Abstract
Antigens expressed on malignant cells in the absence of significant expression on normal tissues are highly desirable targets for therapeutic antibodies. CD70 is a TNF superfamily member whose normal expression is highly restricted but is aberrantly expressed in hematologic malignancies including non-Hodgkin lymphoma (NHL), Hodgkin disease, and multiple myeloma. In addition, solid tumors such as renal cell carcinoma, nasopharyngeal carcinoma, thymic carcinoma, meduloblastoma, and glioblastoma express high levels of this antigen. To functionally target CD70-expressing cancers, a murine anti-CD70 monoclonal antibody was engineered to contain human IgG1 constant domains. The engineered antibody retained the binding specificity of the murine parent monoclonal antibody and was shown to induce Fc-mediated effector functions including antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, and antibody-dependent cellular phagocytosis in vitro. Further, administration of this antibody significantly prolonged survival of severe combined immunodeficient (SCID) mice bearing CD70+ disseminated human NHL xenografts. Survival of these mice was dependent upon the activity of resident effector cells including neutrophils, macrophages, and natural killer (NK) cells. These data suggest that an anti-CD70 antibody, when engineered to contain human IgG1 constant domains, possesses effector cell–mediated antitumor activity and has potential utility for anticancer therapy.
Introduction
Monoclonal antibodies (mAbs) are an important emerging tool in cancer therapy, particularly for the treatment of hematologic malignancies. Therapeutic mAbs can act by blocking growth factors, directly signaling arrest and apoptosis, or inducing elimination of mAb-decorated target cells via activation of host defense mechanisms. Ideally, therapeutic mAbs could differentiate between normal and transformed cells. In reality, the paucity of tumor-specific antigens has led to the development of mAbs directed against receptors that are overexpressed in cancers compared with normal tissues. For example, trastuzumab is FDA approved for the treatment of metastatic breast cancer overexpressing HER2. Alternatively, antibodies have been used to target cell surface molecules characteristic of the lineage from which the malignant cells derive, as is the case with FDA-approved mAbs against CD20 (rituximab), CD52 (alemtuzumab), and CD33 (gemtuzumab ozogamicin). The use of mAbs that collaterally target normal hematopoietic cells can have serious consequences. In the case of alemtuzumab, lymphopenia and neutropenia significantly increase patient risk of contracting opportunistic infections.1-3 Rituximab spares T cells, immature B-cell precursors, and plasma cells, but depletes memory B-cell pools and affects antibody responses against recall antigens.4,5 The long-term effects of these changes are still unknown. It is therefore likely that antibodies targeting cancer-associated antigens with more restricted expression patterns in normal tissues will have better safety profiles.
Diffuse large B-cell lymphoma and follicular lymphoma are the 2 most common non-Hodgkin lymphomas (NHLs), making up greater than 50% of all cases of NHL. The normal counterparts of these lymphomas are thought to be proliferating B cells (centroblasts or immunoblasts) and follicle center B cells (centrocytes and centroblasts). As such, it is not surprising that, aside from pan-B markers such as CD19, CD20, and CD22, many NHLs express markers commonly associated with B-lymphocyte activation. Some of these activation markers could be viable alternative targets for antibody-based therapeutics.
One such activation marker, CD70, is a member of the TNF family whose expression is tightly regulated and only transiently induced on antigen-stimulated B and T cells.6,7 Interaction between CD70 and its ligand CD27 regulates the expansion and differentiation of effector and memory T-cell populations.8-10 In normal B cells, CD70-CD27 costimulation promotes B-cell expansion,11 germinal center formation,10,12 and plasma cell differentiation.13 In addition to activated lymphocytes, CD70 is found on stromal cells of the thymic medulla14 and on mature dendritic cells (DCs),15,16 but is absent from other normal tissues including all vital organs. Of interest, CD70 is expressed by a variety of transformed cells of both hematopoietic and epithelial cell origin. Seventy-one percent of diffuse large B-cell lymphomas, 33% of follicular lymphomas, 50% of B-cell lymphocytic leukemias, 25% of Burkitt and mantle cell lymphomas,17 and 100% of Waldenström macroglobulinemia,18 as well as the majority of Hodgkin disease Reed-Sternberg cells, express CD70.19 In solid tumors, CD70 was first detected on nasopharyngeal carcinoma20 and may be linked to Epstein-Barr virus (EBV) infection. Additionally, CD70 has been found on EBV-negative thymic carcinoma,14 astrocytoma,21,22 and glioblastoma,21,22 as well as renal cell carcinoma.23-25
In this report, we have extended CD70 expression analysis and demonstrate that approximately 60% of myeloma cell lines examined were CD70+. We provide data in support of CD70 as a viable target for an antibody-based therapeutic against B-lineage cancers. We demonstrate that an engineered antihuman CD70 mAb of human IgG1 isotype induces tumor cell lysis via antibody-mediated cellular cytotoxicity (ADCC), complement fixation (CDC), and enhanced uptake by phagocytic cells (ADCP). Administration of this antibody in vivo significantly prolonged survival of mice bearing CD70+ disseminated B-lymphoma xenografts. Using a variant of anti-CD70 mAb with impaired Fc function and animal models in which effector cell subsets were depleted, we further demonstrate that the in vivo antitumor activity of the antibody is dependent on Fc-FcγR interactions.
Materials and methods
Cells and reagents
The human non-Hodgkin lymphoma cell lines IM-9, Raji, Farage, and WIL2-S and renal cell carcinoma (RCC) lines 786-O and Caki-1 were obtained from the American Type Culture Collection (Manassas, VA). The NHL line MHH-PREB-1, Hodgkin disease (HD) cell line L428, and multiple myeloma (MM) lines U266 and LP-1 were from the DSMZ (Braunschweig, Germany). Cells were grown in RPMI 1640 (Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS). For in vivo depletion studies, rabbit anti–asialo-GM-1 was obtained from Wako Pure Chemical Industries (Richmond, VA); rat anti–Gr-1 was from BD Biosciences (San Diego, CA). Liposome-encapsulated clodronate (CEL) was prepared as previously described.26 Clodronate was a gift of Roche Diagnostics (Mannheim, Germany). CD70 expression was determined using murine 1F6 antibody and a DAKO QiFiKiT flow cytometric indirect immunofluorescence assay (DAKO, Glostrup, Denmark). FcγRIII blocking studies were accomplished with sodium azide–free anti-CD16 antibody purchased from Ancell (Bayport, MN). Fluorochrome-conjugated antibodies specific for complement regulatory proteins and Fcγ receptors, and for macrophages and T- and B-cell markers, were purchased from BD Biosciences. All flow cytometry was performed on a FACScan (BD Biosciences), and the data were analyzed with CellQuest analysis software (BD Biosciences).
Anti-CD70 antibody engineering
The murine anti-CD70 hybridoma, clone 1F6, was provided by Dr Rene van Lier, Central Laboratory of the Netherlands Red Cross Blood Transfusion Service (Amsterdam, the Netherlands). A recombinant anti-CD70 (c1F6) based on the VH and VL sequences of 1F6,27 human Cκ, and human IgG1 isotype was constructed. Amino acid substitutions of C226S:C229S:E233P:L234V:L235A were performed on the heavy chain to generate the c1F6 variant (c1F6v) that displayed disrupted FcγR binding and loss of functional activity.28-30 Recombinant c1F6 and c1F6v proteins were expressed in a CHO-DG44 cell line and purified by protein A chromatography.
Antibody-dependent cellular cytotoxicity assay
ADCC activity was measured using a standard 51Cr release assay. Target tumor cells were labeled for 1 hour with 100 μCi (3.7 MBq) Na2[51Cr]O4, washed thoroughly to remove unincorporated radioisotope, then plated in a 96-well plate at a concentration of 5000 cells per well in assay medium (RPMI supplemented with 1% heat-inactivated FBS). Antibody was diluted in assay medium and added 15 to 30 minutes prior to the addition of effector cells. Effector cells were isolated from the leukapheresis product (Lifeblood Biological Services, Memphis, TN) of a normal FcγRIII-158V donor. Leukocytes were enriched by centrifugation over a Ficoll-Paque density gradient (Amersham, Uppsala, Sweden) and cryopreserved. Prior to use, the leukocytes were thawed and cultured overnight in AIM V supplemented with 5% heat-inactivated human serum. Nonadherent cells were collected and enriched for CD16+ cells by negative depletion of CD4+, CD8+, CD20+, and CD14+ cells using immunomagnetic beads (Dynal, Oslo, Norway). The effector cells were added at an effector-target cell ratio of 10 CD16+ cells per target cell. After 4-hour incubation, the 51Cr released from lysed cells was measured and the percent specific lysis calculated as follows: (test sample cpm−spontaneous cpm)/(total cpm−spontaneous cpm) × 100. Spontaneous release of isotope was determined from the supernatant of target cells incubated in medium alone. Total counts were determined from target cells lysed with 1% Triton-X 100.
Complement-dependent cytotoxicity assay
CDC activity was assessed by mixing together target cells and varying concentrations of c1F6 antibody or nonbinding human IgG control in assay medium consisting of RPMI containing 10% heat-inactivated FBS and 2.5% to 10% normal human serum. The human serum was not heat-treated and served as the source of complement in these experiments. After incubation at 37°C for 2 hours, propidium iodide was added to a final concentration of 5 μg/mL and the percent nonviable cells determined by flow cytometry. Spontaneous background lysis of human IgG control samples was subtracted from c1F6-mediated cell lysis to yield specific cell lysis.
Antibody-dependent cellular phagocytosis assay
Tumor cells were labeled red with PKH26, a fluorescent cell membrane dye, according to the manufacturer's instructions (Sigma, St Louis, MO), then precoated with c1F6 in PBS for 30 minutes on ice. The target cells were washed and mixed with monocyte-derived macrophages at a ratio of 4 targets per macrophage in assay medium (RPMI supplemented with 10% Ultra Low IgG FBS [Invitrogen, Carlsbad, CA]). After 1 hour of incubation at 37°C, 5% CO2, the cell mixture was stained with Alexa Fluor 488–conjugated murine anti-CD11b (BD Biosciences) to identify the macrophages. The cells were fixed with 1% paraformaldehyde and analyzed by flow cytometry to detect double fluorescence, an indication of phagocytic activity. Uptake of tumor cells by macrophages was confirmed by fluorescence microscopy using a Leitz Orthoplan Research Microscope (Leica, Wetzler, Germany). For microscopy, tumor cells were labeled green with PKH67, and the macrophages were detected with Alexa Fluor 568–conjugated antibody specific for CD11b. The cells were adhered on poly-d-lysine–coated slides, fixed with 1% paraformaldehyde, and treated with Slowfade Antifade reagent (Invitrogen) prior to visualization. Monocyte-derived macrophages were prepared by adhering Ficoll-Paque density gradient–enriched leukocytes in tissue culture flasks for 1 hour at 37°C, 5% CO2 in Opti-MEM medium (Invitrogen) supplemented with 1% heat-inactivated human serum. Nonadherent cells were removed and adherent cells were cultured for 10 to 15 days in X-VIVO 15 medium (Cambrex BioScience, Walkersville, MD) containing 500 U/mL rhGM-CSF (PeproTech, Rocky Hill, NJ). Cells recovered from these cultures were CD3/CD19− and expressed CD14, CD11b, CD16, CD32, and CD64 as determined by flow cytometry.
Xenograft models of lymphoma
To establish disseminated disease, 1 × 106 Raji or IM-9 cells in 0.2 mL PBS were injected into the lateral tail vein of C.B.-17 severe combined immunodeficient (SCID) mice (Harlan, Indianapolis, IN). After injection, all of the mice were pooled and then placed randomly into the various treatment groups. A single dose of 4 mg/kg c1F6, c1F6v, or control nonbinding antibody was given 1 or 5 days after the cell implantation by intravenous injection into the lateral tail vein. In one experiment, mice were dosed with c1F6 (1 or 4 mg/kg) or nonbinding control antibody by intraperitoneal injection once every 4 days for a total of 4 doses starting one day after cell implantation. Mice were monitored at least twice per week and were killed when they exhibited signs of disease, including weight loss of 15% to 20%, hunched posture, lethargy, cranial swelling, or dehydration. In studies evaluating the mechanism of antibody activity in vivo, tumor-bearing mice were depleted of effector cells using specific antibody or CEL as previously described.26,31,32 Natural killer (NK) cells and neutrophils were depleted by intraperitoneal injection of anti–asialo-GM-1 (1.25 mg/kg) and anti–Gr-1 (4 mg/kg), respectively. Mice were given 3 doses once every 5 days beginning on the day of tumor cell implantation. Macrophages were depleted by intraperitoneal injection of CEL (200 μL/mouse) on the day of tumor injection and every 3 days thereafter for a total of 5 doses. Clodronate, once delivered to macrophages via phagocytosis of liposomes carrying the drug, induces macrophage death by apoptosis.31 Cell depletion was confirmed by flow cytometric analysis of splenocytes, lymph nodes, and blood. Statistical analysis was conducted using the log-rank test provided in the Graphpad Prism Software Package version 4.01 (Graphpad, San Diego, CA). All animal experiments were conducted under Seattle Genetics' IACUC guidelines and approval.
Results
CD70 is expressed on hematologic and epithelial cancer cell lines
Apart from its expression on activated T and B lymphocytes, CD70 has been identified on a number of malignant cell types.17-25 Consistent with prior reports of CD70 expression in primary tumor isolates, more than 67% of NHL, HD, and RCC cell lines examined were CD70+. Table 1 summarizes CD70 receptor numbers determined by quantitative flow cytometry for the cell lines used in this study. Cell lines that expressed CD70 were uniformly positive for this antigen. CD70 copy numbers among the cell lines ranged from approximately 1000 to more than 400 000 copies per cell. We report for the first time CD70 expression on the majority (64%) of multiple myeloma cell lines tested at levels comparable with those found on lymphoma cell lines.
CD70 expression on representative tumor cell lines
| Cancer type . | Frequency of CD70 expression, no. CD70+ cell lines/total no. cell lines* . | Range of receptor copy no.† . | Representative cell line (receptor no.) . |
|---|---|---|---|
| Non-Hodgkin lymphoma | 16/19 | 1 200-120 000 | IM-9 (120 000), Raji (23 000), Farage (31 000), WIL2-S (52 000), MHH-PREB-1 (28 000) |
| Hodgkin disease | 7/9 | 11 000-220 000 | L428 (120 000) |
| Multiple myeloma | 9/14 | 9 000-150 000 | U266 (150 000), LP-1 (64 000) |
| Renal cell carcinoma | 13/14 | 2 400-420 000 | 786-O (160 000), Caki-1 (140 000) |
| Cancer type . | Frequency of CD70 expression, no. CD70+ cell lines/total no. cell lines* . | Range of receptor copy no.† . | Representative cell line (receptor no.) . |
|---|---|---|---|
| Non-Hodgkin lymphoma | 16/19 | 1 200-120 000 | IM-9 (120 000), Raji (23 000), Farage (31 000), WIL2-S (52 000), MHH-PREB-1 (28 000) |
| Hodgkin disease | 7/9 | 11 000-220 000 | L428 (120 000) |
| Multiple myeloma | 9/14 | 9 000-150 000 | U266 (150 000), LP-1 (64 000) |
| Renal cell carcinoma | 13/14 | 2 400-420 000 | 786-O (160 000), Caki-1 (140 000) |
Cell lines expressing greater than 1000 receptors or exhibiting at least 2.5-fold mean fluorescence intensity above that of nonbinding isotype control were considered as positive for CD70.
Receptor copy number was determined by quantitative flow cytometry as described in “Materials and methods.”
Anti-CD70 mediates tumor cell killing via multiple antibody effector functions
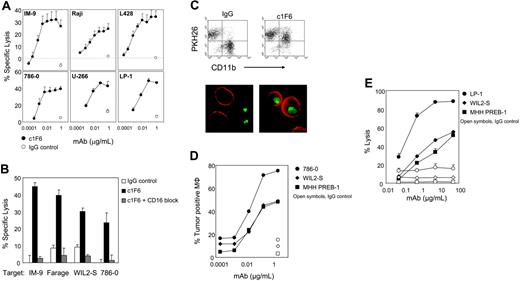
We sought to examine the therapeutic potential of anti-CD70 in targeting CD70+ cancer. The parent mAb 1F6 is a murine IgG1 with limited ability to promote antibody effector functions and does not induce apoptosis in CD70+ cells (data not shown). Chimerized 1F6 (human IgG1) with binding characteristics equivalent to those of the parent antibody (data not shown) was constructed. Similar to the original mouse antibody, c1F6 does not induce apoptosis. However, it demonstrates ADCC, CDC, and ADCP activities. In standard ADCC assays using peripheral blood mononuclear cells (PBMCs) as a source of NK cells, c1F6 induced lysis of CD70+ cell lines in a mAb dose-dependent fashion, while no lysis was achieved with nonbinding control human IgG. Parental murine 1F6 mAb did not demonstrate any ADCC activity in the same assay (data not shown), indicating that the appropriate human IgG isotype was required for this activity. Multiple CD70+ tumor lines representing NHL, HD, RCC, and MM could be efficiently lysed by c1F6 (Figures 1A and 2A), suggesting the mAb would be broadly effective. Maximal lysis was achieved at antibody concentrations of 10 to 100 ng/mL (Figures 1A and 2A), which are significantly lower amounts than that required to saturate target cells in a binding assay (Figure 2A). ADCC activity was completely abrogated when Fc-FcγRIII interaction was blocked by pretreating effector cells with anti-CD16 antibody (Figure 1B), whereas blockade of FcγRI by specific anti-CD64 antibody did not diminish c1F6-mediated ADCC (data not shown). These results indicate that tumor cell destruction in this assay was primarily mediated by CD16+ cells.
c1F6 mediates ADCC, ADCP, and CDC activity. (A) Specific lysis of c1F6-treated (closed symbols) or nonbinding control IgG1–treated (open symbols) target cells was determined by 51Cr release assay. Target cells were mixed with NK-enriched PBMCs at an effector-target ratio of 10:1. (B) Effector cells were untreated (solid bars) or pretreated with saturating amounts of anti-CD16 blocking antibody (hatched bars) prior to incubation with c1F6-coated target cells. Open bars represent target cells that were mixed with nonbinding control IgG1. (C) Representative flow cytometry analysis and fluorescence microscopy of c1F6-mediated phagocytosis. For flow cytometry, 786-O target cells were labeled with PKH26 lipophilic dye, treated with c1F6 or nonbinding control IgG, then mixed with monocyte-derived macrophages (Mφ). Mφ were stained with AF488-conjugated anti-CD11b. Cells present in the top-right quadrant (PKH26+CD11b+) are Mφ that internalized tumor cells. For microscopy, tumor cells were labeled with PKH67 (green), and the macrophages were detected with Alexa Fluor 568–conjugated antibody specific for CD11b (red). Images were taken with a Leitz Orthoplan Research microscope (100×/1.32 NA objective lens) using a Nikon Coolpix 990 camera (Nikon, Tokyo, Japan), then processed with Adobe Photoshop Elements version 2.0 (Adobe Systems, San Jose, CA). (D) Tumor targets were treated with varying concentrations of c1F6 (solid symbols) or 2 μg/mL IgG1 (open symbols) prior to incubation with Mφ. Percent Mφ that engulfed tumor cells was determined by flow cytometry as in panel C. Internalization of tumor cells by Mφ was confirmed by fluorescence microscopy (not shown). (E) c1F6-treated (solid symbols) or control IgG–treated (open symbols) target cells were incubated at 37°C in 10% human serum. The percentage of nonviable cells was identified by flow cytometry after staining with propidium iodide. (A-B,E) Bars represent SD of triplicate samples.
c1F6 mediates ADCC, ADCP, and CDC activity. (A) Specific lysis of c1F6-treated (closed symbols) or nonbinding control IgG1–treated (open symbols) target cells was determined by 51Cr release assay. Target cells were mixed with NK-enriched PBMCs at an effector-target ratio of 10:1. (B) Effector cells were untreated (solid bars) or pretreated with saturating amounts of anti-CD16 blocking antibody (hatched bars) prior to incubation with c1F6-coated target cells. Open bars represent target cells that were mixed with nonbinding control IgG1. (C) Representative flow cytometry analysis and fluorescence microscopy of c1F6-mediated phagocytosis. For flow cytometry, 786-O target cells were labeled with PKH26 lipophilic dye, treated with c1F6 or nonbinding control IgG, then mixed with monocyte-derived macrophages (Mφ). Mφ were stained with AF488-conjugated anti-CD11b. Cells present in the top-right quadrant (PKH26+CD11b+) are Mφ that internalized tumor cells. For microscopy, tumor cells were labeled with PKH67 (green), and the macrophages were detected with Alexa Fluor 568–conjugated antibody specific for CD11b (red). Images were taken with a Leitz Orthoplan Research microscope (100×/1.32 NA objective lens) using a Nikon Coolpix 990 camera (Nikon, Tokyo, Japan), then processed with Adobe Photoshop Elements version 2.0 (Adobe Systems, San Jose, CA). (D) Tumor targets were treated with varying concentrations of c1F6 (solid symbols) or 2 μg/mL IgG1 (open symbols) prior to incubation with Mφ. Percent Mφ that engulfed tumor cells was determined by flow cytometry as in panel C. Internalization of tumor cells by Mφ was confirmed by fluorescence microscopy (not shown). (E) c1F6-treated (solid symbols) or control IgG–treated (open symbols) target cells were incubated at 37°C in 10% human serum. The percentage of nonviable cells was identified by flow cytometry after staining with propidium iodide. (A-B,E) Bars represent SD of triplicate samples.
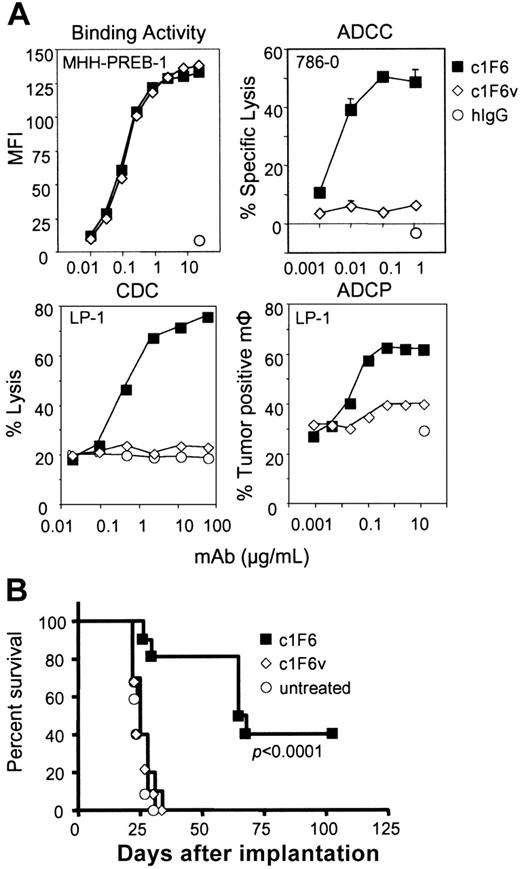
In vitro and in vivo activity of c1F6 requires functional Fc–Fcγ receptor interaction. (A) Binding of c1F6 and c1F6v to CD70+ cells was detected with a fluorochrome-conjugated anti–human IgG antibody by flow cytometry. ADCC, CDC, and ADCP activities of c1F6 (solid squares) compared with c1F6v (open diamonds) or nonbinding IgG1 (open circles) were measured as described in “Materials and methods.” (B) Survival curve of mice injected intravenously with 1 × 106 Raji tumor cells then treated one day later with 4 mg/kg c1F6 or c1F6v (n = 10 per group). The P value indicates difference between the c1F6-treated and untreated groups.
In vitro and in vivo activity of c1F6 requires functional Fc–Fcγ receptor interaction. (A) Binding of c1F6 and c1F6v to CD70+ cells was detected with a fluorochrome-conjugated anti–human IgG antibody by flow cytometry. ADCC, CDC, and ADCP activities of c1F6 (solid squares) compared with c1F6v (open diamonds) or nonbinding IgG1 (open circles) were measured as described in “Materials and methods.” (B) Survival curve of mice injected intravenously with 1 × 106 Raji tumor cells then treated one day later with 4 mg/kg c1F6 or c1F6v (n = 10 per group). The P value indicates difference between the c1F6-treated and untreated groups.
Removal of antibody-coated target cells by the reticuloendothelial system (RES) has increasingly been recognized as key to the therapeutic activity of anti-CD20 mAbs,33,34 suggesting a role for FcγR-mediated phagocytosis in tumor cell elimination. To determine whether engineered anti-CD70 mAb facilitates phagocytic uptake of tumor cells, target cells were labeled with a lipophilic dye, coated with antibody, then mixed with monocyte-derived macrophages. The macrophage surface was subsequently stained with a fluoresceinated anti-CD11b antibody and phagocytosis measured by the appearance of double-labeled coincident events by flow cytometry. Macrophages that engulfed the tumor cells were identified as CD11b+PKH26+ double-positive cells (Figure 1C). Whereas phagocytosis in the presence of nonbinding, control IgG was less than 20% for the target cells tested, macrophages readily phagocytosed tumor cells coated with c1F6 in an antibody dose–dependent manner (Figure 1D). Engulfment of target cells by macrophages was confirmed by fluorescence microscopy. Similar to ADCC, maximal phagocytosis, which ranged from approximately 45% to approximately 75%, was achieved at subsaturating concentrations of anti-CD70 mAb (Figure 1D).
The anti-CD70 mAb was further tested for its ability to induce tumor cell lysis by complement fixation (CDC). A subset of CD70+ cancer cell lines was found to be sensitive to c1F6-mediated CDC. As shown in Figure 1E, LP-1, WIL2-S, and MHH-PREB-1 cells were lysed in an antibody-specific, dose-dependent manner upon addition of c1F6 to target cells in the presence of normal human serum. These cell lines were not protected from cytolysis despite expression of the complement regulatory protein CD46, and in the case of WIL2-S, CD55 (Table 2) Chimeric 1F6-mediated CDC appeared to be inversely correlated to the expression of the complement regulatory receptor CD59 as CD70+ tumor cell lines that were resistant to c1F6-mediated complement-dependent lysis (< 20% lysis at 50 μg/mL of c1F6 mAb) were all CD59+ (Table 2 and data not shown).
Correlation between complement regulatory protein expression and susceptibility to c1F6-mediated CDC
| Cell lines . | Complement regulatory receptors* . | c1F6-mediated CDC (no. experiments)† . | ||
|---|---|---|---|---|
| CD46 . | CD55 . | CD59 . | ||
| IM-9 | ++ | ++ | ++ | 5 ± 1 (4) |
| Raji | + | + | + | 7 ± 0 (2) |
| Farage | ++ | + | +++ | 0 ± 0 (3) |
| WIL2-S | + | + | − | 56 ± 4 (6) |
| MHH-PREB-1 | ++ | − | − | 73 ± 5 (9) |
| L-428 | +++ | + | +++ | 1 (1)‡ |
| U266 | ++ | − | + | 23 (1)‡ |
| LP-1 | ++ | − | − | 64 ± 4 (4) |
| 786-0 | ++ | +++ | ++ | 0 (1)‡ |
| Caki-1 | ++ | +++ | ++ | 0 (1)‡ |
| Cell lines . | Complement regulatory receptors* . | c1F6-mediated CDC (no. experiments)† . | ||
|---|---|---|---|---|
| CD46 . | CD55 . | CD59 . | ||
| IM-9 | ++ | ++ | ++ | 5 ± 1 (4) |
| Raji | + | + | + | 7 ± 0 (2) |
| Farage | ++ | + | +++ | 0 ± 0 (3) |
| WIL2-S | + | + | − | 56 ± 4 (6) |
| MHH-PREB-1 | ++ | − | − | 73 ± 5 (9) |
| L-428 | +++ | + | +++ | 1 (1)‡ |
| U266 | ++ | − | + | 23 (1)‡ |
| LP-1 | ++ | − | − | 64 ± 4 (4) |
| 786-0 | ++ | +++ | ++ | 0 (1)‡ |
| Caki-1 | ++ | +++ | ++ | 0 (1)‡ |
−indicates ratio less than 2; +, 2 to 9; ++, 10 to 49; and +++, at least 50.
Complement regulatory protein (CRP) expression described as ratio of mean fluorescence intensity binding of FITC-anti-CRP antibody to background fluorescence from isotype control antibody.
Percent maximal cell lysis mediated by saturating concentrations of c1F6 in the presence of normal human serum. Mean ± standard error.
Single experiment with triplicate samples.
Anti-CD70 prolongs survival of lymphoma-bearing SCID mice
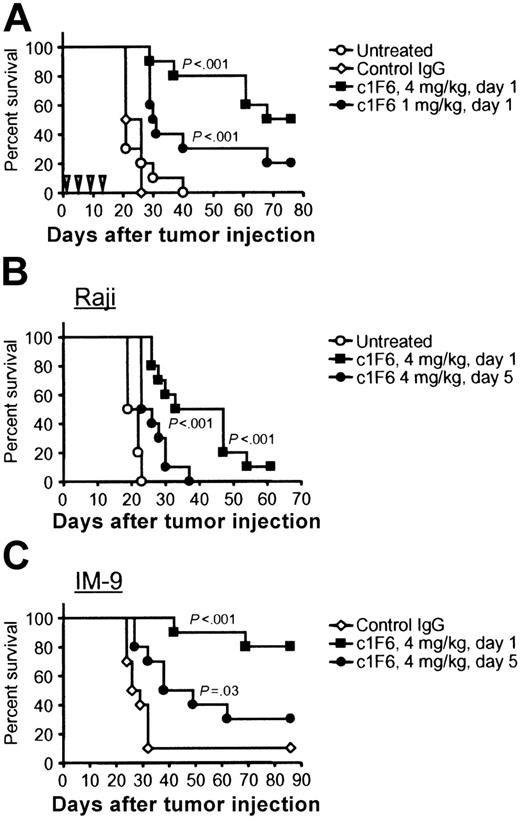
The in vivo antitumor activity of c1F6 was examined in the xenograft IM-9 and Raji disseminated lymphoma models. These cell lines readily establish disease when injected intravenously, such that at least 90% of engrafted mice succumb to disease within 20 to 30 days. Disease is manifested by hunched posture and lack of grooming, weight loss, cranial swelling, and hind limb paralysis, requiring that the animals be killed. In the Raji model, the median survival in untreated mice was 21 days (Figure 3A). Treatment with irrelevant control IgG did not prolong survival. In contrast, median survival was increased to 72 days when Raji-bearing mice were treated with c1F6 on a multidose schedule of 4 mg/kg initiated one day after tumor cell implantation. Survival was also significantly prolonged (median of 31 days) when a lower dose of 1 mg/kg c1F6 was administered using the same schedule (Figure 3A). Using Raji and IM-9 lymphoma models, we further tested the efficacy of c1F6 under more stringent conditions in which tumor-bearing mice received only a single administration of c1F6. In Figure 3B, the median survival of Raji- and IM-9–bearing mice given control IgG was 21 and 28 days, respectively. In both models, a single injection of c1F6 was sufficient to significantly prolong survival. Median survival in Raji-bearing mice increased to 40 days and in IM-9–bearing mice survival increased to more than 86 days when the experiment was terminated. If treatment was delayed to 5 days after tumor cell inoculation, statistically significant survival benefit with c1F6 treatment was still observed, further illustrating the in vivo efficacy of c1F6. Chimeric 1F6 did not show detectable antitumor activity against CD70− xenografts, further supporting the specificity of anti-CD70 targeting (data not shown).
c1F6 prolongs survival of SCID mice bearing B-lymphoma xenografts. (A) Beginning one day after intravenous challenge with Raji cells, mice were given 4 doses of c1F6 at 4 mg/kg or 1 mg/kg once every 4 days as indicated by the inverted triangles. Control mice were untreated or treated with nonbinding IgG1 according to the same schedule. (B) Survival of mice bearing disseminated Raji or IM-9 lymphoma after treatment with a single 4-mg/kg dose of c1F6 administered 1 or 5 days after tumor cell injection (n = 10 per group). P values given are between c1F6-treated groups and either control IgG–treated or untreated group.
c1F6 prolongs survival of SCID mice bearing B-lymphoma xenografts. (A) Beginning one day after intravenous challenge with Raji cells, mice were given 4 doses of c1F6 at 4 mg/kg or 1 mg/kg once every 4 days as indicated by the inverted triangles. Control mice were untreated or treated with nonbinding IgG1 according to the same schedule. (B) Survival of mice bearing disseminated Raji or IM-9 lymphoma after treatment with a single 4-mg/kg dose of c1F6 administered 1 or 5 days after tumor cell injection (n = 10 per group). P values given are between c1F6-treated groups and either control IgG–treated or untreated group.
Antitumor activity of anti-CD70 is Fc dependent and mediated via multiple effector cells in vivo
Having demonstrated that c1F6 can orchestrate tumor cell killing via several Fc-mediated mechanisms in vitro, we sought to assess the relative importance of Fc-FcγR interactions of mAb activity in vivo. A variant of c1F6 (c1F6v) impaired in its ability to bind FcγR was generated with amino acid substitutions described in “Materials and methods.” As shown in Figure 2A, c1F6v retained antigen-binding function identical to that of parent c1F6 but no longer possessed ADCC or CDC activity, and its ability to promote phagocytic uptake of targeted cells by macrophages was substantially limited. In vivo, mice bearing disseminated Raji tumors that were treated with c1F6v succumbed to disease as rapidly as did untreated animals, with a median survival of 25 days. In contrast, treatment with unmodified c1F6 significantly extended median survival to 66 days (Figure 2B). The diminished antitumor activity of c1F6v was not due to increased clearance in vivo as determined by pharmacokinetic studies in mice, since the elimination phase half-lives for c1F6 and c1F6v were estimated to be 6 and 9 days, respectively. These data demonstrate that in vivo Raji tumor cell elimination seen with c1F6 is dependent on an intact Fc.
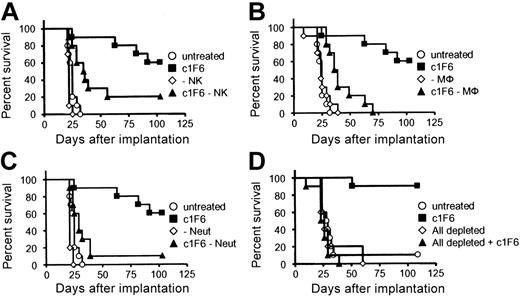
To further define the FcγR-expressing cell types responsible for c1F6-targeted cell clearance in vivo, mice were depleted of NK cells, macrophages, or neutrophils as described in “Materials and methods,” prior to tumor cell inoculation and administration of therapeutic antibody. Depletion of NK cells, macrophages, neutrophils, or a combination of all 3 in the absence of c1F6 did not affect the tumor growth, and tumor-bearing mice all succumbed to disease at the same rate regardless of effector cell depletion (Figure 4). In c1F6-treated mice, the absence of either effector NK cells or macrophages significantly reduced survival (Figure 4A and B, respectively). Still, residual antitumor activity with c1F6 in these mice conferred statistically significant survival benefit compared with similarly depleted mice receiving no therapeutic antibody (Figures 4A-B). Neutrophil depletion impacted survival of antibody-treated mice more profoundly (Figure 4C). Finally, mice depleted of all 3 effector cell subsets prior to treatment with c1F6 died of disease as rapidly as did animals that did not receive the therapeutic antibody (Figure 4D). These data suggest that the presence of NK cells, macrophages, and neutrophils was requisite for the efficacy of c1F6 in the Raji xenograft model.
Host effector cells are required for in vivo antitumor activity of c1F6. Raji-bearing mice were depleted of (A) NK cells, (B) macrophages (Mφ), (C) neutrophils (Neut), or (D) NK, Mφ, and Neut subsets as described in “Materials and methods.” After that, mice were either left untreated or treated with 4 mg/kg c1F6 one day following Raji challenge (n = 10 per group). (A) Untreated versus c1F6 (P < .001), untreated versus c1F6 − NK (P = .002), and c1F6 versus c1F6 − NK (P = .021). (B) Untreated versus c1F6 (P < .001), untreated versus c1F6 − Mφ (P < .001), and c1F6 versus c1F6 − Mφ (P < .001). (C) Untreated versus c1F6 (P < .001), untreated versus c1F6 − Neut (P = .058), and c1F6 versus c1F6 − Neut (P = .002). (D) Untreated versus c1F6 (P < .001), untreated versus all depleted + c1F6 (P = .177), and c1F6 versus all depleted + c1F6 (P < .001).
Host effector cells are required for in vivo antitumor activity of c1F6. Raji-bearing mice were depleted of (A) NK cells, (B) macrophages (Mφ), (C) neutrophils (Neut), or (D) NK, Mφ, and Neut subsets as described in “Materials and methods.” After that, mice were either left untreated or treated with 4 mg/kg c1F6 one day following Raji challenge (n = 10 per group). (A) Untreated versus c1F6 (P < .001), untreated versus c1F6 − NK (P = .002), and c1F6 versus c1F6 − NK (P = .021). (B) Untreated versus c1F6 (P < .001), untreated versus c1F6 − Mφ (P < .001), and c1F6 versus c1F6 − Mφ (P < .001). (C) Untreated versus c1F6 (P < .001), untreated versus c1F6 − Neut (P = .058), and c1F6 versus c1F6 − Neut (P = .002). (D) Untreated versus c1F6 (P < .001), untreated versus all depleted + c1F6 (P = .177), and c1F6 versus all depleted + c1F6 (P < .001).
Discussion
In this report, we confirm that CD70 is expressed in cell lines representing NHL and HD at frequencies similar to those reported for primary tumor samples evaluated by immunohistochemistry (IHC).17,19 We report for the first time CD70 expression in multiple myeloma cell lines. As expression of CD20 is largely absent in plasma cell dyscrasias, rendering them insensitive to CD20-targeted therapies, a therapeutic antibody directed against CD70 represents a novel clinical opportunity for this patient group. Preliminary data suggest that approximately 60% of primary MM tumor isolates are CD70+.35 Apart from hematologic tumors, CD70 is also expressed by a number of solid tumor types including renal cell carcinoma, glioblastoma, astrocytoma, nasopharyngeal carcinoma, and thymic carcinoma.14,20,22-25 Whereas CD70 was uniformly expressed in the cell lines reported herein, less is known about the heterogeneity of CD70 expression from cell to cell within primary tumors. Homogeneous CD70 expression in high-grade B lymphoma17,19 and renal cell carcinoma25 clinical isolates has been described, but the number of samples evaluated was limited. Studies to further evaluate expression of CD70 in primary tumors are certainly required.
We have previously reported that anti-CD70 mAbs can serve as efficient vehicles for delivery of cytotoxic drugs to CD70+ renal cell carcinoma cell lines.25 In this study, we focus on the activity of unconjugated anti-CD70. In contrast to the parent murine 1F6 antibody, chimerization of its VH/VL domains onto a human IgG1 isotype conferred antibody effector functions able to mediate death of all CD70+ tumor cell lines tested (Figure 1). As has been described for other therapeutic antibodies,36 the Fc region of c1F6 was key to its antitumor activities in vitro and in vivo. Modification of the Fc fragment rendered c1F6 unable to fix complement and disrupted interaction with FcγR-bearing cells such that ADCC activity was lost and phagocytic function was profoundly diminished (Figure 2A). Despite binding to target cells, the Fc-modified antibody c1F6v provided no survival benefit when administered to Raji tumor–bearing mice, confirming the importance of Fc-mediated activity of c1F6 in this model (Figure 2B). Clinical data from other mAb therapeutics support the concept that FcγR-mediated mechanisms such as ADCC are operational in patients. Objective clinical response to rituximab is increased significantly in NHL patients who are homozygous for the FcγRIIIa 158V polymorphism. NK cells from these individuals have a higher affinity for human IgG and are more cytotoxic toward target cells sensitized with therapeutic mAbs of human IgG1 isotype.37,38
Depletion of key FcγR-bearing cells responsible for ADCC and phagocytic activity, including neutrophils, macrophages, and NK cells, eliminated the mAb-dependent protective effect of c1F6 (Figure 4). The finding that multiple effector cell types contributed to the antitumor effect of c1F6 is in agreement with data describing rituximab activity in preclinical in vivo models. A predominant role for neutrophils and a secondary role for NK cells in the protection afforded Raji tumor–bearing SCID mice treated with rituximab has been reported.39 However, whereas macrophages did not appear to mediate antitumor activity in that study, macrophage depletion significantly impaired the c1F6 antitumor response in our disseminated Raji model. Consistent with this, 2 recent studies clearly demonstrate the activity of macrophages and the reticuloendothelial system in removing circulating B cells in mice treated with anti–murine CD20 antibody.33,34
The requirement for intact neutrophil, NK, and macrophage compartments and lack of lysis of c1F6-coated tumor cells in mouse serum (data not shown) indicate that cell-mediated mechanisms, rather than CDC, were key for c1F6 antitumor activity in our Raji rodent model. In vitro, CD70+ target cells expressing the complement regulatory protein CD59 were refractory to c1F6-mediated lysis (Figure 1E; Table 2), suggesting that CD59 expression on neoplastic cells could mitigate the CDC activity of c1F6. CD59 expression has been shown to interfere with complement-dependent tumor cell lysis in vitro.40 The Raji cell line used in our study expresses CD59 and is resistant to in vitro c1F6-mediated CDC (Table 2). Although formation of the membrane attack complex and subsequent cell lysis may not occur in CD59+ cancers, it is possible c1F6-mediated complement fixation and deposition of complement components such as C3b could contribute to tumor depletion via opsonization and enhanced phagocytosis in vivo.
The c1F6-mediated CDC activity reported herein agrees with a recent report of in vitro CDC activity by murine IgG2b anti-CD70 antibody, LD6.41 Contrary to our findings, the authors concluded that in vivo antitumor activity of LD6 was due mainly to its CDC activity, as suggested by the lack of inflammatory infiltrate into the tumors in situ. As CD59 expression appears to be important in determining sensitivity to c1F6-mediated CDC (Table 2), it is possible that the cell lines used for xenograft experiments in the Israel et al study41 have reduced CD59 expression and are more sensitive to CDC, or that the murine IgG2b antibody backbone activates complement in mice more efficiently than does antibody of human IgG1 isotype. As the potential contribution of NK or phagocytic cells was not formally tested, it is unclear to what extent cell-mediated mechanisms may have been involved in growth suppression of CD70+ Burkitt lymphoma in their study.
As antibody effector functions of c1F6 appear to be essential for its in vivo antitumor activity, strategies to enhance host immune cell functions may improve the antitumor activity of c1F6. One approach is the use of thalidomide and immunomodulatory drugs (IMiDs) to augment the innate immune response, particularly NK-dependent cytotoxicity.42 This approach has been successfully applied to increase the efficacy of antibodies targeting CD2039 and CD40.43 Cytokines such as IL-244 and GM-CSF45 that enhance ADCC activity mediated by NK cells and neutrophils, respectively, also improve the efficacy of rituximab. Studies are currently ongoing to evaluate whether c1F6 in combination with regimens that stimulate the innate immune response will show enhanced antitumor activity. Improved c1F6-mediated effector functions might also be achieved by altering antibody glycan composition or engineering the Fc amino-acid sequence to improve Fc–Fcγ receptor binding.46
Since CD70 expression on normal cells is rare, targeting B lymphoma with anti-CD70 antibody rather than mAbs targeting pan-lineage markers (eg, CD20 or CD52) will likely have less impact on the resting B-cell repertoire and memory B-cell pools. Targeting CD70 may still have undesirable side effects. Anti-CD70–mediated depletion or blockade of immune cell interaction may result in temporary immune suppression during treatment, since CD70 is expressed on subsets of mature DCs and activated T and B lymphocytes. Treatment of immunocompetent rodents with anti–murine CD70 could shed light on the impact of targeting CD70 on normal immune responses. CD70 lacks defined cytoplasmic motifs to recruit signaling molecules, yet regulation of B- and T-cell functions and proliferation of a subset of B-CLL cells through reverse CD70 signaling has been reported.17,47,48 However, we have not observed proliferation of tumor cell lines, primary CD70+ B or T cells, or augmentation of tumor growth in lymphoma xenograft models as a result of administration of c1F6.
The potential for c1F6 to selectively eliminate activated autoreactive B and T cells or block CD70-CD27 stimulation suggests therapeutic potential in autoimmune diseases. CD70 is present on lymphocytes isolated from joints of rheumatoid and psoriatic arthritis patients49 and on peripheral lymphocytes recovered from systemic lupus erythematosus patients.50,51 In murine models, costimulation blockade using anti–murine CD70 antibody inhibited the development of experimental autoimmune encephalitis52 and prevented cardiac allograft rejection.53,54 The utility of c1F6 in ameliorating autoimmune disease is being studied.
In summary, we provide data in support of CD70 as a target for antibody-based treatment of B-lineage cancers. Other CD70+ hematologic cancers that could be amenable to anti-CD70 therapeutic antibody application include Hodgkin disease, T-cell lymphoma, leukemia, and multiple myeloma. Further, the expression of CD70 on solid tumors including nasopharyngeal carcinoma, glioblastoma, and renal cell carcinoma suggests broader application in carcinoma where treatment options are rare. We provide proof of concept that an engineered anti–human CD70 mAb can eliminate CD70+ tumor cells and prolong survival of tumor-bearing mice via multiple antibody effector functions. For clinical development, we have recently engineered a humanized anti-CD70, SGN-70, that is functionally equivalent to c1F6 reported herein (J.A.M., C.F.M., Leia M. Smith, K.K., E.O., Carol Morris-Tilden, Ivan Stone, Renee McCormick, P.C., Hanspeter Gerber, I.S.G., and C.-L.L., manuscript in preparation). Studies are in progress to evaluate the safety of SGN-70 to enable clinical trials.
Authorship
Contribution: J.A.M. designed and conducted research, analyzed data, and wrote the paper; E.O. designed and conducted research, and analyzed data; K.A.G., I.S., and K.K. conducted research; L.F., C.F.M., E.T., N.v.R., and P.C. contributed vital reagents; A.F.W. and I.S.G. contributed to the design of research; C.-L.L. designed research, analyzed data, and wrote the paper.
Conflict-of-interest statement: J.A.M., E.O., L.F., C.F.M., K.A.G., I.S., K.K., E.T., P.C., I.S.G., and C.-L.L. are full-time employees of Seattle Genetics, Inc. A.F.W. was a full-time employee of Seattle Genetics, Inc, when he conducted the research reported here. A.F.W. is now employed by Trubion Pharmaceuticals Inc. No Trubion Pharmaceuticals products were examined in the study.
Correspondence: Che-Leung Law, Seattle Genetics, Inc, 21823 30th Dr SE, Bothell, WA 98021; e-mail: claw@seagen.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal