Abstract
Ras-related Rho GTPases regulate actin cytoskeletal organization, adhesion, gene transcription, and cell-cycle progression. The Rac subfamily of Rho GTPases and Cdc42 has been shown to play essential roles in hematopoietic stem cell (HSC) engraftment and mobilization. Here, we study the role of RhoA, a related Rho GTPase, in HSC functions. Using retrovirus-mediated gene transfer of a dominant-negative (DN) mutant of RhoA (RhoAN19), we demonstrate that down-regulation of RhoA activity resulted in increased HSC engraftment and self-renewal as measured by competitive repopulation and serial transplantation assays. However, overexpression of RhoAN19 resulted in decreased migration toward SDF-1α and α4β1- and α5β2-integrin–mediated adhesion of hematopoietic progenitor cells in vitro. Low RhoA activity was associated with higher proliferation rate of hematopoietic progenitor cells and increased cells in active phases of cell cycle, most likely via decreasing p21Cip/Waf expression and increasing cyclin D1 levels. Thus, reducing RhoA activity by optimizing the balance between adhesion/migration and proliferation/self-renewal results in a net increase in HSC engraftment. This mechanism could provide a novel therapeutic target to enhance HSC therapies.
Introduction
Hematopoiesis is sustained by proliferation and differentiation of a defined pool of hematopoietic stem cells (HSCs). HSCs reside in the bone marrow of adult animals in contact with components of the hematopoietic microenvironment (HM), including cells and extracellular matrix.1 Cell-cell and cell-matrix interactions within the HM regulate HSC maintenance, proliferation, and differentiation.2 Interruption of these interactions affects retention of HSCs in the bone marrow space, leading to trafficking of these cells to the circulation, a process termed “mobilization.”3 In contrast, on transplantation, HSCs migrate from the blood into the bone marrow cavity and home to a specific site termed the “stem cell niche” proposed to be adjacent to the endosteum.4,5 Disruption of migration or adhesion of HSCs results in impaired localization of these cells and defective interaction with the HM. Using a variety of experimental approaches, α4/β1 and α5/β1 integrins,6-9 CXCR4, a chemokine receptor,10-12 and c-kit, a receptor tyrosine kinase,13,14 have been shown to be important for the HSC engraftment. Understanding the molecular mechanism of signal transduction of these surface receptors may provide important insights into the regulation and maintenance of HSCs in the HM and reveal novel targets to enhance their function.
Rho GTPases are Ras-like molecules that integrate signal transduction pathways linking multiple cell-surface receptors to a variety of intracellular signaling proteins.15,16 Rho GTPases are molecular switches that cycle between an inactive, GDP-bound form and an active, GTP-bound form. Rho GTPase activities are regulated by guanosine exchange factors (GEFs) and GTPase-activating proteins (GAPs) which stimulate GTP loading and accelerate GTP hydrolysis, respectively.17 The GTP-bound Rho GTPases interact with a variety of effectors to control actin cytoskeleton reorganization, cell shape, and cell polarity. To date, the best studied Rho GTPases include RhoA, Rac, and Cdc42. The Rac subfamily of GTPases includes Rac1, Rac2, and Rac3, all highly related at the amino acid sequence level. Studies performed in fibroblasts have shown that RhoA regulates cell shape and adhesion by the assembly of contractile actin microfilaments into stress fibers.18 The related Rac GTPases and Cdc42 regulate the polymerization of actin to form peripheral lamellipodial and filopodial protrusions.18
Rho GTPases also regulate proliferation and cell-cycle progression, especially G1/S transition. Rac1 and Cdc42 regulate the G1/S transition through a number of different mechanisms, both dependent and independent on the mitogen-activated protein kinase (MAPK) pathway.19,20 In fibroblasts, Rac1 can induce expression of cyclin D120 and together with Cdc42 can stimulate Rb phosphorylation.21 The effect of RhoA on cell-cycle progression and proliferation is less clear. In fibroblasts, activation of RhoA has been reported to decrease the expression of Cdk inhibitors and shorten G1.22 Using the same cell types, inactivation of RhoA has been shown to induce the expression of cyclin D–Cdk4 complexes in early G1 phase and promote a rapid G1/S transition.23,24 In mammary gland epithelial cells, TGF-β–induced activation of RhoA stimulates the nuclear translocation of p160 ROCK, a known target of RhoA, which results in cell-cycle arrest by decreasing the activity of Cdc25A phosphatase and decreasing Rb phosphorylation.25 Therefore, the effect of RhoA GTPase activity on cell cycle and proliferation appears both cell-type and agonist specific.
Rho GTPase proteins have been implicated in regulation of a variety of functions in hematopoietic cells. The use of gene-targeted mouse mutants for the study of the role(s) of Rho GTPases in primary cells has provided significant insight into GTPase biology. Gene targeting experiments have shown that Rac1 and Rac2 regulate in distinct and overlapping fashion proliferation, survival, adhesion, and migration of multiple hematopoietic lineages, including HSCs.26-33 Using a genetic approach, Rac2 has been shown to regulate apoptosis via the phosphoinositide 3-kinase (PI3K)/protein-kinase B (Akt/PKB) pathway,32,34 whereas Rac1 predominantly controls cell-cycle progression by regulating the MAPK/cyclin D1 pathway.32 Loss of Rac1 activity by gene deletion prior to transplantation of HSCs is associated with impaired engraftment. Loss of both Rac1 and Rac2 after engraftment leads to massive mobilization of HSCs into the blood likely because of markedly reduced retention of these cells in the HM as a result of the combined interruption of β1 integrin, CXCR4, and c-kit signaling pathways.26,32 Consistent with these in vivo data, hematopoietic stem and progenitor (HSC/P) cells that lack expression of Rac1 or both Rac1 and Rac2 show decreased adhesion via α4β1 and α5β1 integrin in vitro and display markedly decreased migration toward SDF-1α in transwell migration assays.32 In agreement with these data, expression of patient-derived dominant-negative (DN) mutant of Rac2 (Rac2D57N) in murine HSCs resulted in decreased hematopoietic reconstitution of irradiated recipient mice.35
More recently, Cdc42 has also been implicated in the regulation of essential HSC functions.36 Mice demonstrating abnormal activation of Cdc42 because of genetic deletion of Cdc42GAP (a negative regulator of Cdc42) display anemia and reduced erythroid progenitors, including erythroid blast-forming units and erythroid colony-forming units in the bone marrow. HSC/P cells, defined as lineage-negative (lin–) and ckit-positive (ckit+) cells, from these mice showed decreased survival in vitro and have impaired adhesion to fibronectin and reduced SDF-1α–directed migration in vitro. These in vitro defects were associated with decreased engraftment potential of HSCs isolated from Cdc42GAP-deficient mice.
In this study, we analyzed the role of RhoA GTPase in hematopoietic stem and progenitor cell functions by expressing a DN mutant RhoAN19 in HSC/P cells via retrovirus-mediated gene transfer. Inhibition of RhoA GTPase activity led to reduced migration and adhesion of hematopoietic progenitor cells in vitro. Despite this in vitro phenotype of decreased migration and adhesion, reduced RhoA activity was associated with significant enhancement of HSC engraftment and reconstitution in vivo. Increased engraftment of HSCs expressing RhoAN19 was associated with increased cyclin D1 expression and enhanced proliferation and cell-cycle progression of hematopoietic progenitor cells in vitro. Our data show that RhoA GTPase plays a crucial role in HSC engraftment. In the context of previous reports describing Rac GTPase function in HSCs,26,32 these studies suggest that inhibition in Rac activity may enhance mobilization, whereas inhibition of RhoA may augment HSC engraftment. Thus, Rho GTPases provide novel molecular targets to enhance stem cell therapies.
Materials and methods
Retroviral vectors and supernatant
MSCV-based bicistronic retroviral vectors were used to express DN mutants of either Rho GTPase (RhoAN19)37 or Rac GTPase (Rac2D57N).38 The DN mutant proteins were fused in frame at the N-terminus with either 3 hemagglutinin (HA) tandem repeats (RhoAN19) or a flag peptide (Rac2D57N). The transgene expression was linked with enhanced green fluorescent protein (EGFP) expression via an internal ribosome entry site 2 (IRES2) (Figure 1A). The plasmid DNA was used to generate viral supernatant from Phoenix-gp cells as previously described.39 Briefly, Phoenix-gp cells were grown to 70% confluence in a 9-cm tissue-culture dish (Corning, Corning, NY). Eight micrograms of plasmid DNA of interest, 10 μg Moloney leukemia virus (MLV) gag-pol plasmid, and 3 μg ecotropic envelope plasmid were cotransfected using a calcium phosphate transfection kit (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. Eight milliliters of viral supernatant was collected every 12 hours and stored at –80°C until used.
Mouse bone marrow cell culture and retrovirus-mediated transduction
Retrovirus-mediated transduction of bone marrow cells was performed as described.40 Briefly, C57BL/6J mice were injected with 150 mg 5 fluorouracil/kg body weight (American Pharmaceutical Partners, Schaumburg, IL), and low-density mononuclear bone marrow (LDBM) cells were collected 2 days later using Histopaque 1083 (Sigma, St Louis, MO). The cells were cultured for 2 days in Iscove medium (Cellgro, Herndon, VA) supplemented with 10% fetal calf serum (FCS; Tissue Culture Biologicals, Tulare, CA), 100 U/mL penicillin and streptomycin (Cellgro), 1% l-glutamine (Cellgro) (complete medium) and recombinant rat stem cell factor (rrSCF), recombinant human megakaryocyte growth and differentiating factor (rhMGDF), and recombinant human granulocyte-colony stimulating factor (rhG-CSF) all at 100 ng/mL (SMG; all from Amgen, Thousand Oaks, CA). Bone marrow cells were then incubated on recombinant fibronectin, CH296 (Retronectin; Takara Bio, Otsu, Japan) with high-titer viral supernatant in the continued presence of SMG. After 6 hours, the cells were spun down, and the medium was replaced with fresh complete medium. The same procedure was repeated on day 4. For in vivo experiments, 2 days after the second transduction, cells were harvested and sorted for EGFP expression using a fluorescence-activated cell sorter (FACS VantageSEDiVa; Becton Dickinson, San Jose, CA). For in vitro adhesion and migration, proliferation, and cell-cycle progression experiments, 2 days after the second transduction, cells were harvested and stained with phycoerythrin (PE)–conjugated anti–mouse CD117 (c-kit; PharMingen, San Jose, CA), and EGFP+ckit+ cells were sorted by FACS.
Analysis of hematopoiesis
To determine the hematopoietic progenitor content of transduced and sorted cells, colony-forming unit assay was performed as previously described.35 Briefly, 2 × 103 EGFP+ cells were plated in triplicate in methylcellulose (MethocultM3100; StemCell Technology, Vancouver, BC, Canada) in the presence of 10% FCS, 100 ng/mL rrSCF, 20 ng/mL interleukin 3 (IL-3) (PeproTech, Rocky Hill, NJ), 4 U/mL erythropoietin (Amgen). Colonies derived from colony-forming unit (CFU) cells were enumerated 7 days later.
For reconstitution experiments, 2.8 × 105 EGFP+ cells were mixed with 1.2 × 105 freshly isolated total bone marrow cells as competitors and transplanted via lateral tail vein into lethally irradiated (11 Gy [1100 rad], split dose 3 hours apart, using a 137Cs irradiator, Mark-I-68; J. L. Shepherd and Associates, San Fernando, CA) C57BL/6J recipient mice.
To examine hematopoietic reconstitution of mice that received a transplant, chimerism was analyzed by both percentage EGFP+ cells in the peripheral-blood leukocytes as well as percentage of EGFP+ CFU cells in the bone marrow of transplant recipients. To determine the differentiation potential of transduced and engrafted HSCs, peripheral-blood samples were subject to red cell lysis by incubation with ammonium chloride solution (PharMingen) for 10 minutes at room temperature (RT). The remaining cells were then washed once with phosphate-buffered saline (PBS) and incubated with 0.5 μg/100μL anti-B220/CD45R, anti-CD3ϵ, or anti-Gr1, respectively (all PE conjugated; PharMingen) for 15 minutes at RT. After the incubation, cells were washed once with PBS and resuspended in PBS with 3 μg/mL 7-aminoactinomycin D (7-AAD; Molecular Probes, Eugene, OR) and then analyzed for the coexpression of EGFP and lymphoid (B220/CD45R or CD3) or myeloid (Gr1) markers in the viable leukocyte gate (7-AAD negative).
To examine the effect of transgene expression on the stem cell compartment, serial transplantation was performed. In these studies, bone marrow from primary recipient mice were harvested at 16 weeks after transplantation, and 5 × 106 total bone marrow cells from these mice were injected via tail vein into lethally irradiated C57BL/6J secondary recipients. Four months after the secondary transplantation, 5 × 106 total bone marrow cells from the secondary recipients were injected via tail vein into lethally irradiated C57BL/6J tertiary recipients.
Migration and adhesion
The studies of the adhesion and migration of hematopoietic progenitor cells were performed as previously described.7,32 Briefly, for adhesion, nontissue-culture 6-well plates (BD Labware, Franklin Lakes, NJ) were coated with FN fragments (H-296, which contains both the integrin α4β1-binding site and the high-affinity heparin-binding site, and CH-271, which contains the integrin α5β1-binding site and the high-affinity heparin-binding site7 ) at 8 μg/cm2 or bovine serum albumin (BSA; Roche, Indianapolis, IN) overnight at 4°C. The plates were subsequently blocked with 2% BSA for 30 minutes at RT. A total of 1 × 105 EGFP+ckit+ cells (with a frequency of 34 ± 6 CFU/103 cells) in Roswell Park Memorial Institute (RPMI) 1640 medium (Cellgro) containing 10% FCS were then allowed to adhere to the coated plates for 1 hour at 37°C. Nonadherent cells were collected by gently rinsing with medium. Adherent cells were harvested by incubation with cell dissociation buffer (Gibco, Carlsbad, CA) and vigorously rinsing the plates with PBS. The cells were then plated in CFU assay.
For directed migration (chemotaxis), 100 μL serum-free chemotaxis buffer (RPMI 1640 medium, 0.5% crystallized deionized BSA) containing 2 × 105 EGFP+ckit+ cells (with a frequency of 34 ± 6 CFU/103 cells) was added to the upper chamber of a transwell culture plate containing 5-μm pore size filter (Costar, Cambridge, MA), and 0.6 mL serum-free chemotaxis buffer with 100 ng/mL SDF-1α (PeproTech) was added to the lower chamber. After 4 hours of incubation at 37°C in 5% CO2, the upper chamber was carefully removed, and the cells in the bottom chamber were plated in CFU assay as described in “Analysis of hematopoiesis.”
Proliferation and cell cycle
[H3]Thymidine uptake assay was used to measure proliferation of hematopoietic cells in vitro as described by Gu et al.35 Briefly, EGFP+ckit+-sorted cells were cultured overnight in complete medium with SMG and then starved for 6 hours in IMDM containing 1% FCS. The starved cells were resuspended in complete medium with either no cytokines or 100 ng/mL rrSCF or SMG and plated in 6 replicates per condition in 96-well plates with 5 × 104 cells per well. Forty-two hours later, the cells were pulsed with 1 μCi (0.037 MBq) [3H]thymidine/well (Perkin Elmer Life Science, Shelton, CT) for 6 hours and then harvested on a glass fiber filter (Packard Instrument, Boston, MA) using a cell harvester (Packard Instrument). The retained radioactivity on the filter was counted using a scintillation counter (Beckman/Coulter, Brea, CA).
For cell-cycle analysis, the starved cells were cultured for 9 hours in complete medium with no cytokines or 100 ng/mL rrSCF or SMG. At the end of the culture period, cells were stained with propidium iodide (PI) for cell-cycle analysis. For PI staining, 1 × 105 cells were incubated with 0.1 mg/mL PI, 0.6% NP40, 2 mg/mL RNaseA (Invitrogen) in PBS for 30 minutes on ice and then analyzed using flow cytometry and Modfit software (Verity Software House, Topsham, ME).
Immunoblot and RhoGTPase effector pull-down assay
The EGFP+ fraction of transduced bone marrow cells was sorted, and 1 × 105 cells were washed once with PBS and incubated with cell lysis buffer for 30 minutes on ice. The lysate was cleared by centrifugation for 30 minutes at more than 12 000g in a refrigerated centrifuge (Allegra TMX-22R; Beckman Coulter, Palo Alto, CA) and incubated with Laemmli Sample Buffer (Bio-Rad Laboratories, Hercules, CA) for 5 minutes at 95°C. Aliquots of cell lysates were then subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) separation, and the immunoblots were probed with anti-HA antibody (1:1000; Roche Applied Bioscience) for RhoAN19 and anti-flag antibody (1:1000; Sigma) for Rac2D57N.
The specific Rho GTPase activity after expression of the DN mutants was measured by either Rhothekin or Pak effector pull-down assays. Briefly, for Rac the assay was performed using GST-Rac binding domain of Pak, and for RhoA the GST-Rothekin binding domain was used (Upstate, Waltham, MA) following the manufacturer's protocol. The level of GTP-bound GTPase was analyzed by immunoblot using anti-Rac antibody (1:1000; Upstate) and anti-RhoA antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), respectively.
To examine the effect of DN GTPase on proteins known to regulate cell cycle, transduced EGFP+ckit+ bone marrow cells were starved for 6 hours in IMDM 1% FCS and subsequently stimulated for 9 hours with complete medium and 100 ng/mL SCF. The cell pellet was washed with ice-cold PBS and then incubated with cell lysis buffer for 30 minutes on ice. The lysates were cleared as described and incubated with Laemmli Sample Buffer for 5 minutes at 95°C. Aliquots of cell lysates were subjected to SDS-PAGE and immunoblotted with specific polyclonal antibody against p21 (1:200; Santa Cruz Biotechnology), cyclin D1 (1:1000; Cell Signal, Beverly, MA), and specific monoclonal antibody anti–β-actin (1:10 000; Sigma).
Statistical analysis
Statistical analysis was performed using 2-tailed unpaired Student t test. Values of P less than .05 were considered statistically significant.
Results
Expression and function of RhoAN19 and Rac2D57N
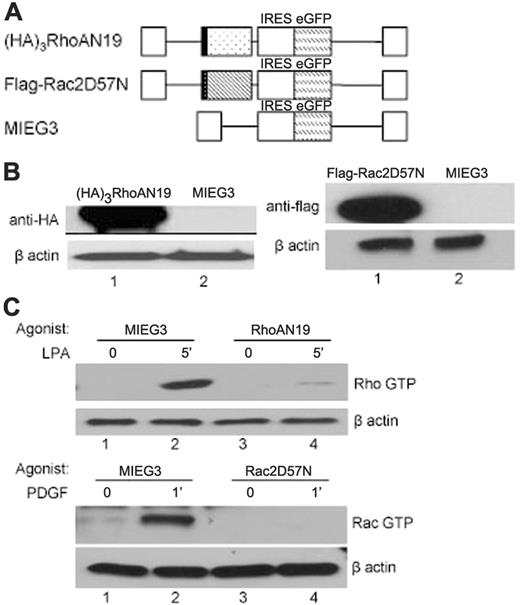
To analyze the roles of RhoA GTPase in HSC/P cells, we transduced LDBM cells with bicistronic MSCV-based retroviral vectors expressing EGFP and HA-tagged DN RhoA (RhoAN19) (Figure 1A). As a negative control, an empty vector (MIEG3) expressing EGFP alone was used, and, as an additional control for experimental methods, we used a retroviral vector expressing EGFP and flag-tagged DN Rac (Rac2D57N), which we have previously characterized in engraftment assays.35 Transduced bone marrow cells were sorted for EGFP and immunoblotted with either anti-HA or anti-flag antibody to confirm the expression of each DN Rho GTPase protein (Figure 1B). The DN function of each mutant was confirmed in EGFP+ cells by effector pull-down assays (Figure 1C). On serum starvation, the level of GTP-bound Rho GTPase was very low in all 3 groups (MIEG3, RhoAN19, and Rac2D57N). LPA, a RhoA-specific agonist, induces significant increase in RhoA GTP levels in control cells, and this effect was significantly blocked by expression of RhoAN19 (Figure 1C, top). Stimulation with PDGF for 1 minute induced a robust increase in Rac GTP levels in MIEG3 cells, and this effect was completely blocked by Rac2D57N (Figure 1C, bottom). In conclusion, the activities of RhoA and Rac GTPases were effectively inhibited in primary bone marrow cells by retroviral-mediated expression of the respective DN mutant.
Retroviral vectors, transgene expression, and function. (A) Bicistronic MSCV-based retroviral vectors expressing EGFP and (HA)3RhoAN19 (▦), or Flag-Rac2D57N (▧). IRES indicates internal ribosome entry site. (B) Immunoblot analysis of transgene expression in the low-density bone marrow transduced cells probed for HA-tag (left), flag-tag (right), or β-actin (loading control). The blots are representative of 3 independent experiments with similar results. (C) Rhothekin/Pak pull-down assays to quantitate active, GTP-bound RhoA (Rho GTP), and Rac (Rac GTP) after specific agonist treatment. The level of β-actin is shown below as loading control. PDGF indicates platelet-derived growth factor; LPA, lysophosphatidic acid. The result is representative of 3 independent experiments.
Retroviral vectors, transgene expression, and function. (A) Bicistronic MSCV-based retroviral vectors expressing EGFP and (HA)3RhoAN19 (▦), or Flag-Rac2D57N (▧). IRES indicates internal ribosome entry site. (B) Immunoblot analysis of transgene expression in the low-density bone marrow transduced cells probed for HA-tag (left), flag-tag (right), or β-actin (loading control). The blots are representative of 3 independent experiments with similar results. (C) Rhothekin/Pak pull-down assays to quantitate active, GTP-bound RhoA (Rho GTP), and Rac (Rac GTP) after specific agonist treatment. The level of β-actin is shown below as loading control. PDGF indicates platelet-derived growth factor; LPA, lysophosphatidic acid. The result is representative of 3 independent experiments.
Expression of RhoAN19 augments engraftment of HSCs
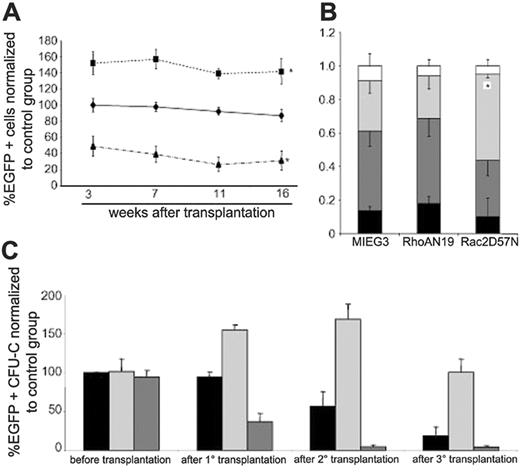
To determine the effect of decreased activity of RhoA on the reconstitution capacity of hematopoietic stem cells, transduced and EGFP+ cells were transplanted together with freshly isolated bone marrow cells as competitors into lethally irradiated C57Bl/6J mice. The mice that received a transplant were bled monthly, and the percentage of EGFP+ cells was measured by flow cytometry to determine the contribution of Rho GTPase-mutant transduced cells to the peripheral-blood chimerism. As shown in Figure 2A, in 3 independent experiments, beginning at 3 weeks after transplantation the normalized percentage of EGFP+ leukocytes in peripheral blood of mice that received a transplant with bone marrow cells expressing RhoAN19 was significantly increased (152% ± 14%) compared with the control group (P < .05). This difference was maintained during the remainder of the initial transplantation period with a peak at 16 weeks after transplantation (142% ± 15% versus 87% ± 7%, RhoAN19 versus vector control, P < .05). As expected based on previous reports,35 expression of Rac2D57N resulted in a significantly lower level of EGFP+ leukocytes in the peripheral blood of recipients at 3 weeks after transplantation (49% ± 12%, P < .05). The chimerism of Rac2D57N-transduced cells continued to decrease during the remainder of the posttransplantation analysis, reaching around 30% of the control group (31% ± 12% versus 87% ± 7%, Rac2D57N versus vector control, P < .05) at 16 weeks after transplantation.
The contribution of HSC/P cells expressing DN mutants to multilineage engraftment was confirmed by using analysis of progeny of transduced cells in the peripheral blood of recipients by flow cytometric immunophenotyping. As seen in Figure 2B, there were equivalent subpopulations of EGFP+ myeloid cells (Gr-1+), B cells (B220+), and T cells (CD3ϵ+) in RhoAN19 recipients when compared with the control group. As previously published,26 decreased levels of Rac activity was associated with a skewing toward myeloid differentiation when compared with the control group (30% ± 1% versus 18% ± 4% Gr-1+, Rac2D57N versus vector control, P < .01).
To more accurately determine the potential effects of expression of DN RhoA GTPase mutant on HSC activity, serial transplantation experiments were conducted. Equal numbers of bone marrow cells harvested at 16 weeks after transplantation from primary recipients were engrafted into secondary- and tertiary-irradiated recipient mice. The number of CFU cells initially infused into each experimental group was identical (Figure 2C). As seen in Figure 2C, the frequency of EGFP+ CFUs in the bone marrow of mice reconstituted with RhoAN19-transduced cells was increased by 1.5-fold compared with the number of EGFP+ CFUs in the control group in the primary recipient mice. A higher level of EGFP+ chimerism was seen from RhoAN19-transduced cells in both secondary- (168% ± 19% versus 57% ± 19%, P < .05) and tertiary-recipient mice (100% ± 18% versus 19% ± 11%, P < .05), respectively, when compared with the control group. In contrast, the level of EGFP+ chimerism from Rac2D57N-transduced cells continued to decrease during serial transplantation to almost undetectable levels. During the entire serial transplantation observation period (4 months primary transplantation, 4 months secondary transplantation, 6 months tertiary transplantation) none of the animals developed EGFP+ hematologic malignancies. Taken together, these data suggested that inhibition of RhoA activity increases multilineage reconstitution of HSCs without obvious effects on differentiation of these cells.
Effect of dominant-negative mutants on long-term repopulating ability of LDBM cells. (A) Evolution of chimerism in the peripheral blood of transplant recipient animals. Data represent percentage change in the peripheral-blood chimerism compared with the control group beginning 3 weeks after transplantation; MIEG3 (♦, control group), RhoAN19 (▪), Rac2D57N (▴). *P < .05 versus control group. The data represent the mean ± SEM of 3 independent experiments (n = 11). See initial engraftment raw data for each independent experiment in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). (B) Lineage analysis of peripheral blood of the recipient animals (shown in panel A), 16 weeks after transplantation; CD3e (▪), B220/CR45R (dark gray bars), Gr1 (light gray bars), other (□). The data are normalized to the level of engraftment in each individual animal and represent the mean ± SEM of 3 independent experiments, *P < .01. See lineage analysis directly related with the proportion of EGFP+ cells from a representative experiment in Figure S1. (C) Analysis of transduction of progenitor (CFU) cells before and after serial transplants; control (▪), RhoAN19 (dark gray bars), and Rac2D57N (light gray bars). Data are represented as percentage change relative to control group before transplantation and represent the mean ± SEM of 3 independent experiments (N = 11), *P < .05 versus control group.
Effect of dominant-negative mutants on long-term repopulating ability of LDBM cells. (A) Evolution of chimerism in the peripheral blood of transplant recipient animals. Data represent percentage change in the peripheral-blood chimerism compared with the control group beginning 3 weeks after transplantation; MIEG3 (♦, control group), RhoAN19 (▪), Rac2D57N (▴). *P < .05 versus control group. The data represent the mean ± SEM of 3 independent experiments (n = 11). See initial engraftment raw data for each independent experiment in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). (B) Lineage analysis of peripheral blood of the recipient animals (shown in panel A), 16 weeks after transplantation; CD3e (▪), B220/CR45R (dark gray bars), Gr1 (light gray bars), other (□). The data are normalized to the level of engraftment in each individual animal and represent the mean ± SEM of 3 independent experiments, *P < .01. See lineage analysis directly related with the proportion of EGFP+ cells from a representative experiment in Figure S1. (C) Analysis of transduction of progenitor (CFU) cells before and after serial transplants; control (▪), RhoAN19 (dark gray bars), and Rac2D57N (light gray bars). Data are represented as percentage change relative to control group before transplantation and represent the mean ± SEM of 3 independent experiments (N = 11), *P < .05 versus control group.
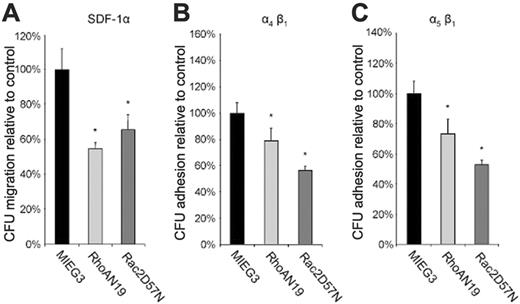
Effect of dominant-negative mutants on migration and adhesion of hematopoietic progenitor (CFU) cells. (A) SDF-1α–directed transwell migration of progenitor (CFU) cells. (B-C) Hematopoietic progenitor cell adhesion via α4β1 and α5β1, respectively, to recombinant fibronectin (see “Materials and methods” for details). In panels A to C, data are normalized to the control group and represent the mean ± SD of 3 independent experiments, *P < .01 versus control group.
Effect of dominant-negative mutants on migration and adhesion of hematopoietic progenitor (CFU) cells. (A) SDF-1α–directed transwell migration of progenitor (CFU) cells. (B-C) Hematopoietic progenitor cell adhesion via α4β1 and α5β1, respectively, to recombinant fibronectin (see “Materials and methods” for details). In panels A to C, data are normalized to the control group and represent the mean ± SD of 3 independent experiments, *P < .01 versus control group.
Inhibition of RhoA reduces adhesion and migration of HPCs
To gain insight into the potential mechanism by which inhibition of RhoA activity increases reconstitution of HSCs, we studied the effect of RhoAN19 on hematopoietic progenitor cell adhesion and migration which have previously been implicated in HSC/P cell homing and engraftment.7-12 Using a transwell progenitor migration assay, both RhoAN19 and, as expected from previous reports, Rac2D57N-transduced CFUs showed reduced migration in response to SDF-1α, a chemokine known to induce migration of HSC/P cells (6% ± 2% and 8% ± 2% for RhoAN19 and Rac2D57N, respectively, versus 12% ± 3% for control group, P < .05) (Figure 3A). We next analyzed the adhesion of transduced CFUs to 2 different recombinant fibronectin (FN) fragments. FN H296 mediates HSC/P cell adhesion via α4β1, whereas FN CH271 mediates adhesion via α5β1.7 The percentage of adherent CFUs to either H296 or CH271 was significantly reduced by expression of RhoAN19 (H296, 29% ± 3% versus 36% ± 4% RhoAN19 versus control, P < .01; CH271, 23% ± 6% versus 31% ± 4% RhoAN19 versus control, P < .01) (Figure 3B and 3C, respectively). As previously noted,35 expression of Rac2D57N also led to reduced adhesion of CFUs to either H296 (21% ± 6% versus 36% ± 4%, Rac2D57N versus control, P < .01) or CH271 (13% ± 2% versus 31% ± 4%, Rac2D57N versus control, P < .01) (Figure 3B and 3C, respectively). In summary, inhibition of RhoA reduced both SDF-1α–induced directed migration and α4β1- and α5β1-mediated adhesion of hematopoietic progenitor cells in vitro.
The effect of expression of dominant-negative RhoA on proliferation and cell-cycle progression. (A) [3H] Thymidine uptake of ckit+ cells expressing dominant-negative RhoA; control (▪), RhoAN19 (□). Data represent the mean ± SEM of 3 independent experiments, *P < .01 versus control group. (B-E) Flow cytometric analysis of cell-cycle status of transduced ckit+ cells. (B-C) Cells expressing the control virus. (D-E) Cells expressing RhoAN19. (C,E) Cells stimulated with SCF for 9 hours. The percentage of cells in each phase of cell cycle is shown at upper right. Data are representative of 4 independent experiments. *P < .05 versus control group.
The effect of expression of dominant-negative RhoA on proliferation and cell-cycle progression. (A) [3H] Thymidine uptake of ckit+ cells expressing dominant-negative RhoA; control (▪), RhoAN19 (□). Data represent the mean ± SEM of 3 independent experiments, *P < .01 versus control group. (B-E) Flow cytometric analysis of cell-cycle status of transduced ckit+ cells. (B-C) Cells expressing the control virus. (D-E) Cells expressing RhoAN19. (C,E) Cells stimulated with SCF for 9 hours. The percentage of cells in each phase of cell cycle is shown at upper right. Data are representative of 4 independent experiments. *P < .05 versus control group.
Expression of RhoAN19 enhances proliferation of hematopoietic cells
Because we demonstrated reduced migration and adhesion of RhoAN19-expressing cells in vitro, an effect likely to lead to reduced homing and engraftment in vivo, we next determined the effect of RhoA inhibition on proliferation of ckit+EGFP+ hematopoietic cells. As shown in Figure 4A, expression of RhoAN19 was associated with increased proliferation of ckit+EGFP+ bone marrow cells after cytokine starvation compared with control transduced cells (1259 ± 233 cpm versus 959 ± 79 cpm, RhoAN19 versus control, P < .01). RhoAN19 also increased proliferation of ckit+EGFP+ cells after stimulation with either SCF alone or with a combination of 3 cytokines (SMG) (20 470 ± 434 cpm versus 12 337 ± 2246 cpm and 81 087 ± 9799 cpm versus 50 086 ± 15 756 cpm, respectively, RhoAN19 versus control, P < .01). Consistent with these data, ckit+EGFP+ bone marrow cells expressing RhoAN19 had a significantly higher percentage of cells in S/G2/M phases of the cell cycle after starvation compared with control (Figure 4D versus 4B, respectively). Modest but reproducible differences in cell-cycle progression were also seen with SCF stimulation alone (34% versus 30%, RhoAN19 versus control, P < .05) (Figure 4E and 4C). In summary, expression of RhoAN19 was associated with increased proliferation of ckit+EGFP+ bone marrow cells in vitro.
Decreased RhoA activity is associated with increased cyclin D1 and decreased p21 levels
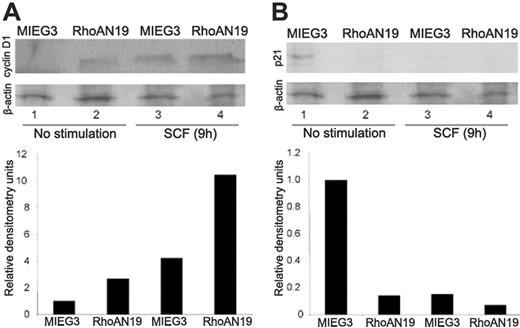
RhoA GTPase and ROCK-LIM kinase, a downstream effector of RhoA, have been implicated in the regulation of cyclin D1 and p21 activity during G1/S transition in nonhematopoietic cells.22-24 We next studied the effect of expression of RhoAN19 on cyclin D1 and p21 levels in sorted ckit+EGFP+ transduced bone marrow cells. As seen in Figure 5A, RhoAN19-expressing cells showed higher levels of cyclin D1 both after starvation as well as on SCF stimulation compared with control cells, respectively (Figure 5A). In control-transduced cells, expression of cyclin D1 is reduced to undetectable levels after serum starvation, and there is a strong induction of cyclin D1 expression after 9 hours of SCF stimulation (Figure 5A). In addition, immunoblot analysis of ckit+ cells expressing RhoAN19 demonstrated consistently reduced levels of p21 when compared with ckit+ control-transduced cells (Figure 5B). Taken together, these data demonstrate that RhoAN19 is a positive regulator of SCF-induced cell-cycle progression machinery in hematopoietic progenitor cells in vitro.
The effect of dominant-negative RhoA on cell-cycle modulators, cyclin D1 and p21. (A) Immunoblot analysis of cyclin D1 expression levels in ckit+ cells transduced with RhoAN19 (lanes 2 and 4) or control vector (lanes 1 and 3). β-Actin levels are used as loading control. Densitometry analysis of cyclin D1 levels normalized to β-actin are shown in the lower panel. One representative blot of at least 3 independent experiments is shown. (B) Immunoblot analysis of p21 expression levels in ckit+ cells transduced with RhoAN19 (lanes 2 and 4) or control vector (lanes 1 and 3). β-Actin levels are used as loading control. Densitometry analysis of p21 levels normalized to β-actin are shown in the lower panel. One representative blot of at least 3 independent experiments is shown. (A-B) The cells were either serum starved (lanes 1-2) or stimulated for 9 hours with SCF (lanes 3-4).
The effect of dominant-negative RhoA on cell-cycle modulators, cyclin D1 and p21. (A) Immunoblot analysis of cyclin D1 expression levels in ckit+ cells transduced with RhoAN19 (lanes 2 and 4) or control vector (lanes 1 and 3). β-Actin levels are used as loading control. Densitometry analysis of cyclin D1 levels normalized to β-actin are shown in the lower panel. One representative blot of at least 3 independent experiments is shown. (B) Immunoblot analysis of p21 expression levels in ckit+ cells transduced with RhoAN19 (lanes 2 and 4) or control vector (lanes 1 and 3). β-Actin levels are used as loading control. Densitometry analysis of p21 levels normalized to β-actin are shown in the lower panel. One representative blot of at least 3 independent experiments is shown. (A-B) The cells were either serum starved (lanes 1-2) or stimulated for 9 hours with SCF (lanes 3-4).
Discussion
Efficient HSC transplantation depends on a complex array of interactions that encompass the migration of circulating HSCs from the peripheral blood into the bone marrow stem cell “niche” and the proliferation and differentiation of these cells in response to multiple local and systemic stimuli to form all terminally differentiated blood cells. A high efficiency of HSC engraftment is especially desirable when the number of donor cells is limited such as in the case of some cord-blood transplants into adult recipients.41,42 In addition, it has been reported that ex vivo manipulation of HSCs, such as in the settings of gene therapy, frequently results in decreased ability of these cells to reconstitute hematopoiesis in recipients that received a transplant.43-45 In this regard, attempts at ex vivo expansion of HSCs have not convincingly demonstrated expansion of the HSC compartment.46,47 Theoretically, a net enhancement in the proliferation of these cells in vitro while preserving engraftment and self-renewal capacity might improve the success rate of HSC transplantation. In light of these challenges, a more thorough understanding of the molecular mechanisms that govern the engraftment and self-renewal properties of HSCs is warranted.
Rho GTPases are well-known regulators of signal transduction pathways that induce cytoskeleton rearrangements, gene expression, and cell-cycle activation in response to multiple extracellular stimuli.48 The signaling pathways downstream of the receptor tyrosine kinase c-kit, the chemokine receptor CXCR4 and β1 integrins, all previously implicated in engraftment11,14,49 have been shown to be integrated at several levels by Rho GTPases.26,32 Members of the Rho GTPase subfamily have been previously shown to play essential roles in hematopoietic progenitor cell functions and to have a dramatic impact on HSC engraftment and marrow retention. Decreased activity of Rac1 and Rac2 results in aberrant microlocalization of HSCs into the HM and either decreased engraftment of HSCs in a transplantation model or increased mobilization of these cells into the peripheral blood in a homeostatic model.26,32
In the current study, dominant-negative inhibition of RhoA GTPase activity resulted in increased HSC engraftment in a competitive repopulation assay. Furthermore, in the presence of decreased RhoA activity, the level of chimerism went from 60% higher than the control group in the primary recipients to 3- and 4-fold higher than the control group in the secondary and tertiary recipients, respectively. This suggests that low RhoA activity is associated with increased HSC self-renewal in vivo. In this respect, RhoA seems to have an opposite effect on HSC transplantation to the well-defined role of Rac1.
In contrast with the published role of RhoA in fate determination and differentiation in mesenchymal stem cells,50 decreased activity of RhoA in HSCs was associated with a normal differentiation profile and the emergence of a complete array of myeloid and lymphoid mature blood elements in the recipient animals. Decreased RhoA activity was not associated with any obvious leukemogenic effects during the follow-up of the transplanted cells over at least 14 months of serial transplantations.
Adhesion and migration of HSCs are considered to be important components of efficient HSC homing and maintenance into the bone marrow “niche.”51-53 Consistent with studies reported in fibroblast cells,48 RhoA was essential for normal adhesion and migration of hematopoietic progenitor cells in vitro. Decreased activity of RhoA GTPase resulted in defective α4β1- and α5β1-integrin–mediated adhesion and impaired SDF-1α–directed migration of hematopoietic progenitor cells in vitro. The results reported here, together with previous studies using Rho GTPase knock-out mice, suggest that the significance of directed cell migration and adhesion, at least as measured by in vitro assays, needs to be reconsidered. Alternatively, the effect of cell-cycle regulation seen with RhoA inhibition provides a dominant mechanism in vivo, overcoming any inefficiency in homing and engraftment because of reduced cell migration and adhesion. This observation could have therapeutic application because previous studies have suggested that alterations of expression and function of adhesion receptors are linked to poor engraftment of cultured cells.54 The exact RhoA-related pathway leading to the in vivo phenotype of HSC/P cells will need to be addressed in future studies by using a genetic knock-out approach.
The quiescence state of HSCs is controlled by genes regulating cell-cycle progression. The reduction or lack of the cell-cycle progression inhibitors, p21Cip1/Waf and p18INK4C, has been associated with an initial augmentation of the HSC pool in vivo under steady-state conditions.55,56 In fibroblast cells, RhoA decreases p21Kip1/Waf1 expression, thus allowing constitutively active Ras to induce cell-cycle progression.22 In contrast, we demonstrate here that hematopoietic progenitor cells expressing reduced levels of RhoA activity have a lower level of p21Kip1/Waf1 expression. It is tempting to speculate that the RhoA-mediated decreased p21Kip1/Waf1 expression that we observed in the progenitor cells is also present in HSCs. This scenario would provide an attractive mechanistic rational for the increased expansion and self-renewal of HSCs observed in RhoAN19-transduced HSCs. Decreased RhoA activity was also associated with increased cyclin D1 levels and a higher frequency of cells in S and G2/M phases of the cell cycle. Previous reports in fibroblast cells have shown that RhoA can block Rac1-induced cyclin D1 expression and delay G1/S transition.23,24 Rac1 has been previously shown to be essential for the proliferation of hematopoietic progenitor cells.32 In the absence of Rac1, cyclin D1 induction and G1/S phase transition are significantly compromised.32 Our data imply that RhoA has an opposing effect to Rac on proliferation and cell-cycle progression in hematopoietic cells.
In the study by Cheng et al,55 p21–/– mice were used to demonstrate the role of the cell-cycle regulator p21 on HSC pool expansion and maintenance. The study demonstrated that in the basal state, 8- to 12-week-old p21–/– adult animals have around 2-fold more week 5 cobblestone area-forming cells (CAFCs) than wild-type controls. Under the stress of serial transplantation, bone marrow cells from p21–/– animals lose radioprotective capacity (consistent with a loss of HSC activity). Although DN RhoA may exert its positive role on HSC expansion by partially releasing the break on cell-cycle progression imposed by the high p21 activity in these cells, we would not expect this effect to be as dramatic as a constitutional loss of p21. In the situation of overexpression of DN RhoA, p21 level (decreased but above background levels) may be low enough to promote HSC expansion but sufficient to maintain HSC pool over serial transplantations. In addition, although the use of DN mutants provides important information, this approach does suffer from some lack of specificity. Sequestering of multiple guanine exchange factors by DN constructs may affect multiple downstream pathways.
In summary, we show that decreased RhoA GTPase activity is associated with increased HSC engraftment, potentially by increased expansion and self-renewal of HSCs. These effects on hematopoietic reconstitution in vivo in a transplantation model occurred despite decreased adhesion and migration of hematopoietic progenitors in vitro. Thus, RhoA is likely to be a molecular target for the rational design of future strategies for HSC expansion that could improve the success rate of HSC transplantation in the clinical settings in which there is a limited number of these cells.
Prepublished online as Blood First Edition Paper, May 18, 2006; DOI 10.1182/blood-2006-02-001560.
Supported by the National Institutes of Health (grants 1P01HL69974 and 1R01DK062757) (D.A.W.).
G.G. designed and performed the research, analyzed the data, and wrote the paper; A.L., J.B., and J.A.C. performed the research; Y.Z. contributed vital new reagents; and D.A.W. designed the research, contributed vital new reagents or analytic tools, analyzed the data, and wrote the paper.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 4. The effect of expression of dominant-negative RhoA on proliferation and cell-cycle progression. (A) [3H] Thymidine uptake of ckit+ cells expressing dominant-negative RhoA; control (▪), RhoAN19 (□). Data represent the mean ± SEM of 3 independent experiments, *P < .01 versus control group. (B-E) Flow cytometric analysis of cell-cycle status of transduced ckit+ cells. (B-C) Cells expressing the control virus. (D-E) Cells expressing RhoAN19. (C,E) Cells stimulated with SCF for 9 hours. The percentage of cells in each phase of cell cycle is shown at upper right. Data are representative of 4 independent experiments. *P < .05 versus control group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/6/10.1182_blood-2006-02-001560/4/m_zh80180601410004.jpeg?Expires=1767701726&Signature=QhtzEHnhe52neWy~G6ymqe4sNMF6Hx0ld0Awq1C~ECq-FQpKx2tCa11uw3eZES2LvNR3BZBqyBlPEHBLnkxgcac0J8ILKROYJsDpEUZiv7x45IuFJWKs49yjxV-sUZPKneH41TjN-yvQKc4B-wRB46K5Xo-BhBkMLDYBEEL~SnDGTkCeT105BUww9JReVI9f-kHyob3qkLzB1D9GX608X4uXdSlQL4mo~YBIPF8ycQwVTPXM4-8BOXMjNBi-1LLfzsDj6bKu5IHmr080U-9Xfy6V6Ccpk~L0P0XTzShPxTXFWqTTO-F~ZAYGja77sZZyhGvz0gJG9lTpm3E-XXWlyg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal