Abstract
After infection of a red blood cell (RBC), the malaria parasite, Plasmodium falciparum, increases the permeability of the host's plasma membrane by inducing new permeability pathways (NPPs). Single-channel patch-clamp experiments have shown the presence in infected RBCs of novel anion-selective channel types with low open-state probabilities at positive membrane potentials. These channels have been postulated to form the NPPs. Here, we have used a range of transport techniques to study whether electroneutral solutes use these channels or altered/separate pathways. Transport of the electroneutral solute sorbitol via the NPPs was found to increase by a small but significant amount after gross membrane depolarization. This is inconsistent with transport via a channel with a reduced open-state probability at positive membrane potentials. As has been demonstrated previously for parasite-induced anion currents, sorbitol transport in infected RBCs was found to be sensitive to the presence of bovine serum albumin (BSA). However, it remains to be shown whether the effect is due to serum/BSA altering a single channel type or activating a new pathway. In addition, the study highlights problems that can occur when using different transport techniques to study the NPPs.
Introduction
Having infected a red blood cell (RBC), the human malaria parasite, Plasmodium falciparum, increases the permeability of its host's plasma membrane to a range of small, structurally unrelated solutes. The pathways responsible for this altered permeability, commonly referred to as the “new permeability pathways” (NPPs), have been postulated to be predominantly anion-selective channels, which also allow the transport of electroneutral and cationic solutes,1 although this hypothesis has been challenged recently.2
The NPPs have been studied for more than 2 decades and are of interest as potential therapeutic targets, but there is still confusion over the number and nature of pathways which form them. Although several groups have put forward the hypothesis that the NPPs can be formed by a single channel type,1,3-6 other groups have suggested that more than one distinct channel type and/or pathway are required to explain the reported transport data.7-12 In addition, both endogenous5,11,12 and parasite-derived pathways3 have been postulated to underlie the NPPs. At present, none of the reports are overwhelmingly conclusive, and there remain a number of discrepant results.2,3,13,14
In collaboration with 2 other groups in this field, we have reported data that explain one of these inconsistencies.15 Although all other groups working on the NPPs, using the patch-clamp technique in the whole-cell configuration, observe only inwardly rectifying whole-cell anion conductances in malaria-infected RBCs,4,5,12 Huber et al11 have reported observing both inwardly and outwardly rectifying conductances. This difference was found to result from the presence of residual serum levels caused by the use of unwashed cells taken directly from culture (causing increased currents at both positive and negative test potentials [VT]) and the use of a negative-holding potential (VH) of –30 mV (leading to a time-dependent inactivation of whole-cell currents at negative VT values).
The aim of the present research was to determine whether different solutes used the same transport pathway to cross the host's plasma membrane in P falciparum–infected RBCs by testing whether factors shown to influence the nature and magnitude of the conductances in P falciparum–infected RBCs (namely membrane potential [Vm] and serum) also affected the transport of other solutes. In particular, the transport of the electroneutral solute sorbitol was investigated, using a number of different transport techniques.
Materials and methods
Materials
Unlabeled solutes (choline, sorbitol, lactate), essentially fatty acid–free bovine serum albumin (BSA), N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), ethylene glycol bis(2-aminoethyl ether)-N, N, N′N′-tetraacetic acid (EGTA), P-chloromercuribenzene sulfonic acid (pCMBS), 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS), and 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) were obtained from Sigma (Dorset, United Kingdom). Radioisotopes ([14C]choline, [14C]sorbitol, [14C]lactate) were obtained from Amersham (Bucks, Untied Kingdom). P falciparum (the A4 line16 )–infected RBCs (using type O donor blood, obtained from National Blood Services, South West, Bristol, United Kingdom) for experimentation (32-38 hours after invasion) were cultured and harvested, as described previously.17
Radio-tracer measurements
The unidirectional influx of sorbitol, choline, and lactate into infected RBCs was estimated from the uptake of [14C]sorbitol, [14C]choline, and [14C]lactate, respectively, using methods described previously.1,18,19 Experiments were carried out at either room temperature (RT; ∼ 20°C) or 37°C as indicated, with cells washed (× 4), then resuspended into iso-osmotic NaCl solution prior to experimentation. Iso-osmotic solutions were prepared by dissolving the compound of interest to a concentration of 155 mM (for NaCl) or 300 mM (for sorbitol and sucrose) in a solution containing 10 mM HEPES and 5 mM glucose (pH 7.4, 310 ± 5 mOsm/kg H2O). Influx measurements were made under conditions that minimized transport via the normal host RBC transport systems, and transport via the NPPs was defined as the NPPB-sensitive influx (the concentration of NPPB used throughout this study was 0.2 mM, which maximally inhibited the NPPs even in the presence of serum or BSA; data not shown).
Uptake time courses for lactate, sorbitol, and choline were performed over 1, 5, and 20 minutes, respectively. The experiments were designed to have a final radio-tracer activity of either 1 μCi/mL (0.037 MBq/mL) (lactate) or 0.3 μCi/mL (0.011 MBq/mL) (sorbitol or choline), a final unlabeled solute concentration of 1 mM, a final cell concentration of 1 to 4 × 108 cells/mL, and a total sample volume of either 0.3 (lactate) or 1 (sorbitol or choline) mL. Human serum was present at a final concentration of either 1% (lactate) or 8.5% (sorbitol or choline) vol/vol, when appropriate. For the experiments shown in Figure 2, in which the cells were exposed to low ionic-strength media, 0.95-mL aliquots of cell suspension were placed in microcentrifuge tubes, and the cells were washed twice in quick succession with ice-cold aliquots of the appropriate solution and then pelleted. Experiments commenced with the addition of an aliquot of appropriate solution at RT to return the volume to 0.95 mL followed immediately by a radio-tracer aliquot (thus stopping any effects of exposing the cells to low ionic-strength conditions before the start of the experiment but removing nearly all extracellular Cl–). Additional influx measurements were taken at 10 minutes for choline, 40 seconds (at 37° C) or 60 seconds (at RT) for sorbitol and 2 seconds for lactate. Note that in lactate transport experiments 0.02 mM DIDS and 0.1 mM pCMBS were used to inhibit endogenous lactate transport19 and that 1% vol/vol human serum did not interfere with their actions (data not shown).
Electrophysiologic measurements
The ruptured patch whole-cell voltage-clamp configuration was used to record membrane currents, as described previously.15 All experiments were performed at RT. Patch pipettes (tip resistances 6 to 12 MΩ) were prepared from borosilicate glass capillaries, pulled, and polished on a Werner Zeitz DMZ programmable puller (Augsburg, Germany). The bath solution contained 155 mM NaCl, 1.4 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 10 mM glucose (pH 7.4, 310 ± 5 mOsm/kg H2O). The pipette solution was of the same composition, with the one exception that 1.4 mM CaCl2 was replaced with 0.5 mM EGTA.
Briefly, a 2-mL aliquot of infected RBCs suspension (0.001% hematocrit) was placed in a glass-bottomed 35-mm Petri dish and left while the RBCs settled. Having obtained a seal (5-15 GΩ) on an infected RBC, the cell was lifted off the bottom of the dish, and the patch was ruptured to attain the whole-cell configuration. Whole-cell currents were recorded using an Axopatch 200B amplifier (digitized at 10 kHz and filtered at 5 kHz with a 4-pole Bessel filter), with voltage command protocols generated, and the currents were analyzed using the pCLAMP software suite (version 9; Axon Instruments, Foster City, CA). Whole-cell current/voltage (I-V) curves were obtained by evoking a series of VT values from –100 to +100 mV in 10-mV steps for 300 milliseconds from a VH of 0 or –30 mV. Data for the construction of I-V curves were measured over the last 50 milliseconds of the current records (ie, 250-300 milliseconds).
Hemolysis measurements
For standard hemolysis assays, hemoglobin release was used to estimate lysis times, as described previously.1,20 Experiments were performed at either RT or 37°C and, when appropriate, in the presence of 0.5% wt/vol BSA. For single-cell lysis assays (designed to mimic the conditions used during patch-clamp experiments), all experiments were performed at RT. Briefly, 100 μL infected RBC suspension (0.001% hematocrit) was placed in a glass-bottomed 35-mm Petri dish and treated as in patch-clamp experiments (described under “Electrophysiologic measurements”). However, having obtained a seal and lifted the infected RBC, 2 mL of either serum-free RPMI 1640 or iso-osmotic sorbitol solution (and, when appropriate, 0.5% wt/vol BSA and/or 0.2 mM NPPB) was added gently to start the lysis experiment. Infected RBCs that lysed during solution addition, which took approximately 20 seconds, were disregarded. An eyepiece camera and WinTV 2000 software (Hauppage, London, United Kingdom) were used to record the time taken for infected RBCs to lyse.
Results
Effect of low ionic-strength media on the Vm and on other homeostatic variables of malaria-infected RBCs
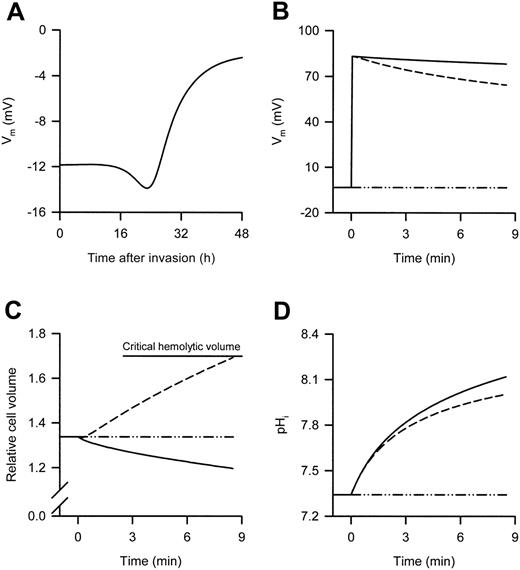
It is generally accepted that the Vm of infected (and uninfected) RBCs is determined by Cl– permeability. Therefore, when infected RBCs are suspended in low ionic-strength media (creating a large outward Cl– gradient because of the removal of external Cl–), it is a simple process of using the concentration difference in the Nernst equation to determine the new Vm (the only unknown in this case is the RBC cytosolic Cl– concentration, although this can be safely assumed to lie between 100 and 140 mM). However, the effects of low ionic-strength are complicated by subsequent changes in a number of homeostatic variables, which differ with the solute used to make the low ionic-strength solution. To approximate these effects, the integrated mathematical model of the P falciparum–infected RBC,21 extended from the earlier Lew-Bookchin model22 of the human RBC was applied to predict the changes in homeostatic variables in infected RBCs (Figure 1). The Vm of 32- to 38-hour postinvasion infected RBCs (as used here) in a physiologic, high NaCl environment is predicted to lie between –7 and –3 mV (Figure 1A), whereas suspension of these infected RBCs in low ionic-strength media is predicted to result in a marked membrane depolarization (Figure 1B).
In the example presented in Figure 1, even with the use of a 5-mM external Cl– concentration, infected RBCs suspended in either iso-osmotic sucrose or sorbitol solution are predicted to have Vm values above +80 mV, which decline slowly. As stated in the previous paragraph, the initial depolarizations are due to the large outward Cl– gradients that form. The slow decline in the Vm of cells suspended in iso-osmotic sucrose solution is due to the slow reduction in the Cl– gradient caused by the loss of internal Cl– (in conjunction with a cation) from the host cytosol to the external medium. A consequence of this phenomenon is the loss of host-cell water and cell shrinkage (Figure 1C). The slightly faster decline in the Vm of cells suspended in isoosmotic sorbitol solution is due to the additional uptake of sorbitol (unlike sucrose, sorbitol is taken up by infected RBCs via the NPPs), which is followed by water. As this process is faster than the process leading to cell shrinkage, there is an increase in host-cell water and thus cell swelling (ultimately leading to lysis [Figure 1C]), which further reduces the Cl– gradient and thus the Vm. The effect of suspending infected RBCs in the different experimental solutions on the host's cytosolic pH (pHi) is shown in Figure 1D and is considered in “Discussion.”
Predicted changes in the Vm of P falciparum–infected RBCs during the asexual reproduction cycle of the parasite and changes in selected homeostatic variables after suspension of 35-hour postinvasion infected RBCs in either high or low ionic-strength media. (A) Predicted changes in the Vm during the asexual reproduction cycle of the parasite. Predicted changes in (B) the Vm, (C) the relative cell volume, and (D) the pHi after suspension of 35-hour postinvasion infected RBCs in iso-osmotic NaCl (dot/dash lines), sucrose (solid lines), and sorbitol (dashed lines) solutions at RT. The experimental protocol used to obtain the data shown in panels B, C, and D was as follows: 35-hour postinvasion infected RBCs were washed into iso-osmotic NaCl solution (pH 7.4) at RT and left for 30 minutes, then the infected RBCs were resuspended for 8.5 minutes in each of the different iso-osmotic solutions at RT. The 8.5-minute time scale depicts the average lysis time for infected RBCs suspended in iso-osmotic sorbitol solution at RT (see Table 2) and was set by adjusting the maximum sorbitol permeability in the model to 2.7 hour–1 (the default values for all other variables were unchanged). Note that the actual NPPB-sensitive rate constant (derived from the sorbitol uptake data measured in iso-osmotic NaCl solution presented in Figure 2A) was 15 ± 3 hour–1 (n = 3) and was not used in the modeling because this resulted in faster than expected lysis rates. A 5-mM external Cl– concentration was used when predicting the effect of low ionic-strength solutions. In panel C, all volumes are expressed relative to the RBC volume at the time of invasion, defined as 1, and the horizontal top line indicates the mean critical hemolytic relative cell volume.
Predicted changes in the Vm of P falciparum–infected RBCs during the asexual reproduction cycle of the parasite and changes in selected homeostatic variables after suspension of 35-hour postinvasion infected RBCs in either high or low ionic-strength media. (A) Predicted changes in the Vm during the asexual reproduction cycle of the parasite. Predicted changes in (B) the Vm, (C) the relative cell volume, and (D) the pHi after suspension of 35-hour postinvasion infected RBCs in iso-osmotic NaCl (dot/dash lines), sucrose (solid lines), and sorbitol (dashed lines) solutions at RT. The experimental protocol used to obtain the data shown in panels B, C, and D was as follows: 35-hour postinvasion infected RBCs were washed into iso-osmotic NaCl solution (pH 7.4) at RT and left for 30 minutes, then the infected RBCs were resuspended for 8.5 minutes in each of the different iso-osmotic solutions at RT. The 8.5-minute time scale depicts the average lysis time for infected RBCs suspended in iso-osmotic sorbitol solution at RT (see Table 2) and was set by adjusting the maximum sorbitol permeability in the model to 2.7 hour–1 (the default values for all other variables were unchanged). Note that the actual NPPB-sensitive rate constant (derived from the sorbitol uptake data measured in iso-osmotic NaCl solution presented in Figure 2A) was 15 ± 3 hour–1 (n = 3) and was not used in the modeling because this resulted in faster than expected lysis rates. A 5-mM external Cl– concentration was used when predicting the effect of low ionic-strength solutions. In panel C, all volumes are expressed relative to the RBC volume at the time of invasion, defined as 1, and the horizontal top line indicates the mean critical hemolytic relative cell volume.
Effect of membrane depolarization on sorbitol transport in malaria-infected RBCs
If the NPPs are formed solely by either of the single-channel types described previously in P falciparum–infected RBCs,4,5 it would be expected that electroneutral solute transport would reduce at positive Vm values because channel openings are reduced. To test this hypothesis, radiotracer uptake experiments, measuring sorbitol transport, were performed in which the Vm of infected RBCs was grossly depolarized by the use of low ionic-strength media (as predicted by the mathematical model). Note that for comparison with data generated by the patch-clamp technique these experiments were performed at RT.
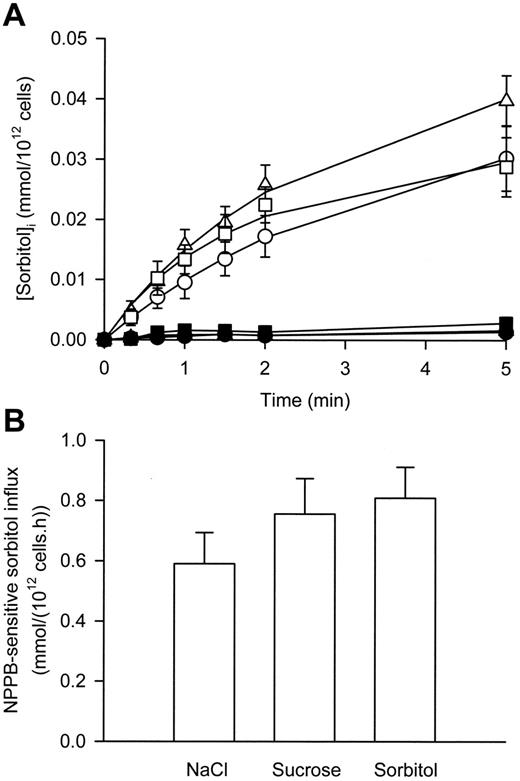
As shown in Figure 2A, in iso-osmotic NaCl solution, sorbitol uptake into P falciparum–infected RBCs was linear for at least 90 seconds and abolished in the presence of NPPB (a known inhibitor of the NPPs1 ). In iso-osmotic sucrose and sorbitol solutions, sorbitol uptake into P falciparum–infected RBCs was linear for at least 60 seconds and also abolished in the presence of NPPB (Figure 2A). However, there was a slight but significant (P < .03, paired, 2-tailed Student t test) increase in the NPPB-sensitive influx of sorbitol in infected RBCs suspended in iso-osmotic sorbitol and sucrose solutions compared with iso-osmotic NaCl solution (Figure 2B). No significant difference was found between the influx data derived from suspension of infected RBCs in either iso-osmotic sorbitol or sucrose solution (P = .07, paired, 2-tailed Student t test).
Effect of serum/BSA on solute transport in malaria-infected RBCs
The presence of blood products (human serum, human plasma, Albumax II [lipid-rich BSA] and essentially fatty acid-free BSA) during whole-cell patch-clamp experiments has been reported to alter the membrane conductance of malaria-infected RBCs markedly.15 To study whether the presence of serum/BSA also affected the transport of other solutes, 2 additional transport measurement techniques were used, namely radio-tracer and hemolysis methodologies.
For radio-tracer experiments, 2 further solutes were tested in addition to sorbitol: the organic cation choline and the organic anion lactate. Table 1 shows the effect of the presence of human serum on solute transport via the NPPs (defined as NPPB-sensitive influx) in P falciparum–infected RBCs. Experiments were performed at RT (for comparison to patch-clamp measurements) and at 37°C for choline and sorbitol transport (it was not possible to measure lactate transport accurately at 37°C because of its speed of uptake). Increasing the temperature from RT to 37°C increased the transport of choline and sorbitol via the NPPs by greater than 3-fold. However, the presence of human serum (at concentrations in excess of those used during patch-clamp experiments to alter membrane conductance15 ) had no significant effect on transport rates via the NPPs of any of the solutes tested at either temperature.
Effect of human serum on the influx of radio-labeled choline, sorbitol, and lactate in malaria-infected RBCs
. | Parasitemia-corrected NPPB-sensitive influx, mmol/(1012 cells/h) . | . | . | |
|---|---|---|---|---|
| Substrate . | Without serum . | With serum . | Student paired, 2-tailed t test . | |
| Choline | ||||
| 37°C | 0.23 ± 0.01 | 0.21 ± 0.01 | 0.33 | |
| RT | 0.064 ± 0.005 | 0.067 ± 0.008 | 0.50 | |
| Ratio37/RT | 3.6 | 3.1 | — | |
| Sorbitol | ||||
| 37°C | 2.3 ± 0.2 | 2.2 ± 0.2 | 0.25 | |
| RT | 0.69 ± 0.08 | 0.68 ± 0.05 | 0.94 | |
| Ratio37/RT | 3.3 | 3.2 | — | |
| Lactate | ||||
| RT | 57 ± 2 | 62 ± 1 | 0.19 | |
. | Parasitemia-corrected NPPB-sensitive influx, mmol/(1012 cells/h) . | . | . | |
|---|---|---|---|---|
| Substrate . | Without serum . | With serum . | Student paired, 2-tailed t test . | |
| Choline | ||||
| 37°C | 0.23 ± 0.01 | 0.21 ± 0.01 | 0.33 | |
| RT | 0.064 ± 0.005 | 0.067 ± 0.008 | 0.50 | |
| Ratio37/RT | 3.6 | 3.1 | — | |
| Sorbitol | ||||
| 37°C | 2.3 ± 0.2 | 2.2 ± 0.2 | 0.25 | |
| RT | 0.69 ± 0.08 | 0.68 ± 0.05 | 0.94 | |
| Ratio37/RT | 3.3 | 3.2 | — | |
| Lactate | ||||
| RT | 57 ± 2 | 62 ± 1 | 0.19 | |
Human serum was present at a concentration of ≥ 1% vol/vol, and the data are presented as the mean ± SEM (n = 3).
— indicates not applicable.
The effect of membrane depolarization on sorbitol transport via the NPP in P falciparum–infected RBCs. (A) Uptake of sorbitol into infected RBCs suspended in iso-osmotic NaCl (circles), sucrose (squares), and sorbitol (triangles) solutions in the absence (open symbols) and presence (closed symbols) of 0.2 mM NPPB. (B) Sorbitol influx (using a 60-second time point) via the NPP in infected RBCs (defined as the NPPB-sensitive influx) in iso-osmotic NaCl, sucrose, and sorbitol solutions. Data are corrected for 100% parasitemia. All values are calculated for an external sorbitol concentration of 1 mM for comparison with previous studies (assuming linear concentration dependence). Data represent averaged values derived from at least 3 separate experiments and are shown as the mean ± SEM.
The effect of membrane depolarization on sorbitol transport via the NPP in P falciparum–infected RBCs. (A) Uptake of sorbitol into infected RBCs suspended in iso-osmotic NaCl (circles), sucrose (squares), and sorbitol (triangles) solutions in the absence (open symbols) and presence (closed symbols) of 0.2 mM NPPB. (B) Sorbitol influx (using a 60-second time point) via the NPP in infected RBCs (defined as the NPPB-sensitive influx) in iso-osmotic NaCl, sucrose, and sorbitol solutions. Data are corrected for 100% parasitemia. All values are calculated for an external sorbitol concentration of 1 mM for comparison with previous studies (assuming linear concentration dependence). Data represent averaged values derived from at least 3 separate experiments and are shown as the mean ± SEM.
Table 2 shows the effect of the presence of BSA on the hemolysis rates of P falciparum–infected RBCs suspended in iso-osmotic solutions of sorbitol (note that sorbitol has no endogenous transport pathway in the RBC plasma membrane but is transported via the NPPs). The data are expressed as the inverse of the half-times of hemolysis of infected RBCs (ie, the inverse of the time taken for lysis, as defined by hemoglobin release, to reach half its maximum value), which provides a measure of the rate of solute influx.23 In this case, increasing the temperature from RT to 37°C resulted in faster hemolysis rates of infected RBCs suspended in iso-osmotic sorbitol solutions by greater than 2-fold. However, the presence of BSA had no significant effect on hemolysis rates at either temperature.
Effect of BSA on the hemolysis of malaria-infected RBCs in iso-osmotic sorbitol solution
. | 1/T50 (min-1) . | . | . | |
|---|---|---|---|---|
| Temperature, °C . | Without BSA . | With BSA . | Student paired, 2-tailed t test . | |
| 37 | 0.28 ± 0.03 | 0.30 ± 0.02 | 0.27 | |
| RT | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.11 | |
| Ratio37/RT | 2.4 | 2.3 | — | |
. | 1/T50 (min-1) . | . | . | |
|---|---|---|---|---|
| Temperature, °C . | Without BSA . | With BSA . | Student paired, 2-tailed t test . | |
| 37 | 0.28 ± 0.03 | 0.30 ± 0.02 | 0.27 | |
| RT | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.11 | |
| Ratio37/RT | 2.4 | 2.3 | — | |
BSA was present at a concentration of 0.5% wt/vol, and the data are presented as the mean ± SEM (n = 3).
— indicates not applicable.
Effect of hematocrit and cell lysate on the whole-cell conductance in malaria-infected RBCs
As serum/BSA had no effect on choline, sorbitol, or lactate transport in infected RBCs measured by radio-tracer and hemolysis protocols, it was hypothesized that the different experimental approaches used to study solute transport might not be comparable directly. Two significant differences between the protocols used for the patch-clamp technique on the one hand and the radio-tracer and hemolysis methodologies on the other hand are that the latter (1) entail the use of much higher hematocrits (leading to cell-cell contact) and (2) entail the presence of significant amounts of RBC lysate in the extracellular medium (as a consequence of the higher hematocrits, with the inevitable lysis of a small fraction of the RBCs, in the radio-tracer assays, and for obvious reasons during the hemolysis assays). In an effort to determine whether these differences in conditions could affect the data produced, the effect of adding RBCs and RBC lysate to the bath solution in whole-cell patch-clamp experiments was tested.
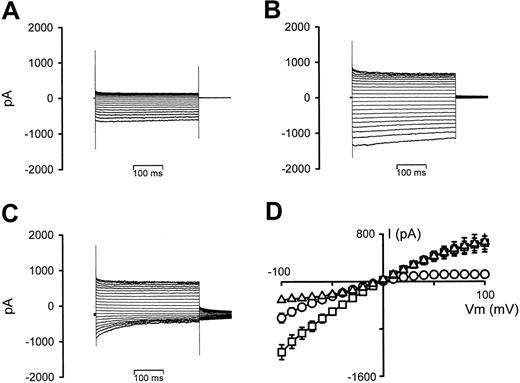
Figure 3 shows the effect on the whole-cell conductance in P falciparum–infected RBCs of increasing the bath hematocrit from 0.001% (typical for patch-clamp experiments) to 5% (typical for radio-tracer experiments). Prewashed infected RBCs held at 0 mV showed typical inwardly rectifying whole-cell conductances (Figure 3A). However, after the addition of washed RBCs (Figure 3B), there were increases in the currents at both negative and positive VT values (with a small degree of time-dependent current inactivation at negative VT values). Furthermore, changing the VH from 0 mV to –30 mV (to allow comparison of this effect with the previous report, in which serum/BSA is shown to affect the whole-cell conductance of infected RBCs15 ) resulted in outwardly rectifying conductances (because of strong time-dependent current inactivation at negative VT values), with additional large, decaying tail currents (Figure 3C). The I-V relationships from 6 experiments are averaged in Figure 3D. Similar results were also observed when using infected RBCs (> 86% parasitemia) in place of uninfected RBCs (data not shown).
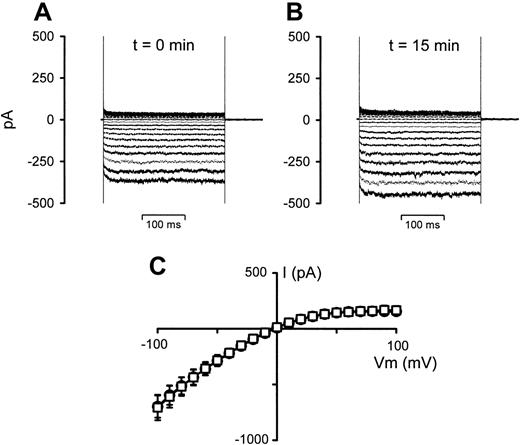
To test whether this effect was due to the presence of a small degree of cell lysate (even though the cells were washed directly prior to use) or to cell-cell contact, aliquots of the stock RBC suspensions used in the experiments presented in Figure 3 (left until after the initial experiments were concluded) were centrifuged, and the supernatant was removed and used in further whole-cell experiments. Figure 4 shows the effect of this supernatant on the whole-cell conductance in P falciparum–infected RBCs. It is clear from the data shown that the supernatant had little, if any, effect on the whole-cell conductances in infected RBCs.
As the previous experiments suggested that cell-cell contact affected whole-cell conductances in infected RBCs, the effect of lysate was tested using membrane-free samples (prepared by centrifuging lysed cells at 18 000g for 30 minutes and using only the very top fraction of the supernatant). The effect of membrane-free lysate on the whole-cell conductance in P falciparum–infected RBCs is shown in Figure 5 and is essentially identical to that observed by cell-cell contact.
The effect of increasing hematocrit on whole-cell current recordings in P falciparum–infected RBCs. (A) Current recording of an infected RBC, VH = 0 mV. (B) Current recording after increasing the hematocrit from 0.001% to 5%, VH = 0 mV. (C) Current recording after changing VH from 0 to –30 mV. (D) I-V curves derived from the data in panels A (○), B (□), and C (▵), averaged with 5 additional experiments. Current data are shown as the mean (SEM) and, where not shown, error bars lie within the symbols.
The effect of increasing hematocrit on whole-cell current recordings in P falciparum–infected RBCs. (A) Current recording of an infected RBC, VH = 0 mV. (B) Current recording after increasing the hematocrit from 0.001% to 5%, VH = 0 mV. (C) Current recording after changing VH from 0 to –30 mV. (D) I-V curves derived from the data in panels A (○), B (□), and C (▵), averaged with 5 additional experiments. Current data are shown as the mean (SEM) and, where not shown, error bars lie within the symbols.
The effect of RBC suspension supernatant on whole-cell current recordings in P falciparum–infected RBCs. (A) Current recording of an infected RBC, VH = 0 mV. (B) Current recording 15 minutes after addition of 0.4 mL supernatant to the 2-mL bath. (C) I-V curves derived from the data in panels A (○) and B(□), averaged with 5 additional experiments. Current data are shown as the mean (SEM) and, where not shown, error bars lie within the symbols.
The effect of RBC suspension supernatant on whole-cell current recordings in P falciparum–infected RBCs. (A) Current recording of an infected RBC, VH = 0 mV. (B) Current recording 15 minutes after addition of 0.4 mL supernatant to the 2-mL bath. (C) I-V curves derived from the data in panels A (○) and B(□), averaged with 5 additional experiments. Current data are shown as the mean (SEM) and, where not shown, error bars lie within the symbols.
Effect of BSA on the lysis rates of single malaria-infected RBCs
If, as the data presented in the previous section suggest, the conditions used during radio-tracer and hemolysis assays produce the same effect on anion conductance as the presence of serum/BSA (even though it is absent) in whole-cell patch-clamp experiments in infected RBCs, and, if this phenomenon also alters electroneutral solute transport, the only way to assess if there is an effect of serum/BSA on sorbitol transport in infected RBCs is to perform assays under identical conditions to those used during patch-clamp experiments. With this in mind, a single-cell hemolysis assay was developed (see “Materials and methods”).
The effect of membrane-free lysate on whole-cell current recordings in P falciparum–infected RBCs. (A) Current recording of an infected RBC, VH = 0 mV. (B) Current recording after the addition of 5% vol/vol membrane-free lysate, VH = 0 mV. (C) Current recording after changing VH from 0 to –30 mV. (D) I-V curves derived from the data in panels A (○), B (□), and C (▵), averaged with 5 additional experiments. Current data are shown as the mean (SEM) and, where not shown, error bars lie within the symbols.
The effect of membrane-free lysate on whole-cell current recordings in P falciparum–infected RBCs. (A) Current recording of an infected RBC, VH = 0 mV. (B) Current recording after the addition of 5% vol/vol membrane-free lysate, VH = 0 mV. (C) Current recording after changing VH from 0 to –30 mV. (D) I-V curves derived from the data in panels A (○), B (□), and C (▵), averaged with 5 additional experiments. Current data are shown as the mean (SEM) and, where not shown, error bars lie within the symbols.
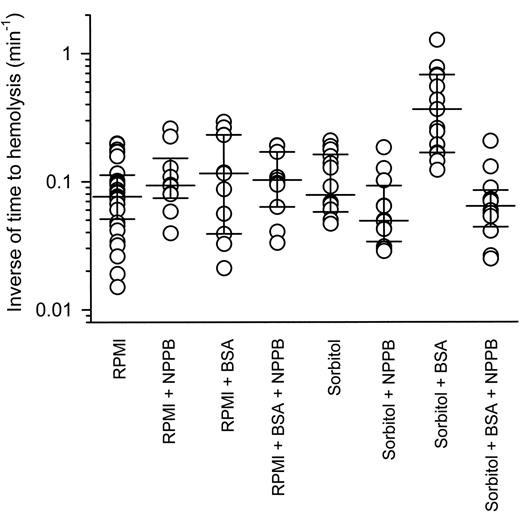
Figure 6 shows the inverse of lysis times for individual P falciparum–infected RBCs sealed to patch pipettes (under patch-clamp conditions) and then suspended in either serum-free RPMI 1640 or iso-osmotic sorbitol solutions (in the presence and absence of 0.5% wt/vol BSA and/or 0.2 mM NPPB). Single infected RBCs suspended from patch pipettes in serum-free RPMI 1640 medium lasted for between 5 and 67 minutes before lysis, although the majority (75%) lasted for fewer than 20 minutes. The average inverse of lysis time was 0.076 (0.057-0.105) minute–1 (median [interquartile range]; n = 32). The presence of BSA and/or NPPB had no significant effect (P > .05, Kruskal-Wallis with Dunn post test).
Infected RBCs suspended in iso-osmotic sorbitol solution lysed with an average inverse of lysis time of 0.078 (0.062-0.153) minute–1 (median [interquartile range]; n = 14), which was not significantly different from cells suspended in RPMI 1640 medium (P > .05, Kruskal-Wallis with Dunn post test). The presence of NPPB seemed to decrease average inverse of lysis times to 0.049 (0.039-0.074) minute–1 (median [interquartile range]; n = 12), but this was not found to be significant compared with cells suspended in iso-osmotic sorbitol solution in the absence of NPPB (P > .05, Kruskal-Wallis with Dunn post test). Infected RBCs suspended in iso-osmotic sorbitol solution lysed faster (∼ 5-fold) in the presence of BSA, with an average inverse of lysis time of 0.37 (0.18-0.67) minute–1 (median [interquartile range]; n = 15), whereas the presence of NPPB decreased the inverse of lysis times back to control levels, with average inverse of lysis times of 0.064 (0.050-0.077) minute–1 (median [interquartile range]; n = 12). The lysis times for infected RBCs suspended in iso-osmotic sorbitol solution in the presence of BSA were significantly faster than cells suspended in iso-osmotic sorbitol solution alone, in iso-osmotic sorbitol solution in the presence of NPPB, in iso-osmotic sorbitol solution in the presence of BSA and NPPB, or in RPMI 1640 medium (P < .05, Kruskal-Wallis with Dunn post test).
The effect of BSA on the lysis time of single P falciparum–infected RBCs. Single infected RBCs were suspended in either serum-free RPMI or iso-osmotic sorbitol solutions in the absence and presence of 0.5% wt/vol BSA and/or 0.2 mM NPPB. Data points represent the inverse of lysis time from single experiments and for each condition the median inverse of lysis time point and 25th and 75th percentile points have been plotted.
The effect of BSA on the lysis time of single P falciparum–infected RBCs. Single infected RBCs were suspended in either serum-free RPMI or iso-osmotic sorbitol solutions in the absence and presence of 0.5% wt/vol BSA and/or 0.2 mM NPPB. Data points represent the inverse of lysis time from single experiments and for each condition the median inverse of lysis time point and 25th and 75th percentile points have been plotted.
Discussion
Are electoneutral solutes using anion-selective channels with a low open-state probability at positive Vm values?
Several electrophysiologic studies have yielded data consistent with the hypothesis that the NPPs are formed from a single anion-selective channel type, having a low open-state probability at positive Vm values.3-5 Egée et al5 reported that the open-state probability of a malarial parasite-induced anion channel is 0.3 at –10 mV and less than 0.1 above +10 mV. Similar values have also been reported for an infection-induced anion channel in Plasmodium knowlesi–infected monkey RBCs.24 Therefore, if electroneutral solutes also use this type of channel (as would be the case if the NPPs are formed by this channel type alone), their transport would be expected to reduce by some degree after membrane depolarization (because of the decreased open-state probability of the channels).
Here, the transport of the electroneutral solute sorbitol via the NPPs in infected RBCs (in conditions that maintain a physiologic Vm negative of 0 mV) is consistent with previous reports (see “Are the data comparable between transport measurement techniques and with data reported previously?”). However, rather than decreasing, sorbitol transport increased slightly after depolarizing the membranes of infected RBCs, with the use of low ionic-strength iso-osmotic sucrose and sorbitol solutions. Duranton et al8 reported that the presence of sucrose inhibits sorbitol uptake in P falciparum–infected RBCs. This is in contrast to the data presented here, although analysis of their sorbitol uptake time-course data suggests uptake is nearly 10 times higher. Given the concordance between the uptake data presented here and previous reports (see “Are the data comparable between transport measurement techniques and with data reported previously?”), it is difficult to rationalize what was actually measured during their study. Nevertheless, the results of the flux experiments imply that sorbitol is not permeating infected RBCs by a channel type with a low open-state probability at positive Vm values. Nor is it permeating via the previously reported NPPB-sensitive solute transporter induced in uninfected RBCs suspended in low ionic-strength media, because this pathway is not permeable to sorbitol.25 It could be argued that other factors, which are altered by suspending infected RBCs in low ionic-strength media, could also affect channel gating. However, other homeostatic variables such as relative cell volume and pHi (see Figure 1) have only low levels of predicted change over the 1-minute duration of the influx experiments presented in Figure 2B.
Are the data comparable between transport measurement techniques and with data reported previously?
The data reported here show that both cell-cell contact and the presence of cell lysate have similar effects to the addition of serum/BSA on whole-cell conductances in P falciparum–infected RBCs (increasing outward currents by 4- to 5-fold and increasing inward currents by approximately 2-fold, with the latter being inactivated by the imposition of negative VH values in a time-dependent manner15 ). These 2 conditions increase the number of different methods that can be used to induce this type of altered whole-cell conductance in infected RBCs but do little to shed any light on the mechanism of action. The fact that heat-treated (to 100°C) serum samples lose their ability to cause this effect suggests that a protein interaction with infected RBCs might be involved.15 The addition of 2 new modulators not containing serum albumin (the common link between the agonists reported previously) suggests that any protein interaction is likely to be more general.
Interestingly, the effects of cell-cell contact and lysate raise questions about the practicality of comparing transport data determined by different methodologies directly. As the hematocrit and levels of lysate present in radio-tracer and hemolysis experiments are far higher than in patch-clamp experiments, the data reported here suggest that the conditions used during the former produce the same effect that the presence of serum/BSA has during whole-cell patch-clamp studies in infected RBCs and support the idea that during radio-tracer and hemolysis assays an effect of serum is not observed because the same effect has already been produced by the experimental conditions. This possibility is further substantiated by the results of the single-cell hemolysis experiments, in which sorbitol transport was affected by the presence of BSA.
The sorbitol lysis data shown in Table 2 are very similar to data presented in an early study by Ginsburg et al,26 in which they reported a T50 value of 10.5 minutes (giving a 1/T50 value of 0.10 minutes–1) for sorbitol-induced infected RBC lysis measured at 22°C and an enthalpy of activation of 10 kcal mol–1 (equivalent to a transport ratio37/22 of 2.39 ). Similar values to those shown here have also been reported since then.1,6 In addition, the sorbitol influx data shown here are similar to data in a previous study. Kirk and Horner18 reported a sorbitol influx value in infected RBCs of approximately 0.70 mmol/(1012 cells/hour) measured at RT in the presence of an external sorbitol concentration of 1 mM, which was inhibited by the NPP blocker furosemide. The choline and lactate influx data are also similar to data in previous reports1,19,20 and taken together are in concordance with the previously reported selectivity9,27 of the NPPs (where sorbitol is 10 times more permeable to the NPPs than choline and where lactate is 100 times more permeable to the NPPs than sorbitol).
There is one obvious difference between the radio-tracer and hemolysis data reported here. Although decreasing the temperature from 37°C to RT during hemolysis experiments decreases hemolysis half-times by just greater than 2-fold, the same change in radio-tracer experiments decreases transport by just greater than 3-fold (for both sorbitol and choline here and for K+ [86Rb+] in a previous report28 ). Because radio-tracer measurements are a more direct way to estimate transport than hemolysis experiments, this suggests that the latter underestimates the effect of temperature on the activity of the NPPs (supporting the semiquantitative nature of hemolysis assays27 ).
It is also possible to compare the data from radio-tracer experiments with the data from patch-clamp experiments by converting the current measurements into influx values. This is performed, as reported previously,9 by dividing the single-cell current at the physiologic Vm by Faraday constant, correcting for the units required for comparison (in this case changing mol/[cell/second] to mmol/[1012 cells/hour]) and correcting for the ion concentration used (in this case 160 mM Cl–). Using the data presented in Figures 3, 4, and 5, the current measured at –10 mV (slightly higher than the predicted Vm values) in infected RBCs in the whole-cell configuration before and after RBCs or lysate were added to the bath are approximately 40 and 80 pA, respectively. This equates to Cl– influx values of approximately 10 and 20 mmol/(1012 cells/hour), respectively, for an external Cl– concentration of 1 mM measured at RT. These values are 3 to 6 times lower than the lactate influx data reported here and, because Cl– is twice as permeable to the NPPs as lactate (when measured by either the radio-tracer1,9 or patch-clamp technique4,8 ), 6 to 12 times lower than total Cl– transport. As the patch-clamp technique only measures electrogenic transport, this difference in measured rates could be explained by electroneutral Cl– transport (radio-tracer methodologies measure both electrogenic and electroneutral transport). However, this would require the presence of an anion exchange mechanism to account for the additional electroneutral Cl– transport.9
Which model for electroneutral transport best fits the data reported here?
A number of transport models have been suggested for the NPPs.14 As mentioned in “Results”, 2 groups3-6 have proposed a single channel model (for the transport of all solutes). The data reported here are not consistent with electroneutral solute transport occurring solely via a channel with a low open-state probability at positive Vm values (unless the channel has an unusual gating system, whereby the channel is closed to anions but not to electroneutral solutes at high positive Vm values). The conductance of anions via either of the single channel types proposed to form the NPPs is also at odds with the total movement of anions (as measured by radio-tracer techniques) unless they have both net and exchange modes of operation.9 However, the data could be explained by a single channel type (with an exchange mode) that is modified in the presence of serum/BSA to alter its functional characteristics (as suggested by Thomas and Lew29 ).
Interestingly, the 5-fold change in the average lysis time of single infected RBCs suspended in iso-osmotic sorbitol solution caused by the presence of BSA is directly comparable with the 4- to 5-fold effect serum/BSA has on whole-cell currents at positive Vm values15 observed in whole-cell patch-clamp experiments (note that during hemolysis assays the cells are suspended in iso-osmotic sorbitol solution, resulting in positive Vm values). Unfortunately, as infected RBCs were not particularly stable when suspended from a pipette (as shown by the fact that the RBCs lyse on average below 20 minutes when suspended in RPMI 1640 medium), the possibility that sorbitol transport does not occur in the absence of BSA in single-cell hemolysis assays cannot be ruled out. The suspension of cells from a pipette may also explain the short lysis times for single cells placed in iso-osmotic sorbitol solution in the presence of BSA (with a median inverse of lysis time of 0.37 minute–1) compared with the lysis profiles of infected RBCs in the same conditions in standard hemolysis assays (with a mean 1/T50 value of 0.13 minute–1).
Having originally proposed a single channel model for the NPPs, the group of Bouyer et al30 has recently reported evidence for the presence of 3 novel anion channels on the host's plasma membrane in P falciparum–infected RBCs. Huber et al13 have reported evidence for at least 3 channel types underlying the NPPs: endogenous ClC-2 channels,10 a nonspecific cation channel,7 and an anion-selective channel type, which produces outwardly rectifying whole-cell currents and which is also permeable to electroneutral solutes.8,31 In addition, Ginsburg and Stein2,9 have suggested that there are 2 types of pathways underlying the NPPs: a high-copy number pathway, which mediates the transport of anions and nucleosides predominantly, and a low-copy number pathway, which mediates the transport of all other solutes. All 3 of these models could accommodate the data reported here, but further questions will need to be addressed before the correct model, if any, is ascertained (eg, models based around anion channels will need to account for the high solute uptakes, measured by using radio-tracer techniques9 ).
Summary
The results of the present study are not consistent with the transport of both anions and electoneutral solutes (in this case sorbitol) via a single channel type with a low open-state probability at positive Vm values. In addition, like whole-cell conductances,15 sorbitol transport in P falciparum–infected RBCs was shown to be sensitive to serum/BSA, suggesting a common pathway or regulator for both electrogenic and electroneutral solutes. However, it was not possible to extrapolate any further information about the nature and number of pathways that underlie the NPPs.
Prepublished online as Blood First Edition Paper, July 13, 2006; DOI 10.1182/blood-2006-02-001693.
Supported by the Wellcome Trust, United Kingdom (grants 071662 and 076441). H.M.S. is a Wellcome Trust Research Career Development Fellow (sponsor S. Krishna, St. George's, University of London).
H.M.S. designed the research, performed the research, analyzed the data, and wrote the paper; S.A., H.F., and J.M. performed the research and analyzed the data; T.P. and J.C.E. designed the research and analyzed the data.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Prof K. Kirk for advice while preparing this manuscript, Dr V. L. Lew for help with the modeling, and Mrs V. Harris for her assistance with the parasite culture.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal