Abstract
Trogocytosis is the uptake of membrane fragments from one cell by another and has been described for immune cells in mice and humans. Functional consequences of trogocytosis are emerging, but a dramatic immune function has still to be associated with it. Here we show that some resting, and most activated, CD4+ and CD8+ T cells acquire immunosuppressive HLA-G1 from antigen-presenting cells (APCs) in a few minutes. Acquisition of HLA-G through membrane transfers does not change the real nature of the T cells but immediately reverses their function from effectors to regulatory cells. These regulatory cells can inhibit allo-proliferative responses through HLA-G1 that they acquired. These data demonstrate that trogocytosis of HLA-G1 leads to instant generation of a new type of regulatory cells, which act through cell-surface molecules they temporarily display but do not express themselves. Such regulatory cells whose existence is most likely limited in space and time might constitute an “emergency” immune suppression mechanism used by HLA-G–expressing tissues to protect themselves against immune aggression. In addition, T cells acquire from HLA-G–expressing APCs their HLA-G–dependent capability to induce the slower differentiation of regulatory cells that act independently of HLA-G. These data re-emphasize the significance of HLA-G expression in normal and pathologic situations.
Introduction
“Trogocytosis” is a new name for “fast, cell-to-cell contact-dependent uptake of membranes and associated molecules.”1 Trogocytosis has been documented in T and B lymphocytes, natural killer (NK) cells, antigen-presenting cells (APCs), and tumor cells (reviewed in Hudrisier and Bongrand2 ). Most of the work on trogocytosis by T cells was done in the murine system, in which it was shown that CD4+ and CD8+ T cells acquired APC major histocompatibility complex (MHC) class II and MHC class I molecules, respectively, in an antigen-specific manner3–7 : for trogocytosis to occur, T-cell receptor (TCR) engagement was necessary, and activation or anti-CD3 increased trogocytosis efficiency.8,9 Yet, there is no strict dependence of trogocytosis on TCR engagement: (1) in some systems, trogocytosis was shown to depend on CD28 engagement5 ; and (2) transfer of MHC class II antigens from APCs to T cells can occur in an all-autologous system.8 Even though most of the work on APC material acquisition by T cells was done on MHC molecules, it was shown that costimulatory molecules B7-1 (CD80), B7-2 (CD86), and ICAM-1 (CD54) are also acquired by murine antigen-specific T cells.5,10 Trogocytosis is a transfer of membrane fragments, not of individual molecules. Consequently, all molecules contained within a certain membrane area are transferred from one cell to another during trogocytosis, including some that might have nothing to do with the acquirer cell, or participate in cell-to-cell cross-talk. Thus, during trogocytosis, some molecules transfer passively. This was clearly evidenced by showing that CD8+ T cells can acquire MHC class II molecules along with the MHC class I complexes they are specific for5,11 and that the reverse is true for CD4+ T cells.11 Recently, trogocytosis of HLA-DR and CD80 from APCs by T cells was evidenced in humans and was shown to follow the same rules as in the murine system.8,12
Functionally, it was shown that CD8+ T cells that had acquired their cognate MHC class I ligands became susceptible to “fratricide” antigen-specific cytolysis.3,6 The authors speculated that this type of trogocytosis might contribute to effector clearance. Furthermore, after acquisition of HLA-DR and CD80, T cells could stimulate resting T cells in an antigen-specific manner and thus behaved as APCs themselves.8,10,12,13 Transfer of stimulatory membrane portions, which have also been called “presentasomes,”14 might constitute a cheap and efficient way of increasing presentation/stimulation capabilities of the immune system. Yet, APC-like T cells that arose via trogocytosis might not functionally compete with the professional APCs they took HLA-DR from, and this makes their in vivo functional contribution to immune responses unresolved. Finally, MHC class II and CD80 acquired from APCs by CD4+ T cells regulated T-cell proliferative signals and sustained their activation in the absence of APCs.14 In this case, trogocytosis might provide added value to an immune response because it directly affects the biology of the cell that has acquired CD80 by providing proactivation intracellular signals. However, this might not have a major impact on immune responses because, although it might be crucial on a quantitative standpoint as an immune-response booster, it should not induce qualitative changes (ie, in the repertoire or in the function of antigen-selected cells).
We reasoned that trogocytosis would be most useful if, instead of HLA-DR, which is expressed by every APC, it concerned a molecule that would be unusual (ie, rarely expressed) and/or functionally atypical. In this case, trogocytosis would confer unusual phenotypical, and possibly functional, characteristics to T cells. HLA-G is one such molecule: it is a nonclassical HLA class I molecule characterized by a strong immunosuppressive function and a tissue-restricted expression in nonpathologic conditions and a neoexpression in some cancers, transplantations, viral infections, and inflammatory and autoimmune diseases. HLA-G inhibits the functions of NK cells and cytotoxic T-lymphocytes (CTLs; reviewed in Carosella et al15 ), inhibits allogeneic responses,16,17 induces regulatory cells,16–18 up-regulates inhibitory receptor expression,19 and inhibits dendritic-cell maturation.18 This has positioned HLA-G as a molecule capable of significantly contributing to tolerance of allografts17,20,21 and immune escape of tumors22–26 and virus-infected cells.27,28 In pathologic contexts, HLA-G is often expressed by antigen-presenting cells that infiltrate lesions (reviewed in Carosella et al15 ). HLA-G pathologic expression has been proposed as a mechanism that cells use to protect themselves from destruction by cytotoxic effectors and/or as a mechanism aimed at dampening the extent of an immune reaction or even stopping it.
Here, we investigated whether HLA-G1 could be transferred from APCs to T cells and whether trogocytosis of HLA-G1 had functional significance.
Materials and methods
Cells and cell lines
Blood was obtained from healthy volunteers after informed consent according to the Declaration of Helsinki under a protocol approved by the Institutional Review Board of the St Louis, Paris. Peripheral-blood mononuclear cells (PBMCs) from healthy volunteer donors were used as is (resting PBMCs) or after activation. Different activation protocols were used: (1) 48-hour PHA activation (4 μg/mL phytohemagglutinin; Sigma, St Louis, MO) followed or not by a 24- to 48-hour culture in medium supplemented with 100 IU IL-2 (Sigma), thereafter referred to as PHA/IL-2 activation; (2) 48-hour CD3/CD28 activation using magnetic beads coated with equal amounts of anti-CD3 and anti-CD28; (3) 3-day activation with 25 ng/mL of anti-CD3 (OKT3 kindly provided by Janssen-Cilag); or (4) 5-day stimulation by allogeneic LCL-RSV cells.
Lymphoblastoid LCL 721.221 cells (LCL; ATCC, Manassas, VA) transfected with the pRc/RSV vector (Invitrogen, Carlsbad, CA) alone (LCL-RSV) or containing the HLA-G1 cDNA (LCL–HLA-G1) have been described19 and were used as “HLA-G1 donor cells” and their controls in trogocytosis assays.
Monocytes were obtained by adherence on plastic plates, and HLA-G1–expressing monocytes were obtained by a 48-hour stimulation by 500 IU of IFNG, as described elsewhere.29
Trogocytosis assays
For trogocytosis assays, resting or activated PBMCs (“acquirer” cells) were cocultured with LCL-RSV or LCL–HLA-G1 cells (“donor” cells) at a 0.5:1 donor-acquirer ratio, a total concentration of 106 to 107 cells/mL, and at 37°C in a 5% CO2, humidified incubator. At the end of the coincubation, cells were placed on ice and all further steps were performed at less than 4°C. When indicated in the text, purification of CD4+ or CD8+ T cells was subsequently performed. Depending on the experimental requirements, cells were labeled with anti-CD4–PE and then positively separated using anti-PE magnetic beads (BD Biosciences, San Jose, CA) according to the manufacturer's specifications. Purifications were systematically checked by flow-cytometry analysis, and purified fractions were used only if more than 95% pure. Acquisition of donor cell–derived molecules by acquirer cells was investigated by flow cytometry. If required, T cells purified after coincubation with APCs were maintained in culture in medium supplemented with 100 IU IL-2.
Antibodies and flow cytometry
Classical antibodies used in this study are described in Document S1 (available on the Blood website; see the Supplemental Documents link at the top of the online article). Anti–HLA-G1 MEMG/09 monoclonal antibody (mAb) conjugated to FITC or PE and blocking anti–HLA-G1 mAb 87G, which is known to block the interaction of HLA-G1 with ILT2,30 were obtained from Exbio (Prague, Czech Republic). Anti–HLA-G mAb 4H84 (kind gift of M. Mc Master, University of California, San Francisco) was used in Western blot and confocal analyses.
For blocking experiments, donor cells or CD4+HLA-Gacq+ T cells were preincubated for 30 minutes at 37°C with 10 μg/mL of anti–HLA-G 87G or isotypic control, or with 5 μg/mL anti-TCRα/β, anti-CD3, anti–pan–HLA class I W6-32, anti-CD85j/ILT2, anti-CD28, anti-CD54, or isotypic controls, and then used.
For flow-cytometry analyses, Fc receptors were blocked by a 30-minute incubation in PBS containing 20% human serum, and isotype-matched control antibodies were systematically used to evaluate nonspecific binding. Flow-cytometry analyses were performed on an Epics XL cytometer (Beckman Coulter, Hialeah, FL) using EXPO32 software (Beckman Coulter).
Transwell experiments
Transwell experiments were performed using Transwell culture system (Greiner Bio-One, Kremsmünster, Austria): T cells were cultured in the upper chamber of a 12-well plate and separated from the LCL-RSV or LCL-HLA-G1 cells by a 0.4-μm–pore-size membrane.
Surface biotinylation experiments
LCL-RSV or LCL–HLA-G1 cells (20 × 106) were biotinylated in PBS containing 200 μg/mL sNHS-Biotin (Interchim, Montluçon, France) for 30 minutes at 4°C, and the reaction was quenched by addition of 50 mM glycine (Sigma) for 5 minutes before PBS wash. Half (10 × 106 cells) of the biotinylated LCL-RSV or LCL–HLA-G1 cells were then incubated with PBMCs precoated with anti-CD4–PE and the other half were incubated with PBMCs precoated with anti-CD8–PE in a 1-hour trogocytosis assay. Subsequently, CD4+ or CD8+ T cells were purified using anti-PE magnetic beads (BD Biosciences) as described above under “Trogocytosis assay” and lysed in 400-μL lysis buffer (100 mM Tris-HCl, pH 7.4; 1% Triton X-100; 150 mM NaCl; 5 mM EDTA; Complete, Roche Diagnostics, Meylan, France). The negative fractions, which contained LCL-RSV or LCL–HLA-G1 cells, were labeled with anti-ILT3–PC5 or MEMG/09-PE, coated with anti-PE magnetic beads, and lysed. HLA-G and ILT3 bound to anti-PE beads were then extracted using magnetic separation to serve as positive controls for biotinylated HLA-G and biotinylated ILT3. All lysates were washed in PBS prior to separation on a 6% polyacrylamide gel, transferred to nitrocellulose membranes, and Western blotted using streptavidin-HLR (Amersham Biosciences; LCL-containing wells) or anti–HLA-G 4H84 followed by goat anti–mouse HRP (DAKO, Carpinteria, CA; wells containing immunoprecipitated fractions of CD4+ and CD8+ cells). After washing, membranes were treated with enhanced chemiluminescence reagent (Amersham Pharmacia Biotech, Cambridge, United Kingdom) and exposed to X-ray film (Hyperfilm; Kodak, Rochester, NY).
Confocal microscopy
T cells and LCL-HLA-G1 cells were loaded for 10 minutes at 37°C with either 0.5 μM BODIPY 630 or 0.5μM Orange-CMTMR Cell Tracker (Molecular Probes, Leiden, The Netherlands), respectively. T cells were then conjugated with LCL-HLA-G1 at 37°C for different times and left adhered on poly-L-lysine-coated slides for 1 minute at 37°C. The cells were then fixed for 10 minutes with 3% paraformaldehyde and permeabilized for 5 min with 0.1% Triton X-100. Staining was performed with anti–HLA-G1 (4H84) mAb followed by FITC-labeled goat antimouse mAb (Immunotech, Marseille, France). The samples were mounted in 90% glycerol–PBS containing 2.5% 1-4-diazabicyclo (2.2.2) octane (DABCO; Sigma, St. Louis, MO). Images were acquired by confocal microscopy on a Zeiss LSM 510 META confocal microscope (Zeiss, Jena, Germany) with a Plan Apochromat 63×/1.4 NA objective using LSM510 software version 3.2 (Zeiss). Images were then assembled and RGB format changed to CMJN using Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
T-cell stimulation assays
For stimulation of already activated CD4+ T cells before trogocytosis and CD4+HLA-G1acq+ T cells and their CD4+HLA-G1acq− controls, we used the following.
Cytokine stimulation.
CD4+ T cells (5 × 105) taken prior to trogocytosis, CD4+HLA-G1acq+ T cells, and their CD4+HLA-G1acq− controls were cultured in a 96-well plate with addition or not of 100 IU IL-2 immediately after trogocytosis followed by purification. Proliferation was measured by tritiated thymidine incorporation (1 μCi [0.037 MBq]/well; Amersham Biosciences) 0, 24, 48, and 72 hours after addition of IL-2.
Allo-stimulation.
Responder cells (105; purified CD4+HLA-G1acq+ or CD4+HLA-G1acq− T cells) were cocultured with 105 γ-irradiated allogeneic stimulator PBMCs (25 Gy) or 5 × 104 γ-irradiated allogeneic stimulator LCL-RSV cells (75 Gy) and plated in a final volume of 150 μL per well. All samples were run in triplicate, and for each allogeneic combination, responder cells alone, irradiated stimulated cells alone, and autologous controls were included. After 4 days, cultures were pulsed with tritiated thymidine (1 μCi [0.037 MBq]/well; Amersham Biosciences). 3 H thymidine incorporation into DNA was quantified 18 hours later on a β-counter (Wallac 1450; Amersham Biosciences).
Tetanus-toxin stimulation.
Fresh PBMCs (2 × 105) were stimulated for 5 days in a 96-well plate in culture medium containing 5 μg/mL Tetanus Toxin C fragment (Sigma), and their antigen-specific response was evaluated by thymidine incorporation as described above.
Suppressor assays
To evaluate the regulatory function of T cells after trogocytosis, T cells of interest and their controls were purified and γ irradiated (25 Gy). These purified cells were then used as irradiated third-party cells autologous to responder cells in mixed-lymphocyte reaction (MLR) at various suppressor-effector-stimulator ratios or as autologous third-party cells in tetanus-toxin stimulation assay at a 1:1 responder PBMC–suppressor ratio.
Statistical analyses
Data are presented as means ± standard deviation (SD). Student t test was used and a P value less than .05 was taken to be significant. For figures showing representative experiments, error bars represent SD of triplicates.
Results
T cells acquire HLA-G1 from APCs by trogocytosis
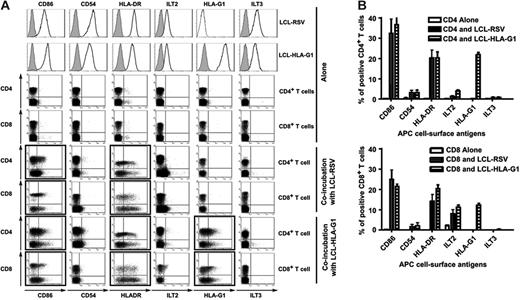
We investigated whether membrane-bound HLA-G1 could be acquired by T cells from HLA-G1–expressing APCs by trogocytosis. We chose this model because membrane transfers from APCs to T cells are well characterized2 and because HLA-G1–expressing APCs are described in various pathologies.21,28,31 We used HLA-G1–transfected LCL-721.221 cells (LCL–HLA-G1) and their mock-transfected controls (LCL-RSV) as HLA-G1–expressing APCs. In our experiments, APCs are “donor” cells, and T cells from PBMCs of healthy volunteer donors are “acquirer” cells. Thus, we set up 1-hour cocultures between “donor” APCs and “acquirer” allogeneic T cells, following which T-cell surface expression of molecules originally present only on APCs was investigated by flow cytometry. Figure 1A shows raw data obtained for 1 representative experiment, and Figure 1B shows the results obtained for 10 independent experiments expressed as means ± SD. As can be seen prior to coincubation, LCL-RSV and LCL–HLA-G1 cells but not CD4+ or CD8+ T cells expressed CD86, CD54, HLA-DR, ILT2, and ILT3, and only LCL–HLA-G1 expressed HLA-G1. However, after 1 hour of contact with either APCs, about 35% of CD4+ T cells and 25% of CD8+ T cells displayed CD86, 20% of CD4+ and CD8+ T cells displayed HLA-DR, 20% of CD4+ and 15% of CD8+ T cells displayed HLA-G (only when coincubated with LCL–HLA-G1), 5% of CD4+ and 10% of CD8+ T cells displayed ILT2, 3% to 5% of CD4+ or CD8+ T cells displayed CD54, and no T cells displayed ILT3. Longer coincubation times did not significantly increase these proportions. The fact that ILT3 was never found on T cells allowed us to use this molecule as an APC-specific marker in the rest of our experiments.
Cell-surface expression of HLA-G1 and APC-produced molecules on T cells cultured with LCL and LCL–HLA-G1 cells. Phenotype of T cells prior to and after a 1-hour coincubation with LCL and LCL–HLA-G1 cells. Cell-surface expression of the indicated molecules was investigated by flow cytometry for CD4+ and CD8+ T cells and HLA-G1–negative LCL-RSV and HLA-G1–positive LCL–HLA-G1 cells alone; for CD4+ and CD8+ T cells incubated with LCL-RSV cells; and for T cells incubated with LCL–HLA-G1 cells. (A) Raw data from 1 representative experiment. T cells were identified by size and CD3 expression. Filled histograms represent isotypic controls. (B) Results obtained for n = 6 independent experiments. Results expressed as percentage of CD4+ or CD8+ T cells expressing the indicated molecules (mean ± SD).
Cell-surface expression of HLA-G1 and APC-produced molecules on T cells cultured with LCL and LCL–HLA-G1 cells. Phenotype of T cells prior to and after a 1-hour coincubation with LCL and LCL–HLA-G1 cells. Cell-surface expression of the indicated molecules was investigated by flow cytometry for CD4+ and CD8+ T cells and HLA-G1–negative LCL-RSV and HLA-G1–positive LCL–HLA-G1 cells alone; for CD4+ and CD8+ T cells incubated with LCL-RSV cells; and for T cells incubated with LCL–HLA-G1 cells. (A) Raw data from 1 representative experiment. T cells were identified by size and CD3 expression. Filled histograms represent isotypic controls. (B) Results obtained for n = 6 independent experiments. Results expressed as percentage of CD4+ or CD8+ T cells expressing the indicated molecules (mean ± SD).
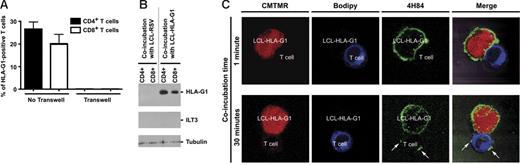
We then investigated whether the new cell-surface expression of HLA-G1 by T cells after coincubation with HLA-G1–expressing APCs was due to trogocytosis. To assess cell-to-cell contact dependence, we set up transwell experiments in which LCL–HLA-G1 and T cells were separated by a semipermeable 0.4-μM–pored membrane or were in contact with each other for 1 hour. Figure 2A shows that prevention of cell-to-cell contact between LCL–HLA-G1 cells and T cells also prevented HLA-G1 cell-surface neoexpression by T cells. This means that cell-to-cell contact is necessary for HLA-G1 to neodisplay by T cells. Next, we investigated whether HLA-G1 found on T cells after coincubation with LCL–HLA-G1 cells was of LCL–HLA-G1 origin. For this purpose, we biotinylated the surface molecules of LCL–HLA-G1 cells and their controls prior to coincubation with allogeneic T cells. After coincubation, T cells were purified and the biotinylation status of the HLA-G1 molecules at their cell surface was determined by Western blotting. Since ILT3 was never found on T cells after coincubation, this molecule was used to assess the purity of the T cells and possible contamination with biotinylated HLA-G1 from LCL–HLA-G1 cells. As can be seen in Figure 2B, no ILT3 was recovered from the T-cell populations analyzed, which shows that contamination by LCL cells, if any, was below detection. Furthermore HLA-G1 recovered from purified CD4+ and CD8+ T cells after coincubation with LCL–HLA-G1 cells was biotinylated, which proves its APC–HLA-G1 origin. These experiments demonstrated that HLA-G1 molecules had been transferred from APC–HLA-G1 to T cells. We next ensured that all T-cell surface HLA-G1 was of APC–HLA-G1 origin and not in part endogenously produced. This was a possibility since T cells might express HLA-G mRNA, as previously reported.28,32 For this purpose, we observed HLA-G1 neoexpression by T cells coincubated with LCL–HLA-G1 by confocal microscopy (Figure 2C). Prior to coincubation, LCL–HLA-G1 cells, whose cytoplasms had been labeled in red, expressed HLA-G1 (in green) both intracellularly and at their cell surface. By contrast, CD4+ T cells, whose cytoplasms had been labeled in blue, did not express endogenous HLA-G1 intracellularly or at their cell surface. After one minute of contact between LCL–HLA-G1 and CD4+ T cells, contact areas between LCL–HLA-G1 cells and T cells were clearly visible but expression of HLA-G by CD4+ T cells was not evident, and no intracellular HLA-G was detected in these cells. After 30 minutes of contact, multiple HLA-G1–positive areas were clearly seen on the surface of CD4+ T cells, some of which were located outside of the contact area with LCL–HLA-G1 cells, whereas intracellular HLA-G was still not detectable. These additional patches might indicate multiple contacts with LCL–HLA-G1 cells and/or membrane movements. Taken together, these data show that upon contact between LCL–HLA-G1 cells and T cells, membrane fragments that include HLA-G1 are transferred from the APCs onto the T cells by trogocytosis. These T cells will thereafter be referred to as HLA-G1acq+ T cells.
HLA-G1 is acquired by T cells from APCs by trogocytosis. (A) Transfer of HLA-G1 from APCs to T cells is cell-to-cell contact dependent. CD4+ and CD8+ T cells were coincubated with HLA-G1–transfected LCL–HLA-G1 cells (No transwell) or separated by a 0.4-μM–pored membrane (Transwell) for 1 hour, prior to flow-cytometry analysis of HLA-G1 expression. Results are expressed as mean ± SD of n = 8 independent experiments. (B) HLA-G1 that is newly displayed by CD4+ and CD8+ T cells is of APC origin and not endogenously produced. Biotinylation of LCL-RSV and LCL–HLA-G1 cell surface was performed prior to coincubating them for 1 hour with resting T cells from PBMCs. CD4+ and CD8+ T cells were then purified and lysed, and the obtained lysates were analyzed by Western blotting. For each sample, protein content was controlled using antitubulin, and the absence of contamination by LCL cells was controlled using anti-ILT3. Results shown are representative of 3 independent experiments. (C) Visualization of trogocytosis of HLA-G from APCs by T cells by confocal microscopy. Red is cytoplasmic label of LCL–HLA-G1 cells with CMTMR. Blue is cytoplasmic labeling of T cells with Bodipy. Green is HLA-G labeling using 4H84 mAb and then FITC-conjugated goat antimouse secondary antibody. Arrows indicate HLA-G transferred from APCs to T cells after the indicated time of coincubation.
HLA-G1 is acquired by T cells from APCs by trogocytosis. (A) Transfer of HLA-G1 from APCs to T cells is cell-to-cell contact dependent. CD4+ and CD8+ T cells were coincubated with HLA-G1–transfected LCL–HLA-G1 cells (No transwell) or separated by a 0.4-μM–pored membrane (Transwell) for 1 hour, prior to flow-cytometry analysis of HLA-G1 expression. Results are expressed as mean ± SD of n = 8 independent experiments. (B) HLA-G1 that is newly displayed by CD4+ and CD8+ T cells is of APC origin and not endogenously produced. Biotinylation of LCL-RSV and LCL–HLA-G1 cell surface was performed prior to coincubating them for 1 hour with resting T cells from PBMCs. CD4+ and CD8+ T cells were then purified and lysed, and the obtained lysates were analyzed by Western blotting. For each sample, protein content was controlled using antitubulin, and the absence of contamination by LCL cells was controlled using anti-ILT3. Results shown are representative of 3 independent experiments. (C) Visualization of trogocytosis of HLA-G from APCs by T cells by confocal microscopy. Red is cytoplasmic label of LCL–HLA-G1 cells with CMTMR. Blue is cytoplasmic labeling of T cells with Bodipy. Green is HLA-G labeling using 4H84 mAb and then FITC-conjugated goat antimouse secondary antibody. Arrows indicate HLA-G transferred from APCs to T cells after the indicated time of coincubation.
Parameters of HLA-G1 trogocytosis by T cells
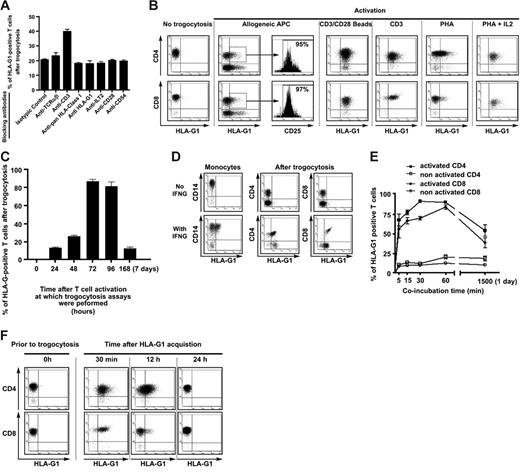
In order to gain insight into the mechanisms underlying the transfer of HLA-G from APCs to T cells, we used monoclonal antibodies to selectively block TCR-HLA interaction, CD28-B7 interaction, and CD54-LFA interaction. We chose to block these interactions because it was reported that they drove trogocytosis in other systems.3,5,10 Furthermore, we used monoclonal antibodies to block the interaction of HLA-G with its receptors to investigate whether HLA-G1 transferred passively or actively. As shown in Figure 3A, blocking these interactions did not reduce trogocytosis of HLA-G1. These data show that since blocking the HLA-G1–LILRB1/ILT2 interaction using anti–HLA-G1 or anti-ILT2 mAbs had no effect, HLA-G1 does not need to interact with its receptors to be transferred. Rather, HLA-G1 seems to be dragged from one cell to another because it is part of the transferred membrane fragment. Furthermore, blocking data show that HLA-G1 trogocytosis is not exclusively mediated by CD28-B7, or MHC-TCR interaction, as was reported in other systems.3 Yet, since addition of anti-CD3 actually increased HLA-G1 transfer to T cells by 2-fold, these data also indicate that TCR engagement is likely to be required for trogocytosis of HLA-G1 by T cells.
Parameters of HLA-G1 trogocytosis. (A) Analysis of HLA-G1 transfer to resting T cells after blocking HLA-TCR, CD28-B7, and HLA-G–HLA-G receptor interactions. HLA-TCR interactions were blocked by anti-TCR, anti-CD3, and, to a certain extent, anti–pan–HLA class I blocking antibodies. CD28-B7 interactions were blocked by anti-CD28 antibodies. Interaction of HLA-G1 with its receptors was blocked by anti–HLA-G1, anti–pan–HLA class I, or anti-ILT2 antibodies. Results are expressed as mean ± SD of at least 3 independent experiments for each antibody. (B) HLA-G1 trogocytosis capabilities of activated CD4+ and CD8+ T cells. T cells were activated by the indicated methods, and their capability to acquire HLA-G1 from LCL–HLA-G1 cells was investigated by flow cytometry. Results shown are representative of more than 10 independent experiments. (C) Kinetics of trogocytosis capability acquisition by T cells upon PHA activation. Resting T cells were stimulated by PHA and then by IL-2 after the second day. The capability of T cells to acquire HLA-G1 from LCL–HLA-G1 cells was investigated by flow cytometry at the indicated times. Results shown are expressed as mean ± SD of more than 10 independent experiments. (D) Capabilities of activated CD4+ and CD8+ T cells to acquire HLA-G1 from nontransfected HLA-G1–positive autologous monocytes. T cells were activated by PHA+IL-2 treatment (see “Cells and cell lines” under “Materials and methods”), and their capability to acquire HLA-G1 from IFNG-stimulated, HLA-G1–positive autologous monocytes (With IFNG) and their HLA-G1–negative controls (No IFNG) was investigated by flow cytometry. Results shown are representative of 3 independent experiments. (E) Kinetics of HLA-G1 acquisition by resting and activated CD4+ and CD8+ T cells. Resting and activated T cells from the same donor were incubated with LCL–HLA-G1 cells and their cell-surface expression of HLA-G1 was investigated at the indicated times. Results are expressed as mean ± SD of 4 independent experiments. (F) Lifetime of acquired HLA-G at the T-cell surface. Flow-cytometry analysis of acquired HLA-G1 cell-surface expression by CD4+ and CD8+ T cells at the indicated times corresponding to culture time after purification. Results shown are representative of 3 independent experiments.
Parameters of HLA-G1 trogocytosis. (A) Analysis of HLA-G1 transfer to resting T cells after blocking HLA-TCR, CD28-B7, and HLA-G–HLA-G receptor interactions. HLA-TCR interactions were blocked by anti-TCR, anti-CD3, and, to a certain extent, anti–pan–HLA class I blocking antibodies. CD28-B7 interactions were blocked by anti-CD28 antibodies. Interaction of HLA-G1 with its receptors was blocked by anti–HLA-G1, anti–pan–HLA class I, or anti-ILT2 antibodies. Results are expressed as mean ± SD of at least 3 independent experiments for each antibody. (B) HLA-G1 trogocytosis capabilities of activated CD4+ and CD8+ T cells. T cells were activated by the indicated methods, and their capability to acquire HLA-G1 from LCL–HLA-G1 cells was investigated by flow cytometry. Results shown are representative of more than 10 independent experiments. (C) Kinetics of trogocytosis capability acquisition by T cells upon PHA activation. Resting T cells were stimulated by PHA and then by IL-2 after the second day. The capability of T cells to acquire HLA-G1 from LCL–HLA-G1 cells was investigated by flow cytometry at the indicated times. Results shown are expressed as mean ± SD of more than 10 independent experiments. (D) Capabilities of activated CD4+ and CD8+ T cells to acquire HLA-G1 from nontransfected HLA-G1–positive autologous monocytes. T cells were activated by PHA+IL-2 treatment (see “Cells and cell lines” under “Materials and methods”), and their capability to acquire HLA-G1 from IFNG-stimulated, HLA-G1–positive autologous monocytes (With IFNG) and their HLA-G1–negative controls (No IFNG) was investigated by flow cytometry. Results shown are representative of 3 independent experiments. (E) Kinetics of HLA-G1 acquisition by resting and activated CD4+ and CD8+ T cells. Resting and activated T cells from the same donor were incubated with LCL–HLA-G1 cells and their cell-surface expression of HLA-G1 was investigated at the indicated times. Results are expressed as mean ± SD of 4 independent experiments. (F) Lifetime of acquired HLA-G at the T-cell surface. Flow-cytometry analysis of acquired HLA-G1 cell-surface expression by CD4+ and CD8+ T cells at the indicated times corresponding to culture time after purification. Results shown are representative of 3 independent experiments.
Given the results obtained with anti-CD3 above, and since it had been reported that a 24-hour anti-CD3 treatment increased PBMCs' trogocytic capabilities,8 we investigated whether activation of the acquirer cell was crucial to HLA-G1 trogocytosis. For this purpose, we stimulated PBMCs from healthy donors by a 5-day allogeneic stimulation, or by anti-CD3 (48 hours), CD3-CD28 beads (48 hours), PHA (48 hours), or PHA (48 hours) followed by IL-2 treatment (48-72 hours), and evaluated their capabilities to uptake HLA-G1. These times corresponded to full activation and active proliferation of T cells based on CD25 expression and cluster formation in culture. For all antigen nonspecific stimulations, CD25 was expressed on more than 90% of CD3+ cells. As can be seen in Figure 3B, HLA-G1 was acquired by a significant proportion of allo-activated T cells, which were all CD25+ cells, indicating that these cells were indeed activated. Similarly, T-cell activation through TCR/CD3 cross-linking rendered all CD4+ and CD8+ T cells capable of capturing HLA-G1 from APCs. The same results were obtained with PHA+IL-2 activation, whereas PHA alone was less efficient (Figure 3B). These results show that trogocytosis of HLA-G1 depends on the activation status of the acquirer cell and, that once activated, T cells efficiently capture APC-produced molecules regardless of their antigen specificity. This is in accordance with results obtained in all-autologous murine systems.8
However, we found that T-cell populations reached maximal trogocytic capability only during the late stages of activation. As was observed for PHA-activated T cells in Figure 3B and as can be seen in Figure 3C, populations of PHA-activated T cells became of high trogocytic capability only if exogenous IL-2 was added to the culture and only if used for trogocytosis assays 72 to 96 hours after stimulation. If used before that time, T cells activated with PHA+IL-2 were not much more trogocytic than resting T cells. Similarly, it took 2 days of stimulation by anti-CD3 beads for T cells to reach maximum trogocytic capability. In addition to antigen challenge and TCR cross-linking, full activation seems to be a key factor to antigen nonspecific trogocytosis. Indeed, 7 days after activation, which corresponds to an almost complete return to resting stage after activation, T cells were no longer trogocytic (Figure 3C) unless their activation status was maintained by addition of exogenous IL-2 (data not shown). Since there was no difference between anti-CD28–coated bead activation and PHA+IL-2 activation of T cells with respect to trogocytic capability, we used these 2 means of generation indiscriminately. Results obtained were the same with either method. These results indicate that trogocytosis capability of T cells seems to occur in a strict time frame and at a precise activation stage. In fine, antigen-specific trogocytosis seems to concern only antigen-specific naive T cells, whereas antigen nonspecific trogocytosis concerns just any fully activated/differentiated effector T cell. Yet, since in vivo antigen specificity is required for full activation, it means that antigen specificity is always required at some point for trogocytosis.
The HLA-G1 transfectant cells that we used as donor cells expressed HLA-G at high levels that might not be physiologically relevant. In order to ensure that trogocytosis of HLA-G1 could happen in a more physiologic setting, we performed experiments using HLA-G1 naturally expressing IFNG-stimulated monocytes. Figure 3D shows that activated CD4+ and CD8+ T cells acquired HLA-G1 from monocytes to almost the same extent as from LCL–HLA-G1 cells, despite much lower cell-surface HLA-G1 expression levels on donor cells. This is consistent with a heterogeneous localization at the cell surface and acquisition of membrane portions of high HLA-G content such as rafts, as postulated elsewhere.2,33
One final difference between antigen-specific trogocytosis by resting T cells and antigen nonspecific trogocytosis by activated effectors is efficiency; Figure 3E shows that activated CD4+ and CD8+ T cells acquired more HLA-G1 from LCL–HLA-G1 cells with faster kinetics than their resting counterparts. Indeed, while the number of resting T cells that acquired HLA-G1 reached its maximum after 1 hour of coincubation with LCL–HLA-G1 cells, 70% of activated CD4+ and 60% of activated CD8+ T cells acquired HLA-G in less than 5 minutes. Given the fast kinetics of trogocytosis, T cells prior to and after coincubation with LCL–HLA-G1 or LCL-RSV phenotypically differed only by the display of APC-generated molecules and HLA-G1 (compare controls and day-0 T cells in Figure 6A and Document S2).
Thus, trogocytosis induces phenotypical changes that are almost instantaneous because they do not involve endogenous expression changes. However, for that very reason, the duration of these changes should depend on the lifetime of the transferred molecules at the surface of the acquirer cell. We found that HLA-G1acq+ T cells no longer displayed cell-surface HLA-G1 24 hours after they had been generated and purified (Figure 3F). This is compatible with the 14-hour half-life of HLA-G1 at the cell surface.34 Given the immunosuppressive functions of HLA-G1, we next investigated whether trogocytosis of HLA-G had functional relevance.
CD4+HLA-G1acq+ effector T cells no longer respond to stimulation
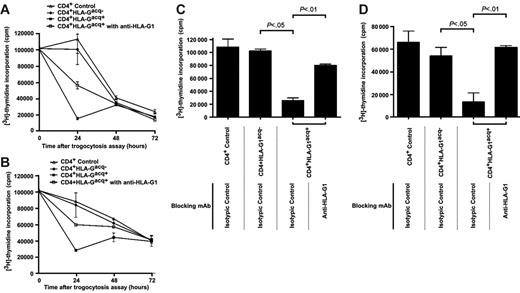
We investigated the proliferative capabilities of CD4+ T cells that had acquired HLA-G1 from APCs (CD4+HLA-G1acq+ T cells). Since the cells used for trogocytosis were already proliferating, we investigated whether acquisition of HLA-G1 inhibited their ongoing proliferation. Thus, we generated and purified CD4+HLA-G1acq+ T cells and their CD4+HLA-Gacq− counterparts, put them back in culture, and evaluated their proliferation at this time, 24 hours, and again 72 hours later (Figure 4). We found that the proliferation of control CD4+ T cells and CD4+HLA-Gacq− T cells was identical, decreasing over time, indicating that coincubation of already activated CD4+ T cells with LCL-RSV cells had no effect on their proliferation within the 72-hour time frame. By contrast, proliferation of CD4+HLA-G1acq+ was dramatically reduced 24 hours after acquisition of HLA-G1 and remained so thereafter. This shows that acquisition of HLA-G1 stopped CD4+ T-cell proliferation. Next, we masked HLA-G at the surface of LCL–HLA-G1 cells prior to coincubation with CD4+ T cells. This did not prevent HLA-G uptake by CD4+ T cells but blocked HLA-G function. As shown in Figure 4A, masking HLA-G prevented proliferation inhibition of CD4+HLA-G1acq+, showing that acquired HLA-G1 was responsible for CD4+HLA-G1acq+ proliferation inhibition.
CD4+HLA-G1acq+ T cells no longer respond to stimulation. (A) Proliferation analysis of purified CD4+ T cells taken prior to trogocytosis, CD4+HLA-G1acq− T cells, and CD4+HLA-G1acq+ T cells at the indicated times after purification. Uptake of HLA-G1 by T cells stopped their proliferation, which was partially maintained by masking HLA-G1 at the CD4+HLA-G1acq+ T-cell surface. Results shown are representative of 3 independent experiments. (B) Proliferation analysis of purified CD4+ T cells taken prior to trogocytosis; CD4+HLA-G1acq− and CD4+HLA-G1acq+ T cells in medium supplemented with IL-2. Proliferation was measured at the indicated times after purification. Unlike masking HLA-G1 at the cell surface of CD4+HLA-G1acq+ T cells, stimulation by exogenous IL-2 did not counteract HLA-G1–dependent proliferation inhibition. Results shown are representative of 3 independent experiments. (C) Analysis of allo-proliferative response of purified CD4+ T cells taken prior to trogocytosis, CD4+HLA-G1acq− T cells, and CD4+HLA-G1acq+ T cells using LCL-RSV as stimulator cells. HLA-G1 was masked when indicated. Results shown are representative of 3 independent experiments. (D) Analysis of allo-proliferative response of purified CD4+ T cells taken prior to trogocytosis; CD4+HLA-G1acq− and CD4+HLA-G1acq+ T cells using allogeneic PBMCs as stimulator cells. HLA-G1 was masked when indicated. Results shown are representative of 3 independent experiments.
CD4+HLA-G1acq+ T cells no longer respond to stimulation. (A) Proliferation analysis of purified CD4+ T cells taken prior to trogocytosis, CD4+HLA-G1acq− T cells, and CD4+HLA-G1acq+ T cells at the indicated times after purification. Uptake of HLA-G1 by T cells stopped their proliferation, which was partially maintained by masking HLA-G1 at the CD4+HLA-G1acq+ T-cell surface. Results shown are representative of 3 independent experiments. (B) Proliferation analysis of purified CD4+ T cells taken prior to trogocytosis; CD4+HLA-G1acq− and CD4+HLA-G1acq+ T cells in medium supplemented with IL-2. Proliferation was measured at the indicated times after purification. Unlike masking HLA-G1 at the cell surface of CD4+HLA-G1acq+ T cells, stimulation by exogenous IL-2 did not counteract HLA-G1–dependent proliferation inhibition. Results shown are representative of 3 independent experiments. (C) Analysis of allo-proliferative response of purified CD4+ T cells taken prior to trogocytosis, CD4+HLA-G1acq− T cells, and CD4+HLA-G1acq+ T cells using LCL-RSV as stimulator cells. HLA-G1 was masked when indicated. Results shown are representative of 3 independent experiments. (D) Analysis of allo-proliferative response of purified CD4+ T cells taken prior to trogocytosis; CD4+HLA-G1acq− and CD4+HLA-G1acq+ T cells using allogeneic PBMCs as stimulator cells. HLA-G1 was masked when indicated. Results shown are representative of 3 independent experiments.
We investigated whether CD4+HLA-G1acq+ T cells could still respond to stimulation. For restimulation, we used cytokine stimulation by addition of exogenous IL-2 and stimulation through TCR by irradiated allogeneic LCL-RSV cells or allogeneic PBMCs. Figure 4B shows that stimulation by 100 IU/mL of IL-2 was not sufficient to bypass HLA-G1–induced proliferation inhibition of CD4+HLA-Gacq+ T-cell proliferation during the first 24 hours of culture but did induce their renewed proliferation at later time points. It has to be noted that 100 IU/mL of IL-2 is the dose used to generate trogocytic CD4+ T cells. This means that addition of IL-2 to CD4+ T-cell cultures actually means keeping these cells in IL-2–supplemented medium. Figure 4 also shows that while CD4+HLA-Gacq− T cells could be restimulated by allogeneic APCs (Figure 4C) or allogeneic PBMCs (Figure 4D), CD4+HLA-G1acq+ could not. However, masking HLA-G1 at their cell surface restored response, showing that acquired HLA-G1 was directly responsible for proliferation inhibition.
Inability of a polyclonal CD4+ T-cell population to respond to allo-stimulation might be anergy. However, anergic T cells are usually generated through maturation and not in 5 minutes. It is then unlikely that CD4+HLA-G1acq+ T cells were anergic but rather prevented from proliferating by the presence of HLA-G1 at their surface. In this case, each CD4+HLA-G1acq+ T cell would inhibit others directly or indirectly via inhibition of stimulatory APCs. Either way, such a function characterizes a regulatory T cell.
HLA-G1acq+ T cells are regulatory cells
We investigated whether CD4+HLA-G1acq+ T cells were regulatory cells by using them as third-party cells in functional assays. We first found that irradiated CD4+HLA-G1acq+ T cells generated and purified as described above inhibited the proliferation of autologous, resting T cells in response to allo-stimulation by LCL cells (Figure 5A) or stimulation with tetanus toxin (not shown), whereas their irradiated CD4+HLA-G1acq− counterparts did not. The suppressive/regulatory function of CD4+HLA-G1acq+ T cells was directly mediated by acquired HLA-G1, since masking it at the surface of CD4+HLA-G1acq+ T cells in the experiments above abrogated their suppressive function. Thus, CD4+HLA-G1acq+ T cells are effector cells that act as regulatory cells through HLA-G1, a molecule they do not endogenously express. As can be seen in Figure 5B, regulatory function was directly proportional to the suppressor-responder ratio.
Regulatory/suppressive functions of CD4+HLA-G1acq+ T cells. The regulatory function of CD4+HLA-G1acq+ T cells was investigated by adding them as irradiated third-party cells in functional assays. (A) Irradiated CD4+HLA-G1acq− and CD4+HLA-G1acq+ T cells were added to mixed-lymphocyte reactions between autologous PBMCs and allogeneic LCL-RSV cells. When indicated, HLA-G1 was masked at the CD4+HLA-G1acq+ T cells by blocking anti–HLA-G1 mAb. CD4+HLA-G1acq+ but not CD4+HLA-G1acq− T cells inhibited resting T-cell allo-proliferation. This inhibition was abrogated by blocking anti–HLA-G1 mAb. Results shown are representative of 5 independent experiments. (B) Irradiated CD4+HLA-G1acq− and CD4+HLA-G1acq+ T cells were added to mixed-lymphocyte reactions between autologous PBMCs and allogeneic LCL-RSV cells at different third-party–responder–stimulator ratios. Inhibition of resting T-cell allo-response was directly proportional to the amount of CD4+HLA-G1acq+ used. Results shown are representative of 3 independent experiments.
Regulatory/suppressive functions of CD4+HLA-G1acq+ T cells. The regulatory function of CD4+HLA-G1acq+ T cells was investigated by adding them as irradiated third-party cells in functional assays. (A) Irradiated CD4+HLA-G1acq− and CD4+HLA-G1acq+ T cells were added to mixed-lymphocyte reactions between autologous PBMCs and allogeneic LCL-RSV cells. When indicated, HLA-G1 was masked at the CD4+HLA-G1acq+ T cells by blocking anti–HLA-G1 mAb. CD4+HLA-G1acq+ but not CD4+HLA-G1acq− T cells inhibited resting T-cell allo-proliferation. This inhibition was abrogated by blocking anti–HLA-G1 mAb. Results shown are representative of 5 independent experiments. (B) Irradiated CD4+HLA-G1acq− and CD4+HLA-G1acq+ T cells were added to mixed-lymphocyte reactions between autologous PBMCs and allogeneic LCL-RSV cells at different third-party–responder–stimulator ratios. Inhibition of resting T-cell allo-response was directly proportional to the amount of CD4+HLA-G1acq+ used. Results shown are representative of 3 independent experiments.
HLA-G1acq+ T cells differentiate into HLA-G1− regulatory cells
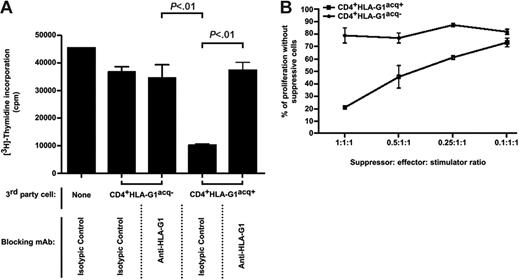
Given the limited half-life of acquired HLA-G1 at the cell surface of CD4+HLA-G1acq+ T cells, we investigated whether these cells lost their regulatory functions along with HLA-G1 surface expression (CD4+[HLA-G1acq+→HLA-G1−] T cells). As can be seen in Figure 6A for selected markers and as indicated in Document S2 for others, 2 days after trogocytosis all T cells were phenotypically identical. These cells still expressed significant amounts of HLA-DR, CD86, CD54, and ILT2, whereas they no longer expressed HLA-G1. This is most likely due to endogenous neoexpression of these molecules by activated T cells. Furthermore, these cells still expressed high levels of CD25 and had started expressing CD45RO. Yet, CD4+[HLA-G1acq+→HLA-G1−] and CD4+[HLA-G1acq−→HLA-G1−] cells were functionally very different; using them as irradiated third-party cells in MLR, we found that CD4+[HLA-G1acq+→HLA-G1−] T cells were still suppressive (Figure 6B). As expected for lack of HLA-G1 cell-surface expression, anti–HLA-G1 blocking antibody had no effect on CD4+[HLA-G1acq+→HLA-G1−] T-cell regulatory function. These data show that regulatory CD4+HLA-G1acq+ T cells acting through borrowed HLA-G1 had evolved into CD4+[HLA-G1acq+→HLA-G1−] regulatory T cells that act independently on HLA-G1. Yet, HLA-G1 had been involved in the making of CD4+ [HLA-G1acq+ → HLA-G1−], since blocking HLA-G1 at the surface of HLA-G1–APCs prior to trogocytosis prevented their subsequent differentiation (Figure 6B). These data indicate that T cells acquired from HLA-G1–APCs not only directly immunosuppressive HLA-G1 but also the previously described function of HLA-G1–APCs to induce the differentiation of HLA-G–negative suppressor T cells.
HLA-G1acq+ T cells evolve from HLA-G1+, HLA-G1–dependent regulatory cells into HLA-G1−, HLA-G1–independent regulatory cells. The phenotype and regulatory functions of CD4+HLA-G1acq+ T cells after they had lost HLA-G1 expression (CD4+[HLA-G1acq+→HLA-G1−] cells) were investigated by flow cytometry and by adding them as irradiated third-party cells in functional assays, respectively. (A) Phenotype of control T cells and T cells after a 1-hour coincubation with LCL and LCL–HLA-G1 cells, analyzed either at the time of trogocytosis assay (Day 0) or 2 days afterward (Day 2). Cell-surface expression of the indicated molecules was investigated by flow cytometry for CD4+ T cells, CD4+ T cells incubated with LCL-RSV cells, and T cells incubated with LCL–HLA-G1 cells at the time of trogocytosis assay (day 0) and 2 days afterward (day 2). Results shown are representative of 3 independent experiments. Analysis for phenotypical markers unaffected by the coincubation is in Document S2. (B) Irradiated CD4+ T cells and CD4+HLA-G1acq− and CD4+HLA-G1acq+ T cells taken immediately after trogocytosis (day 0) or 2 days afterward (day 2) were added to mixed-lymphocyte reactions between autologous PBMCs and allogeneic LCL-RSV cells. When indicated, HLA-G1 was masked at the CD4+HLA-G1acq+ T cells by blocking anti–HLA-G1 mAb, either during the proliferation assay itself or during trogocytosis only. Day 0: CD4+HLA-G1acq+ but not CD4+HLA-G1acq− T cells inhibited resting autologous T-cell allo-proliferation. This inhibition was abrogated by blocking anti–HLA-G1 mAb. Day 2: CD4+[HLA-G1acq+→HLA-G1−] but not CD4+[HLA-G1acq−→HLA-G1−] T cells inhibited resting autologous T-cell allo-proliferation. This inhibition was not abrogated by blocking anti–HLA-G1 mAb when added during the proliferation assay but was abrogated if HLA-G was blocked during trogocytosis assay itself, showing that HLA-G1 drives CD4+[HLA-G1acq+→HLA-G1−] regulatory cell differentiation but does not mediate their function. Results shown are representative of 5 independent experiments.
HLA-G1acq+ T cells evolve from HLA-G1+, HLA-G1–dependent regulatory cells into HLA-G1−, HLA-G1–independent regulatory cells. The phenotype and regulatory functions of CD4+HLA-G1acq+ T cells after they had lost HLA-G1 expression (CD4+[HLA-G1acq+→HLA-G1−] cells) were investigated by flow cytometry and by adding them as irradiated third-party cells in functional assays, respectively. (A) Phenotype of control T cells and T cells after a 1-hour coincubation with LCL and LCL–HLA-G1 cells, analyzed either at the time of trogocytosis assay (Day 0) or 2 days afterward (Day 2). Cell-surface expression of the indicated molecules was investigated by flow cytometry for CD4+ T cells, CD4+ T cells incubated with LCL-RSV cells, and T cells incubated with LCL–HLA-G1 cells at the time of trogocytosis assay (day 0) and 2 days afterward (day 2). Results shown are representative of 3 independent experiments. Analysis for phenotypical markers unaffected by the coincubation is in Document S2. (B) Irradiated CD4+ T cells and CD4+HLA-G1acq− and CD4+HLA-G1acq+ T cells taken immediately after trogocytosis (day 0) or 2 days afterward (day 2) were added to mixed-lymphocyte reactions between autologous PBMCs and allogeneic LCL-RSV cells. When indicated, HLA-G1 was masked at the CD4+HLA-G1acq+ T cells by blocking anti–HLA-G1 mAb, either during the proliferation assay itself or during trogocytosis only. Day 0: CD4+HLA-G1acq+ but not CD4+HLA-G1acq− T cells inhibited resting autologous T-cell allo-proliferation. This inhibition was abrogated by blocking anti–HLA-G1 mAb. Day 2: CD4+[HLA-G1acq+→HLA-G1−] but not CD4+[HLA-G1acq−→HLA-G1−] T cells inhibited resting autologous T-cell allo-proliferation. This inhibition was not abrogated by blocking anti–HLA-G1 mAb when added during the proliferation assay but was abrogated if HLA-G was blocked during trogocytosis assay itself, showing that HLA-G1 drives CD4+[HLA-G1acq+→HLA-G1−] regulatory cell differentiation but does not mediate their function. Results shown are representative of 5 independent experiments.
Discussion
The results presented here describe a new type of immune suppression and its actors, which are regulatory cell-surface–positive HLA-G1 T cells. Immune suppression such as that presented here differs from other cell-mediated immune suppression because (1) although it is regulatory-cell mediated, it does not involve a specific subset of regulatory cells but regular immune effectors; (2) it does not require specific cell maturation but membrane transfers between cells, which induce an almost instant switch from effector to regulatory function. Hence, this type of immune suppression is mediated by immune effectors that only act as immune suppressors. Trogocytosis-based immune suppression also differs from other cell-mediated immune suppression because it is controlled by the effector's environment and not by the immune system itself during antigen-specific activation/maturation.
Trogocytosis of HLA-G1 looks like a first line of defense against immune aggression (emergency immune suppression) for tissues that express HLA-G normally (fetal tissues) or pathologically (eg, tumors). The aim of this emergency immune suppression would be to (1) quickly increase the number of regulatory HLA-G1–positive cells locally without time-consuming and potentially hazardous unlocking of HLA-G expression; (2) spread HLA-G1 presence to a larger area than that covered by the few HLA-G1–expressing cells; and (3) block the function of any effector cell that would be recruited to that area, including that of the HLA-G1acq+ cells themselves, in order to dampen/stop the local reaction and prevent damage to/destruction of tissues in the vicinity of HLA-G1–expressing cells. This type of regulation might be temporary, due to the limited lifespan of borrowed HLA-G1 at the cell surface. However, it might also be sufficient to delay immune reactions and give time to real regulatory cells to differentiate and take over, as we could show 2 days after HLA-G1 transfer. In the case presented here, trogocytosis was even responsible for the induction of “real” regulatory cells because HLA-G1–APCs transferred to T cells their HLA-G1–dependent capability to differentiate regulatory cells, albeit of unknown phenotype.16
Finally, this is the first demonstration that trogocytosis can have a major impact on immune responses, that highly efficient regulatory T cells can be generated immediately by reversing the function of effector immune cells and without the requirement of a maturation process (“emergency” suppression mechanism), and that a regulatory-cell population exists whose very existence, by the nature of its making, is most likely limited in time and space.
The physiopathologic relevance of the regulatory mechanism described above might come from the fact that HLA-G–expressing APCs do exist in vivo during the course of various pathologies,21,27,28,35–37 which include cancer,35,36 and that HLA-G–expressing APCs are found specifically within lesions and not within surrounding healthy tissues. In these situations, trogocytosis of HLA-G1 leading to instant transformation of immunocompetent cells into tolerogenic cells is likely to occur in the vicinity of HLA-G–expressing APCs (ie, within lesions or tumors). Thus, all conditions are set in vivo to make of immune suppression through trogocytosis of HLA-G1 a de facto lesion/tumor-specific immune escape mechanism. This re-emphasizes the need for HLA-G expression monitoring in pathologic contexts and for incorporation of HLA-G blocking strategies into immunotherapies.
Authorship
Contribution: J.L. designed experiments and wrote the manuscript; J.C. designed and performed experiments and assisted in manuscript preparation; M.D., B.F., S.L., and A.G. performed experiments; and E.D.C. assisted in data analysis and manuscript preparation.
Conflict of interest disclosure: The authors declare no competing financial interests.
J.L. and J.C. contributed equally to this work.
Correspondence: Service de Recherches en Hemato-Immunologie, CEA-DSV-DRM, Institut Universitaire d'Hematologie, Hopital Saint Louis, 1 Avenue Claude Vellefaux, 75010 Paris, France
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by the Commissariat a l'Energie Atomique, France.
We would like to thank the Confocal Imagery Department (IFR 105) of the Institut Universitaire d'Hematologie, Hopital Saint-Louis, Paris, France, for technical help.

![Figure 6. HLA-G1acq+ T cells evolve from HLA-G1+, HLA-G1–dependent regulatory cells into HLA-G1−, HLA-G1–independent regulatory cells. The phenotype and regulatory functions of CD4+HLA-G1acq+ T cells after they had lost HLA-G1 expression (CD4+[HLA-G1acq+→HLA-G1−] cells) were investigated by flow cytometry and by adding them as irradiated third-party cells in functional assays, respectively. (A) Phenotype of control T cells and T cells after a 1-hour coincubation with LCL and LCL–HLA-G1 cells, analyzed either at the time of trogocytosis assay (Day 0) or 2 days afterward (Day 2). Cell-surface expression of the indicated molecules was investigated by flow cytometry for CD4+ T cells, CD4+ T cells incubated with LCL-RSV cells, and T cells incubated with LCL–HLA-G1 cells at the time of trogocytosis assay (day 0) and 2 days afterward (day 2). Results shown are representative of 3 independent experiments. Analysis for phenotypical markers unaffected by the coincubation is in Document S2. (B) Irradiated CD4+ T cells and CD4+HLA-G1acq− and CD4+HLA-G1acq+ T cells taken immediately after trogocytosis (day 0) or 2 days afterward (day 2) were added to mixed-lymphocyte reactions between autologous PBMCs and allogeneic LCL-RSV cells. When indicated, HLA-G1 was masked at the CD4+HLA-G1acq+ T cells by blocking anti–HLA-G1 mAb, either during the proliferation assay itself or during trogocytosis only. Day 0: CD4+HLA-G1acq+ but not CD4+HLA-G1acq− T cells inhibited resting autologous T-cell allo-proliferation. This inhibition was abrogated by blocking anti–HLA-G1 mAb. Day 2: CD4+[HLA-G1acq+→HLA-G1−] but not CD4+[HLA-G1acq−→HLA-G1−] T cells inhibited resting autologous T-cell allo-proliferation. This inhibition was not abrogated by blocking anti–HLA-G1 mAb when added during the proliferation assay but was abrogated if HLA-G was blocked during trogocytosis assay itself, showing that HLA-G1 drives CD4+[HLA-G1acq+→HLA-G1−] regulatory cell differentiation but does not mediate their function. Results shown are representative of 5 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/5/10.1182_blood-2006-05-024547/4/m_zh80050708930006.jpeg?Expires=1763489661&Signature=DMc6kCGF-qrvt8Obr06-swEC8CZCtwTt5YnZywt1pd1zlUVDzyShMkBnxAvwwVdlVjjWzWgwWKVX~SlRo1Jmq8Ym3M~nK3fTDCPsRcHWSu1lhfLC2tYvvgS3s~7JOJy4iIUx8rqagUpEg2YaksD0Gh6aVq6VNt-9lcD5L8WrYPc1NqOs9a0yMKKwNwwzRJYXOQwBasL6~-rIvUWIHIuxaXlRXqyM8u4e6cG-wNusNaZlPZSSuy09w0NrKFpsik9Bd5oVvVCu8DfSUHzNrAzQwSZN1uF8~tPIkhsEE3btLXdbVLK2kGj5tXRA6RDgcigvysa8dp1V5mTQGZhu7cThaA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal