Abstract
Early allogeneic hematopoietic stem cell transplantation (HSCT) has been proposed as primary treatment modality for patients with chronic myeloid leukemia (CML). This concept has been challenged by transplantation mortality and improved drug therapy. In a randomized study, primary HSCT and best available drug treatment (IFN based) were compared in newly diagnosed chronic phase CML patients. Assignment to treatment strategy was by genetic randomization according to availability of a matched related donor. Evaluation followed the intention-to-treat principle. Six hundred and twenty one patients with chronic phase CML were stratified for eligibility for HSCT. Three hundred and fifty four patients (62% male; median age, 40 years; range, 11-59 years) were eligible and randomized. One hundred and thirty five patients (38%) had a matched related donor, of whom 123 (91%) received a transplant within a median of 10 months (range, 2-106 months) from diagnosis. Two hundred and nineteen patients (62%) had no related donor and received best available drug treatment. With an observation time up to 11.2 years (median, 8.9 years), survival was superior for patients with drug treatment (P = .049), superiority being most pronounced in low-risk patients (P = .032). The general recommendation of HSCT as first-line treatment option in chronic phase CML can no longer be maintained. It should be replaced by a trial with modern drug treatment first.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) has been recommended as first-line treatment in chronic myeloid leukemia (CML) since it is considered to be the only treatment modality with curative potential.1,2 HSCT is most successful if it is done early within the first 2 years after diagnosis.3 Progress with drug treatment4 and persisting transplantation mortality5 have challenged the concept of first-line transplantation. In view of the improved survival times after the introduction of interferon α (IFN)6,7 and imatinib,8,9 the question came up of whether first-line transplantation is still justified in all suitable patients with a donor, or whether drug treatment should precede transplantation as long as remission is maintained. No randomized study has yet compared outcome of treatment strategies of HSCT versus drug treatment. In a simulation of such a study, survival of IFN and hydroxyurea (HU)–treated patients of the German CML Study I10 had been retrospectively compared with that of a matched cohort of patients who had undergone transplantation and who were registered with the IBMTR.11 Transplantation did not achieve a survival advantage in this historical analysis before year 6 after diagnosis in all patients and not at all in low-risk patients during the observation period of up to 8 years from diagnosis. Based on 5-year observation, survival with imatinib seems to be even better than that with IFN-based therapy.12 This has led to the expert recommendation of a trial with imatinib first before proceeding to HSCT.13
In order to verify the data obtained from the retrospective study,11 a prospective randomized study was designed to compare treatment outcome in a cohort of patients predefined by eligibility for transplantation. Since randomization had to consider the availability of a donor, availability of a matched related donor was used as a random criterion (genetic randomization). The main goal of the study was to describe and compare survival times in patients treated with HSCT in early chronic phase versus best available drug treatment. Prognostic score at diagnosis and transplantation risk were taken into account.14,15 We here report the outcome 11 years after the start of the study.
Patients and methods
Study protocol
All patients with Philadelphia chromosome (Ph)– and/or BCR-ABL–positive CML in chronic phase were examined for primary HSCT (age < 55 years, no serious comorbidity, no other contraindications, informed consent). Patients eligible for HSCT were then genetically randomized according to availability of a matched related donor to primary HSCT or best available drug treatment. A matched related donor was defined as HLA-identical sibling donor, or if a sibling donor was not available, another fully matched family donor.
Patients
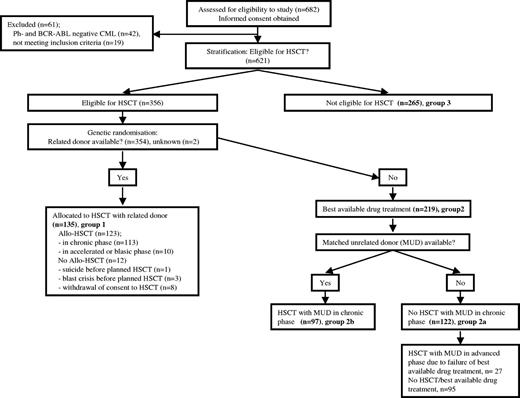
Enrollment, allocation, follow-up, and analysis of patients are depicted in the flow diagram in Figure 1. In total, 682 patients fulfilling the inclusion criteria of, and consenting to, the protocol were consecutively entered into the study by the participating centers between January 1995 and December 2001 and analyzed as of May 15, 2006. A list of centers that contributed patients to this study is available on the Blood website (see the Supplemental Appendix link at the top of the online article). Survival documentation was complete except for one patient who was lost to follow-up. Nineteen patients were excluded by the central data quality control. Forty two patients had Ph- and BCR-ABL–negative CML and will be analyzed separately. Six hundred and twenty one Ph- and/or BCR-ABL–positive patients in chronic phase CML were therefore registered and stratified according to eligibility for primary HSCT. Median time from diagnosis to registration was 19 days. Three hundred and fifty six of the 621 patients were eligible for transplantation, and 354 were randomized to receive either allogeneic HSCT from a related donor (group 1; n = 135) or best available drug treatment (group 2; n = 219). In 2 patients, the donor status remained unknown. Three hundred and fifty four eligible patients were thus used for analysis and comparison. Two hundred and sixty five patients were not eligible for transplantation (group 3), due to age (n = 213; median age, 63 years; range, 47-90), comorbidity (n = 19), other or unknown reasons (n = 21), and no consent (n = 12). They are included here to allow comparison with other studies (eg, concerning patients' characteristics or survival).
Flow diagram of enrollment, allocation, follow-up, and analysis of patients.
Patients' initial characteristics of all 3 groups are depicted in Table 1. There were no differences between the groups eligible for HSCT with or without donor with most variables available for all randomized patients including prognostic score at diagnosis determined according to Hasford et al,14 which takes into account age, spleen size, platelet count, and percentages of blasts, basophils, and eosinophils in the peripheral blood. Fourteen patients were younger than 20 years, 5 in the transplantation and 9 in the drug treatment group. There were significant differences between patients eligible for HSCT (groups 1 and 2) and those not (group 3). Differences mainly concerned age, symptoms due to organomegaly, white blood cell (WBC) count, and differential, hemoglobin, and prognostic score. Transplantation risk (EBMT score) was determined according to Gratwohl et al.15
Baseline characteristics
| Variable . | Group 1: eligible for HSCT and related donor available . | Group 2: eligible for HSCT and no related donor available . | Group 3: not eligible for HSCT . | Total . | P . | |
|---|---|---|---|---|---|---|
| Group 1 vs group 2 . | Groups 1 and 2† vs group 3 . | |||||
| No. patients | 135 | 219 | 265 | 621* | ||
| Median age, y (range) | 39 (11-59) | 40 (14-58) | 61 (25-90) | 49 (11-90) | .800 | < .001 |
| Younger than 20 y, % | 3.7 | 4.1 | 0 | 2.3 | ||
| 20 to 40, % | 49.6 | 48.0 | 1.1 | 28.2 | ||
| Older than 40, % | 46.7 | 48.0 | 98.9 | 69.6 | ||
| Median prognostic score, %; | .371 | < .001 | ||||
| Low | 63.0 | 58.9 | 17.8 | 42.3 | ||
| Intermediate | 32.6 | 32.9 | 64.0 | 46.1 | ||
| High | 4.4 | 8.2 | 18.2 | 11.6 | ||
| Sex, % male | 60.7 | 62.1 | 56.6 | 59.6 | .798 | .192 |
| Fatigue, % | 44.4 | 47.5 | 51.5 | 48.6 | .577 | .203 |
| Symptoms due to organomegaly, % | 23.7 | 24.7 | 15.2 | 20.3 | .839 | .006 |
| Weight loss of more than 10%, % | 14.1 | 13.3 | 18.9 | 15.8 | .837 | .068 |
| Fever, % | 6.7 | 6.4 | 3.8 | 5.3 | .928 | .141 |
| Extramedullary manifestation, % | 0.7 | 2.7 | 2.7 | 2.3 | .259 | .570 |
| Median hemoglobin, g/dL (range) | 11.7 (6.8-16.5) | 11.8 (7.2-16.0) | 12.1 (5.1-18.8) | 12.0 (5.1-18.8) | .989 | .018 |
| Median WBC count, × 109/L (range) | 116 (4-560) | 122 (1-560) | 82 (4-650) | 101 (1-650) | .874 | < .001 |
| Median PB eosinophils, % (range) | 2 (0-15) | 2 (0-15) | 2 (0-51) | 2 (0-51) | .22 | .429 |
| Median PB basophils, % (range) | 3 (0-23) | 3 (0-22) | 3 (0-40) | 3 (0-40) | .188 | .96 |
| Median PB blasts, % (range) | 1 (0-15) | 1 (0-25) | 1 (0-33) | 1 (0-33) | .728 | .001 |
| Median BM blasts, % (range) | 3 (0-25) | 2 (0-17) | 2 (0-25) | 3 (0-25) | .358 | .891 |
| Median platelet count, × 109/L (range) | 409 (63-1880) | 373 (90-2343) | 371 (49-2694) | 381 (49-2694) | .858 | .633 |
| Variable . | Group 1: eligible for HSCT and related donor available . | Group 2: eligible for HSCT and no related donor available . | Group 3: not eligible for HSCT . | Total . | P . | |
|---|---|---|---|---|---|---|
| Group 1 vs group 2 . | Groups 1 and 2† vs group 3 . | |||||
| No. patients | 135 | 219 | 265 | 621* | ||
| Median age, y (range) | 39 (11-59) | 40 (14-58) | 61 (25-90) | 49 (11-90) | .800 | < .001 |
| Younger than 20 y, % | 3.7 | 4.1 | 0 | 2.3 | ||
| 20 to 40, % | 49.6 | 48.0 | 1.1 | 28.2 | ||
| Older than 40, % | 46.7 | 48.0 | 98.9 | 69.6 | ||
| Median prognostic score, %; | .371 | < .001 | ||||
| Low | 63.0 | 58.9 | 17.8 | 42.3 | ||
| Intermediate | 32.6 | 32.9 | 64.0 | 46.1 | ||
| High | 4.4 | 8.2 | 18.2 | 11.6 | ||
| Sex, % male | 60.7 | 62.1 | 56.6 | 59.6 | .798 | .192 |
| Fatigue, % | 44.4 | 47.5 | 51.5 | 48.6 | .577 | .203 |
| Symptoms due to organomegaly, % | 23.7 | 24.7 | 15.2 | 20.3 | .839 | .006 |
| Weight loss of more than 10%, % | 14.1 | 13.3 | 18.9 | 15.8 | .837 | .068 |
| Fever, % | 6.7 | 6.4 | 3.8 | 5.3 | .928 | .141 |
| Extramedullary manifestation, % | 0.7 | 2.7 | 2.7 | 2.3 | .259 | .570 |
| Median hemoglobin, g/dL (range) | 11.7 (6.8-16.5) | 11.8 (7.2-16.0) | 12.1 (5.1-18.8) | 12.0 (5.1-18.8) | .989 | .018 |
| Median WBC count, × 109/L (range) | 116 (4-560) | 122 (1-560) | 82 (4-650) | 101 (1-650) | .874 | < .001 |
| Median PB eosinophils, % (range) | 2 (0-15) | 2 (0-15) | 2 (0-51) | 2 (0-51) | .22 | .429 |
| Median PB basophils, % (range) | 3 (0-23) | 3 (0-22) | 3 (0-40) | 3 (0-40) | .188 | .96 |
| Median PB blasts, % (range) | 1 (0-15) | 1 (0-25) | 1 (0-33) | 1 (0-33) | .728 | .001 |
| Median BM blasts, % (range) | 3 (0-25) | 2 (0-17) | 2 (0-25) | 3 (0-25) | .358 | .891 |
| Median platelet count, × 109/L (range) | 409 (63-1880) | 373 (90-2343) | 371 (49-2694) | 381 (49-2694) | .858 | .633 |
PB indicates peripheral blood; BM, bone marrow.
Including 2 patients eligible for HSCT but with missing data on availability of related donor. These patients are not part of groups 1, 2, or 3.
The 2 eligible patients with missing data on availability of related donor are added to groups 1 and 2 for comparison with group 3.
One hundred and twenty three of 135 patients randomized to undergo HSCT indeed underwent transplantation (91%; 113 in chronic phase, and 10 patients had progressed to accelerated or blastic phase by the time of transplantation) and 12 patients (9%) did not (4 because of death prior to transplantation: 3 blast crises, 1 suicide; and 8 due to secondary withdrawal of consent). Of 219 patients (62% of 354) without a related donor, 97 patients (44% of 219) received a matched unrelated donor (MUD) transplant during chronic phase (81 patients were considered to have an insufficient response to drug treatment, 12 patients on request, 4 patients due to unknown reasons) and 27 patients during accelerated or blastic phase. The comparison of risk profiles between patients who underwent MUD transplantation and patients who did not undergo transplantation indicated that patients who underwent MUD transplantation had a better risk profile (low risk: n = 62 [64%]; intermediate risk: n = 30 [31%]; high risk: n = 5 [5%]) than patients who did not undergo transplantation (low risk: n = 67 [55%]; intermediate risk: n = 42 [34%]; high risk: n = 13 [11%]), but this difference did not achieve statistical significance.
Allogeneic HSCT/transplantation cohort
Of the 354 patients eligible for HSCT, a total of 247 patients underwent HSCT, 210 in chronic phase and 37 in accelerated and blastic phases. Eleven of the 265 patients who were not eligible (3 older than 55 years) also underwent HSCT later on. In total, 258 (42%) of 621 patients underwent transplantation. HSCT was performed at 29 accredited centers in Germany, Switzerland, Austria, and Poland. The source of stem cells was peripheral blood in 56 patients (23%) and marrow in all others. The median time from diagnosis to transplantation from a related donor was 10 months (range, 2-106 months). The recommended treatment prior to HSCT was HU. IFN therapy had to be terminated not later than 90 days before HSCT.17 The 113 transplantations with related donors in first chronic phase were performed in 1995 (n = 4), 1996 (n = 33), 1997 (n = 29), 1998 (n = 25), 1999 (n = 9), 2000 (n = 9), 2001 (n = 3), and 2004 (n = 1). The conditioning regimen basically consisted of busulfan 16 mg/kg by mouth, 4 mg/kg daily for 4 days with or without cyclophosphamide 30 mg/kg daily for 4 days (n = 110), cyclophosphamide plus total body irradiation (TBI) 12 Gy (n = 135), or other drug combinations (n = 2). Graft-versus-host disease (GvHD) prophylaxis and supportive therapy were conducted according to the standard practice of the individual center.
Drug treatment
At the time of recruitment to this study, the recommended primary drug treatment consisted of IFN in combination with HU.18 Therapy was started with HU (40 mg/kg a day). After cytoreduction, IFN was given at a dose of 5 × 106 IU/m2 (in general, 9 × 106 IU per day). IFN dosage was adjusted to maintain a WBC count of 2 to 4 × 109/L. The platelet count was to be kept above 50 × 109/L. In most cases, the IFN dose required for maintenance was less than the initial IFN dose, on average 2 to 3 × 106 IU/d from years 3 to 4 on. HU was continued only if the desired white blood cell (WBC) count could not be maintained with IFN alone. Low-dose AraC was added in the case of IFN/HU failure.
If no complete hematologic remission was achieved by months 3 to 9 or no cytogenetic response by months 12 to 18, treatment intensification with AraC (2 × 100 mg/m2 per day over 5 days per month) and idarubicin 10 mg/m2 intravenously on days 3 and 4 (8 mg/m2 in patients older than 60 years; n = 51, 10 in group 2) was offered in a randomized fashion. These data will be analyzed separately. In qualified hospitals, high-dose chemotherapy with subsequent autologous SCT were offered as well to this patient group. With the availability of imatinib from 1999 on, imatinib was offered in the case of IFN failure. One hundred and ninety six of 621 patients received imatinib at some time, 15 in group 1 (11%), 62 in group 2 (43% of 122 patients who did not undergo transplantation and 9% of 97 patients who underwent MUD transplantation), and 119 in group 3 (45%).
Patients who did not achieve a cytogenetic remission on IFN (< 35% Ph+ metaphases) within 12 to 18 months had the option of a MUD transplant.
Statistics
The study had 2 main goals. First, patients with consent and eligibility to HSCT were to be compared between transplantation with a transplant from a related donor and best available drug treatment; second, subject to having received conservative drug treatment and not achieving cytogenetic response within 12 months, patients were then randomized between HU/IFN and idarubicin/AraC/plus IFN maintenance. Sample size was determined in alliance with the second goal under the assumption to simultaneously enter also enough patients to be able to investigate the first goal with sufficient power.
As in the study by Archimbaud et al,19 all patients eligible for HSCT and with a suitable related donor were scheduled to undergo HSCT. The result of HLA family typing was considered to be equivalent to genetic randomization between HSCT and best available drug treatment. All patients were analyzed following the intention-to-treat principle. Thus, “time to transplantation” bias could be avoided (ie, patients assigned to undergo HSCT appropriately had to carry the risk of death while waiting for the day of transplantation). The statistical comparison between both groups benefited from all advantages of statistical randomization: comparable patient characteristics, best possible reduction of selection bias, and identical observation periods within both treatment arms.
Primary end point was survival time from diagnosis to cut point of survival curves. In the drug treatment group, survival times of patients who received a MUD transplant were censored at the day of transplantation, if patients were still in first chronic phase because the outcome could not be related to drug treatment anymore. Patients undergoing transplantation in accelerated or blastic phase were not censored, since drug treatment had failed before. Prior to the study, it was assumed that survival probabilities of patients who underwent transplantation would be less favorable in the beginning, but would be better than those of drug-treated patients after an extended period of time. Hence, survival times to first cut point (and overall survival) were compared by Kaplan-Meier estimation and Wilcoxon-Gehan test,16 which is to be applied if survival curves are nonproportional and cross (ie, if they are logistic-function rather than exponential-function related).11 The significance level α was chosen to be .05 2-sided. Patients' characteristics at baseline were descriptively compared using chi-square test, Student t test, or Wilcoxon 2-sample test, as appropriate. All analyses were performed with the program package SAS (SAS Institute, Cary, NC).
Molecular analysis
BCR-ABL transcript levels were determined by nested and quantitative reverse-transcriptase–polymerase chain reaction (RQ-PCR) following current international expert recommendations.20 Quantification of transcripts was achieved by measuring the BCR-ABL/ABL ratio according to the international scale.20 A major molecular response was defined by a BCR-ABL/ABL ratio of 0.1 or lower, and a complete molecular response, by undetectable BCR-ABL transcripts using normal abl as an internal sensitivity control.20
Ethics
The protocol followed the Declaration of Helsinki and was approved by the ethics committee of the Fakultät für Klinische Medizin Mannheim of the University of Heidelberg and by local ethics committees of participating centers. Written informed consent was obtained from all patients prior to entering the study.
Results
Survival
All patients.
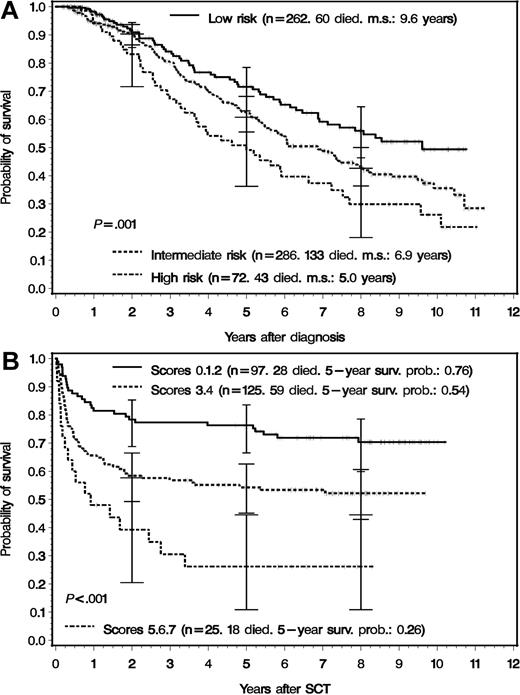
Median survival of all 621 patients was 7.5 years when patients were censored at the time of transplantation in first chronic phase and 8.1 years without censoring. The median observation time for living patients was 8.9 years (range, 4.2-11.2 years). Survival according to prognostic score at diagnosis is depicted in Figure 2A. Five-year (10-year) survival probabilities were 72% (49%) in low-risk, 62% (36%) in intermediate-risk, and 49% (26%) in high-risk patients. Five-year survival probabilities of the 354 patients eligible for transplantation were 81% (n = 214) in low-risk, 58% (n = 116) in intermediate-risk, and 56% (n = 24) in high-risk patients. Survival of all 247 patients who underwent transplantation according to EBMT score is shown in Figure 2B. Five-year survival probabilities were 76% (n = 97) for EBMT scores 0, 1, and 2; 54% (n = 125) for scores 3 and 4; and 26% (n = 25) for scores 5, 6, and 7.15 Survival of drug-treated patients and patients who underwent transplantation is in line with published data.14,15
Survival by risk profile at diagnosis and by transplantation risk. (A) Survival of 620 registered Ph- or BCR-ABL–positive patients with CML in chronic phase categorized by risk profile at diagnosis.14 For one patient, the prognostic score was not available. The survival times of patients who received an allogeneic transplant in first chronic phase were censored at the day of transplantation. The 620 patients were later stratified according to eligibility of receiving a transplant from a related donor. The survival differences between the 3 curves were significant (log-rank test: P = .001). m.s. indicates median survival. The error bars signify 95% confidence intervals.16 (B) Survival of 247 patients who actually received an allogeneic transplant stratified for transplantation risk according to the EBMT score.15 The survival differences between the 3 curves were significant (log-rank test: P < .001). The error bars signifiy 95% confidence intervals.
Survival by risk profile at diagnosis and by transplantation risk. (A) Survival of 620 registered Ph- or BCR-ABL–positive patients with CML in chronic phase categorized by risk profile at diagnosis.14 For one patient, the prognostic score was not available. The survival times of patients who received an allogeneic transplant in first chronic phase were censored at the day of transplantation. The 620 patients were later stratified according to eligibility of receiving a transplant from a related donor. The survival differences between the 3 curves were significant (log-rank test: P = .001). m.s. indicates median survival. The error bars signify 95% confidence intervals.16 (B) Survival of 247 patients who actually received an allogeneic transplant stratified for transplantation risk according to the EBMT score.15 The survival differences between the 3 curves were significant (log-rank test: P < .001). The error bars signifiy 95% confidence intervals.
Randomized patients.
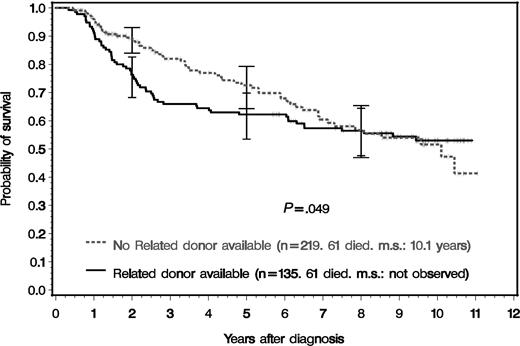
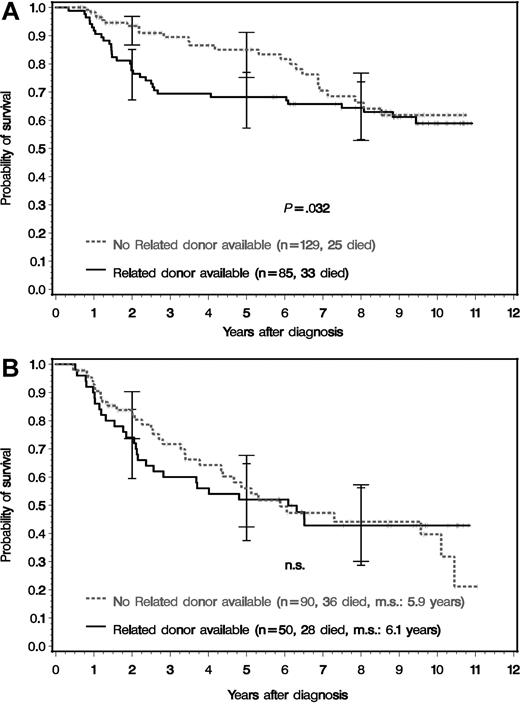
Figure 3 shows the survival of the 354 randomized patients by presence or absence of a matched related donor. Survival was better for drug-treated patients (no related donor) both for the time until the curves converge (cut point) at year 8 (P = .041) and for the entire observation period up to year 11 (P = .049) and most marked at 3 years after diagnosis. At 8 years after diagnosis, survival curves are no longer distinct. Survival differences were most pronounced in patients with low-risk features at diagnosis (Figure 4A) both for the time to the cut point at year 8 (P = .027) and for the entire observation period of 11 years (P = .032), with the same pattern of convergence. No survival difference was observed between intermediate- or high-risk patients with or without a related donor (Figure 4B). Intermediate- and high-risk patients were combined, since their survival curves were similar in this study. The survival probabilities after 2, 5, 8, and 10 years of groups 1 to 3 and of all patients, overall and according to risk profile at diagnosis, are shown in Table 2.
Survival of all 354 Ph- or BCR-ABL–positive CML patients that were eligible for transplantation and genetically randomized according to availability of a related donor. The survival times of patients who received an unrelated transplant in first chronic phase were censored at the day of transplantation. The survival differences were significant for the entire period and for the time until the curves converge (first cut point, year 8) (Wilcoxon-Gehan test: P = .049 and P = .041, respectively). For patients at risk see Table 2. m.s. indicates median survival. The error bars signify 95% confidence intervals.16
Survival of all 354 Ph- or BCR-ABL–positive CML patients that were eligible for transplantation and genetically randomized according to availability of a related donor. The survival times of patients who received an unrelated transplant in first chronic phase were censored at the day of transplantation. The survival differences were significant for the entire period and for the time until the curves converge (first cut point, year 8) (Wilcoxon-Gehan test: P = .049 and P = .041, respectively). For patients at risk see Table 2. m.s. indicates median survival. The error bars signify 95% confidence intervals.16
Survival of the 354 patients eligible for transplantation and genetically randomized according to risk profile. (A) Low-risk and (B) non–low-risk patients. The survival times of patients who received an unrelated transplant in first chronic phase were censored at the day of transplantation. The survival differences in the low-risk group were significant for the entire period and for the time until the curves converge (first cut point at year 8) (Wilcoxon-Gehan test: P = .032 and P = .027, respectively). For patients at risk, see Table 2. m.s. indicates median survival. The error bars indicate 95% confidence intervals.16
Survival of the 354 patients eligible for transplantation and genetically randomized according to risk profile. (A) Low-risk and (B) non–low-risk patients. The survival times of patients who received an unrelated transplant in first chronic phase were censored at the day of transplantation. The survival differences in the low-risk group were significant for the entire period and for the time until the curves converge (first cut point at year 8) (Wilcoxon-Gehan test: P = .032 and P = .027, respectively). For patients at risk, see Table 2. m.s. indicates median survival. The error bars indicate 95% confidence intervals.16
Survival probabilities
| . | At diagnosis, no. . | After 2 y . | After 5 y . | After 8 y . | After 10 y . | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. . | % (95% CI) . | No. . | % (95% CI) . | No. . | % (95% CI) . | No. . | % (95% CI) . | ||
| Group 1: eligible for HSCT and related donor available | |||||||||
| Total | 135 | 103 | 76 (68-83) | 84 | 62 (53-70) | 62 | 56 (48-64) | 23 | 53 (44-61) |
| Low risk | 85 | 66 | 78 (67-85) | 58 | 68 (57-77) | 45 | 64 (53-74) | 16 | 59 (47-69) |
| Nonlow risk | 50 | 37 | 74 (59-84) | 26 | 52 (37-65) | 17 | 43 (29-56) | 7 | 43 (29-56) |
| Group 2: eligible for HSCT and no related donor available | |||||||||
| Total | 219 | 130‡ | 89 (84-93) | 81 | 73 (64-79) | 43 | 57 (47-65) | 13 | 52 (41-61) |
| Low risk | 129 | 81 | 93 (87-97) | 54 | 85 (75-91) | 30 | 66 (53-77) | 7 | 62 (48-73) |
| Nonlow risk | 90 | 49 | 84 (74-90) | 27 | 56 (42-68) | 13 | 44 (30-57) | 6 | 40 (25-54) |
| Group 3: patients not eligible for HSCT | |||||||||
| Total | 265* | 241 | 91 (87-94) | 152 | 60 (54-66) | 70 | 40 (34-46) | 22 | 32 (25-39) |
| Low risk | 47 | 42 | 91 (79-97) | 24 | 58 (42-71) | 12 | 43 (28-57) | 4 | 32 (16-49) |
| Nonlow risk | 217 | 198 | 91 (87-94) | 128 | 61 (54-67) | 58 | 40 (33-47) | 18 | 32 (24-40) |
| All patients | |||||||||
| Total | 621† | 476 | 87 (84-90) | 317 | 64 (59-67) | 175 | 48 (44-53) | 58 | 42 (37-47) |
| Low risk | 262 | 190 | 87 (82-91) | 136 | 72 (66-78) | 87 | 61 (54-68) | 27 | 55 (47-62) |
| Nonlow risk | 358 | 285 | 87 (83-90) | 181 | 58 (53-63) | 88 | 41 (35-46) | 31 | 35 (29-41) |
| . | At diagnosis, no. . | After 2 y . | After 5 y . | After 8 y . | After 10 y . | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. . | % (95% CI) . | No. . | % (95% CI) . | No. . | % (95% CI) . | No. . | % (95% CI) . | ||
| Group 1: eligible for HSCT and related donor available | |||||||||
| Total | 135 | 103 | 76 (68-83) | 84 | 62 (53-70) | 62 | 56 (48-64) | 23 | 53 (44-61) |
| Low risk | 85 | 66 | 78 (67-85) | 58 | 68 (57-77) | 45 | 64 (53-74) | 16 | 59 (47-69) |
| Nonlow risk | 50 | 37 | 74 (59-84) | 26 | 52 (37-65) | 17 | 43 (29-56) | 7 | 43 (29-56) |
| Group 2: eligible for HSCT and no related donor available | |||||||||
| Total | 219 | 130‡ | 89 (84-93) | 81 | 73 (64-79) | 43 | 57 (47-65) | 13 | 52 (41-61) |
| Low risk | 129 | 81 | 93 (87-97) | 54 | 85 (75-91) | 30 | 66 (53-77) | 7 | 62 (48-73) |
| Nonlow risk | 90 | 49 | 84 (74-90) | 27 | 56 (42-68) | 13 | 44 (30-57) | 6 | 40 (25-54) |
| Group 3: patients not eligible for HSCT | |||||||||
| Total | 265* | 241 | 91 (87-94) | 152 | 60 (54-66) | 70 | 40 (34-46) | 22 | 32 (25-39) |
| Low risk | 47 | 42 | 91 (79-97) | 24 | 58 (42-71) | 12 | 43 (28-57) | 4 | 32 (16-49) |
| Nonlow risk | 217 | 198 | 91 (87-94) | 128 | 61 (54-67) | 58 | 40 (33-47) | 18 | 32 (24-40) |
| All patients | |||||||||
| Total | 621† | 476 | 87 (84-90) | 317 | 64 (59-67) | 175 | 48 (44-53) | 58 | 42 (37-47) |
| Low risk | 262 | 190 | 87 (82-91) | 136 | 72 (66-78) | 87 | 61 (54-68) | 27 | 55 (47-62) |
| Nonlow risk | 358 | 285 | 87 (83-90) | 181 | 58 (53-63) | 88 | 41 (35-46) | 31 | 35 (29-41) |
95% CI indicates 95% confidence interval.
For one patient, the prognostic score was not available.
Additional to groups 1, 2, and 3, 2 patients eligible for HSCT were added for whom information on donor availability was missing.
Between diagnosis and 2 years, reduction in patient number was mainly due to MUD transplantations in first chronic phase.
At the time of evaluation, 74 (55%) of the 135 patients with related donor and 128 (60%) of the 219 patients without related donor (including 67 recipients of MUD transplants in chronic phase) were still alive. These patients were analyzed for their state of health (signs and symptoms of CML relapse such as fatigue, spleen related symptoms, weight loss, fever, anemia, thrombocytopenia and leukopenia, or adverse drug effects). No differences were found between the 2 groups.
Causes of death
The causes of death are listed and assigned to groups 1 to 3 in Table 3. Blast crisis, as expected, was with 42.5% the most frequent cause of death particularly in groups 2a (no related donor available, no MUD transplantation) and 3 (not eligible for HSCT). This was followed by transplantation-related mortality (26.1%) in groups 1 (related donor available) and 2b (no related donor available, MUD transplantation in second line) and by other CML-related causes (12.6%). It is noteworthy that with the long survival times observed in this study, 17.6% of all causes of death were not directly CML related.
Causes of death
| Reported main cause . | All patients, no. (%) . | Eligible for HSCT, no.* (%) . | Group 1: with related donor, no. (%) . | Group 2: without related donor . | Group 3: not eligible for HSCT, no. (%) . | |
|---|---|---|---|---|---|---|
| 2A: no transplantation in chronic phase, no. (%) . | 2B: MUD transplantation in chronic phase, no. (%) . | |||||
| No. dead/total | 318/621 | 154/356 | 61/135 | 61/122 | 30/97 | 164/265 |
| Blast crisis | 135 (42.5) | 50* (32.5) | 12 (19.7) | 35 (57.4) | 2 (6.7) | 85 (51.8) |
| Transplant related† | 83 (26.1) | 78 (50.7) | 39 (63.9) | 15 (24.6) | 24 (80.0) | 5 (3.1) |
| CML related other than blast crisis | 40 (12.6) | 10* (6.5) | 4 (6.6) | 4 (6.6) | 1 (3.3) | 30 (18.3) |
| Not directly CML related‡ | 56 (17.6) | 16 (10.4) | 6 (9.8) | 7 (11.5) | 3 (10.0) | 40 (24.4) |
| Unknown | 4 (1.3) | — | — | — | — | 4 (2.4) |
| Reported main cause . | All patients, no. (%) . | Eligible for HSCT, no.* (%) . | Group 1: with related donor, no. (%) . | Group 2: without related donor . | Group 3: not eligible for HSCT, no. (%) . | |
|---|---|---|---|---|---|---|
| 2A: no transplantation in chronic phase, no. (%) . | 2B: MUD transplantation in chronic phase, no. (%) . | |||||
| No. dead/total | 318/621 | 154/356 | 61/135 | 61/122 | 30/97 | 164/265 |
| Blast crisis | 135 (42.5) | 50* (32.5) | 12 (19.7) | 35 (57.4) | 2 (6.7) | 85 (51.8) |
| Transplant related† | 83 (26.1) | 78 (50.7) | 39 (63.9) | 15 (24.6) | 24 (80.0) | 5 (3.1) |
| CML related other than blast crisis | 40 (12.6) | 10* (6.5) | 4 (6.6) | 4 (6.6) | 1 (3.3) | 30 (18.3) |
| Not directly CML related‡ | 56 (17.6) | 16 (10.4) | 6 (9.8) | 7 (11.5) | 3 (10.0) | 40 (24.4) |
| Unknown | 4 (1.3) | — | — | — | — | 4 (2.4) |
— indicates that no patient was classified as eligible for HSCT (columns 2 to 5).
For 2 patients, availability of a related donor remained unknown. Thus, the patients could not be classified into groups 1, 2A, or 2B.
Underwent transplantation in accelerated or blastic phase: group 1 (n = 4), group 2a (n = 15), group 2b (n = 0), group 3 (n = 1), all patients (n = 20).
Not directly CML-related causes of death were organ failure (heart, kidney, liver, lung; n = 20), thromboembolism (n = 9), other neoplasia (n = 9), infection (n = 8), suicide (n = 4), other (accident, hemorrhage [2], aortic aneurysm, seizures, Creutzfeld-Jakob; n = 6).
Current drug treatment
At the time of evaluation 20 (37%) of 54 living patients in group 2 (no related donor available, no MUD transplantation) still received IFN or HU, but 31 patients (57%) had been changed to imatinib and other BCR-ABL tyrosine kinase inhibitors afterward (nilotinib, n = 1; dasatinib, n = 2),21,22 mostly after IFN failure. When patients were censored at the start of imatinib treatment, survival curves did not change, indicating that these patients represented a group with more advanced disease and limited response to imatinib.
Cytogenetic and molecular responses
Differences between the transplantation and drug treatment groups were found regarding cytogenetic and molecular remissions. All patients surviving at least 5 years and evaluable (group 1: n = 113; group 2, no MUD: n = 92) were analyzed for cytogenetic and molecular responses (Table 4). Significantly higher proportions of complete cytogenetic remissions (91% vs 48%, P = .002) and of major molecular responses (81% vs 45%, P = .001) were found in group 1, indicating higher levels of residual disease in group 2 receiving drug treatment.
Current cytogenetic and molecular responses of patients of groups 1 (eligible for HSCT, donor available; n = 113) and 2 (no related donor available, no MUD transplantation in any phase; n = 92) surviving at least 5 years
| . | Group 1: eligible for HSCT, related donor available . | Group 2: eligible for HSCT, no related donor available, no MUD transplantation . |
|---|---|---|
| No. patients | 113 | 92 |
| Median follow-up (range), y | 6.6 (4.1-10.8) | 8.1 (4.6-10.0) |
| Evaluable for cytogenetic response, no. (%) | 55 (49) | 52 (57) |
| Median proportion of Ph+ metaphases, % | 0 | 8 |
| Complete cytogenetic response, Ph+ of 0%, no. (%) | 50 (91) | 25 (48) |
| Major cytogenetic response, Ph+ less than 35%, no. (%) | 50 (91) | 32 (61) |
| Evaluable for molecular response, no. (%) | 58 (51) | 40 (43) |
| Median ratio BCR-ABL/ABL, % | 0 | 0.19 |
| Undetectable BCR-ABL, no. (%) | 42 (72) | 4 (10) |
| Major molecular response, BCR-ABL/ABL ratio less than 0.1%, no. (%) | 47 (81) | 18 (45) |
| . | Group 1: eligible for HSCT, related donor available . | Group 2: eligible for HSCT, no related donor available, no MUD transplantation . |
|---|---|---|
| No. patients | 113 | 92 |
| Median follow-up (range), y | 6.6 (4.1-10.8) | 8.1 (4.6-10.0) |
| Evaluable for cytogenetic response, no. (%) | 55 (49) | 52 (57) |
| Median proportion of Ph+ metaphases, % | 0 | 8 |
| Complete cytogenetic response, Ph+ of 0%, no. (%) | 50 (91) | 25 (48) |
| Major cytogenetic response, Ph+ less than 35%, no. (%) | 50 (91) | 32 (61) |
| Evaluable for molecular response, no. (%) | 58 (51) | 40 (43) |
| Median ratio BCR-ABL/ABL, % | 0 | 0.19 |
| Undetectable BCR-ABL, no. (%) | 42 (72) | 4 (10) |
| Major molecular response, BCR-ABL/ABL ratio less than 0.1%, no. (%) | 47 (81) | 18 (45) |
Discussion
This is the first trial that quantifies survival after drug treatment and transplantation in CML by randomized controlled comparison. Ninety-one percent of the patients randomized for transplantation indeed underwent transplantation, demonstrating protocol feasibility and compliance. Four patients (3%) died prior to planned HSCT. The main reason for the high compliance rate was the acceptance of the curative potential of transplantation by most patients. It was ascertained that censoring of MUD patients would not introduce a bias against the HSCT group. MUD patients had even slightly better prognostic scores than the rest of the patients without a related donor. In total, transplantation was available to 42% of all patients. Results of transplantation outcome in this study were in line with data of concurrent transplantations in the literature.15
The superiority of drug treatment in all, and particularly in low-risk patients during the first 8 years after diagnosis is evident and significant. Although IFN was used as primary treatment in this study, the results are valid and relevant also in the imatinib era, since survival with primary imatinib treatment is even better. There is no evidence that the situation is different in very young patients (< 20 years old). There is no hint so far that the years lost early due to transplant-related mortality will be compensated in the course of the transplantation group later on. Long-term observations of CML patients who underwent transplantation5 demonstrate that survival curves continuously decline at a rate of 1% per year due to late transplant-related mortality or relapse.
Transplantation procedures have improved since the start of this study.23,24 In this study, patients who underwent transplantation between 1995 and 1998 had no significantly different survival from patients who underwent transplantation between 1999 and 2004 (5-year survival 64% and 68%, respectively). In a comparison of transplantation results between 1995 to 1998 versus 1999 to 2002 by the EBMT, 5-year survival increased by 6% (from 57% to 63%, respectively).5 Such an increase in survival after transplantation would not alter the conclusion of this study.
The study shows that both approaches, drug therapy and HSCT, are potent treatment forms for patients with CML, with a high potential for good long-term outcome and specific advantages and disadvantages. It remains open whether the higher rate of major cytogenetic and molecular responses after HSCT will translate into a survival advantage some time in the future. Some form of immunotherapy25 might be necessary for durable control of leukemic stem cells after drug treatment. Improvements can occur in either arm. Reassessment after 20 years would therefore be of interest. In the meantime, transplant-related mortality and morbidity and early years of life lost due to transplantations justify a change in policy.
This prospective randomized comparison of primary HSCT versus best available drug treatment provides clear results. On the basis of up to 11 years of follow-up, the general recommendation of HSCT for all patients as first-line treatment in chronic phase CML can no longer be maintained. It should be replaced by a trial with modern drug treatment first. Exceptions may be patients' preference, very low transplantation risk, and economic reasons. HSCT is regarded as an important salvage therapy in patients without optimal response to drug therapy or in early relapse.
The online version of this manuscript contains a data supplement.
An Inside Blood analysis of this article appears at the front of this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The study was supported by the Competence Network “Akute und chronische Leukämien,” sponsored by the German Bundesministerium für Bildung und Forschung (Projektträger Gesundheitsforschung, DLR e.V.–01 GI9980/6).
The assistance of M. Dumke, G. Lalla, C. Folz, R. Pleil-Lösch, C. Willersinn, M. Müller, L. Sadikaj, C. Schneider, T. Lorenz, and M. Kripp and free idarubicin from Pharmacia are acknowledged.
Clinical trials number: National Cancer Institute, Physician Data Query Database no: GER-CML-3, EU-95042
Authorship
Contribution: All authors made a major contribution to this paper as follows, and have approved the submitted paper. R.H. wrote the paper and initiated the project; M.P. performed the final analyses and was supported by J.H. All authors performed the trial, contributed data, and commented on the paper. A.G. performed data checking and checking of preliminary analyses; A.H., U.B., A.R., T.L., and O.M. collected the data and corresponded with the contributors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the SAKK and German CML Study Group is provided in Document S1, available on the Blood website; see the Supplemental Document link at the top of the online article.
Correspondence: Rüdiger Hehlmann, III. Medizinische Universitätsklinik, Medizinische Fakultät Mannheim der Universität Heidelberg, Wiesbadener Straße 7-11, 68305 Mannheim, Germany; e-mail: r.hehlmann@urz.uni-heidelberg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal