Abstract
Labile iron in hemosiderotic plasma and tissue are sources of iron toxicity. We compared the iron chelators deferoxamine, deferiprone, and deferasirox as scavengers of labile iron in plasma and cardiomyocytes at therapeutic concentrations. This comprised chelation of labile plasma iron (LPI) in samples from thalassemia patients; extraction of total cellular iron; accessing labile iron accumulated in organelles and preventing formation of reactive-oxidant species; and restoring impaired cardiac contractility. Neonatal rat cardiomyocytes were used for monitoring chelator extraction of LCI (labile cell iron) as 59Fe; assessing in situ cell iron chelation by epifluorescence microscope imaging using novel fluorescent sensors for iron and reactive oxygen species (ROS) selectively targeted to organelles, and monitoring contractility by time-lapse microscopy. At plasma concentrations attained therapeutically, all 3 chelators eliminated LPI but the orally active chelators rapidly gained access to the LCI pools of cardiomyocytes, bound labile iron, attenuated ROS formation, extracted accumulated iron, and restored contractility impaired by iron overload. The effect of deferoxamine at therapeutically relevant concentrations was primarily by elimination of LPI. The rapid accessibility of the oral chelators deferasirox and deferiprone to intracellular labile iron compartments renders them potentially efficacious for protection from and possibly reversal of cardiac damage induced by iron overload.
Introduction
Siderotic cardiac disease is the complication responsible for 71% of the mortality in thalassemia,1-7 largely preventable by intensive chelation,8-10 like continuous infusion with deferrioxamine (DFO).9 This and the reversal of cardiac arrhythmia achieved following DFO treatment led to the concept of depletion of toxic labile plasma iron (LPI) as the primary chelation target.9-13 LPI appears after the transferrin iron binding capacity is surpassed due to excessive output of catabolic iron, with a tendency to rebound during falls in plasma DFO concentrations.12,14,15 Thus, uninterrupted chelation of circulating LPI may be essential for preventing labile iron from entering cells via pathways that bypass the highly regulated transferrin-mediated iron uptake.3,12,14 Recent studies indicated that optimal cardioprotection is achieved by sequential nightly treatments of subcutaneous DFO combined with daily oral deferiprone (DFP) and that even daily monotherapy with DFP is significantly more cardioprotective than nightly DFO.16-24 Although DFP's membrane permeation properties seemingly contribute to its cardioprotective effects in siderotic heart,25-27 its use in iron chelation is limited by hematotoxicity in 1% to 5% of patients.2,3
The advent of bis-hydroxyphenyl thiazole (DFR) has improved the prospects for good patient compliance in iron overload.28 A single daily oral dose of DFR can apparently maintain a plasma level sufficient for reducing daily LPI for about 20 hours29-32 and possibly also for reducing labile iron in heart31 and liver.28 However, direct experimental demonstration that DFR can permeate into cardiac cells, safely bind and remove accumulated toxic iron, and thereby restore impaired cell functionality is still scanty3 and repeatedly questioned.33
In this work, we compared DFR, DFP, and DFO for their ability to (1) eliminate LPI of thalassemic sera at concentrations attained clinically; (2) penetrate into primary rat cardiomyocytes and chelate iron from the labile cell iron (LCI) pool in organelle (cytosol, endosomes, nucleus, and mitochondria); (3) extract cell-accumulated iron, and (4) restore cardiac contractility affected by toxic iron accumulation. The results indicate that at therapeutic plasma levels the 3 chelators can efficiently eliminate LPI, provided their regimen of administration affords their maintenance in circulation above a basal level during most hours of the day. The oral chelators significantly surpass DFO in their capacity to (1) access cardiomyocytes within short time periods and chelate labile iron accumulated in various organelles, thus reducing iron-evoked formation of ROS; (2) partially extract radiolabeled iron accumulated transitorily in labile pools of cardiomyocytes, and (3) restore cardiomyocyte contractility impaired by iron overload.
Materials and methods
Materials and cells
Calcein green (CALG) 3,3′-bis[N, N-bis(carboxymethyl) aminomethyl]fluorescein, calcein blue (CALB) 4-methylumbelliferone-8-methyleneiminodi-acetic acid and their acetomethoxy (AM) precursors, CALG-AM and CALB-AM, were obtained from Molecular Probes (Eugene, OR); dihydrorhodamine 123, dihydrochloride salt (DHR) from Biotium (Hayward, CA); human serum albumin (HSA) was from Kamada (Kiryat Weizman, Rehovot Israel); DTPA (diethylene-triamine-pentaacetic acid), ferric ammonium citrate (FAC), and ferrous ammonium sulfate (FAS) as well as MES and core histone mixture from calf thymus were from Sigma Chemical (St Louis, MO); rhodamine isothiocyanate from Fluka AG (Buchs, Switzerland). SIH (salicylaldehyde isonicotynoyl hydrazone), a gift from Prof Premysl Ponka (Lady Davis Institute of Medical Research, Montreal, Canada), deferiprone (DFP; sold as Ferriprox) from Apopharm (Toronto, Canada), and deferasirox (DFR; sold as ICL670 and Exjade) and deferrioxamine (DFO) were from Novartis-Pharma (Basel, Switzerland).
Synthesis
Calcein-histone-rhodamine B (CALG-H-R). We dissolved 7.5 mg core histone mixture from calf thymus in 5.5 mL of 50 mM sodium borate, pH 9.4, to which 0.165 mL of 20 mM rhodamineB-isothiocyanate in dimethylsulfoxide was added with mixing. The precipitate formed after 2 hours at 37°C, was removed by centrifugation (1500 g × 10′), and the supernatant loaded on a 20 mL Bio-Gel P-2 column (BioRad, Hercules, CA) equilibrated in HEPES-buffered saline (HBS) (150 mM NaCl, 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] pH 7.4). Unreacted rhodamine B-isothiocyanate was retained on the column while the histone-rhodamine B (H-R) eluted in the void volume (3-4 rhodamine B per histone were determined spectrophotometrically).
CALG-H-R conjugate. The CALG-H-R conjugate was prepared by cross-linking CALG to H-R using EDC (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide. To 4 mL H-R (90 μM) were added 0.1 mL of 1 M Na-MES, pH 5.5, and 0.256 mL of 5 mM calcein-green pre-incubated with 5.5 mM CoCl2 in HBS. Coupling (2 hours at room temperature) was initiated by adding 9 mg dry EDC (12 mM) followed by exhaustive dialysis against HBS. In order to remove CALG-bound Co(II), 11 mM diethylenetri-aminepentaacetic acid (DTPA), pH 8.0, was added for 30 minutes, followed by dialysis (final CALG-H-R preparation: 1:1:3.5 CAL:H:R). The CALG fluorescence was quenched by 95% and rhodamine by 20% at saturating Fe(II) concentrations.
Cell cultures
Primary cultures from 1-day-old rats (Hebrew University strain) were obtained as previously described.34,35 Cultures were kept at 37°C in an atmosphere of 5% CO2 and 95% air in Ham F-10 medium containing added 1 mM CaCl2, 10% fetal calf serum, and 10% horse serum. The rat cardiomyocyte H9C2 cell line was grown in 5% CO2 in air in Dulbecco modified Eagle (DMEM) medium supplemented with 10% fetal calf serum, 4.5 g/L D-glucose, glutamine, and antibiotics (all from Biological Industries, Kibbutz Bet Haemek, Israel).
Labeling of cells with radioiron (59Fe) for 3 or 24 hours and time-dependent (0-24 hours) extraction by chelators were done essentially as described elsewhere.26,31 The total radioactivity remaining in cells was expressed as mean values (n = 4) per plate or as percent remaining prior to adding chelator (time 0). Cell viability (routinely > 90%) was documented by supravital dye exclusion, absence of lactate dehydrogenase leakage into the culture medium after 24-hour iron loading, and cell protein concentration (BCA method; Pierce Chemicals, Dallas, TX).26,31
LPI measurements
Samples of blood were obtained from thalassemia major patients (with informed consent) who participated in a previously published study14 approved by the local ethical committee of the Unità Day Hospital Talassemici, Ospedale Sant' Eugenio, Rome Italy. LPI was measured using the method described elsewhere.36
FMS (fluorescent metalosensors) and fluorescence analysis
CALG or CALB loading into the cytosol. Cells were exposed to CALG-AM 0.25 μM or CALB-AM 1 μM at 37°C for 10 minutes in DMEM containing 10 mM Na-HEPES (loading medium) and washed first with 37°C HBS pH 7.4 and subsequently perfused with either HBS or DMEM-HEPES containing 0.5 mM probenecid (to minimize probe leakage; Glickstein et al37 ).
CALG-Fe(III) (1:1) complexes as FMS for endosomes. Cells were exposed to 50 to 100 μM preformed 1:1 CALG-Fe complex (prepared with ferrous ammonium sulfate, which was allowed to oxidize in air to ferric iron) in loading medium for 30 minutes at 37°C and washed extensively with the same probe-free medium and subsequently with HBS.
CALG-histone-rhodamine (CALG-H-R) (as FMS for nucleus) was loaded by incubating cells in DMEM-HEPES containing the probe (equivalent to 12 μM CALG and 40 μM R) for 1 hour at 20°C, followed by washing with HBS and resuspension in DMEM-HEPES.
Mitochondrial ROS formation was determined in cells preloaded for 10 minutes in HBS at 37°C with 50 μM dihydrorhodamine 123 (DHR), which accumulates in the mitochondria and is oxidized to the fluorescent rhodamine 123 (R123) and is analyzed either in a fluorescent plate reader (exc 488, em 517 nm) or under a microscope as described above (fluorescein settings). At indicated times, H2O2 (20-50 μM final) and/or chelator were added.
All fluorescence imaging was done by epifluorescence microscopy with a Zeiss Axiovert 35 microscope (Carl Zeiss, Jena, Germany) attached to a Polychrome V image system (Till Photonics, Grafelfing, Germany). The samples were inspected with a 60×/1.3 NA oil objective and recorded with a Sensicon PCO modified CCD camera (adapted by Till Photonics), as recently described.37 Image analysis was performed with Till Photonics software in conjunction with the Image J program, version 1.34 (National Institutes of Health, Bethesda, MD).38
Cardiomyocyte contractility was recorded by phase microscopy (20× objective) on beating cell clusters at 2 images per second and with histone-rhodamine B (H-R) by fluorescence microscopy and frequency and amplitude analyzed with Origin (v 7.5).
Results
Extraction of iron from iron-loaded cardiomyocytes
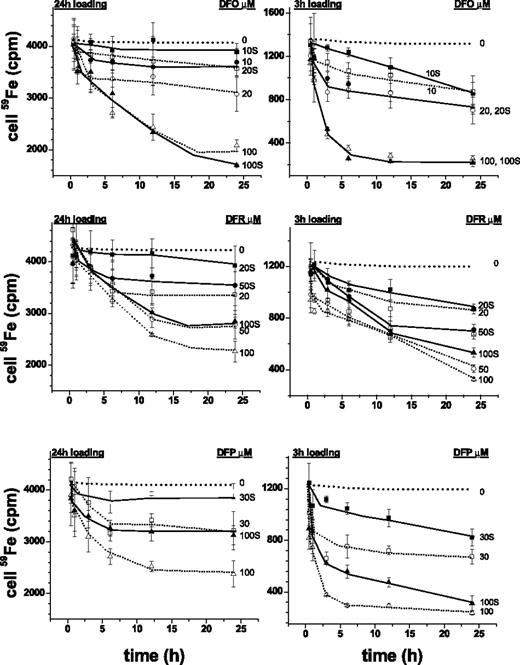
The rat cardiomyocyte model of iron overload26,31,34,39 was used following short (3-hour) and long (24-hour) loading periods of incubations with radioiron, so as to attain different levels of LCI. Extraction by iron chelators was carried out at concentrations corresponding to those attained in plasma during treatment and variable chelation periods. Figure 1 depicts the time profiles of 59Fe retained in cardiomyocytes after 3 hours' and 24 hours' loading with 59Fe-citrate. The initial radioactivity attained in cells following 3-hour and 24-hour loading corresponded to total cell iron of 250 to 300 μM and 1000 to 1200 μM, respectively, namely 5- and 20-fold higher than in nonloaded cells.37 The cells retained 59Fe when incubated for an additional 24-hour period in growth medium with or without 30% fetal calf serum (Figure 1) or in culture medium supplemented with 4% HSA or bovine serum albumin (BSA) (not shown). Exposures to DFO for up to 24 hours, which at 10 μM is only slightly over the range attained therapeutically (6-8 μM, Hershko and Weatherall4 ) significantly reduced radio-iron in cells loaded for the short 3-hour period. DFR and DFP removed the cell accumulated radiolabel substantially faster and with greater efficacy than DFO at concentrations representing peak, and for DFR, also trough plasma concentrations. Moreover, following 24-hour iron loading, DFR and DFP were still effective in their iron extraction ability, particularly in the upper range of plasma concentrations (50-100 μM) attained therapeutically.28-30 These results indicate that all 3 chelators act on labile iron pools that are relatively more accessible because they were generated by relatively short iron-loading period. As to the apparent inability of 10 μM DFO to significantly reduce cardiac cells' labile iron, we found it is not necessarily related to DFO's limited permeation properties but rather to the relatively low therapeutically relevant concentration used. Raising DFO to a concentration, comparable to those by plasma DFR or DFP, caused cell iron to be efficiently extracted. It is noteworthy that the presence of 30% serum in the medium markedly reduced the efficacy of the chelators applied at therapeutic doses. While this consideration would be expected to gain in significance as plasma chelator concentrations taper off, it does not apply to DFO, which is maintained continually by subcutaneous infusion.
Cell-associated radioiron following exposure of cardiomyocytes to chelators. Primary cardiomyocytes were loaded with 0.36 mM 59Fe (prepared from 59Fe-chloride Amersham Radiochemical Centre, Amersham, England, and sterile ferric ammonium citrate to provide a concentration of 100 mg/mL elemental iron). Loading was for either 3 hours (right graphs) or 24 hours (left graphs) in medium with no serum and subsequently washed sequentially with cold medium containing serum, medium containing 100 μM DTPA for 30′, and resuspended either in medium with or without 30% fetal calf serum and the indicated concentrations of chelators (labels next to curves indicate chelator concentrations; S denotes presence of serum). After incubation at 37°C for various periods of time, the cells were washed extensively with cold medium and processed for counting radioactivity and protein, as described in “Materials and methods.” Each point represents the mean radioactivity ± SE (n = 3) associated with cells (in cpm) at the indicated time of incubation (h). The dotted lines represent the experimental lines of control (no chelator) with SD of ± 7% obtained identically as those treated with chelator.
Cell-associated radioiron following exposure of cardiomyocytes to chelators. Primary cardiomyocytes were loaded with 0.36 mM 59Fe (prepared from 59Fe-chloride Amersham Radiochemical Centre, Amersham, England, and sterile ferric ammonium citrate to provide a concentration of 100 mg/mL elemental iron). Loading was for either 3 hours (right graphs) or 24 hours (left graphs) in medium with no serum and subsequently washed sequentially with cold medium containing serum, medium containing 100 μM DTPA for 30′, and resuspended either in medium with or without 30% fetal calf serum and the indicated concentrations of chelators (labels next to curves indicate chelator concentrations; S denotes presence of serum). After incubation at 37°C for various periods of time, the cells were washed extensively with cold medium and processed for counting radioactivity and protein, as described in “Materials and methods.” Each point represents the mean radioactivity ± SE (n = 3) associated with cells (in cpm) at the indicated time of incubation (h). The dotted lines represent the experimental lines of control (no chelator) with SD of ± 7% obtained identically as those treated with chelator.
Identification of cellular sites of iron loading and removal by chelators
We assessed here the ability of ferric salts to raise the LCI pools of primary cardiomyocytes and that of chelators to lower them and compared this form of imposed iron overload with the more patho-physiologically relevant LPI-containing serum from an iron overloaded patient. For that purpose, we incubated cardiomyocytes for 24 hours with 30% serum from an iron-overloaded patient that contained 6.5 μM LPI, determined as previously described.14,36 Higher human serum concentrations were found to be toxic to primary rat cardiomyocytes and, to an even greater degree, to H9C2 cells in culture. For controls, we used the same human serum but depleted of LPI by addition of DFO at a concentration approximately equivalent to the LPI content (6.5 μM).
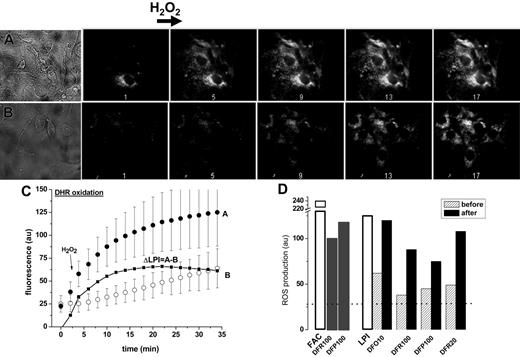
Effect of chelators on mitochondrial ROS production in iron-loaded cells. LCI of cardiomyocytes were initially assessed by their ability to promote formation of ROS following a brief exposure to H2O2 (50 μM). A rise in rhodamine 123 fluorescence resulting from oxidation of the nonfluorescent precursor dihydrorhodamine-123 was confined to mitochondria, as was independently confirmed by colocalization with other markers.37 As shown in Figure 2, the basal and the H2O2-evoked ROS production (initial rate and final levels) were markedly higher in cells exposed to iron, but also rose significantly in those exposed to LPI-containing serum relative to cells treated with LPI-depleted serum. The net contribution of LPI to the mitochondrial ROS production in cardiomyocytes is calculated as ΔLPI, the difference in final plateau levels of ROS attained with LPI-containing serum as compared to LPI-depleted serum (Figure 2C). The effect of chelators on mitochondrial ROS production in cells exposed to FAC (in culture medium containing 10% fetal calf serum and 10% horse serum) or to serum containing LPI as compared to LPI-depleted serum is shown in Figure 3D. Chelator action was examined in 2 modes: long-term exposure, where chelators were added to the iron-overloaded media (either LPI or FAC) at the start of the 24-hour incubation and short-term exposure, where chelators were added at the conclusion of the 24-hour incubation with iron-loaded media. At therapeutic concentrations attained in plasma (in the range of 10 μM), DFO markedly reduced ROS-production after long-term, but not after short-term, exposure. The failure of DFO to produce a significant effect when added after cells were exposed to FAC or LPI implies that DFO's action on iron accumulation in cardiac cells is more preventive than corrective in these experimental conditions. Unlike DFO, both DFR and DFP reduced mitochondrial ROS production after long-term as well as short-term exposure, when applied at concentrations attained clinically in plasma (20-100 μM). These results indicate that whereas the 3 chelators are efficacious at preventing iron loading by LPI over the long term, only DFR and DFP efficiently reduce cellular ROS production at the level of intracellular LCI, after its elevation by prolonged incubation with iron-loaded sera.
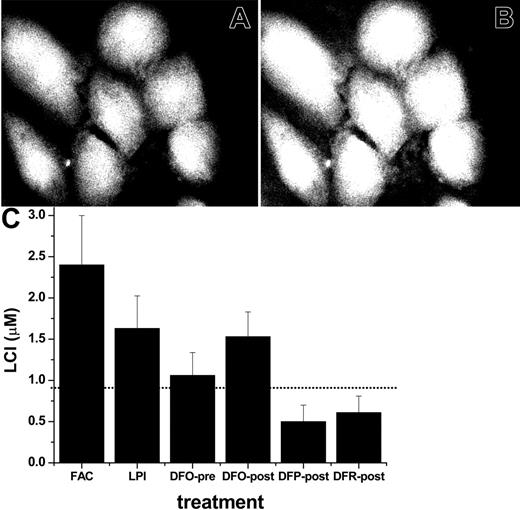
Effect of chelators on total LCI pools in iron-loaded cells. The ability of FAC and of LPI-containing serum to raise LCI were assessed with different FMS targeted to different cell compartments. First, we used CALB, which distributes uniformly throughout the cell, for LCI measurements in a fluorescent plate reader and by epifluorescence microscopy imaging.15,40 The former technique has the advantage of providing quantitative fluorescence data, while the latter allows pinpointing of the cellular localization of the fluorescent signal. CALB binding of LCI leads to a reversible stoichiometric quenching of fluorescence, which is revealed by adding a permeant and strong iron chelator (DFR or SIH) that restores the original LCI fluorescence.37 The extent of dequenching provides a measure of the LCI,40 as demonstrated in Figure 3 for CALB-labeled cardiomyocytes before (Figure 3A) and after (Figure 3B) addition of an excess amount of permeant chelator. The calculated LCI values in untreated cells and in cells exposed to sera containing LPI are depicted in Figure 3C. Overnight incubation with sera containing FAC or LPI led to a significant rise in the cytosolic labile cell iron pool of cardiomyocytes. It is noteworthy that 20 hours of incubation with LPI-containing serum in the continued presence of a therapeutically attainable concentration of DFO (10 μM) largely abrogated the LCI pool increase, which is consistent with the results in Figure 2 and reinforces the presumed role of LPI in cell iron overload. However, adding 10 μM DFO after overnight loading with LPI-containing serum evoked essentially no reduction in LCI, though significant reductions were obtained with 100 μM DFR or DFP. Lower concentrations (20 μM) of the latter also were effective. These results indicate that the increase in cytosolic LCI by iron-loaded sera can be prevented by DFO if given access to extracellular LPI. However, once LCI is elevated, it is removed by DFP and DFR more efficiently than by DFO at therapeutically relevant levels.
Effect of LPI on mitochondrial ROS production in primary cardiomyocytes. Primary cardiomyocytes were preincubated for 24 hours in medium with LPI-containing serum from an iron-overloaded patient (A) or with the same serum depleted of LPI by addition of equimolar DFO (B). The final LPI in panel B was less than 0.5 μM and in panel A 2.0 μM. After washing, the cells were loaded with 50 μM DHR for 10 minutes at 37°C and washed, and ROS production was monitored by epifluorescence microscopy and recorded at 2-minute intervals. The base line fluorescence was established, and 50 μM H2O2 was added at 4 minutes (indicated by arrow). The montage depicts snapshots of phase contrast images (A: serum with LPI, and B: serum depleted of LPI) and 5 (of 17) epifluorescence images of the same time sequence before and after H2O2 addition, taken at 2, 10, 18, 26, and 34 minutes. (C) Scatter plots of A and B showing average fluorescence density of 3 to 5 cells in each field indicating treatment with 30% LPI-containing human serum (curve A; • or 30% human serum depleted of LPI (curve B; ○. The line graph ΔLPI (▪) is the difference between curve B and curve A. (D) Relative ROS production levels in cardiomyocytes (determined with DHR and fluorescence microscopy) after incubation overnight with FAC (100 μM) in growth medium containing 10% fetal calf serum and 10% horse serum or in growth medium containing 30% human LPI-containing serum (□). The growth media were supplemented with the indicated concentrations (10, 20, or 100 μM) of chelators either present during the 24-hour preincubation (before, ▨) or added only 20 minutes before ROS determination (after, ▪). The dotted line represents the basal level of ROS production (27 au, arbitrary units of fluorescence) obtained by addition of excess iron chelator (200 μM DFR).
Effect of LPI on mitochondrial ROS production in primary cardiomyocytes. Primary cardiomyocytes were preincubated for 24 hours in medium with LPI-containing serum from an iron-overloaded patient (A) or with the same serum depleted of LPI by addition of equimolar DFO (B). The final LPI in panel B was less than 0.5 μM and in panel A 2.0 μM. After washing, the cells were loaded with 50 μM DHR for 10 minutes at 37°C and washed, and ROS production was monitored by epifluorescence microscopy and recorded at 2-minute intervals. The base line fluorescence was established, and 50 μM H2O2 was added at 4 minutes (indicated by arrow). The montage depicts snapshots of phase contrast images (A: serum with LPI, and B: serum depleted of LPI) and 5 (of 17) epifluorescence images of the same time sequence before and after H2O2 addition, taken at 2, 10, 18, 26, and 34 minutes. (C) Scatter plots of A and B showing average fluorescence density of 3 to 5 cells in each field indicating treatment with 30% LPI-containing human serum (curve A; • or 30% human serum depleted of LPI (curve B; ○. The line graph ΔLPI (▪) is the difference between curve B and curve A. (D) Relative ROS production levels in cardiomyocytes (determined with DHR and fluorescence microscopy) after incubation overnight with FAC (100 μM) in growth medium containing 10% fetal calf serum and 10% horse serum or in growth medium containing 30% human LPI-containing serum (□). The growth media were supplemented with the indicated concentrations (10, 20, or 100 μM) of chelators either present during the 24-hour preincubation (before, ▨) or added only 20 minutes before ROS determination (after, ▪). The dotted line represents the basal level of ROS production (27 au, arbitrary units of fluorescence) obtained by addition of excess iron chelator (200 μM DFR).
Effects of chelators on LCI pools in subcellular compartments of iron loaded cells. In an attempt to define potential sites of chelatable iron accumulation in cardiomyocytes, we designed FMS molecules with organelle-targeting properties. Core histones were used as plasma membrane and nuclear membrane translocating devices,41 to which metallosensor moieties such as CALG were coupled covalently (yielding CALG-H). Rhodamine (R) was additionally coupled to the CALG-H conjugate (yielding CALG-H-R), in order to attain improved photostability while retaining the same iron-binding capacity. Thus, in CALG-H and in CALG-H-R conjugates the ability to reversibly bind iron and undergo quenching-dequenching is attributed to CALG. In the CALG-H-R conjugates, the proximity of the rhodamine units to the metal-binding CALG is sufficient to cause them to undergo fluorescence quenching together with CALG.
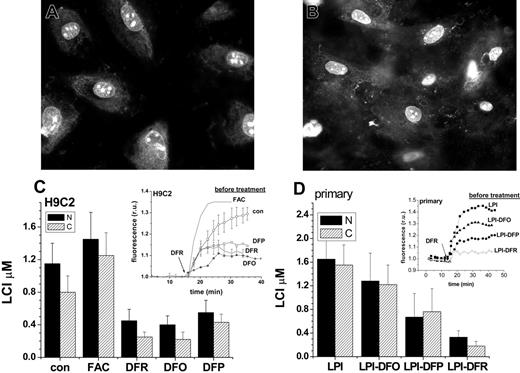
When applied at room temperature to minimize endocytosis/pinocytosis, the histone conjugates gained rapid access to the cytosol followed by accumulation in the nucleus of H9C2 cells (Figure 4A) and of primary cardiomyocytes (Figure 4B). The cytosolic fluorescence was relatively low and spread unevenly, while it was intense in the nucleus and highly concentrated in particular areas of the nucleus, possibly representing nucleoli and the nuclear membrane. Importantly, the cellular distribution of CALG-H-R remained stable for more than 24 hours. As seen previously with CALB (Figure 2), addition of the permeant DFR revealed the total iron-quenched fluorescence, which reflects the labile iron concentration in that area of the cell. Figure 4 depicts the mean fluorescence values in the cytosol and nucleus of H9C2 cells pretreated for 24 hours with excess iron or with chelators (Figure 4C) and primary cardiomyocytes pretreated for 24 hours with LPI-containing serum with and without chelators (Figure 4D) and dequenched by excess DFR. The insets in Figure 4C, D show the amplitude and kinetics of increase in nuclear fluorescence after addition of excess DFR, from which the nuclear LCI concentrations were estimated. The cytosolic LCI values (kinetics not shown) followed a pattern closely parallel to nuclear LCI, indicating a qualitatively similar response of nuclear and cytosolic LIP to cell iron loading (with FAC or iron overloaded serum containing LPI) or iron deprivation (pretreatment of cells with chelators for 24 hours). In H9C2 cells, prolonged incubations with 10 μM DFO had no significant effects on LCI (not shown), therefore, the concentration of chelator was raised by 10-fold to 100 μM, leading to a major reduction in LCI. This further supports the view that DFO is intrinsically inefficient in permeating into cultured cardiomyocytes and to the extent that this model is therapeutically relevant, DFO may fail to attain sufficiently high intracellular levels for efficiently chelating LCI.
Effect of iron-loaded sera and chelators on cytosolic labile cell iron pools of primary cardiomyocytes. Cardiomyocytes grown for 6 days in culture were incubated for 20 hours in media containing either 30% normal human serum or 30% serum from an iron-overloaded patient containing (final) LPI of approximately 2 μM or in medium containing 10% fetal calf and 10% horse serum and 50 μM FAC. Cells were loaded with CALB-AM in DMEM medium without serum or pH indicator dye as described in “Materials and methods,” and LCI was either estimated by epifluorescence microscopy and digital image analysis (A, B) or quantified in a fluorescence plate reader (C). The upper panel depicts CALB fluorescence in cells pre-incubated for 20 hours with 50 μM FAC in growth medium, then loaded with CALB and photographed before (A) and after (B) addition of 50 μM SIH. The 45% rise in fluorescence elicited by SIH corresponds to a LCI pool of 2.5 ± 0.8 μM, determined in a fluorescence plate reader, as described previously.41 The lower panel (C) depicts the values of LCI pools based on measurements in a fluorescence plate reader. The bars represent LCI pools in cells incubated for 20 hours in growth medium containing FAC: 50 μM in standard growth medium with 10% fetal calf serum, 10% horse serum; LPI: 30% LPI-containing serum from an iron-overloaded patient with no additions; DFO-pre: 30% LPI-containing serum supplemented with 10 μM DFO from the onset of 20 hours of incubation; DFO-post, DFP-post, and DFR-post: 30% LPI-containing serum, which was supplemented with 10 μM DFO or 100 μM DFP or 100 μM DFR at the conclusion of the 20-hour preincubation, 20 minutes before loading with CALB-AM. LCI denotes mean labile cell iron pool values with standard deviations obtained with 5 experimental cell systems run in parallel, calculated as mean intensity values immediately before and 10 minutes after addition of 50 μM SIH, as described elsewhere.41 The dotted line represents the LCI of cells exposed to standard growth medium containing 10% fetal calf serum and 10% horse serum.
Effect of iron-loaded sera and chelators on cytosolic labile cell iron pools of primary cardiomyocytes. Cardiomyocytes grown for 6 days in culture were incubated for 20 hours in media containing either 30% normal human serum or 30% serum from an iron-overloaded patient containing (final) LPI of approximately 2 μM or in medium containing 10% fetal calf and 10% horse serum and 50 μM FAC. Cells were loaded with CALB-AM in DMEM medium without serum or pH indicator dye as described in “Materials and methods,” and LCI was either estimated by epifluorescence microscopy and digital image analysis (A, B) or quantified in a fluorescence plate reader (C). The upper panel depicts CALB fluorescence in cells pre-incubated for 20 hours with 50 μM FAC in growth medium, then loaded with CALB and photographed before (A) and after (B) addition of 50 μM SIH. The 45% rise in fluorescence elicited by SIH corresponds to a LCI pool of 2.5 ± 0.8 μM, determined in a fluorescence plate reader, as described previously.41 The lower panel (C) depicts the values of LCI pools based on measurements in a fluorescence plate reader. The bars represent LCI pools in cells incubated for 20 hours in growth medium containing FAC: 50 μM in standard growth medium with 10% fetal calf serum, 10% horse serum; LPI: 30% LPI-containing serum from an iron-overloaded patient with no additions; DFO-pre: 30% LPI-containing serum supplemented with 10 μM DFO from the onset of 20 hours of incubation; DFO-post, DFP-post, and DFR-post: 30% LPI-containing serum, which was supplemented with 10 μM DFO or 100 μM DFP or 100 μM DFR at the conclusion of the 20-hour preincubation, 20 minutes before loading with CALB-AM. LCI denotes mean labile cell iron pool values with standard deviations obtained with 5 experimental cell systems run in parallel, calculated as mean intensity values immediately before and 10 minutes after addition of 50 μM SIH, as described elsewhere.41 The dotted line represents the LCI of cells exposed to standard growth medium containing 10% fetal calf serum and 10% horse serum.
Chelator access to nuclear and cytosolic labile cell iron pools as sensed by histone-conjugated CALG and rhodamine B (CALG-H-R). H9C2 (A) and primary cardiomyocytes (B) were initially loaded for 1 hour with CALG-H-R (12 μM) at room temperature and washed as described in “Materials and methods.” The fluorescence microscopy images show the predominantly nuclear and partially cytosolic distribution of the probe, which was found to be maintained for over 24 hours. The cells loaded with CALG-H-R were incubated for 24 hours in the presence of the indicated additives. (C) CALG-H-R loaded H9C2 cells in complete growth medium supplemented with FAC (50 μM), DFR (50 μM), DFO (100 μM)), DFP (50 μM), or with no addition (con). (D) CALG-H-R loaded primary cardiomyocytes incubated in 30% human serum containing 2 μM LPI final concentration (LPI), which was supplemented with 10 μM DFO (LPI-DFO) or 50 μM DFR (LPI-DFR) or 50 μM DFP (LPI-DFP). Panels C and D depict the LCI values for nuclear (N, ▪) and cytosolic (C, ▨) compartments estimated by analysis of images obtained before and after addition of excess permeant chelator (DFR 200 μM, applied for the purpose of attaining maximal recovery of fluorescence) and normalized to the initial fluorescence. The insets show the kinetics of the changes in nuclear fluorescence in response to 200 μM DFR (indicated by arrow), based on a mean of 3 to 5 cells in a field. The various preincubation conditions are indicated for each trace. Error bars indicate SD (n = 4).
Chelator access to nuclear and cytosolic labile cell iron pools as sensed by histone-conjugated CALG and rhodamine B (CALG-H-R). H9C2 (A) and primary cardiomyocytes (B) were initially loaded for 1 hour with CALG-H-R (12 μM) at room temperature and washed as described in “Materials and methods.” The fluorescence microscopy images show the predominantly nuclear and partially cytosolic distribution of the probe, which was found to be maintained for over 24 hours. The cells loaded with CALG-H-R were incubated for 24 hours in the presence of the indicated additives. (C) CALG-H-R loaded H9C2 cells in complete growth medium supplemented with FAC (50 μM), DFR (50 μM), DFO (100 μM)), DFP (50 μM), or with no addition (con). (D) CALG-H-R loaded primary cardiomyocytes incubated in 30% human serum containing 2 μM LPI final concentration (LPI), which was supplemented with 10 μM DFO (LPI-DFO) or 50 μM DFR (LPI-DFR) or 50 μM DFP (LPI-DFP). Panels C and D depict the LCI values for nuclear (N, ▪) and cytosolic (C, ▨) compartments estimated by analysis of images obtained before and after addition of excess permeant chelator (DFR 200 μM, applied for the purpose of attaining maximal recovery of fluorescence) and normalized to the initial fluorescence. The insets show the kinetics of the changes in nuclear fluorescence in response to 200 μM DFR (indicated by arrow), based on a mean of 3 to 5 cells in a field. The various preincubation conditions are indicated for each trace. Error bars indicate SD (n = 4).
The nuclear and cytosolic labile iron in primary cardiomyocytes showed a similar response to iron loading and chelation by DFO, DFR, and DFP as in H9C2 cells. DFO (10 μM) added to serum containing LPI (LPI-DFO) only prevented the rise over control values of LCI (LCI ∼0.9 μM, as determined with CALB, Figure 3) evoked by incubation with iron-overloaded serum. However, DFR and DFP (both 50 μM) added to the serum also reduced LCI in the nucleus by 80% and 41% and in the cytosol by 88% and 46%, respectively, due to their ability to permeate into both cell compartments and chelate their LCI. The fact that LCI changes elicited by incubating cardiomyocytes with FAC, LPI, or with permeant chelators such as DFR or DFP are similarly reflected in both cytosolic and nuclear compartments, suggests that whenever labile iron appears in the cytosol, it is also likely to diffuse into the nucleus.
Effect of chelators on LCI pools in endosomes. Since a significant portion of labile iron in extracellular fluids likely exists in stable complexes with macromolecules or small negatively charged ligands (citrate, phosphate, etc), a plausible route of its entry into cells is by adsorptive endocytosis. In order to simulate this process, we added to cells preformed CALG-Fe complexes generated by stoichiometrically mixing CALG with Fe:NTA to produce CALG/Fe ratio of 1:1. As the CALG-Fe complexes are approximately 90% fluorescence quenched, low-level, basal fluorescence can be initially detected and subsequently fully revealed in cell compartments by adding permeant chelators, as seen in Figure 5. Exposure of H9C2 cells to CALG-Fe for 1 hour at 37°C showed that both the basal fluorescence and that revealed by addition of chelators (DFR and DFP at 50 μM) were localized exclusively in particulate structures corresponding to intracellular vesicles, for instance, endosomes. The rate and extent of fluorescence recovery elicited by the chelators provides a measure of their ability to permeate into cells and endosomes and scavenge bound iron. In protein-free medium, DFR was the fastest and most efficient among the 3 chelators tested. DFO showed essentially no detectable effects on endosomal LCI pools over a 30-minute period, although after prolonged time periods it could permeate into cells by endocytosis and affect the endosomal labile iron pools (not shown). The inclusion of 30% fetal calf serum reduced the rates of dequenching by 50 μM DFR and DFP by about 40% and 28%, respectively (H.G. and Z.I.C., unpublished observations, December 2005). The relative permeability of the chelators into endosomes roughly parallels their patterns of permeability into the cytosol, mitochondria, and nuclei.
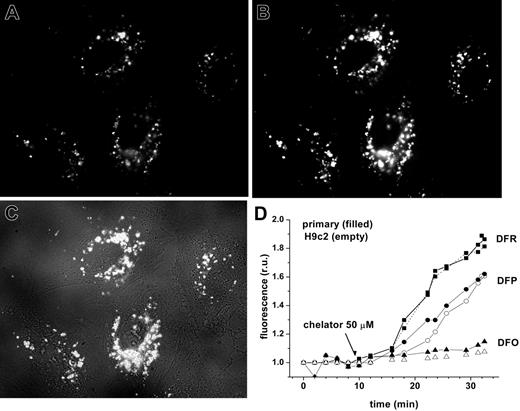
Chelator access to endosomal labile iron in H9C2 cells and cardiomyocytes, as revealed by endocytosed CALG-Fe complexes. H9C2 or cardiomyocytes were incubated with fluorescence-quenched CALG/Fe (1:1 ratio, 30 μM) for 1 hour at 37°C in HEPES-buffered DMEM medium, then washed and incubated at 37°C in the same medium. Fluorescence (485 nm excitation, 520 nm emission) was monitored by fluorescent microscopy (×60 objective). The images shown are of H9C2 cells, selected from a time sequence of 15 images recorded at 2-minute intervals: A was taken at 2 minutes (prior to chelator addition) and B at 28 minutes (18 minutes after addition of 50 μM DFR). C is a merged picture of fluorescence and phase contrast of image B. D represents mean fluorescence values of 4 cells/field calculated for each time-point image and normalized to the basal fluorescence prior to addition of chelator, in relative units (ru). The chelators DFO (triangles), DFP (circles), and DFR (squares) were added at 50 μM concentrations at 10 minutes, as indicated by arrow. H9C2 cells are indicated by closed symbols; primary cardiomyocytes, by open symbols.
Chelator access to endosomal labile iron in H9C2 cells and cardiomyocytes, as revealed by endocytosed CALG-Fe complexes. H9C2 or cardiomyocytes were incubated with fluorescence-quenched CALG/Fe (1:1 ratio, 30 μM) for 1 hour at 37°C in HEPES-buffered DMEM medium, then washed and incubated at 37°C in the same medium. Fluorescence (485 nm excitation, 520 nm emission) was monitored by fluorescent microscopy (×60 objective). The images shown are of H9C2 cells, selected from a time sequence of 15 images recorded at 2-minute intervals: A was taken at 2 minutes (prior to chelator addition) and B at 28 minutes (18 minutes after addition of 50 μM DFR). C is a merged picture of fluorescence and phase contrast of image B. D represents mean fluorescence values of 4 cells/field calculated for each time-point image and normalized to the basal fluorescence prior to addition of chelator, in relative units (ru). The chelators DFO (triangles), DFP (circles), and DFR (squares) were added at 50 μM concentrations at 10 minutes, as indicated by arrow. H9C2 cells are indicated by closed symbols; primary cardiomyocytes, by open symbols.
Recovery of impaired cardiomyocyte contractility caused by iron loading. The association of iron toxicity with excess iron loading of cardiomyocytes is manifested as impaired contractility that is amenable to reversal by chelation.26 In a given culture of rat cardiomyocytes, more than 50% of the cells contract rhythmically, each culture with a particular rate and amplitude that are largely dictated by the age of cells and culture media. Following iron loading, the contraction becomes irregular and markedly lower in amplitude, while beating occasionally accelerates by up to 50% and in most cases ceases almost completely, as shown in Figure 6. Effects of labile iron could be evoked by 24 hours' incubation with 360 μM FAC, but more reliably by exposure to 10 μM of the permeant 1:1 Fe(III)–hydroxyquinoline for 1 hour. The reduced contractility was partially restored following 1-hour treatment with 100 μM DFR or DFP, but not with up to 100 μM DFO. DFP (100 μM) gave inconsistent results, causing variable degrees of recovery in different heart cell preparations. Control cells, namely cells not loaded with iron, were essentially unaffected by 1-hour exposure to 10 or 100 μM DFO in growth medium supplemented with 30% calf serum or 4% bovine serum albumin and marginally (< 10%) inhibited by 100 μM DFR in the same medium (not shown). The chelating activity of DFP and DFR are retained throughout the 24-hour incubation period, while that of DFO is lost after 12 hours in culture conditions (W.B. and Z.I.C., unpublished data, May 2006), indicating that extraction of radioactive iron by DFO is largely accomplished during the initial hours of incubation. In the in vivo situation, the gradual loss of activity of DFO is probably less of a drawback as the drug is continually replenished by infusion.
Restoration of contractile properties of iron-loaded cardiomyocytes by chelators. A 7-day culture of cardiomyocytes kept in DMEM medium at 37°C was followed microscopically by phase contrast and contractility assessed by monitoring changes in light intensity within selected cellular areas lying in the plane of contraction. A shows in sequence (top to bottom) the tracings in terms of optical density (OD, in arbitrary units [au]) of cells followed for 120 seconds at the end of treatment with (1) medium (+none) to which was added in sequence: (2) 10 μM Fe-hydroxyquinoline for 60 minutes (+FHQ); (3) deferrioxamine (100 μM) for 60minutes (+FHQ+DFO) and either (4) deferiprone (100 μM) (+FHQ+DFO+DFP) for 60 minutes, or (5) deferasirox (100 μM) for 60 minutes (+FHQ+ DFO+DFR). B shows beating frequency, expressed as beats/min, representing the frequency of spikes (solid line) and mean amplitude, calculated from the mean heights of the spikes in each trace shown in the top graph and expressed in arbitrary units (au; broken line). The various agents were added sequentially at the time indicated by the respective arrow, and contractility was measured 60 minutes later.
Restoration of contractile properties of iron-loaded cardiomyocytes by chelators. A 7-day culture of cardiomyocytes kept in DMEM medium at 37°C was followed microscopically by phase contrast and contractility assessed by monitoring changes in light intensity within selected cellular areas lying in the plane of contraction. A shows in sequence (top to bottom) the tracings in terms of optical density (OD, in arbitrary units [au]) of cells followed for 120 seconds at the end of treatment with (1) medium (+none) to which was added in sequence: (2) 10 μM Fe-hydroxyquinoline for 60 minutes (+FHQ); (3) deferrioxamine (100 μM) for 60minutes (+FHQ+DFO) and either (4) deferiprone (100 μM) (+FHQ+DFO+DFP) for 60 minutes, or (5) deferasirox (100 μM) for 60 minutes (+FHQ+ DFO+DFR). B shows beating frequency, expressed as beats/min, representing the frequency of spikes (solid line) and mean amplitude, calculated from the mean heights of the spikes in each trace shown in the top graph and expressed in arbitrary units (au; broken line). The various agents were added sequentially at the time indicated by the respective arrow, and contractility was measured 60 minutes later.
The fact that functional recovery of cardiac cells from iron toxicity may precede the mass extraction of iron (compare to Figure 1) suggests either that (1) only a small subfraction of rapidly chelatable iron is associated with toxicity, or (2) egress of iron chelates proceeds at a relatively slow rate compared to the fast complexation and neutralization of intracellular labile iron caused by added chelators, or (3) functional recovery demands additional biochemical processes independent of the rate of chelation or egress of iron-chelator complexes.42 Possible ways to discern between the different explanations may be by defining the correlations between the intracellular sites of iron loading in cardiomyocytes and the neutralization of labile cell iron forms by chelators in conditions analogous to those used in this study.
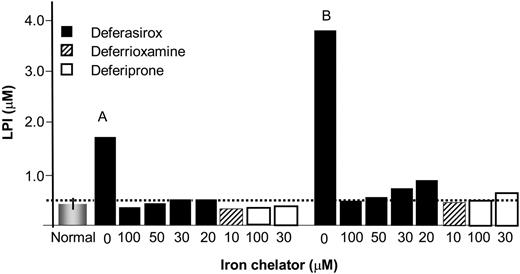
Effect of chelators on LPI. The ability of DFR, DFP, and DFO to eliminate LPI was assessed in sera from thalassemia patients, using chelator concentrations therapeutically attainable in the plasma. The sera samples were from a previous study14 and were taken from patients treated with DFP at 75 mg/kg/d. Blood samples were taken in the morning, at least 10 hours after the last daily dose of DFP, so as to allow sufficient time for DFP to wash out and LPI to raise.14 The data depicted in Figure 7 represent sera from 2 individuals with LPI values of 1.75 μM and 3.8 μM. The sera were treated with chelators at concentrations representing (1) peak to trough plasma levels of DFR attained therapeutically with a 40 mg/k/d oral dose (20-100 μM),30 (2) plateau levels attained during subcutaneous administration of DFO (10 μM),4 and (3) range of concentrations of plasma DFP generally attained 2 hours and 3 hours following intake of a single (of 3 daily) 25 mg/kg oral dose of DFP.43 LPI was reduced effectively to near-basal levels by concentrations of DFO attained during infusion and by peak-trough plasma concentrations attained with the oral chelators DFR and DFP. The data indicate that continual maintenance of a basal level of any of the 3 chelators in plasma might suffice for effective suppression of LPI.
Effect of therapeutic concentrations of chelators on LPI in sera of thalassemia major patients. Iron chelators were added at the indicated concentrations to sera from 2 thalassemia major patients (A and B) undergoing therapy with orally administered DFP (75 mg/kg/d) and showing substantial levels of LPI after more than 10-hour washout period (since last intake of DFP). The bars denote: the mean LPI value of 0.4 ± 0.2 μM of 10 normal individuals (normal = no iron overload); LPI values of serum samples from thalassemia patient A and B to which had been added the concentrations of chelators indicated under each bar. The respective initial LPI values for A and B were 1.75 and 4.85 μM, representing 2 different classes of LPI levels that are attained in the plasma following more than 10-hour drug washout. The chelators were allowed to act on sera for 1 hour at 37°C before LPI was analyzed, as described elsewhere.36 The broken line represents the empirical background level of the LPI assay. Deferasirox: ▪; deferrioxamine: ▨; deferiprone: □.
Effect of therapeutic concentrations of chelators on LPI in sera of thalassemia major patients. Iron chelators were added at the indicated concentrations to sera from 2 thalassemia major patients (A and B) undergoing therapy with orally administered DFP (75 mg/kg/d) and showing substantial levels of LPI after more than 10-hour washout period (since last intake of DFP). The bars denote: the mean LPI value of 0.4 ± 0.2 μM of 10 normal individuals (normal = no iron overload); LPI values of serum samples from thalassemia patient A and B to which had been added the concentrations of chelators indicated under each bar. The respective initial LPI values for A and B were 1.75 and 4.85 μM, representing 2 different classes of LPI levels that are attained in the plasma following more than 10-hour drug washout. The chelators were allowed to act on sera for 1 hour at 37°C before LPI was analyzed, as described elsewhere.36 The broken line represents the empirical background level of the LPI assay. Deferasirox: ▪; deferrioxamine: ▨; deferiprone: □.
Discussion
We focused this study on the comparative ability of the clinically relevant DFR, DFP, and DFO to suppress the labile iron forms implicated in siderosis-associated damage in primary cardiomyocytes subjected to acute or chronic iron loading. These included extracellular LPI and intracellular LCI resulting from LPI uptake into cardiomyocytes and ensuing functional impairment. We examined the chelators' effects under conditions that approximate those found in vivo, namely in the presence of patient's 30% serum or medium containing albumin as in full serum, thus filling essential information regarding the basic properties of chelator accessibility to cells.44
Both cell iron extraction and functional restoration of iron-impaired contractily were more efficiently attained with plasma peak and trough levels attained with DFR and peak and one third peak values for DFP with the plateau values for DFO. At therapeutic concentrations DFR and DFP extracted 30% to 50% of the short-term radiolabeled iron, whereas DFO required 8 to 12 hours of exposures to extract at most 10% to 20%. On the other hand, radiolabeled iron loaded over long term was poorly extractable by DFO, DFP, or DFR at trough plasma levels even over a 12-hour exposure time. In all the latter experimental conditions, DFR was relatively more efficacious in extracting iron at therapeutic concentrations.
The physiological implications of the comparative data of radiolabeled iron extraction should be extended with caution to the clinical setting. Factors such as drug administration regimens vis a vis drug pharmacokinetics including stability determine the time windows of drug exposure to cells and hence their chelation efficacy. DFO plasma levels are relatively steady during infusion,5,9,45 and although it might not retain its full chelation potential over time (W.B. and Z.I.C., unpublished data, May 2006), it is continuously replenished by infusion. For DFP, the peak-to-trough periods are relatively short (< 3 hours)43 and for DFR more than 8 to 10 hours.29,30 These apparent advantages of DFR pharmacokinetics also should be weighed against the possible effects of plasma proteins in limiting the cell availability of the oral chelators but apparently not in suppressing LPI. In the case of DFR, the effect of serum may become significant at trough concentrations attained with the standard daily dose of 20 mg/kg (Figure 7), but not with higher doses used in highly iron-overloaded patients. The iron extraction profiles of chelators obtained with primary cardiomyocytes were reproduced with rat cardiac H9C2 cells (G.L., unpublished observations, January 2006).
The major limitation of studies based on radiolabeled iron is the inability to associate the label with the sites of cell iron accumulation.44 The application of fluorescence metal sensors (FMS) and microscopic fluorodetection paved the road for assessing (1) whether the LCI accumulated in cardiomyocytes from exposure to high concentrations of organic iron complexes are germane to those generated by long-term exposure to serum containing LPI, the alleged source of cardiac iron overload,45 and (2) whether chelators applied at therapeutically relevant doses have the ability to modulate the levels of LCI from either source. The focus was on LCI, since this is the redox active and chelatable form of the metal and the one experimentally amenable to dynamic tracing in medium and cells.15 The FMS employed for LCI tracing differed in their mode of iron detection and localization in the cells: (1) iron-dependent generation of ROS in mitochondria was measured using the membrane potential–driven DHR (Figure 2); (2) whole-cell LCI was measured with CALB, which fluor responds to labile iron in chelator-reversible manner by quenching/dequenched (Figure 3); (3) for long-term monitoring of LCI we used histone-conjugated FMS (target to cytosol and nucleus) retainable for more than 24 hours; (4) for LCI in pino/endocytic vesicles, we used quenched CALG-Fe complexes that get endocytosed by cells and their fluorescence revealed by add permeant chelators (Figure 5). These probes enabled us to monitor the access of chelators to specific compartments within cells as well as the accumulation of LCI resulting from exposure of cells to LPI-containing sera from iron-overloaded patients.
Our studies indicate that incubations with medium containing LPI or FAC lead to increased levels of total LCI that is both redox-active and chelatable by permeant chelators and preventable by pre-complexation of the LPI-containing sera with either chelator (Figure 2). The LCI generated by LPI and FAC was detected in the cytosol (Figures 3, 4), nucleus (Figure 4), and mitochondria (Figure 2), indicating relatively efficient cellular uptake and distribution of LPI. Assuming that endosomal CALG-Fe complexes are analogous to endocytosed forms of LPI (Figure 5), it is likely that endocytosis contributes significantly to cellular uptake of LPI. This is in accord with recent studies37 that also suggested that a major iron source of LCI can be attributed to the endocytosis of iron complexes and followed by diffusion of labile iron to other compartments. At this point a possible contribution of L-type Ca channels to LPI entry into cardiomyocytes46 cannot be dismissed, despite the difficulty in accepting a mechanism operating in a pathophysiological milieu with LPI of 3 orders of magnitude lower concentration than Ca(II) and virtually all the metal bound to organic polyanions.46
The possible influence of serum proteins on reducing the activity of chelators cannot be overlooked in in vitro studies of chelator action on cells. We found that the presence of plasma proteins may reduce, but not abrogate, that efficiency, by lowering the concentration of available chelator. This effect gains in significance at low chelator concentrations. Nonetheless, all 3 chelators effectively eliminated serum LPI (Figure 7) at therapeutically achieved concentrations. For DFR, it might even confer an advantage by prolonging the chelator's half-life in the plasma. As for DFO, although it attains sufficient plasma levels for eliminating LPI, it is apparently not as stable as DFR47 or DFP over time (W.B. and Z.I.C., unpublished data, May 2006) and inefficient in accessing cells.44 While it is clear that DFO can slowly permeate into cardiac cells (Figure 4C) and efficiently extract iron (Figure 1), when applied at 100 μM, it is not effective at therapeutic doses (< 10 μM) in accessing cells and extracting LCI from any LCI pool in cardiomyocytes.
We also attempted to simulate a situation where LCI levels become toxic to the point of interfering with cardiac function, using the rapidly penetrating complex Fe-hydroxyquinoline to generate acute iron overload. DFR, and to some extent, also DFP, but not DFO, caused a partial recovery of contractility within 2 hours of exposure (Figure 6), consistent with DFR's rapid cell penetration and efficient chelation of the various LCI pools. DFP effects were inconsistent, with partial recovery in some experiments and no observable changes in others, possibly because of the short exposure time or the form of iron overload used. Nonetheless, the results obtained with this model corroborate the conclusion that the 2 oral chelators, in particular DFR, can rapidly restore iron balance in iron-overloaded cardiomyocytes.
The studies presented here have both basic and clinical implications for understanding the pathophysiology of iron overload in relation to the heart and the selection of an appropriate chelation regimen. It has been proposed that a daily maintenance of a basal level of chelator in the plasma might confer cardiac protection by eliminating LPI, the form of plasma iron implicated in iron overload.11,12 This work shows that what is attainable in culture conditions with continuous 24-hour DFO infusion or with sequential administration of DFP and DFO9 is likely attainable with DFR, offered orally in a single dose. Preliminary indications are that the same applies apparently with DFR-treated patients.31 Mechanistically, it shows that in addition to abrogating LPI and reducing cardiomyocyte labile iron burden (Figure 3), DFR and DFP at therapeutic levels can reduce LCI accumulated in organelles and, in doing so, reduce iron redox activity and ensuing cardiotoxicity. To the extent that loading cardiomyocytes with radioiron in the form of citrate complexes simulates qualitatively the loading patterns of LPI implies that DFR and DFP at therapeutic levels also can extract long-term accumulated iron with comparable dose efficacy. Importantly, the possibility of maintaining basal DFR plasma levels for most day hours with a single daily drug intake might also confer better extraction potential of labile iron accumulated in the heart.
Prepublished online as Blood First Edition Paper, July 11, 2006; DOI 10.1182/blood-2006-05-020867.
Supported by the European Economic Community and by Novartis (Basel).
Part of this work and H.V.'s salary in specifics were covered by a contract with Novartis Pharma Basel.
H.G. performed contractility and LCI measurements in endosomes, cytosol, and nuclei; R.B.E. equally contributed to H.G. but on measurements in mitochondria and LPI loading; G.L. did all the radiolabeled work; W.B. did LPI measurements on thalassemia sera and the synthesis of fluorescent probes; A.M.K., C.H., and H.P. helped with the design of the experiments; Z.I.C. designed and supervised the project and wrote the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 6. Restoration of contractile properties of iron-loaded cardiomyocytes by chelators. A 7-day culture of cardiomyocytes kept in DMEM medium at 37°C was followed microscopically by phase contrast and contractility assessed by monitoring changes in light intensity within selected cellular areas lying in the plane of contraction. A shows in sequence (top to bottom) the tracings in terms of optical density (OD, in arbitrary units [au]) of cells followed for 120 seconds at the end of treatment with (1) medium (+none) to which was added in sequence: (2) 10 μM Fe-hydroxyquinoline for 60 minutes (+FHQ); (3) deferrioxamine (100 μM) for 60minutes (+FHQ+DFO) and either (4) deferiprone (100 μM) (+FHQ+DFO+DFP) for 60 minutes, or (5) deferasirox (100 μM) for 60 minutes (+FHQ+ DFO+DFR). B shows beating frequency, expressed as beats/min, representing the frequency of spikes (solid line) and mean amplitude, calculated from the mean heights of the spikes in each trace shown in the top graph and expressed in arbitrary units (au; broken line). The various agents were added sequentially at the time indicated by the respective arrow, and contractility was measured 60 minutes later.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/9/10.1182_blood-2006-05-020867/4/m_zh80210603200006.jpeg?Expires=1769115683&Signature=t~XCwG3bW-vrEH-QHSOBAliHWSR5AFx7Ku-9j7ZIou3HL-TF4oe1l0Amzcup0a-gMnNbzrhenZwLIfvZhd-Nn0fpBHPQtHZh1GfcIybVBnJbZFIayjScmOG8eMPZVnfilC~qxis771JkHsWRtOkzS8vLhj37meHB1pQBD910p1CLSe2qc5MmZdj5FgeySIrzDwa2YTKg8SZOIuGiamBP4-cB3sgO1VHsbQ53A-stMgKn0aG9vQUqPDI~2ketXwn3zKlU2nUHLScVswCXJo0gTnABVYa1S39A6i-MFIBG5MMBHN-iExV3SzEAmzvbqRR9ae~g75a1ZEQTm2~NHsj9XQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal