Abstract
Inhibition of endothelial cell proliferation and angiogenesis is emerging as an important strategy in cancer therapeutics. Kringle 5 (K5) of human plasminogen is a potent angiogenesis inhibitor. Previous studies have shown K5 exposure promotes caspase activity and apoptosis in endothelial cells. Here we report that K5 treatment evokes an autophagic response in endothelial cells that is specific and initiated even in the absence of nutritional stress. Endothelial cells exposed to K5 up-regulated Beclin 1 levels within a few hours. Furthermore, progressively increasing amounts of antiapoptotic Bcl-2 were found to be complexed with Beclin 1, although total levels of Bcl-2 remained unchanged. Prolonged exposure to K5 ultimately led to apoptosis via mitochondrial membrane depolarization and caspase activation in endothelial cells. Knocking down Beclin 1 levels by RNA interference decreased K5 induced autophagy but accelerated K5-induced apoptosis. These studies suggest that interfering with the autophagic survival response can potentiate the antiangiogenic effects of Kringle 5 in endothelial cells.
Introduction
The sprouting of new blood vessels from preexisting vasculature, angiogenesis, is necessary for tumor growth and metastasis. Several angiogenic growth factors are secreted into the tumor microenvironment that can induce endothelial cell proliferation, migration, and capillary tube formation. Intervention of any one of these processes can affect angiogenesis and, consequently, inhibit tumor growth.1 A number of endogenous inhibitors of angiogenesis have been identified. Among them, 2 prominent groups of angiogenic inhibitors have been extensively studied. One of the groups of inhibitors is generated by the proteolytic cleavage of blood coagulation related proteins, and the other is generated by the proteolysis of extracellular matrix proteins such as collagen.2–4 Kringle fragments of plasminogen K1, K2, K3, and the multiple kringle domains of K1-4 (angiostatin), K1-3, K2-3, and K1-5 have been shown to inhibit endothelial cell growth.5,6 K1-5 specifically inhibits endothelial cell growth via its interaction with endothelial cell–surface ATP synthase, which sequentially triggers the activation of caspases -8, -9, and -3.7 Kringle 5 (K5), an internal proteolytic fragment of plasminogen, is also an inhibitor of angiogenesis although the molecular mechanism by which K5 affects endothelial cell proliferation is not completely understood.8 K5 has also been shown to induce apoptosis in endothelial cells.9 Glucose-regulated protein 78 (GRP 78), an endoplasmic reticulum (ER) stress response protein up-regulated by glucose starvation, acidosis, and hypoxia,10 was identified as one of the targets of K5. The binding of K5 to GRP 78 was shown to enhance caspase-7 activity in cells under nutritional stress.11
Stress-inducing stimuli that promote apoptotic cell death also activate autophagy, an adaptive response that may prolong cell survival.12 In fact, it is evident that the 2 pathways also referred to as type I programmed cell death (PCD I; apoptotic cell death) and type II programmed cell death (PCD II; autophagic cell death), interact at a number of levels.13 Autophagy (self-consumption) is an evolutionarily conserved pathway that plays a major role in bulk degradation of most long-lived proteins and defective or superfluous organelles. Autophagy is characterized by the formation of double- or multimembrane cytoplasmic vesicles called autophagosomes that subsequently fuse with late endosomes or lysosomes.13 A protein complex containing the class III PI-3 kinase, a p150 myristylated protein kinase, and Beclin 1 is essential for autophagosome generation.14,15 Beclin 1, a mammalian homolog of the yeast protein ATG6, is believed to function as a tumor suppressor protein.16 The cross-talk between apoptotic and autophagic stress response pathways became evident from the observation that Bcl-2 when overexpressed was found to bind Beclin 1 and inhibit starvation-induced autophagy.17 We were interested in determining whether autophagy played any role during the treatment of endothelial cells with an angiogenesis inhibitor, K5.
Our present studies show that recombinant K5 (rK5) induces both autophagy and apoptosis in endothelial cells. The K5-induced autophagy was specific and initiated even in the presence of nutrient-rich medium. Beclin 1 was up-regulated while Bcl-2 levels remained unchanged in endothelial cells treated with the angiogenesis inhibitor. Our studies suggest that endothelial cells initiate autophagic survival response early on during the treatment with K5 and that prolong exposure activates intrinsic apoptotic cell death pathway. Therefore, interfering with autophagic survival response can potentiate the biological activity of K5.
Materials and methods
Materials
Tetramethyl rhodamine ethylester (TMRE), 3-methyladenine (3-MA), and β-actin antibody were from Sigma (St Louis, MO). MitoTracker Deep red 633, Mitotracker Green FM, Alexa Fluor 647 anti–mouse IgG antibody, and Alexa Fluor 488 anti–rabbit IgG antibody were from Molecular Probes (Eugene, OR). Lipofectamine 2000 and monoclonal anti-His antibody were from Invitrogen (San Diego, CA). Antibody to cleaved caspase-3 (Asp 175) was from Cell Signaling Technology (Danvers, MA). Protein A/G Plus-Agarose and Bcl-2 polyclonal antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). Vinculin antibody was from Abcam Inc (Cambridge, MA). Recombinant hVEGF (VEGF165) was from R&D Systems (Minneapolis, MN). zVAD-fmk was from Calbiochem (San Diego, CA). GFP-LC3 is a gift from Drs T. Yoshimori (National Institute of Genetics, Shizuoka-ken, Japan) and N. Mizushima (The Tokyo Metropolitan Institute of Medical Science, Japan). Lysosomal-associated membrane protein 1–green fluorescent protein (LAMP1-GFP) construct is a generous gift from Dr Dell'Angelica (University of California, Los Angeles).
Cell culture
MA148, OVCAR-3, and OVCAR-5 cells were grown in RPMI-1640 medium (Life Technologies, Grand Island, NY) supplemented with 10% FBS. Ad HEK-293 cells (Stratagene, La Jolla, CA) were grown in DMEM medium supplemented with 10% FBS. Human umbilical vein cells (HUVECs), human microvascular endothelial cells (HMVECs), and human foreskin fibroblasts were provided by Dr Vercellotti (University of Minnesota, Minneapolis). Endothelial cells were grown in tissue-culture flasks precoated with 0.2% gelatin (Sigma) and maintained in complete endothelial growth medium (EGM; Clonetics, San Diego, CA).
Construction of rAAV-K5 and characterization of soluble rK5
K5 cDNA was cloned into rAAV plasmid (Stratagene) pCMV-MCS that had been modified to contain the IgG kappa light-chain signal peptide. pAAV-GFP was used as a control. For the production of soluble rK5 (henceforth rK5), rAAV-K5–transduced Ad HEK-293 cells were maintained in gene therapy serum-free media (Sigma). rK5 was purified from the conditioned medium using Ni-NTA affinity column (Qiagen, Valencia, CA), and was further characterized by mass spectroscopy and NH2-terminal sequencing. To verify the specific effect of rK5, conditioned media from rAAV-GFP–transduced HEK-293 cells were passed through the same affinity purification column. Fractions eluted by 100 mM imidazole (Sigma) were concentrated and used as a negative control (purified rAAV-GFP CM).
Effect of rK5 on endothelial cell proliferation
HUVEC proliferation assay was carried out as previously described.18 Adenovirus-expressed P125A endostatin was used as a positive control. HUVEC proliferation was determined 72 hours after treatment with rK5 by BrdU Assay (Roche Diagnostics, Indianapolis, IN). VEGF-induced cell proliferation was considered as a 100% response to calculate relative inhibition elicited by rK5 and P125A endostatin. BrdU incorporation in the absence of VEGF was considered the basal level of proliferation.
Assessment of apoptosis
rK5-induced apoptosis in the first 24 hours of treatment was determined by the annexin V–FLUOS assay kit (Roche Diagnostics). After 72 hours of rK5 treatment, apoptosis was quantified by TUNEL assay (Roche Diagnostics). Images from triplicate samples were recorded by a PixCell II LCM system (Arcturus, Mountain View, CA) at 100× magnification (UPlanFl 10×/0.30 NA) or 40× magnification (UPlanFl 4×/0.13 NA). The images were processed by the Image Processing Toolkit and Adobe Photoshop (Adobe Inc, Mountain View, CA) software. The apoptotic index was determined by the ratio of annexin V+/TUNEL+ cells to the total number of nuclei per field.
Flow cytometry
Caspase activation in HUVECs treated with rK5 was assessed by flow cytometry using a carboxyfluorescein fluorochrome-labeled inhibitor of caspases (FLICA) apoptosis detection kit (Immunochemistry Technologies, LLC). Endothelial cells were cotransfected with plasmid expressing either scrambled shRNA or shRNA specific for Beclin 1 and a DsRed 2 expression construct. Transfectants were treated with rK5 (1.5 μg/mL) and then stained with FAM-VAD-FMK and 7-AAD. Cells positive for activated caspases were detected by a FACSCanto flow cytometer (BD Biosciences, Rockville, MD) according to the manufacturer's protocol.
Confocal and immunofluorescence microscopy
Immunofluorescence staining was carried out as previously described19 using antibodies against LAMP1 (Affinity Bioreagents, Golden, CO) and Beclin 1 (BD Transduction Laboratories, Lexington, KY). Alexa Fluor 488 anti–rabbit IgG antibody and Alexa Fluor 647 anti–mouse IgG antibody were used as the secondary antibody for the detection of LAMP1 and Beclin 1, respectively. Mitotracker Red or DAPI (Vector Laboratories Inc, Burlingame, CA) was used to visualize mitochondria and the nucleus, respectively. Fields were chosen randomly from various sections to ensure objectivity of sampling. Digital images at 600× magnification (Plan Apo N 60×/1.42 NA oil) were acquired using a Bio-Rad MRC Single Photon Confocal (1024) System via Bio-Rad Laser Sharp 3.0 imaging software (Bio-Rad, Hercules, CA). Images were converted to TIFF format using Confocal Assistant software version 4.02 (a free 2- and 3-dimensional animation program, created and copyrighted by T. J. Brelje). To quantify the number of autophagy vesicles per cell, confocal microscopy images of the monodansyl cadaverine (MDC)–stained vesicles (red) or LAMP1-GFP vesicles (green) were binarized to black and white images by Adobe Photoshop (Adobe Systems, San Jose, CA) and then converted to centroids for scoring automatically by the Image Processing Tool Kit.
shRNA-mediated knockdown of Beclin 1 expression in endothelial cells
HUVECs were transiently transfected with shRNA against Beclin 1 and GFP-LC3 or LAMP1-GFP by using Lipofectamine 2000 (Invitrogen) and Opti-MEM as previously described.20 Scrambled shRNA was used as a negative control. The reduction in Beclin 1 protein was estimated by Western blot analysis using mouse anti–human Beclin 1 monoclonal antibody.
Results
rK5 inhibits endothelial cell proliferation and tube formation
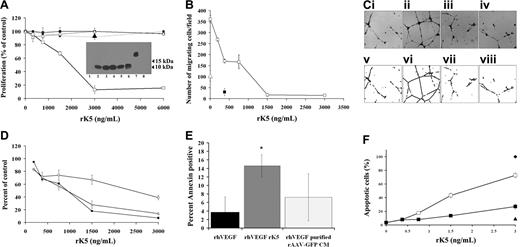
Angiogenesis is characterized by endothelial cell proliferation, migration, and tube formation. At the outset, rK5 was tested for its biological activity in HUVEC proliferation, migration, and tube formation. BrdU cell proliferation assays indicated that rK5 inhibited VEGF-induced HUVEC proliferation in a dose-dependent manner. HUVEC proliferation was suppressed to the basal level at a concentration of 1.5 μg/mL of rK5 (Figure 1A), while nontarget cells such as OVCAR-3, OVCAR-5, and fibroblasts derived from human foreskin were not inhibited. rK5 inhibited HUVEC migration induced by VEGF in a Boyden chamber in a concentration dependent manner with an IC50 of approximately 0.375 μg/mL. At a higher concentration, rK5 completely suppressed HUVEC migration (Figure 1B).
Effects of rK5 on endothelial cells. (A) The effect of rK5 on VEGF-induced cell proliferation. BrdU incorporation was used as an index of proliferation. Each data point represents a mean of 3 independent experiments using triplicate cultures. HUVECs (□), human foreskin fibroblast (■), OVCAR-5 (●), and OVCAR-3 (▵). Purified rAAV-GFP CM (3 μg/mL)–treated HUVECs (▴) was used as negative control. Inset: anti-His Tag immunoblot analysis of purified recombinant AAV-K5 (rK5) in fractions eluted by 100 mM imidazole (lanes 2-5) and CM before purification (lane 6). A major band of approximately 10 kDa was detected. Lane 1 shows CM of nontransduced HEK-293 cells; lane 7 shows endostatin with a C-terminal poly-His–tagged (positive control); and lane 8 shows purified rAAV-GFP CM. (B) The effect of rK5 on endothelial cell migration in Boyden chamber assay. The migration of endothelial cells was determined by a method previously described.18 Endothelial cells were prelabeled with 5.0 μM 56-CFDA and induced to migrate toward the bottom chamber containing 40 ng/mL VEGF. The number of cells migrated to the bottom side of the membrane was counted using a PixCell II LCM system at 40× magnification. Data represent values from 3 independent experiments using 3 wells per concentration. M199 medium (5% FBS; ▵) was used to determine basal level of migration. P125A endostatin (■) was used as a positive control. rK5 induced concentration-dependent inhibition of cell migration (□). (C) The effect of rK5 on angiogenesis was evaluated by using VEGF-stimulated tube formation in a Matrigel assay.18 Representative images of endothelial cell tube formation are shown. (Ci) Basal level of tube formation. (Cii-iv) Tube formation induced by 40 ng/mL VEGF. (Ciii) rK5. (Civ) P125A endostatin (300 ng/mL). Bright-field images were recorded at 40× magnification (top row) and processed for analysis (Cv-viii) as described in “Materials and methods” (bottom row). (D) Morphometric analysis of tube formation. Ends (□), branch points (■), and tube length (▵) are shown. Values represent data from 3 independent experiments using 3 wells per sample. VEGF-induced tube formation was considered as 100% tube formation. Values are shown as means ± SE. (E) rK5 induced endothelial cell apoptosis under nutrient-rich culture conditions. HUVECs were cultured in the presence of 40 ng/mL VEGF. Cells were treated with 1.5 μg/mL rK5 for 24 hours and then labeled with annexin V FLUOS and DAPI. Images were recorded at 40× magnification and processed by the Image Processing Toolkit and Adobe Photoshop. Percentage of apoptotic cells was determined by the ratio of annexin V+ cells over the total number of nuclei per field. Purified rAAV-GFP CM was used as a negative control at a similar concentration. Values represent data from 2 independent experiments using 3 wells per sample. Error bars denote SE (*P < .05). (F) HUVECs were stimulated with VEGF and treated with different concentrations of rK5. In one set of cultures, rK5 was withdrawn after 24 hours of exposure (■) and replaced with fresh media. The second set of cultures was continuously exposed to rK5 (□). Cells from both treatment groups were labeled with TUNEL concurrently with DAPI after 72 hours of treatment. Percentage of apoptotic HUVECs was determined by the ratio of TUNEL+ cells over the total number of nuclei observed per field. Purified rAAV-GFP CM (3 μg/mL; ▴) and 0.02% H2O2 (♦) were used as a negative and positive control, respectively. Values represent data from 3 independent experiments using triplicate wells per concentration. Values are shown as means ± SE.
Effects of rK5 on endothelial cells. (A) The effect of rK5 on VEGF-induced cell proliferation. BrdU incorporation was used as an index of proliferation. Each data point represents a mean of 3 independent experiments using triplicate cultures. HUVECs (□), human foreskin fibroblast (■), OVCAR-5 (●), and OVCAR-3 (▵). Purified rAAV-GFP CM (3 μg/mL)–treated HUVECs (▴) was used as negative control. Inset: anti-His Tag immunoblot analysis of purified recombinant AAV-K5 (rK5) in fractions eluted by 100 mM imidazole (lanes 2-5) and CM before purification (lane 6). A major band of approximately 10 kDa was detected. Lane 1 shows CM of nontransduced HEK-293 cells; lane 7 shows endostatin with a C-terminal poly-His–tagged (positive control); and lane 8 shows purified rAAV-GFP CM. (B) The effect of rK5 on endothelial cell migration in Boyden chamber assay. The migration of endothelial cells was determined by a method previously described.18 Endothelial cells were prelabeled with 5.0 μM 56-CFDA and induced to migrate toward the bottom chamber containing 40 ng/mL VEGF. The number of cells migrated to the bottom side of the membrane was counted using a PixCell II LCM system at 40× magnification. Data represent values from 3 independent experiments using 3 wells per concentration. M199 medium (5% FBS; ▵) was used to determine basal level of migration. P125A endostatin (■) was used as a positive control. rK5 induced concentration-dependent inhibition of cell migration (□). (C) The effect of rK5 on angiogenesis was evaluated by using VEGF-stimulated tube formation in a Matrigel assay.18 Representative images of endothelial cell tube formation are shown. (Ci) Basal level of tube formation. (Cii-iv) Tube formation induced by 40 ng/mL VEGF. (Ciii) rK5. (Civ) P125A endostatin (300 ng/mL). Bright-field images were recorded at 40× magnification (top row) and processed for analysis (Cv-viii) as described in “Materials and methods” (bottom row). (D) Morphometric analysis of tube formation. Ends (□), branch points (■), and tube length (▵) are shown. Values represent data from 3 independent experiments using 3 wells per sample. VEGF-induced tube formation was considered as 100% tube formation. Values are shown as means ± SE. (E) rK5 induced endothelial cell apoptosis under nutrient-rich culture conditions. HUVECs were cultured in the presence of 40 ng/mL VEGF. Cells were treated with 1.5 μg/mL rK5 for 24 hours and then labeled with annexin V FLUOS and DAPI. Images were recorded at 40× magnification and processed by the Image Processing Toolkit and Adobe Photoshop. Percentage of apoptotic cells was determined by the ratio of annexin V+ cells over the total number of nuclei per field. Purified rAAV-GFP CM was used as a negative control at a similar concentration. Values represent data from 2 independent experiments using 3 wells per sample. Error bars denote SE (*P < .05). (F) HUVECs were stimulated with VEGF and treated with different concentrations of rK5. In one set of cultures, rK5 was withdrawn after 24 hours of exposure (■) and replaced with fresh media. The second set of cultures was continuously exposed to rK5 (□). Cells from both treatment groups were labeled with TUNEL concurrently with DAPI after 72 hours of treatment. Percentage of apoptotic HUVECs was determined by the ratio of TUNEL+ cells over the total number of nuclei observed per field. Purified rAAV-GFP CM (3 μg/mL; ▴) and 0.02% H2O2 (♦) were used as a negative and positive control, respectively. Values represent data from 3 independent experiments using triplicate wells per concentration. Values are shown as means ± SE.
The matrigel tube formation assay serves as an ideal in vitro model system to study the formation of the vascular loop during angiogenesis.21 Upon seeding on matrigel, endothelial cells are able to form a tubelike network (Figure 1C) in the presence of growth factors such as VEGF or bFGF. Consistent with its effects on proliferation and migration, rK5 reduced the number of branch points, ends, and total tube length in VEGF-induced endothelial cell tube formation with an IC50 of approximately 0.75 μg/mL (Figure 1D). In contrast, purified conditioned media from rAAV-GFP–transduced HEK-293 cells (purified rAAV-GFP CM) did not inhibit endothelial cell proliferation, migration, or tube formation. Together, these results indicate that the inhibitory effect of rK5 is specific to endothelial cell growth and function.
rK5 induces cell death in endothelial cells under nutrient-rich culture conditions
Previous studies showed that K5 induced endothelial cells to undergo apoptosis9,11 under nutritional stress condition; cells were maintained in reduced serum media (1% FBS) prior to being stimulated by growth factors. In the present study, HUVECs were seeded in a less stringent condition (5% FBS) and supplemented with 40 ng/mL VEGF. As shown in Figure 1E, approximately 15% of the HUVECs stained positive for annexin V after 24 hours of rK5 treatment. Chromosomal DNA fragmentation assay also showed distinct fragmentation in 72 hours of rK5 treatment compared with that of 24 hours of treatment (data not shown). To determine whether continuous exposure of endothelial cells to rK5 was essential to induce apoptosis, HUVECs were exposed to rK5 either continuously or washed out after a 24-hour exposure (Figure 1F). When cells were treated continuously with rK5, pronounced endothelial cell apoptosis was observed. rK5 treatment (1.5 μg/mL) resulted in 43% TUNEL+ cells after 72 hours. The efficacy of rK5 in short-term treatment, however, was greatly reduced compared with the continuous treatment, suggesting that continuous exposure to rK5 was essential for optimal inhibition of endothelial cells. These data also suggest that early physiologic responses of endothelial cells to rK5 influence their rate of commitment to cell death.
rK5 transduces apoptotic signal via an intrinsic pathway
The mechanism underlying the apoptotic effect of the K5 domain is not completely understood. Previous studies have suggested that rK5 activates caspase-7 indirectly by interacting with its binding partner, GRP 78.11 Significant caspase-3 activation was also detected in tissues of grafted mouse hepatomas treated with K5.22 We were interested in identifying the apoptotic pathway activated by rK5 on endothelial cells. Angiostatin has been shown to induce p53 expression and activates Fas-mediated apoptotic pathway in part via the down-regulation of c-Flip and the activation of caspase-3 in VEGF-stimulated endothelial cells.23 The tumor suppressor p53 has been implicated in the induction of both extrinsic and intrinsic apoptotic pathways.24 However, it is unclear whether p53 is involved in rK5-induced apoptosis, since we observed only a small, and transient, increase in p53 expression 12 hours after rK5 exposure (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). Activation of caspases via the intrinsic apoptotic pathway is preceded by mitochondrial depolarization and cytochrome c release.25 To determine whether rK5 affected the mitochondrial membrane potential (ΔψM), rK5-treated HUVECs were stained with the mitochondrial pH-sensitive fluorescent dye TMRE and analyzed by flow cytometry. The reduction in fluorescence intensity shown in Figure 2A demonstrates that rK5 treatment promotes mitochondrial depolarization. Similar reduction in ΔψM was also detected in HUVECs treated with 1.5 μg/mL rK5 in complete medium (10% FBS; Figure S2). Mitochondrial depolarization events and cytochrome c release promote the activation of a caspase cascade that includes effectors caspases-3, -6, and -7.26 Consistent with a previous study demonstrating that rK5 enhances caspase-7 activity through surface-expressed GRP78,11 the Western blot in Figure 2B shows procaspase-7 cleavage in HUVECs within 4 hours of rK5 treatment, although it is unclear whether the early low level of caspase-7 processing is significant. However, the processing of caspase-7 as well as caspase-3 was clearly enhanced at later times following rK5 treatment, suggesting that both of these effectors were contributing to the amplification of an apoptotic caspase cascade (Figure 2C-D). Altogether, these data suggest that rK5 induces apoptosis in endothelial cells via the activation of an intrinsic pathway.
rK5 transduces apoptotic signal via an intrinsic pathway. (A) Mitochondrial membrane depolarization following 24-hour treatment of endothelial cells with rK5 in the presence or absence of VEGF. HUVECs were incubated with the ΔψM-sensitive mitochondrial dye, TMRE (40 nM), for 15 minutes prior to harvesting and immediately analyzed by FACS. The profiles of unstained and etoposide (10 μg/mL)–treated HUVECs are included with each histogram. (B) Western blot analysis of full-length caspase-7 and cleaved caspase-7 in rK5-treated HUVECs. Total cell lystates from rK5-treated HUVECs were collected at different time points and subjected to Western blot analysis. β-actin was used to normalize the amount of loaded proteins. (C) Representative Western blot analysis of cleaved caspase-3 and β-actin in rK5-treated HUVECs. Total cell lystates from rK5-treated HUVECs were collected at different time points and subjected to Western blot analysis. (D) Densitometric analysis was used to quantify the levels of caspase-3 activation. Values were normalized against β-actin and presented as fold increase compared with the basal level (0 hours of treatment). Data are shown as means ± SE.
rK5 transduces apoptotic signal via an intrinsic pathway. (A) Mitochondrial membrane depolarization following 24-hour treatment of endothelial cells with rK5 in the presence or absence of VEGF. HUVECs were incubated with the ΔψM-sensitive mitochondrial dye, TMRE (40 nM), for 15 minutes prior to harvesting and immediately analyzed by FACS. The profiles of unstained and etoposide (10 μg/mL)–treated HUVECs are included with each histogram. (B) Western blot analysis of full-length caspase-7 and cleaved caspase-7 in rK5-treated HUVECs. Total cell lystates from rK5-treated HUVECs were collected at different time points and subjected to Western blot analysis. β-actin was used to normalize the amount of loaded proteins. (C) Representative Western blot analysis of cleaved caspase-3 and β-actin in rK5-treated HUVECs. Total cell lystates from rK5-treated HUVECs were collected at different time points and subjected to Western blot analysis. (D) Densitometric analysis was used to quantify the levels of caspase-3 activation. Values were normalized against β-actin and presented as fold increase compared with the basal level (0 hours of treatment). Data are shown as means ± SE.
rK5 induces the formation of acidic compartments in HUVECs
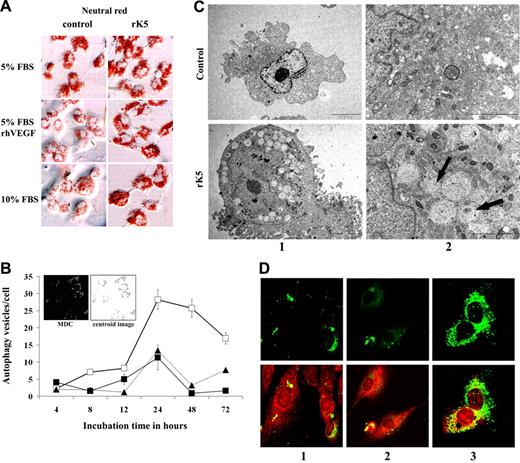
Following rK5 treatment, HUVECs were observed under a bright-field phase-contrast microscope. These studies showed the appearance of large vesicles that increased in number and size over a period of time. Vesicles were readily visible as early as 8 hours following treatment (Figure S3). To understand the nature of these vesicles, rK5-treated cells were exposed to neutral red, a weak cationic cell-permeable dye that accumulates in lysosomes and acidic compartments. rK5 treatment increased both the intensity and the quantity of red stained vesicles in treated HUVECs compared with controls in different culture conditions (ie, complete serum medium, reduced serum medium, and VEGF-supplemented medium) within 24 hours of treatment (Figure 3A). HUVECs treated with rK5 (1.5 μg/mL) for various times were also exposed to MDC, an autofluorescent probe reported to label acidic autophagolysosome and acidic compartments.28,29 Figure 3B shows an increase of MDC-labeled vesicles over the course of rK5 treatment that reached a plateau after 24 hours of treatment. The accumulation of MDC-labeled vesicles also correlated with the increase of rK5 concentration (not shown). Similarly, rK5 also induced acidic vesicles in HMVECs. A treatment of 1.5 μg/mL rK5 induced an approximately 3.5-fold increase in MDC-labeled vesicles in HMVECs compared with nontreated cells (P < .001; data not shown). These findings prompted us to address the possibility that the acidic compartments were indeed autophagolysosomes, and that rK5 induced an autophagic as well as an apoptotic response in endothelial cells.
rK5 induces autophagy in HUVECs. (A) HUVECs cultured in different conditions (5% FBS in M199, 5% FBS in M199 supplemented with 40 ng/mL VEGF, and 10% FBS in M199) were treated with 1.5 μg/mL rK5 for 24 hours and stained with neutral red as described previously.27 Phase-contrast images were recorded at 400× magnification using an Olympus BX60 upright microscope (Olympus, Center Valley, PA). There was an increase in the intensity and size of neutral red–positive vesicles in rK5-treated cells compared with control cultures. (B) Kinetic study of rK5-induced autophagy in endothelial cells. HUVECs were treated with 1.5 μg/mL of rK5 for the indicated time periods (□). Autophagic vesicles were labeled by 0.05 mM MDC and recorded by confocal microscopy at 600× magnification at different time points. The number of autophagic vesicles per cell was determined by an image analysis program as described in “Materials and methods.” Untreated cultures (■) and cultures treated with 1.5 μg/mL purified rAAV-GFP CM (▴). Values are shown as means ± SE. Inset: representative grayscale image of MDC-labeled HUVECs; digital centroid images are shown. Cells were treated by rK5 in the presence of VEGF. Images were recorded after 24 hours of treatment with rK5. (C) TEM analysis of HUVECs treated with 1.5 μg/mL rK5. Treated and control HUVECs were processed for TEM as described previously.20 Images were recorded at 5000× magnification (column 1) and 20 000× magnification (column 2) using a JEOL 1200 EX transmission electron microscope (JEOL, Peabody, MA). Note the presence of double-membrane autophagosomes and single-membrane autolysosomes that contained disintegrated materials clustering at the perinuclear sites (arrows). The nucleus and the cellular membrane structures of both control and treated cell remained intact. (D) rK5-induced autophagy was detected by LC3-GFP. HUVECs were transfected with LC3-GFP using Lipofectamine 2000 (Invitrogen). Transiently transfected cells were treated by 1.5 μg/mL rK5 in the presence of VEGF (40 ng/mL; column 3). Transfected HUVECs cultured with 40 ng/mL VEGF (column 2) or without (column 1) was used as control. After 24 hours of treatment, LC3-GFP–labeled cells (top row) were colocalized with mitochondria labeled with Mitotracker Red (bottom row). Confocal images were obtained at 600× magnification.
rK5 induces autophagy in HUVECs. (A) HUVECs cultured in different conditions (5% FBS in M199, 5% FBS in M199 supplemented with 40 ng/mL VEGF, and 10% FBS in M199) were treated with 1.5 μg/mL rK5 for 24 hours and stained with neutral red as described previously.27 Phase-contrast images were recorded at 400× magnification using an Olympus BX60 upright microscope (Olympus, Center Valley, PA). There was an increase in the intensity and size of neutral red–positive vesicles in rK5-treated cells compared with control cultures. (B) Kinetic study of rK5-induced autophagy in endothelial cells. HUVECs were treated with 1.5 μg/mL of rK5 for the indicated time periods (□). Autophagic vesicles were labeled by 0.05 mM MDC and recorded by confocal microscopy at 600× magnification at different time points. The number of autophagic vesicles per cell was determined by an image analysis program as described in “Materials and methods.” Untreated cultures (■) and cultures treated with 1.5 μg/mL purified rAAV-GFP CM (▴). Values are shown as means ± SE. Inset: representative grayscale image of MDC-labeled HUVECs; digital centroid images are shown. Cells were treated by rK5 in the presence of VEGF. Images were recorded after 24 hours of treatment with rK5. (C) TEM analysis of HUVECs treated with 1.5 μg/mL rK5. Treated and control HUVECs were processed for TEM as described previously.20 Images were recorded at 5000× magnification (column 1) and 20 000× magnification (column 2) using a JEOL 1200 EX transmission electron microscope (JEOL, Peabody, MA). Note the presence of double-membrane autophagosomes and single-membrane autolysosomes that contained disintegrated materials clustering at the perinuclear sites (arrows). The nucleus and the cellular membrane structures of both control and treated cell remained intact. (D) rK5-induced autophagy was detected by LC3-GFP. HUVECs were transfected with LC3-GFP using Lipofectamine 2000 (Invitrogen). Transiently transfected cells were treated by 1.5 μg/mL rK5 in the presence of VEGF (40 ng/mL; column 3). Transfected HUVECs cultured with 40 ng/mL VEGF (column 2) or without (column 1) was used as control. After 24 hours of treatment, LC3-GFP–labeled cells (top row) were colocalized with mitochondria labeled with Mitotracker Red (bottom row). Confocal images were obtained at 600× magnification.
rK5 induces autophagy in HUVECs under serum starvation and in nutrient-rich growth conditions
Autophagy is an adaptive response activated in cells under stresses.13,30,31 Autophagic vesicles are formed de novo through nucleation assembly and elongation of small membrane structures around large proteins and, often, whole organelles. Transmission electron microscopy (TEM) revealed an accumulation of numerous large autophagic vesicles within the cytoplasm of rK5-treated HUVECs. At an ultrastructural level, both double-membrane and single-membrane vesicles containing intact and disintegrating materials were observed in treated cells, but not in the control (Figure 3C).
To further confirm that rK5 induced autophagy in HUVECs, GFP-LC3–transfected cells were analyzed by confocal microscopy. Microtubule-associated protein-light chain 3 (MAP-LC3) has been used extensively as a biomarker for autophagy.13 This protein normally exhibits diffuse cytosolic distribution, but is modified and localized exclusively to autophagosomes during autophagy. Figure 3D shows that GFP distribution changed from largely diffuse to punctate structures following a 24-hour treatment with rK5. These results demonstrate that rK5 induces autophagy in endothelial cells. To determine whether rK5 induction of autophagy was specific to endothelial cells, we treated MA148 ovarian cancer cells and human foreskin fibroblasts with the antiangiogenic inhibitor. Recombinant K5 did not induce autophagy in either MA148 or human foreskin fibroblasts (Figure S4). However, a 24-hour treatment of etoposide (10 μg/mL) resulted in the formation of a robust number of MDC-labeled vesicles in MA-148. In contrast, little effect was observed on fibroblasts treated with etoposide. Altogether, these results indicated that rK5 selectively promoted autophagy in endothelial cells.
rK5-induced autophagy in HUVECs is independent of the nutrient deprivation–induced stress response
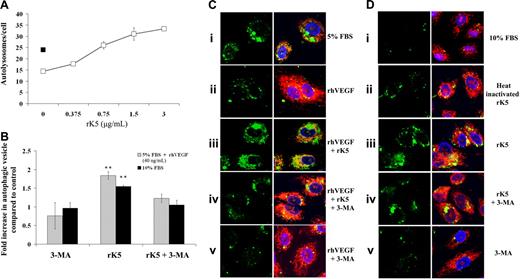
Autophagy is a stress-associated pathway normally induced in response to growth factor nutrient starvation. To confirm that rK5-induced autophagy in HUVECs was independent of the nutrient deprivation–induced stress response, HUVECs were transfected with LAMP1-GFP and cultured either in low serum supplemented with VEGF (Figure 4B) or complete medium (Figure 4D). LAMP1 is a marker for intermediate autophagosomes and autolysosomes.32 Figure 4A shows a dose-dependent increase in LAMP1-GFP–labeled vesicles in rK5-treated HUVECs in VEGF-supplemented conditions. Immunohistochemistry using a polyclonal anti–human LAMP1 also showed a high level of LAMP1 expression in rK5-treated HUVECs (Figure S5). Unpublished data from our laboratory (T.M.B.N., I.V.S., A.K., and S.R., November 2006) indicate that VEGF can substantially reduce rK5-induced autophagy in HUVECs at higher concentrations. In contrast, the withdrawal of VEGF further potentiated the induction of autophagy in rK5-treated HUVECs. After a 24-hour rK5 exposure, the number of LAMP1-GFP labeled autolysosomes in VEGF-treated HUVECs increased by approximately 1.5-fold when compared with the control (Figure 4B-C). Similarly, rK5 also induced autophagy in HUVECs cultured in complete serum media (Figure 4Ciii,Diii). As an additional control, we used heat-inactivated rK5. The biological activity of rK5, however, was disrupted by heat inactivation (Figure 4Dii).
rK5-induced autophagy in HUVECs is independent of nutrient deprivation-induced stress response. (A) LAMP1-GFP–transfected HUVECs were treated with different concentrations of rK5 cultured in M199 containing 5% FBS and supplemented with 40 ng/mL VEGF (□). After 24 hours of treatment, random fields of confocal microscopy images were recorded at 600× magnification and processed as described in “Materials and methods” to determine the number of autolysosomes per cell. Transfected HUVECs in serum-reduced medium (■) was used as a positive control. Values are shown as means ± SE. (B) LAMP1-GFP–transfected HUVECs cultured either in 5% FBS supplemented with VEGF or 10% FBS medium were treated with rK5 in the presence or absence of 3-MA. The number of LAMP1-GFP+ vesicles per cell was quantified. Values are presented as fold increase when compared with the control group (**P < .001; n = 3). The number of vesicles per cell in the control group was considered the basal level (1.0). Values are shown as means ± SE. (C) Representative confocal images of LAMP1-GFP–transfected HUVECs cultured in 5% FBS with (Cii-v) or without (Ci) VEGF. Transfected cells were treated with rK5 (1.5 μg/mL) in the presence (Ciii) or absence (Civ) of 5 mM 3-MA. LAMP1-GFP+ vesicles (left column) were colocalized with mitochondria (Mitotracker Red staining). Confocal images were recorded at 600× magnification. (D) Representative confocal images of HUVECs cultured in 10% FBS in the absence (Di) or presence (Diii) of rK5. As a control, heat-inactivated rK5 (Dii) was used. Control (Dv) and rK5-treated cultures (Div) were exposed to 5 mM 3-MA. LAMP1-GFP–labeled cells from different treatment groups (left column) were merged with the DAPI and Mitotracker Red images.
rK5-induced autophagy in HUVECs is independent of nutrient deprivation-induced stress response. (A) LAMP1-GFP–transfected HUVECs were treated with different concentrations of rK5 cultured in M199 containing 5% FBS and supplemented with 40 ng/mL VEGF (□). After 24 hours of treatment, random fields of confocal microscopy images were recorded at 600× magnification and processed as described in “Materials and methods” to determine the number of autolysosomes per cell. Transfected HUVECs in serum-reduced medium (■) was used as a positive control. Values are shown as means ± SE. (B) LAMP1-GFP–transfected HUVECs cultured either in 5% FBS supplemented with VEGF or 10% FBS medium were treated with rK5 in the presence or absence of 3-MA. The number of LAMP1-GFP+ vesicles per cell was quantified. Values are presented as fold increase when compared with the control group (**P < .001; n = 3). The number of vesicles per cell in the control group was considered the basal level (1.0). Values are shown as means ± SE. (C) Representative confocal images of LAMP1-GFP–transfected HUVECs cultured in 5% FBS with (Cii-v) or without (Ci) VEGF. Transfected cells were treated with rK5 (1.5 μg/mL) in the presence (Ciii) or absence (Civ) of 5 mM 3-MA. LAMP1-GFP+ vesicles (left column) were colocalized with mitochondria (Mitotracker Red staining). Confocal images were recorded at 600× magnification. (D) Representative confocal images of HUVECs cultured in 10% FBS in the absence (Di) or presence (Diii) of rK5. As a control, heat-inactivated rK5 (Dii) was used. Control (Dv) and rK5-treated cultures (Div) were exposed to 5 mM 3-MA. LAMP1-GFP–labeled cells from different treatment groups (left column) were merged with the DAPI and Mitotracker Red images.
Class III PI-3 kinase/p150/Beclin complex is essential for the initiation of the autophagy. 3-MA, an PI-3 kinase inhibitor, has been widely used to prevent the generation of autophagic precursors.13,33 In order to investigate whether 3-MA would inhibit rK5-induced autophagy in endothelial cells, we treated LAMP1-GFP–transfected HUVECs with rK5 for 24 hours and added 3-MA (5 mM) to the treated culture 12 hours prior to harvesting. The PI3K inhibitor 3-MA reduced rK5-induced autophagy under both culture conditions (Figure 4Civ and 4Div). Analysis of variance (ANOVA) showed that the mean number of autolysosomes per cell in the rK5-treated group was significantly higher than in the other 3 treatment groups (P < .001), while there were no significant differences in the mean number of autolysosomes per cell among rK5 treatment in the presence of 5 mM 3-MA, heat-inactivated rK5, and the nontreatment group. These results suggest that rK5 induced autophagy through a Beclin 1/PI-3 kinase pathway that was independent of nutrient/growth factor deprivation in endothelial cells.
Beclin 1 is essential for K5-induced autophagy in endothelial cells
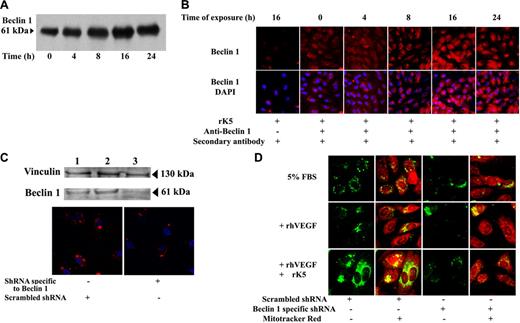
The expression of Beclin 1 correlates directly with autophagosome formation.16 Beclin 1 is part of a class III PI-3 kinase complex that is essential in mediating the localization of other autophagy proteins to autophagic vesicles.13 We observed an increase of Beclin 1 level after 8 hours of rK5 treatment that reached peak levels by 16 hours (Figure 5A). Confocal microscopy images further illustrated the up-regulation of Beclin 1 in treated HUVECs (Figure 5B). To determine whether Beclin 1 was essential for the induction of the autophagic response of HUVECs to rK5, we down-regulated its expression using an RNA interference approach. Figure 5C shows a representative Western blot of Beclin 1 expression in untransfected HUVECs or cells transiently transfected with either Beclin 1–specific shRNA or a scrambled control. Transfectants were exposed to rK5 for 24 hours. The knock-down in Beclin 1 expression led to a reduction of GFP-LC3–labeled vesicles (Figure 5D). Thus, Beclin 1 is an essential component of the K5-activated autophagy pathway.
rK5-induced autophagy is Beclin 1 dependent. (A) Representative immunoprecipitation of Beclin 1 in rK5-treated HUVECs. Cell lysates (200 μg/mL) collected at different time points were immunoprecipitated by anti–Beclin 1 antibody and blotted with anti–Beclin 1 antibody. (B) Confocal microscopy images of HUVECs showing endogenous Beclin 1 levels at different time points after rK5 treatment. Nuclei were labeled by DAPI. Beclin 1 expression (red) was detected using an anti–Beclin 1 monoclonal antibody. Alexa Fluor 647 goat anti–mouse IgG was used as secondary antibody. rK5-treated HUVEC (16 hours) sample without the primary antibody treatment was used as a negative control. (C) HUVECs were grown to subconfluent conditions. Scrambled shRNA or shRNA specific to Beclin 1 were transfected using Lipofectamine 2000. Top row shows total-cell lysates of untransfected HUVECs (lane 1), scrambled shRNA-transfected HUVECs (lane 2), and shRNA specific to Beclin 1–transfected HUVECs (lane 3) that were analyzed to determine Beclin 1 levels by Western blot. Bottom row shows confocal images of shRNA-transfected HUVECs showing Beclin 1 levels (red) 36 hours after transfection. Nuclei were stained with DAPI (blue). (D) HUVECs were cotransfected with LC3-GFP (green) and shRNA. Transfected HUVECs were cultured in 5% FBS in M199 with or without 40 ng/mL VEGF. Scrambled shRNA and shRNA specific to Beclin 1–transfected cells were exposed to 1.5 μg/mL rK5 for 24 hours. Treated HUVECs were labeled with Mitotracker Red. Confocal microscopy images were obtained at 600× magnification.
rK5-induced autophagy is Beclin 1 dependent. (A) Representative immunoprecipitation of Beclin 1 in rK5-treated HUVECs. Cell lysates (200 μg/mL) collected at different time points were immunoprecipitated by anti–Beclin 1 antibody and blotted with anti–Beclin 1 antibody. (B) Confocal microscopy images of HUVECs showing endogenous Beclin 1 levels at different time points after rK5 treatment. Nuclei were labeled by DAPI. Beclin 1 expression (red) was detected using an anti–Beclin 1 monoclonal antibody. Alexa Fluor 647 goat anti–mouse IgG was used as secondary antibody. rK5-treated HUVEC (16 hours) sample without the primary antibody treatment was used as a negative control. (C) HUVECs were grown to subconfluent conditions. Scrambled shRNA or shRNA specific to Beclin 1 were transfected using Lipofectamine 2000. Top row shows total-cell lysates of untransfected HUVECs (lane 1), scrambled shRNA-transfected HUVECs (lane 2), and shRNA specific to Beclin 1–transfected HUVECs (lane 3) that were analyzed to determine Beclin 1 levels by Western blot. Bottom row shows confocal images of shRNA-transfected HUVECs showing Beclin 1 levels (red) 36 hours after transfection. Nuclei were stained with DAPI (blue). (D) HUVECs were cotransfected with LC3-GFP (green) and shRNA. Transfected HUVECs were cultured in 5% FBS in M199 with or without 40 ng/mL VEGF. Scrambled shRNA and shRNA specific to Beclin 1–transfected cells were exposed to 1.5 μg/mL rK5 for 24 hours. Treated HUVECs were labeled with Mitotracker Red. Confocal microscopy images were obtained at 600× magnification.
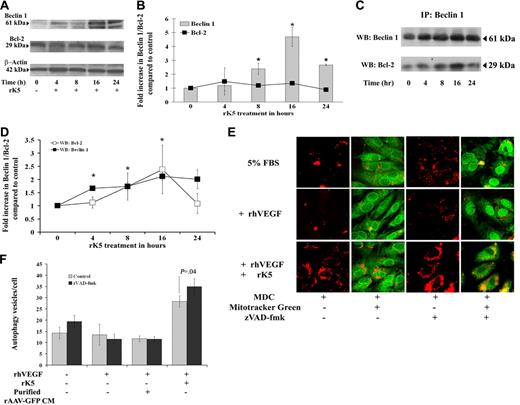
rK5 effects on endothelial cells are mediated via Beclin 1/Bcl-2 interactions
Previous studies have shown that a Beclin 1/Bcl-2 complex may function as a rheostat that regulates autophagic capacity and contributes to homeostasis.17 To determine whether rK5 treatment affected Beclin 1 and Bcl-2 expression, total cellular levels of each protein were analyzed by Western blots (Figure 6A-B). While Bcl-2 levels remained unchanged over the period of rK5 treatment, Beclin 1 levels increased more than 2-fold (P = .017) after 8 hours of rK5 treatment, reaching peak levels (4.5-fold) by 16 hours. To address the possibility that Beclin 1 interaction with Bcl-2 regulates rK5-induced autophagy, endogenous Beclin 1/Bcl-2 complexes were immunoprecipitated with antibodies against Beclin 1. The immunoprecipitation (IP)–Western blots in Figure 6C and 6D show that the levels of Bcl-2 coimmunoprecipitating with Beclin 1 steadily increased (P < .05) with increasing levels of Beclin 1 for 16 hours, but dropped to basal levels by 24 hours of rK5 treatment. Immunoprecipitation for Bcl-2 indicated little change in Bcl-2 levels (data not shown) and confirmed the Western blot data (Figure 6A-B). Together, these results suggest that rK5 induces autophagy in endothelial cells by up-regulating Beclin 1 expression and disrupting the balance between Beclin 1 and Bcl-2. Interestingly, the small but consistent reduction in Beclin 1 levels at 24 hours of rK5 treatment coincided with the enhancement of caspase-3 and caspase-7 activation. Exposure of HUVECs to 1.5 μg/mL rK5 in the presence and absence of 25 μM zVAD-fmk, a pancaspase inhibitor, resulted in increased number of MDC-labeled autophagic vesicles compared with the control (P = .045; Figure 6E-F). Altogether, the data suggest that increased sequestration of Bcl-2 by induced Beclin 1 delays the onset of apoptosis for at least 10 to 16 hours. Ultimately, the autophagic stress response may be supplanted by autophagic and apoptotic PCD.
Effects of rK5 on endothelial cells are mediated via Beclin 1/Bcl-2 interactions. (A) Representative Western blot analysis for Beclin 1, Bcl-2, and β-actin in rK5-treated HUVECs. Total-cell lysates from rK5-treated HUVECs were collected at different time points and subjected to Western blot analysis. (B) Densitometric analysis of Beclin 1 and Bcl-2 (■) levels. Values were normalized against β-actin and presented as fold increase compared with the basal level (0 hours of treatment). Data represent means of 3 independent blots. Error bars denote SE (*P < .05). (C) Coimmunoprecipitation of Beclin 1 and Bcl-2 in HUVECs. Cells were grown in 5% FBS in M199 medium and supplemented with 40 ng/mL VEGF in the presence of 1.5 μg/mL rK5. Cell lysates from rK5-treated HUVECs were collected at different time points. The lysates (200 μg per sample) were incubated with anti–Beclin 1 antibodies covalently coupled to protein A/G Plus–Sepharose. The immunoprecipitates were analyzed for Beclin 1 and Bcl-2 by immunoblotting. (D) Densitometric analysis of Bcl-2 (□) and Beclin 1 (■) levels. Coimmunoprecipitation experiments were repeated 3 times. Values are presented as fold increase compared with the basal level (0 hours of treatment; *P < .05). Data are shown as means ± SE. (E) HUVECs were treated with 1.5 μg/mL rK5 in the presence of 25 μM zVAD-fmk. Cells were labeled with 0.05 mM MDC (red) and 1 μM Mitotracker Green. Images were recorded using confocal microscopy at 600× magnification and processed as described previously to quantify the number of autophagy vesicles per cell. (F) Values of MDC+ vesicles in rK5-treated HUVECs in the presence or absence of 25 μM zVAD-fmk. Data represent values from 2 independent experiments using triplicate cultures; bars denote SE.
Effects of rK5 on endothelial cells are mediated via Beclin 1/Bcl-2 interactions. (A) Representative Western blot analysis for Beclin 1, Bcl-2, and β-actin in rK5-treated HUVECs. Total-cell lysates from rK5-treated HUVECs were collected at different time points and subjected to Western blot analysis. (B) Densitometric analysis of Beclin 1 and Bcl-2 (■) levels. Values were normalized against β-actin and presented as fold increase compared with the basal level (0 hours of treatment). Data represent means of 3 independent blots. Error bars denote SE (*P < .05). (C) Coimmunoprecipitation of Beclin 1 and Bcl-2 in HUVECs. Cells were grown in 5% FBS in M199 medium and supplemented with 40 ng/mL VEGF in the presence of 1.5 μg/mL rK5. Cell lysates from rK5-treated HUVECs were collected at different time points. The lysates (200 μg per sample) were incubated with anti–Beclin 1 antibodies covalently coupled to protein A/G Plus–Sepharose. The immunoprecipitates were analyzed for Beclin 1 and Bcl-2 by immunoblotting. (D) Densitometric analysis of Bcl-2 (□) and Beclin 1 (■) levels. Coimmunoprecipitation experiments were repeated 3 times. Values are presented as fold increase compared with the basal level (0 hours of treatment; *P < .05). Data are shown as means ± SE. (E) HUVECs were treated with 1.5 μg/mL rK5 in the presence of 25 μM zVAD-fmk. Cells were labeled with 0.05 mM MDC (red) and 1 μM Mitotracker Green. Images were recorded using confocal microscopy at 600× magnification and processed as described previously to quantify the number of autophagy vesicles per cell. (F) Values of MDC+ vesicles in rK5-treated HUVECs in the presence or absence of 25 μM zVAD-fmk. Data represent values from 2 independent experiments using triplicate cultures; bars denote SE.
Down-regulation of Beclin 1 in endothelial cells potentiates the effects of rK5
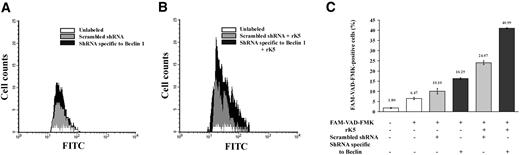
Autophagy has been shown to prolong cell survival under pharmacologic stress conditions.32,34 Since endothelial cells responded to rK5 treatment with the up-regulation of Beclin 1, we determined whether apoptotic cell death could be potentiated by reducing the levels of Beclin 1. HUVECs were transiently cotransfected with shRNA specific to Beclin 1 and a transposon expressing DsRed 2 plasmid. Treated transfectants were gated for DsRed+ cell populations (approximately 25% of the total number of cells) and analyzed for caspase activation. Data in Figure 7A and 7B show the levels of activated caspases in HUVECs. K5-treated HUVECs showed an increase in the activity of caspases upon knocking down the levels of Beclin 1. A summary of these results is shown in Figure 7C. Untransfected control cells showed low levels of caspase activation. Transfection with scrambled shRNA caused about 10% of cells to be positive for activated caspases. Beclin 1 down-regulation showed a marginal increase in caspase-positive cells (16%). K5 treatment of scrambled shRNA-transfected cells showed an increased number of positive cells for activated caspases (24%). However, when shRNA specific for Beclin 1 was used and then treated with rK5, the number of activated caspase-positive cells increased to 40%. Parallel experiments with TUNEL staining (Figure S6B) again showed an increase in apoptosis of unsorted HUVECs (transfected and nontransfected) treated with rK5 when the Beclin 1 levels were down-regulated. Discernible activation of caspases was observed after 16 hours of rK5 treatment (*P < .05; Figure S6A), while a higher rate of endothelial cell apoptosis was induced by rK5 in Beclin 1–down-regulated HUVECs as early as 12 hours following rK5 treatment (Figure S6B) in the total cell population (both transfected and nontransfected HUVECs). In addition, confocal microscopy studies further suggested that reduced Beclin 1 coincided with higher levels of activated caspase-3 (data not shown). Together, these data suggest that interfering with the autophagic responses in endothelial cells can potentiate the apoptotic effects of rK5.
Flow cytometric analysis of HUVECs showing increased levels of activated caspases. HUVECs were cotransfected with a transposon expressing DsRed 2 plasmid and shRNA specific to Beclin 1 or a scrambled shRNA, using Lipofectamine 2000. After 36 hours, transfected HUVECs were treated with 1.5 μg/mL rK5. Treated cells were labeled with green fluorescent-labeled inhibitor of caspases (FLICA) and analyzed by FACS. Transfected HUVECs were gated for DsRed+ cell populations and scored for FAM-VAD-FMK+ cells. (A) Histogram shows FAM-VAD-FMK+ cells in the control, untransfected, scrambled shRNA-transfected, and shRNA specific to Beclin 1–transfected HUVECs. (B) Histogram of rK5-treated HUVECs that were transfected with either shRNA specific to Beclin 1 or scrambled shRNA. (C) Summary of the data from the flow cytometric analyses is depicted as a percentage (mean ± SE) of transfected HUVECs (DsRed 2) positive for the activation of caspases. Each value is a mean of triplicate determinations.
Flow cytometric analysis of HUVECs showing increased levels of activated caspases. HUVECs were cotransfected with a transposon expressing DsRed 2 plasmid and shRNA specific to Beclin 1 or a scrambled shRNA, using Lipofectamine 2000. After 36 hours, transfected HUVECs were treated with 1.5 μg/mL rK5. Treated cells were labeled with green fluorescent-labeled inhibitor of caspases (FLICA) and analyzed by FACS. Transfected HUVECs were gated for DsRed+ cell populations and scored for FAM-VAD-FMK+ cells. (A) Histogram shows FAM-VAD-FMK+ cells in the control, untransfected, scrambled shRNA-transfected, and shRNA specific to Beclin 1–transfected HUVECs. (B) Histogram of rK5-treated HUVECs that were transfected with either shRNA specific to Beclin 1 or scrambled shRNA. (C) Summary of the data from the flow cytometric analyses is depicted as a percentage (mean ± SE) of transfected HUVECs (DsRed 2) positive for the activation of caspases. Each value is a mean of triplicate determinations.
Discussion
Kringle domains of plasminogen and antithrombin are some of the known inhibitors of endothelial cell proliferation and angiogenesis.2,35 While K5 has been well characterized for its ability to inhibit tumor growth, its mechanism of action on endothelial cell viability is not well understood. In the present study, we have investigated the effect of K5 on the biology of endothelial cells. rK5 treatment of human endothelial cells resulted in the inhibition of growth factor–induced proliferation, migration, and tube formation. These results are consistent with other published reports.8,9,11 In addition to these known effects, treatment with K5 induced specific morphologic changes, resembling autophagy, in the endothelial cells. The connection between angiogenic inhibitors and autophagy had not previously been established, and the role of autophagy induced by angiogenic inhibitors in endothelial cells was largely undefined.
Autophagy has been shown to prolong cell survival under physiologic, pathologic, and pharmacologic stress conditions.32,34 Deregulation of autophagy is associated with liver diseases, neurodegenerative diseases, cardiomyopathies, and cancer.13,32 Nutrient starvation and hypoxia are autophagy-inducing stimuli;36,37 radiation therapy and chemotherapeutic compounds such as etoposide and camptothecin are also known to trigger autophagy in tumor cells.20,31 A number of studies have suggested that reduced autophagic activity confers a survival advantage on tumor cells.12,17,38,39 The role of autophagy in cell growth and death remains controversial. Although autophagy is initiated as a protective response to stress, persistent autophagy can lead to cell death.
In this study, TEM and immunofluorescence microscopy revealed the presence of autophagic vesicles harboring whole cellular organelles, including mitochondria, in rK5-treated endothelial cells. The accumulation of these vesicles was both time and concentration dependent and limited to endothelial cells. Inhibiting PI-3 kinase activity by 3-MA, knocking down Beclin 1 expression by shRNA, or heat-inactivating K5 resulted in a reduction in autophagy. Interestingly, the K5-induced autophagic response was independent of nutritional stress and was observed in endothelial cells cultured either in complete medium or in low serum supplemented with growth factor. The supplementation of low-serum medium with VEGF, however, rescued HUVECs from rK5-induced autophagy in a concentration dependent manner (data not shown). VEGF is a survival factor for endothelial cells and signals through 2 major forms of receptor tyrosine kinases, VEGFR1 and VEGFR2.40,41 The presence of VEGF has been shown to induce the activation of ERK 1/2 and the up-regulation of Bcl-2. It was possible that increased Bcl-2 levels were leading to greater sequestration of Beclin 1 and that this sequestration, in turn, was accounting for VEGF-mediated inhibition of autophagy. Our studies indicated, however, that K5 treatment did not increase Bcl-2 expression over 24 hours even in the presence of VEGF (Figure 6B). It is possible that rK5 interferes with the activation of VEGF receptors or at an earlier step upstream of ERK 1/2; this connection will be investigated. Meanwhile, blocking the access of K5 to its putative cell-surface target GRP 78 caused insignificant effects on the induction of autophagy (data not shown). These results suggest that GRP 78 is not the sole target for K5. Indeed, the voltage-dependent anion channel and GRP 94 have also been identified as the putative binding proteins for K5.11,42
GRP 78 is a chaperone protein associated with ER stress response.10 A GRP 78–procaspase-7 complex is believed to prevent apoptosis.43 Binding of rK5 may interrupt the complex between GRP 78 and procaspase-7, allowing the release and activation of caspase 7 leading to apoptotic cell death.11 In the current study, we have shown that rK5 can induce caspase-7 activation in primary endothelial cells. This induction was observed as early as 4 hours after K5 treatment. It is unclear, however, whether the low levels of caspase-7 activation at this early stage of the treatment are sufficient to induce the cell death observed at 24 hours. The mitochondrial membrane depolarization (Figure 2A; Figure S2) observed 24 hours after treatment strongly suggests that K5 induces apoptosis via the intrinsic pathway. Coinciding with the rK5-induced reduction in ΔψM, we also observed high levels of caspase-7 and caspase-3 activation at later times. The delay in caspase activation may also be attributable to the protective effects of autophagy.
Bcl-2 prevents the release of cytochrome c from the mitochondrial intermembrane space, thereby inhibiting the activation of a caspase cascade and subsequent downstream apoptotic events.44,45 A number of studies have shown that Bcl-2 imparts a growth advantage to tumors either by sustaining mitotic activities in cancer cells or by enhancing tumor angiogenesis.46 The interaction of proapoptotic BH3 domain Bcl-2 family proteins with Bcl-2 abrogates its protective function and promotes mitochondrial depolarization and cytochrome c release.47,48 The identification of Beclin 1 as a novel binding partner for Bcl-2 established a new direction in the study of Bcl-2 and its involvement in apoptotic and autophagic PCD.49 More recently, Bcl-2 has been shown to inhibit Beclin 1–dependent autophagy. The complex of Bcl-2/Beclin 1 was modeled as a rheostat controlling the delicate balance between autophagy and apoptosis.17 Bcl-2 is now believed to function both as an antiapoptotic protein and an antiautophagy protein via its association with Beclin 1.39 Our studies show that rK5 treatment induced early up-regulation of Beclin 1, while Bcl-2 levels were not significantly affected. However, there was a steady increase in the levels of the Beclin 1/Bcl-2 complex for a limited time (Figure 6C-D). The increased sequestration of Beclin 1 by Bcl-2 supports the rheostat hypothesis and confirms the dual antiapoptotic and antiautophagic role of the Bcl-2 protein. The limited availability of Bcl-2 ultimately switched the response from being protective to promoting cell death due to high levels of free Beclin 1 and excessive autophagy. In addition, a marked reduction in Bcl-2 coimmunoprecipitating with Beclin 1 at 24 hours despite high Beclin 1 levels (Figure 6B,D) further suggests a concomitant switch to apoptotic pathways that promote cytochrome c release and caspase activation. This may be due to interaction of Bcl-2 with activated proapoptotic BH3 domain proteins. Further studies to address the interaction of Beclin 1, Bcl-2, and other Bcl-2–binding partners in the presence of rK5 may help to explain the events regulating autophagy and apoptosis in endothelial cells.
In conclusion, we have, for the first time, presented evidence that angiogenesis inhibitors such as K5 induce autophagy in endothelial cells. The study provides important insight into endothelial cell response to an angiogenesis inhibitor. Although several essential connections among autophagy and apoptosis need to be elucidated, our studies suggest that blocking the autophagic response of endothelial cells will potentiate K5-induced apoptotic cell death.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to thank Dr Dell'Angelica for the LAMP1-GFP plasmid, and Drs T. Yoshimori and N. Mizushima for the GFP-LC3 plasmid. We also thank Jerry Sedgewick and John Oja, Dr Robert Hafner, Dr Md Joynal Abedin, Julia Nguyen, Dr Sabita Roy, Phan Nguyen, Yan Zhang, Dr Michael Olin, Dr Paul H. Marker, Matthew L. Hillestad, Hemant Joshi, Dr Yumi Yokoyama, and Dr Gregory J. Connell for technical support. Transposon DsRed2 plasmid was a gift from Dr B. Blazar.
Supported in part by NIH CA114340, NIH DA 11806, Academic Health Center, Cancurable Foundation, and the Sparboe Endownment; A.K. was supported by a grant from the University of Minnesota Cancer Center, a Comprehensive Cancer Center designated by the National Cancer Institute and the Regis Foundation.
Authorship
T.M.B.N. designed and performed research, analyzed data, and wrote the manuscript. This work is a part of a research project carried out toward a graduate degree. I.V.S. performed research, analyzed data, and revised the manuscript. A.K. designed research, contributed vital new reagents, analyzed data, and revised the manuscript. S.R., the graduate advisor for T.M.B.N., designed the research; contributed vital reagents, instruments, and analytical tools; analyzed data; and revised the manuscript. A.K. and S.R. contributed equally to this manuscript.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Sundaram Ramakrishnan, Department of Pharmacology, University of Minnesota Medical School, 6-120 Jackson Hall, 321 Church St Southeast, Minneapolis, MN 55455; e-mail: sunda001@umn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal