Abstract
Extracellular signal-regulated kinase-1/2 (ERK1/2) is frequently found constitutively activated (p-ERK1/2) in hematopoietic diseases, suggesting a role in leukemogenesis. The aim of this study was to assess the expression and clinical role of p-ERK1/2 in adult acute lymphoblastic leukemia (ALL). In 131 primary samples from adult de novo ALL patients enrolled in the Gruppo Italiano per le Malattie Ematologiche dell'Adulto (GIMEMA) Leucemia Acute Linfoide (LAL) 2000 protocol and evaluated by flow cytometry, constitutive ERK1/2 activation was found in 34.5% of cases; these results were significantly associated with higher white blood cell (WBC) values (P = .013). In a multivariate analysis, p-ERK1/2 expression was an independent predictor of complete remission achievement (P = .027). Effective approaches toward MEK inhibition need to be explored in order to evaluate whether this may represent a new therapeutic strategy for adult ALL patients.
Introduction
Studies on the treatment of adult acute lymphoblastic leukemia (ALL) have shown only modest improvements over the last 2 decades, with the actual cure rate remaining in the range of 15% to 40%.1,2 Novel treatment modalities have been directed toward inappropriately activated cell-signaling pathways that may be responsible for the proliferation and/or apoptosis escape of leukemic blasts.3
The mitogen-activated protein kinase (MAPK) signaling cascade is one of the pathways that mediates the proliferation and differentiation of hematopoietic cells.4 Among the MAPK pathways, extracellular signal-regulated kinase (ERK1/2) is activated in response to various mitogenic signals, participating in a wide range of cellular programs including cell-cycle progression and differentiation.5
In recent years, it has been reported that constitutive ERK1/2 activation (p-ERK1/2) is frequently found in solid tumors6,7 as well as in hematologic neoplastic diseases, particularly acute myeloid leukemia (AML). Milella et al8 demonstrated the constitutive ERK1/2 activation in 74% of primary AML cells. Ricciardi et al,9 using a flow cytometric technique, similarly found constitutive p-ERK1/2 in 83.3% of AML cases. In addition, it has been reported10 that p-ERK1/2 may represent an independent prognostic factor for survival in AML patients.
The availability of low–molecular-weight, cell membrane–permeable agents targeting the MAPK pathway by inhibiting MEK-dependent ERK1/2 phosphorylation has suggested a potential role of therapeutic strategies based on MEK inhibition in diseases characterized by its constitutive activation.11,12 Limited information is available, at this time, on the presence and role of constitutive ERK1/2 signaling activation in ALL. Constitutive ERK1/2 activation in ALL cell lines and in a limited number of clinical ALL specimens has been reported.13,14 The aim of our study was to evaluate the frequency of p-ERK1/2 expression in primary blasts from adult ALL cases enrolled in the Gruppo Italiano per le Malattie Ematologiche dell'Adulto (GIMEMA) Leucemia Acute Linfoide (LAL) 2000 multicenter study and to correlate p-ERK1/2 expression with different clinical and biologic prognostic factors.
Patients, materials, and methods
The study was approved by the Institutional Review Board of the Department of Cellular Biotechnologies and Hematology, University “La Sapienza” of Rome. Bone marrow (BM) and/or peripheral blood (PB) samples for p-ERK1/2 studies were obtained at initial diagnosis from adult ALL patients enrolled in the GIMEMA LAL2000 multicenter study. This study was performed on 518 enrolled patients, of which 455 were evaluated for response to chemotherapy. Because of cell limitation, sufficient amounts of fresh material for p-ERK1/2 expression were available from 131 patients. The correlation between p-ERK1/2 expression and the clinical response was possible in 111 of 131 of cases. Blast cells from fresh centralized material were enriched by Ficoll-Hypaque (1077 g/mL; Sigma, Milan, Italy) gradient centrifugation. Assessment of cell number and viability was performed by trypan-blue exclusion; only 0.5% of samples could not be further processed because of poor viability (< 80% of viable cells).
According to the GIMEMA LAL2000 study protocol, therapy consisted of a 7-day prephase with prednisone (PDN; 20 mg/m2) followed by standard induction chemotherapy. The protocol is described in Document S2 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Response to the steroid prephase (peripheral blast cell count at day 0 < 1000/μL) was also evaluated in the study. Postinduction treatment and response criteria have been described elsewhere.15,16
p-ERK1/2 expression was preliminarily evaluated in leukemic cell lines and in unstimulated and PMA-activated normal PB and leukemic primary samples (Document S2). For the propose of this study, clinical samples were evaluated by a flow cytometric technique as previously described.9,17 Cell-cycle distribution was evaluated as previously described.18 p-ERK1/2 flow cytometric expression was analyzed using the Kolmogorov-Smirnov statistic test (D value).16 Protein expression was represented as either a continuous or a dichotomized variable; samples with a D value of 0.10 or greater were considered positive. In order to assess the differences between groups, chi-square and Fisher exact tests were performed. The SAS software (SAS Institute, Cary, NC) was used for the analysis.
Results and discussion
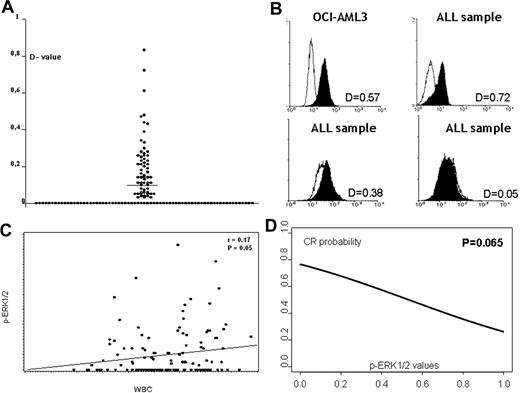
This study was conducted to determine whether the MAPK pathway, specifically ERK1/2, is constitutively activated in adult ALL cells and may therefore represent a potential therapeutic target.19 In the present study, we have evaluated the largest series of clinical samples reported to date. Table 1 reports the clinical and pathologic characteristics of the 131 patients with evaluable cell material for the p-ERK1/2 study. The clinical characteristics and outcome of this population differed from the remaining LAL2000 enrolled patients because of a higher WBC count (P = .021) and blast percentage (P = .001), lower response to PDN measured by decreased peripheral blast cell count (P = .032), and lower complete response (CR) rate (P < .001). In the 131 de novo ALL samples analyzed, p-ERK1/2 levels were expressed as D values and ranged between 0 and 0.83. ERK1/2 activation (D value ≥ 0.10) was observed in 45 (34.5%) of 131 samples. Three examples of primary ALL characterized by negative, intermediate, and high p-ERK1/2 expression are shown in Figure 1. Table 2 reports the clinical and biologic characteristics of the 131 patients according to p-ERK1/2 expression. No significant association was observed between p-ERK1/2 expression and age or immunophenotype. Interestingly, the expression of p-ERK1/2 was significantly associated with the WBC counts (Table 2). Higher levels of p-ERK1/2, in fact, directly correlated with a higher WBC count expressed as logarithmic scale (r = 0.17; P = .05; Figure 1), while cell-cycle distribution did not (Table S1). We also noticed that ALL samples expressing the CD34 antigen were inversely associated with p-ERK1/2 expression (P = .003). No significant association was observed between p-ERK1/2 expression and cytogenetic and molecular groups; in particular, BCR/ABL+ patients were not significantly associated with p-ERK1/2, both when analyzed by dichotomized variable (Table 2) and by continuous variable (P = not significant [NS]). Of relevance, p-ERK1/2 expression (dichotomized variable) was found in 44.1% (15/34) of steroid nonresponder patients versus 27.7% (18/65) of cases with day-0 blasts less than 1000 (responders; P = .099). We then evaluated in the study population (Table S2) the prognostic role of known clinical/biologic parameters (age, WBC counts, blast reduction following the steroid prephase, phenotype, and BCR/ABL translocation) and p-ERK1/2 expression. Blast reduction significantly affected CR achievement (P = .015) and a trend-significant association was found between lower p-ERK1/2 (continuous variable) and higher probability of achieving CR (P = .069; Figure 1). The BCR/ABL translocation was not a prognostic factor for CR achievement in this series of patients (P = NS). Multivariate analysis was performed to evaluate the impact of p-ERK1/2 expression on achievement of CR in a model that included WBC values and blast reduction following the steroid prephase. Among 99 patients evaluable for these parameters, p-ERK1/2 was an independent predictor of CR (P = .027; odds ratio 0.040; 95% confidence interval [CI] 0.002-0.693), together with blast reduction (P = .009), whereas WBC counts were not. Conversely, p-ERK1/2 expression failed to predict continuous complete remission (CCR), overall survival (OS), and event-free survival (EFS). Multivariate analysis confirmed these results showing that in our population, only blast reduction and age influenced EFS (P = .005 and P = .038, respectively), whereas WBC count (P = .89), immunophenotype (P = .58), BCR/ABL (P = .54), and p-ERK1/2 (P = .19) did not.
Clinical and biologic characteristics of ALL patients treated according to the GIMEMA LAL2000
| . | p-ERK1/2 study population, n = 131 . | P . | Nonstudy population, n = 387 . |
|---|---|---|---|
| WBC, ×109/L | <.001* | ||
| Median | 39.0 | 11.0 | |
| Range | 0.9-872.3 | 0.3-800 | |
| Age, y | NS* | ||
| Median | 36.2 | 33.6 | |
| Range | 15.4-59.9 | 14.1-59.9 | |
| Blasts, % | .001* | ||
| Median | 91 | 90 | |
| Range | 65-99 | 30-99 | |
| Sex, no. (%) | NS† | ||
| Male | 74 (56.5) | 222 (57.4) | |
| Female | 57 (43.5) | 165 (42.6) | |
| Phenotype, no. (%) | NS† | ||
| B-ALL | 95 (72.5) | 310 (80.1) | |
| T-ALL | 34 (26.0) | 72 (18.6) | |
| Biphenotypic | 2 (1.5) | 5 (1.3) | |
| Molecular characteristics, no. (%) | NS† | ||
| BCR/ABL negative | 90 (73.2) | 236 (70.4) | |
| BCR/ABL positive | 33 (26.8) | 99 (29.6) | |
| NS‡ | |||
| t(4;11) negative | 94 (94.0) | 245 (95.7) | |
| t(4;11) positive | 6 (6.0) | 11 (4.3 | |
| Pretreatment responders, no. (%) | .032† | ||
| Blasts less than 1000 | 67 (66.3) | 232 (77.1) | |
| Blasts 1000 or greater | 34 (33.7) | 69 (22.9) | |
| Response to treatment | .001‡ | ||
| % CR | 72.1% | 86.3% |
| . | p-ERK1/2 study population, n = 131 . | P . | Nonstudy population, n = 387 . |
|---|---|---|---|
| WBC, ×109/L | <.001* | ||
| Median | 39.0 | 11.0 | |
| Range | 0.9-872.3 | 0.3-800 | |
| Age, y | NS* | ||
| Median | 36.2 | 33.6 | |
| Range | 15.4-59.9 | 14.1-59.9 | |
| Blasts, % | .001* | ||
| Median | 91 | 90 | |
| Range | 65-99 | 30-99 | |
| Sex, no. (%) | NS† | ||
| Male | 74 (56.5) | 222 (57.4) | |
| Female | 57 (43.5) | 165 (42.6) | |
| Phenotype, no. (%) | NS† | ||
| B-ALL | 95 (72.5) | 310 (80.1) | |
| T-ALL | 34 (26.0) | 72 (18.6) | |
| Biphenotypic | 2 (1.5) | 5 (1.3) | |
| Molecular characteristics, no. (%) | NS† | ||
| BCR/ABL negative | 90 (73.2) | 236 (70.4) | |
| BCR/ABL positive | 33 (26.8) | 99 (29.6) | |
| NS‡ | |||
| t(4;11) negative | 94 (94.0) | 245 (95.7) | |
| t(4;11) positive | 6 (6.0) | 11 (4.3 | |
| Pretreatment responders, no. (%) | .032† | ||
| Blasts less than 1000 | 67 (66.3) | 232 (77.1) | |
| Blasts 1000 or greater | 34 (33.7) | 69 (22.9) | |
| Response to treatment | .001‡ | ||
| % CR | 72.1% | 86.3% |
NS indicates not significant.
Wilcoxon statistic test.
Chi-square statistic test.
Fisher statistic test.
Expression and role of p-ERK1/2 in adult ALL samples. (A) Distribution of p-ERK1/2 expression in 131 ALL samples. (B) Three examples of primary ALL characterized by negative or intermediate and high p-ERK1/2–positive expression (flow-cytometry assay). (C) Correlation between p-ERK1/2 expression and WBC count in 131 ALL samples (P = .026; r = 0.20). (D) Correlation between p-ERK1/2 expression and response to treatment. A trend (P = .069) was found between lower phosphoprotein level (continuous variable) and higher probability of achieving CR.
Expression and role of p-ERK1/2 in adult ALL samples. (A) Distribution of p-ERK1/2 expression in 131 ALL samples. (B) Three examples of primary ALL characterized by negative or intermediate and high p-ERK1/2–positive expression (flow-cytometry assay). (C) Correlation between p-ERK1/2 expression and WBC count in 131 ALL samples (P = .026; r = 0.20). (D) Correlation between p-ERK1/2 expression and response to treatment. A trend (P = .069) was found between lower phosphoprotein level (continuous variable) and higher probability of achieving CR.
Frequency distribution of clinical and biologic features with respect to p-ERK1/2 expression
| . | p-ERK1/2 positive/total, no. (%) . | P . |
|---|---|---|
| Age, y | NS | |
| 16–30 | 14/45 (31.1) | |
| 31–60 | 31/86 (36.1) | |
| WBC | .013 | |
| Less than 50 × 109/L | 18/72 (25.0) | |
| 50 × 109/L or greater | 27/59 (45.8) | |
| Immunophenotype | NS | |
| B-ALL | 31/95 (32.6) | |
| T-ALL | 13/34 (38.2) | |
| .003 | ||
| CD34− less than 10% | 11/17 (64.7) | |
| CD34+ 10% or greater | 19/72 (26.4) | |
| Cytogenetic risk group | NS | |
| Standard low risk | 14/27 (51.9) | |
| Intermediate risk | 4/14 (28.6) | |
| High risk | 9/24 (37.5) | |
| Failure | 11/50 (22.0) | |
| Molecular genetics | NS | |
| BCR/ABL negative | 30/90 (33.3) | |
| BCR/ABL positive | 12/33 (36.4) | |
| t(4;11) negative | 34/94 (36.2) | |
| t(4;11) positive | 1/6 (16.7) | |
| Pretreatment responders | .099 | |
| Blasts less than 1000 | 18/65 (27.7) | |
| Blasts 1000 or greater | 15/34 (44.1) |
| . | p-ERK1/2 positive/total, no. (%) . | P . |
|---|---|---|
| Age, y | NS | |
| 16–30 | 14/45 (31.1) | |
| 31–60 | 31/86 (36.1) | |
| WBC | .013 | |
| Less than 50 × 109/L | 18/72 (25.0) | |
| 50 × 109/L or greater | 27/59 (45.8) | |
| Immunophenotype | NS | |
| B-ALL | 31/95 (32.6) | |
| T-ALL | 13/34 (38.2) | |
| .003 | ||
| CD34− less than 10% | 11/17 (64.7) | |
| CD34+ 10% or greater | 19/72 (26.4) | |
| Cytogenetic risk group | NS | |
| Standard low risk | 14/27 (51.9) | |
| Intermediate risk | 4/14 (28.6) | |
| High risk | 9/24 (37.5) | |
| Failure | 11/50 (22.0) | |
| Molecular genetics | NS | |
| BCR/ABL negative | 30/90 (33.3) | |
| BCR/ABL positive | 12/33 (36.4) | |
| t(4;11) negative | 34/94 (36.2) | |
| t(4;11) positive | 1/6 (16.7) | |
| Pretreatment responders | .099 | |
| Blasts less than 1000 | 18/65 (27.7) | |
| Blasts 1000 or greater | 15/34 (44.1) |
NS indicates not significant.
In conclusion, our data, obtained in this large series of patients uniformly enrolled in a multicenter clinical trial, indicate that p-ERK1/2 is consistently expressed in a proportion of adult ALL, is associated with poor prognostic factors (higher WBC counts and failure to respond to steroid prephase), and independently affects achievement of CR. Small-molecule inhibitors effectively targeting the MAPK pathway and/or other potentially involved signaling pathways, such as the PI3K/Akt/mTOR cascade,20,21 must be evaluated to establish whether this targeted therapeutic modality may add benefit (antiproliferative/proapoptotic) to the current treatment of adult ALL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Stefania Gubbiotti for biostatistical analysis and Sandra De Simone for data management. The authors are grateful to the GIMEMA group for cooperation in this study.
This work was supported by the Italian Association for Cancer Research (AIRC).
Authorship
Contribution: C.G. designed the experiments, performed research, and wrote the paper. M.R.R. performed research and provided experimental advice. M.T.P. provided experimental advice. M.C.S. and F.D.C. performed research. P.F. and M.V. analyzed data. A.V. supervised the centralized core facility. M. Mancini performed cytogenetic studies. G.C. performed molecular studies. S.P., F.D.R., G.S., F.F., N.C., F. Mosna, A.C., M.L., L.A., E.M., G.F., F.R., and G.M. performed clinical activities and provided samples. F.M. reviewed the manuscript. M.A. provided project advice. M. Milella provided important analytical tools. R.F. critically discussed the results and reviewed the manuscript. A.T. designed the project, supervised the work, wrote the paper, and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the institutions that included samples for the GIMEMA Acute Leukemia Working Party, Italy in this study appears in Document S1.
Correspondence: Agostino Tafuri, Division of Hematology, Department of Cellular Biotechnologies and Hematology, University “La Sapienza” of Rome, Via Benevento 6, 00161 Rome, Italy; e-mail: agotaf@bce.uniroma1.it.