-kDa phosphoprotein, we immunoprecipitated ZAP-70 and Syk from stimulated and unstimulated CLL B cells and performed immunoblotting analysis with the phospho-ZAP-70Tyr319/SykTyr352 antibody (Figure 1B). To control for antibody specificity, we analyzed in the same manner ZAP-70 immunoprecipitated from the T-cell lymphoma Jurkat and Syk immunoprecipitated from the ZAP-70− B-cell lymphoma BJAB. As shown in Figure 1B, no signal was detected with the phospho-ZAP-70Tyr319/SykTyr352 antibody in the immunoprecipitation of ZAP-70 from CLL B cells, whereas a strong band of 72 kDa and a very weak band of 70 kDa was present in the immunoprecipitation of Syk. The lower molecular size protein was also seen as a minor band in the immunoprecipitation of Syk from anti-IgM–stimulated BJAB B cells, along with the predominant 72-kDa species. In Jurkat T cells the phospho-ZAP-70Tyr319/SykTyr352 antibody reacted only with a 70-kDa protein that underwent additional phosphorylation after stimulation with anti-CD3, as expected for ZAP-70. Taken together, these data indicate that the lower molecular size protein is SykB and that ZAP-70 is not phosphorylated at Tyr319 in CLL B cells, whereas it becomes strongly phosphorylated in antigen receptor-stimulated T cells.
The second step in ZAP-70 activation involves phosphorylation of Tyr493 in the kinase domain. To further investigate the capacity of ZAP-70 to become activated in CLL B cells, we performed immunoblotting with an antibody developed against a phosphopeptide spanning the Tyr493 residue. In Jurkat T cells, this antibody reacted with a 70-kDa protein that became phosphorylated on CD3 ligation, consistent with ZAP-70 activation (Figure 1C). However, in BCR-stimulated, ZAP-70− CLL and BJAB B cells this antibody detected a strong band of slightly higher molecular weight, indicating that it cross-reacts with the corresponding Tyr526 in the kinase domain of Syk. A band of 70 kDa was not observed in the ZAP-70+ CLL sample, further suggesting that ZAP-70 does not become activated in CLL B cells. This was confirmed by the subsequent immunoprecipitation experiment. As shown in Figure 1D, ZAP-70 that was immunoprecipitated from anti-IgM–stimulated CLL B cells did not become phosphorylated at Tyr493, in contrast to ZAP-70 that was immunoprecipitated from anti-CD3–stimulated Jurkat T cells. Moreover, a strong BCR-induced signal was detected with the same antibody when Syk was immunoprecipitated from CLL B cells, confirming that this antibody also recognizes Syk phosphorylated at Tyr526. Taken together, these experiments indicate that of the 2 homologous kinases, only Syk becomes activated in CLL B cells following BCR stimulation.
ZAP-70 is inefficiently phosphorylated on the activating tyrosine residues when overexpressed in BJAB lymphoma B cells
In a recent study we observed that in the majority of ZAP-70+ CLL cases, the levels of ZAP-70 do not reach the levels of Syk and on average are 50% lower.19 Therefore, to exclude the possibility that quantitative differences in the levels of the 2 PTKs may have biased toward detection of phosphorylated tyrosine residues in Syk only, we decided to overexpress ZAP-70 in BJAB B cells. In 2 of the 3 stable transfectants that we obtained the levels of ZAP-70 mRNA were up to 3-fold higher than the levels of Syk (clones A and D in Figure S2A). Moreover, the amount of ZAP-70 protein produced by these clones was several-fold higher than the amount of ZAP-70 expressed in Jurkat T cells (Figure S2B).
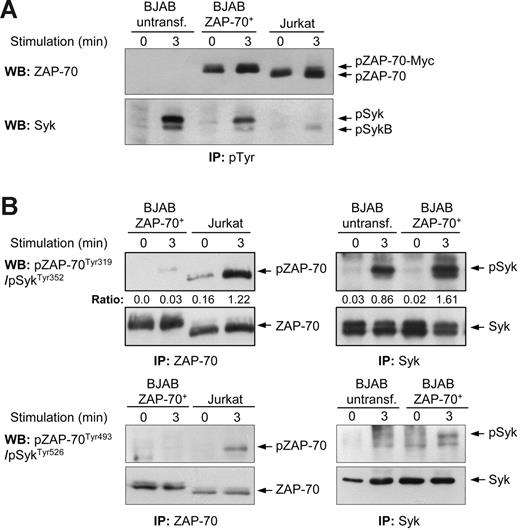
To determine whether ZAP-70 becomes tyrosine-phosphorylated in the transfectant cell lines, we first performed immunoprecipitation experiments with an anti–phospho-Tyr antibody (P-Tyr-100), followed by immunoblotting with antibodies against total ZAP-70 and total Syk (Figure 2A). This experiment revealed that certain tyrosine residues are constitutively phosphorylated in ZAP-70, with a moderate increase in signal intensity following BCR stimulation. Because the increase in tyrosine phosphorylation could also be accounted for by tyrosines that are not involved in ZAP-70 activation, we immunoprecipitated ZAP-70 from the BJAB transfectants and performed immunoblotting with the phospho-specific antibodies. ZAP-70 immunoprecipitated from anti-CD3–stimulated Jurkat T cells was used as a control for Tyr319 and Tyr493 phosphorylation. As shown in the upper left panel of Figure 2B, phosphorylation of ZAP-70 at Tyr319 was extremely weak in BJAB B cells, whereas a strong signal was induced in Jurkat T cells. Densitometric analysis showed that phosphorylation of ZAP-70 was 30 times less efficient in BJAB B cells than in Jurkat T cells, but this difference is likely an underestimate, considering that the intensity of the signal in Jurkat cells had to reach saturation for the signal in BJAB cells to become visible. In addition, Tyr493 phosphorylation was only detected in ZAP-70 that was immunoprecipitated from anti-CD3–stimulated Jurkat T cells, although the amount of ZAP-70 protein that was immunoprecipitated from the BJAB transfectant was considerably higher (Figure 2B lower left panel). Interestingly, analysis of Syk revealed increased phosphorylation of this kinase at the corresponding tyrosines residues (Tyr352 and Tyr526) in BJAB transfectants as compared to parental BJAB B cells, indicating that ZAP-70 may be enhancing activation of Syk (Figure 2B right panels).
Tyr319 and Tyr493 in ZAP-70 are inefficiently phosphorylated following IgM ligation in stable BJAB transfectants. (A) Immunoblotting analysis of immunoprecipitated tyrosine-phosphorylated proteins from parental and ZAP-70 transfectant BJAB B cells (unstimulated or anti-IgM stimulated) and Jurkat T cells (unstimulated or anti-CD3 stimulated). Membranes were probed with anti–ZAP-70 (top panel) and anti-Syk (bottom panel). The band corresponding to ZAP-70 in BJAB is 1 kDa bigger than the band corresponding to endogenous ZAP-70 in Jurkat T cells due to the presence of the Myc tag. The expected positions of ZAP-70, ZAP-70-Myc, Syk, and SykB are indicated by arrows. (B) ZAP-70 and Syk were immunoprecipitated from parental and ZAP-70–transfected BJAB B cells and from Jurkat T cells and analyzed by immunoblotting with phospho-ZAP-70Tyr319/SykTyr352 (top panels) or phospho-ZAP-70Tyr493/SykTyr526 antibody (bottom panels). The ratio of ZAP-70 phosphorylated at Tyr319 and total ZAP-70 was quantified by densitometry.
Tyr319 and Tyr493 in ZAP-70 are inefficiently phosphorylated following IgM ligation in stable BJAB transfectants. (A) Immunoblotting analysis of immunoprecipitated tyrosine-phosphorylated proteins from parental and ZAP-70 transfectant BJAB B cells (unstimulated or anti-IgM stimulated) and Jurkat T cells (unstimulated or anti-CD3 stimulated). Membranes were probed with anti–ZAP-70 (top panel) and anti-Syk (bottom panel). The band corresponding to ZAP-70 in BJAB is 1 kDa bigger than the band corresponding to endogenous ZAP-70 in Jurkat T cells due to the presence of the Myc tag. The expected positions of ZAP-70, ZAP-70-Myc, Syk, and SykB are indicated by arrows. (B) ZAP-70 and Syk were immunoprecipitated from parental and ZAP-70–transfected BJAB B cells and from Jurkat T cells and analyzed by immunoblotting with phospho-ZAP-70Tyr319/SykTyr352 (top panels) or phospho-ZAP-70Tyr493/SykTyr526 antibody (bottom panels). The ratio of ZAP-70 phosphorylated at Tyr319 and total ZAP-70 was quantified by densitometry.
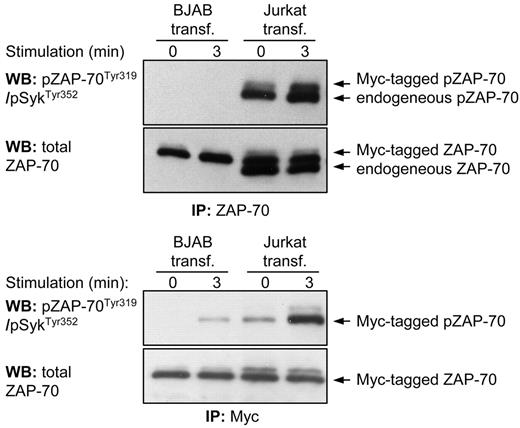
To exclude the possibility that inefficient BCR-induced activation of ZAP-70 was a consequence of artificial expression of the protein, we performed transient transfection of Myc-tagged ZAP-70 in Jurkat T cells and compared Tyr319 phosphorylation of this protein with endogenous ZAP-70. Immunoprecipitation of endogenous and Myc-tagged ZAP-70, which differ in size by approximately 1 kDa, showed that both proteins respond to CD3 ligation by an increase in Tyr319 phosphorylation (Figure 3 top panel). In contrast, no signal could be detected in ZAP-70 immunoprecipitated from transiently transfected BJAB B cells either before or after BCR stimulation, even though the amount of ZAP-70 immunoprecipitated from BJAB B cells was higher than the amount of ZAP-70 immunoprecipitated from Jurkat T cells. A very faint band was observed when the Myc-tagged ZAP-70 protein was selectively immunoprecipitated from BCR-stimulated BJAB cells, which was consistent with the marginal Tyr319 phosphorylation previously noted in the stable transfectants (Figure 3 lower panel). Taken together, these experiments confirm that the efficiency of activation of ZAP-70 is strikingly different in antigen receptor-stimulated B and T cells, and this is not due to artificial expression of the protein.
Phosphorylation of Tyr319 in ectopically expressed ZAP-70 is significantly less efficient in BJAB B cells than in Jurkat T cells. Immunoblotting analysis of immunoprecipitated ZAP-70 protein from transiently transfected BJAB B cells and Jurkat T-cells was done with the phospho-ZAP-70Tyr319/SykTyr352 antibody. The pcDNA3-ZAP-70-Myc construct was introduced into both cell lines by nucleofection. In the top panels both Myc-tagged and endogenous ZAP-70 proteins were immunoprecipitated with an anti–ZAP-70 antibody. The 2 proteins differ in size by 1 kDa because of the Myc tag. The bottom panels show analysis only of Myc-tagged ZAP-70 that was immunoprecipitated with an anti-Myc antibody.
Phosphorylation of Tyr319 in ectopically expressed ZAP-70 is significantly less efficient in BJAB B cells than in Jurkat T cells. Immunoblotting analysis of immunoprecipitated ZAP-70 protein from transiently transfected BJAB B cells and Jurkat T-cells was done with the phospho-ZAP-70Tyr319/SykTyr352 antibody. The pcDNA3-ZAP-70-Myc construct was introduced into both cell lines by nucleofection. In the top panels both Myc-tagged and endogenous ZAP-70 proteins were immunoprecipitated with an anti–ZAP-70 antibody. The 2 proteins differ in size by 1 kDa because of the Myc tag. The bottom panels show analysis only of Myc-tagged ZAP-70 that was immunoprecipitated with an anti-Myc antibody.
ZAP-70 associates with PI3K, Cbl-b, c-Cbl, and Shc in CLL and BJAB B cells
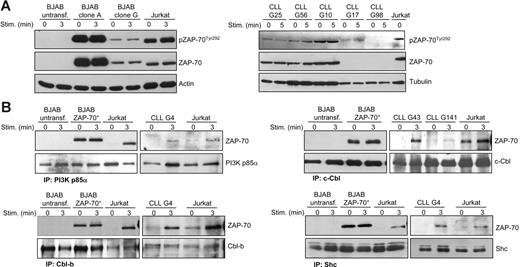
ZAP-70 contains additional sites of tyrosine phosphorylation that are not involved in the regulation of its catalytic activity, but instead regulate the recruitment of downstream signaling molecules and adaptor proteins. In particular, Tyr292 is required for the interaction of ZAP-70 with the p85 regulatory subunit of PI3K and the E3-ubiquitin ligases c-Cbl and Cbl-b, whereas Tyr474 is required for the association of ZAP-70 with the adaptor protein Shc.21,23,24,31,32 To investigate whether ZAP-70 can interact with these proteins in B cells, we first performed immunoblotting analysis with an antibody that recognizes phosphorylated Tyr292. This site was constitutively phosphorylated in ZAP-70+ BJAB and CLL B cells, with a signal intensity that largely reflected the total amount of ZAP-70 protein (Figure 4A). A similar level of Tyr292 phosphorylation was observed in Jurkat T cells, indicating that phosphorylation at this site is regulated in the same manner in B and T cells. Interestingly, BCR or TCR stimulation did not induce a significant change in the level of Tyr292 phosphorylation, suggesting that the interactions mediated by this site do not require additional phosphorylation.
Tyr292 phosphorylation and interaction of ZAP-70 with PI3K p85α, c-Cbl, Cbl-b, and Shc. (A) Immunoblotting analysis with phospho-ZAP-70Tyr292 antibody of total cellular extracts from parental BJAB B cells, 2 BJAB clones expressing different levels of ZAP-70 (clone A and clone G), Jurkat T cells, and ZAP-70+ (G25, G56, and G10) or ZAP-70− (G17 and G98) CLL B cells. Cells were stimulated with anti-IgM or anti-CD3, as applicable. (B) Immunoblotting analysis with anti–ZAP-70 antibody of protein complexes immunoprecipitated with PI3K p85α, c-Cbl, Cbl-b, and Shc antibodies. Immunoprecipitations were performed before and after antigen receptor stimulation on parental and ZAP-70–transfected BJAB B cells, Jurkat T cells, and CLL B cells, as indicated. Membranes were reprobed with antibodies against p85α, c-Cbl, Cbl-b, and Shc to evaluate the quantity of immunoprecipitated proteins.
Tyr292 phosphorylation and interaction of ZAP-70 with PI3K p85α, c-Cbl, Cbl-b, and Shc. (A) Immunoblotting analysis with phospho-ZAP-70Tyr292 antibody of total cellular extracts from parental BJAB B cells, 2 BJAB clones expressing different levels of ZAP-70 (clone A and clone G), Jurkat T cells, and ZAP-70+ (G25, G56, and G10) or ZAP-70− (G17 and G98) CLL B cells. Cells were stimulated with anti-IgM or anti-CD3, as applicable. (B) Immunoblotting analysis with anti–ZAP-70 antibody of protein complexes immunoprecipitated with PI3K p85α, c-Cbl, Cbl-b, and Shc antibodies. Immunoprecipitations were performed before and after antigen receptor stimulation on parental and ZAP-70–transfected BJAB B cells, Jurkat T cells, and CLL B cells, as indicated. Membranes were reprobed with antibodies against p85α, c-Cbl, Cbl-b, and Shc to evaluate the quantity of immunoprecipitated proteins.
To directly examine whether in B cells ZAP-70 interacts with the proteins that are recruited by Tyr292, we immunoprecipitated p85, c-Cbl, and Cbl-b from BJAB, CLL, and Jurkat cells, and analyzed the association of these proteins by immunoblotting. As shown in Figure 4B, ZAP-70 coimmunoprecipitated with p85, c-Cbl, and Cbl-b in antigen receptor-stimulated CLL B cells and Jurkat T cells, whereas in BJAB B cells the interactions were constitutive, presumably due to the significant overexpression of ZAP-70. In addition, in all cell types ZAP-70 associated with Shc, which requires phosphorylation at Tyr474 for recruitment. Therefore, these experiments demonstrate that the ability of ZAP-70 to interact with downstream effector molecules is preserved in B cells.
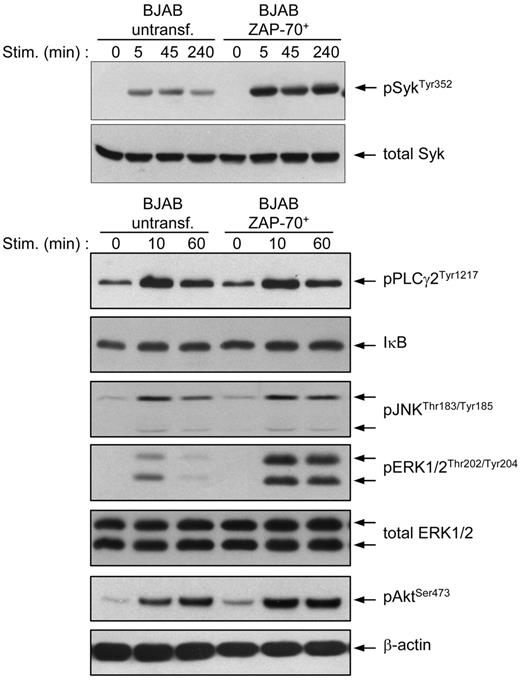
Expression of ZAP-70 in BJAB and CLL B cells induces stronger and prolonged activation of the Syk, ERK, and Akt kinases
We next investigated the effect of ZAP-70 on the activation of several key mediators of BCR signaling. Immunoblotting analysis of BCR-stimulated parental and transfected BJAB B cells confirmed the previous observation that phosphorylation of Syk at Tyr352 is stronger and prolonged in the presence of ZAP-70 (Figure 5). Moreover, the ZAP-70 transfectants also showed a significant increase in the intensity and duration of ERK and Akt activation, whereas no significant differences were observed in terms of PLCγ2 and JNK phosphorylation or degradation of the NF-κB inhibitor IκB.
BJAB B cells transfected with ZAP-70 show stronger and prolonged activation of Syk, ERK, and Akt after BCR stimulation. Parental and ZAP-70–transfected BJAB B cells were stimulated with anti-IgM for the indicated times and analyzed by immunoblotting with phospho-ZAP-70Tyr319/SykTyr352, phospho-PLCγ2Tyr1217, IκB-α, phospho-JNKThr183/Tyr185, phospho-ERK1/2Thr202/Tyr204, and phospho-AKTSer493 antibodies. To ensure equal loading, membranes were stripped and reprobed with anti-Syk (top) or anti-ERK1/2 and anti–β-actin (bottom).
BJAB B cells transfected with ZAP-70 show stronger and prolonged activation of Syk, ERK, and Akt after BCR stimulation. Parental and ZAP-70–transfected BJAB B cells were stimulated with anti-IgM for the indicated times and analyzed by immunoblotting with phospho-ZAP-70Tyr319/SykTyr352, phospho-PLCγ2Tyr1217, IκB-α, phospho-JNKThr183/Tyr185, phospho-ERK1/2Thr202/Tyr204, and phospho-AKTSer493 antibodies. To ensure equal loading, membranes were stripped and reprobed with anti-Syk (top) or anti-ERK1/2 and anti–β-actin (bottom).
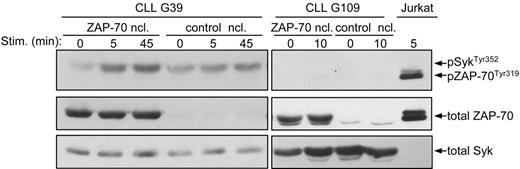
To verify that ZAP-70 also enhances BCR signaling in CLL B cells, we introduced by nucleofection a ZAP-70 expression vector into ZAP-70− CLL B cells. In repeated experiments this procedure allowed us to achieve high levels of ZAP-70 expression in more than 60% of primary CLL B cells. BCR ligation of the transiently transfected CLL B cells showed a substantial increase in the phosphorylation of SykTyr352 in the absence of ZAP-70 activation (CLL sample G39 in Figure 6). However, this effect was not observed in all experiments and, in particular, was not observed with samples where no BCR-induced Syk activation could be detected following transfection with the control vector (CLL sample G109 in Figure 6). Considering that cell viability after nucleofection was always above 50%, these experiments suggest that ZAP-70 can enhance activation of Syk only in CLL B-cell samples that are capable of responding to IgM ligation.
Analysis of ZAP-70 and Syk activation in transiently transfected primary CLL B cells. Primary CLL B cells lacking endogenous ZAP-70 were transiently transfected by nucleofection (ncl) with ZAP-70 or with empty vector as control. Sixteen hours after nucleofection the cells where stimulated for the indicated times with anti-IgM. Immunoblotting was performed with phospho-ZAP-70Tyr319/SykTyr352 antibody, followed by anti–ZAP-70 and anti-Syk.
Analysis of ZAP-70 and Syk activation in transiently transfected primary CLL B cells. Primary CLL B cells lacking endogenous ZAP-70 were transiently transfected by nucleofection (ncl) with ZAP-70 or with empty vector as control. Sixteen hours after nucleofection the cells where stimulated for the indicated times with anti-IgM. Immunoblotting was performed with phospho-ZAP-70Tyr319/SykTyr352 antibody, followed by anti–ZAP-70 and anti-Syk.
ZAP-70 down-regulates ligand-mediated BCR internalization in BJAB B cells
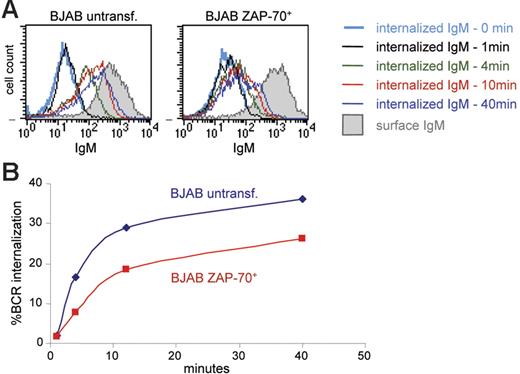
The prolonged and enhanced activation of Syk, ERK, and Akt, despite negligible or absent catalytic activation of ZAP-70, raised the possibility that ZAP-70 may be affecting events that regulate the duration of the signaling response, such as the rate of BCR internalization. This possibility was examined by analyzing the percentage of BCR molecules internalized at various time points following IgM ligation in parental and ZAP-70+ BJAB B cells. Cell-surface BCRs were first stained on ice with R-PE–coupled F(ab′)2 anti-IgM and then incubated at 37°C to allow BCR internalization to proceed. Internalization was stopped by placing the samples on ice, and the fluorescent F(ab′)2 remaining on the cell surface was stripped with a brief acid wash. The amount of internalized IgM was then quantified by flow cytometry, based on the R-PE fluorescence intensity of the internalized F(ab′)2-IgM complex, and the rate of internalization was calculated as the portion of internalized signal relative to the signal from surface IgM labeling. Analysis of parental and transfected BJAB B cells showed that the fluorescence from the intracellular F(ab′)2-IgM complexes increases over time, consistent with internalization of the BCR complex (Figure 7A). However, the MFI at all time points was considerably lower in ZAP-70+ BJAB cells. Quantification of the percentage of internalized BCR molecules from 3 independent experiments revealed that BCR internalization is reduced by approximately 30% to 40% in the presence of ZAP-70 (Figure 7B). These data suggest that the enhancement in BCR signaling related to ZAP-70 expression may be mediated by the ability of ZAP-70 to down-modulate BCR internalization.
Decreased BCR internalization in ZAP-70+ BJAB B cells. (A) Histograms show IgM staining with R-PE F(ab′)2 in parental and ZAP-70 transfected BJAB B cells. Open histograms show staining of cells that were acid treated to inactivate the fluorescence of cell surface R-PE and thus represent the signal of internalized BCR; shaded histograms correspond to untreated cells stained at 4°C and provide a measure of the starting amount of R-PE F(ab′)2 bound to surface IgM. (B) Percentage of BCR internalization at various time points in wild-type BJAB B cells and ZAP-70+ stable transfectant clones, calculated as described in “Patients, materials, and methods.” Values represent means from 3 independent experiments that were performed with 2 different ZAP-70 transfectant clones (clone A and clone D).
Decreased BCR internalization in ZAP-70+ BJAB B cells. (A) Histograms show IgM staining with R-PE F(ab′)2 in parental and ZAP-70 transfected BJAB B cells. Open histograms show staining of cells that were acid treated to inactivate the fluorescence of cell surface R-PE and thus represent the signal of internalized BCR; shaded histograms correspond to untreated cells stained at 4°C and provide a measure of the starting amount of R-PE F(ab′)2 bound to surface IgM. (B) Percentage of BCR internalization at various time points in wild-type BJAB B cells and ZAP-70+ stable transfectant clones, calculated as described in “Patients, materials, and methods.” Values represent means from 3 independent experiments that were performed with 2 different ZAP-70 transfectant clones (clone A and clone D).
Discussion
This study demonstrates that activation of ZAP-70 is extremely inefficient in BCR-stimulated B cells that express normal levels of the homologous kinase Syk. In ZAP-70+ CLL B cells we could not detect phosphorylation of the Tyr319 and Tyr493 residues that are required for catalytic activation of ZAP-70, although phosphorylation of the corresponding Tyr352 and Tyr526 residues in Syk was readily detectable with the same phospho-specific antibodies. Phosphorylation of Tyr319 was also not seen when ZAP-70− CLL B cells were transiently transfected with a ZAP-70 expression vector. In addition, we observed only weak phosphorylation of Tyr319 in transfected BJAB lymphoma B cells in which ZAP-70 was overexpressed at several-fold higher levels than in CLL B cells or in Jurkat T cells. However, even in these cells phosphorylation of Tyr319 in response to IgM ligation was at least 30 times less efficient than phosphorylation of the corresponding Tyr352 in Syk. Moreover, phosphorylation of Tyr493 was not detected in anti-IgM–stimulated BJAB transfectants but was efficiently induced by CD3 ligation in Jurkat T cells. Together, these data show that activation of ZAP-70 is very inefficient in CLL and lymphoma B cells and is negligible when compared to activation of Syk.
The inefficient phosphorylation of the activating tyrosines in ZAP-70 was unexpected, considering that in Syk-deficient avian lymphoma B cells ZAP-70 has been shown to reconstitute several proximal signaling events that require its catalytic activation, such as tyrosine phosphorylation of cellular proteins or mobilization of intracellular calcium.14 The possibility that the inefficient activation of ZAP-70 in BJAB B cells was somehow related to the expression vector was excluded by showing that transfected ZAP-70 is equally phosphorylated as the endogenous protein in Jurkat T cells. We also considered the possibility that BJAB and CLL B cells may lack a signaling molecule required for ZAP-70 activation. A possible candidate was the Src family kinase Lck, which in T cells phosphorylate Tyr319 following recruitment of ZAP-70 to phosphorylated ITAM sequences. Most B cells do not express Lck and instead express the homologous kinase Lyn, whose capacity to activate ZAP-70 is uncertain. However, transient expression of Lck in ZAP-70+ BJAB cells did not induce phosphorylation of Tyr319 following BCR stimulation (Figure S3). Moreover, previous studies have shown that CLL B cells express Lck, and this was confirmed in the majority of our cases (Majolini et al33 and data not shown). Another possible explanation for the inefficient phosphorylation of the activating tyrosines in ZAP-70 is that this protein does not acquire the correct spatial orientation following recruitment to the BCR, reminiscent to what is observed in T cells stimulated with altered peptide ligands.34 Consistent with this possibility, it was recently shown that ZAP-70 is associated with the chaperone protein Hsp90 in CLL B cells but not in T cells, indicating that the folding and conformation of ZAP-70 may differ between these cell types.35 Finally, the inefficient activation of ZAP-70 in the presence of Syk may reflect a greater capacity of the latter kinase to associate with the phosphorylated ITAMs, which could be a limiting step in the activation of these kinases.30,36
In T cells, ZAP-70 interacts with several signaling molecules or adaptor proteins that propagate or regulate the BCR-derived signal. These interactions are mediated by phosphorylation of other tyrosine residues, such as Tyr292 and Tyr474, which are required for recruitment of PI3K, c-Cbl, Cbl-b, and Shc. ZAP-70 interacted with these proteins in CLL B cells following IgM ligation, whereas in transfected BJAB B cells the interactions were constitutive. Presumably, these constitutive associations could have been provoked by the significant overexpression of ZAP-70, although some constitutive association with c-Cbl and Cbl-b was also observed in Jurkat T cells. Regardless, these results show that ZAP-70 associates with antigen receptor-signaling molecules in B lymphocytes, which is consistent with previous observations that ZAP-70 is recruited to the BCR complex in CLL B cells.11
Despite the inefficient activation of ZAP-70, we observed stronger and prolonged BCR-induced phosphorylation of positive regulatory residues in Syk, ERK, and Akt in BJAB B cells transfected with ZAP-70. Moreover, phosphorylation of Syk at Tyr352 was stronger and prolonged when ZAP-70 was transiently transfected in primary CLL B cells. This enhancement of BCR signaling is consistent with the work of Chen et al who observed increased BCR-induced phosphorylation of Syk, BLNK, and PLCγ in CLL B cells transduced with ZAP-70.15 Considering that ZAP-70 does not become fully activated in these cells, it is not readily apparent how ZAP-70 enhances BCR signaling. One possible explanation is that ZAP-70 functions as an adaptor protein that facilitates the recruitment of other signaling molecules to the activated BCR. The associations with PI3K and Shc are noteworthy in this respect because these proteins are involved in the activation of Akt and ERK, respectively.
A second possibility is that catalytically inactive ZAP-70 could decrease activation of negative regulators of BCR signaling or inhibit events that terminate the signaling response. This hypothesis is intriguing in view of the associations between ZAP-70 and the c-Cbl and Cbl-b ubiquitin ligases, which negatively regulate BCR and TCR signaling by promoting ligand-induced down-modulation of the antigen receptors and by targeting for degradation specific BCR and TCR components.22,29,37–39 Consistent with this hypothesis, we observed that ligand-induced BCR internalization was reduced by approximately one third in ZAP-70+ BJAB B cells. Decreased ligand-mediated BCR internalization has already been shown to increase the magnitude and duration of BCR signaling, including activation of the Akt and ERK kinases.40,41
We investigated the effect of ZAP-70 on BCR internalization only in BJAB B cells, and therefore it remains to be demonstrated that ZAP-70 also down-modulates BCR internalization in CLL B cells. However, it should be noted that ZAP-70 is expressed not only in CLL B cells, but rather in a wide range of normal and malignant human B-cell subsets.6,7,42 Therefore, the effect of ZAP-70 on BCR internalization is likely to represent a more general mechanism through which this protein modulates the magnitude and duration of BCR signaling.
Authorship
Contribution: S.G. and D.G.E. designed the study, performed research, and analyzed the data; P.G.L. performed research and analyzed data; L.L., S.S., and G.L. provided patient material, data, and critical suggestions; and D.G.E. supervised the study and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dimitar Efremov, International Centre for Genetic Engineering and Biotechnology (ICGEB) Outstation-Monterotondo, Consiglio Nazionale delle Ricerche (CNR) Campus “Adriano Buzzati-Traverso,” Via E. Ramarini 32, I-00016 Monterotondo Scalo, Rome, Italy; e-mail: efremov@icgeb.org.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by the Leukemia and Lymphoma Society grant 6043-06 (D.G.E.).
The authors would like to thank Dr Michele Pelosi and Dr Egidio Romano for useful suggestions and critical reading of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal