Abstract
Current models predict that mouse plasmacytoid dendritic cells (PDCs) derive from lymphoid progenitors. However, we show PDCs arise exclusively from common myeloid progenitors (CMPs) characterized by low-level expression of several lymphoid-associated genes, including a RAG2/GFP reporter transgene. This conclusion is supported by both adoptive transfer experiments and an estrogen treatment strategy that led to marked depletion of very early lymphoid progenitors without affecting RAG2/GFP+ CMPs or the developmental kinetics, RAG-mediated recombinase activity, and cytokine production of PDCs. These data suggest that PDCs arise exclusively from early myeloid progenitors and that promiscuous low-level expression of lymphoid-associated genes is a general feature of PDC progenitors among CMPs.
Introduction
Mouse plasmacytoid dendritic cells (PDCs) were first described in 2001.1-3 Similar to human PDCs, mouse PDCs are relatively inefficient at presenting pathogenic peptides to immune effector cells,4 but are potent producers of type I interferons, which can induce TH1 polarization in CD8+ T cells and stimulate natural killer (NK) cell-mediated cytotoxicity.5,6 These functions are mediated through the Toll-like receptors (TLRs) specific for viral single-stranded RNA and CpG-containing motifs, TLR-7 and TRL-9, respectively.7
Little is known about the mechanisms underlying PDC differentiation from hematopoietic stem cells (HSCs); however, several lines of evidence suggest significant overlap between the molecular circuitry required for PDC and lymphocyte development. First, PDCs express an array of genes associated with lymphocyte development or function,8-10 and a small fraction of PDCs contain DH-JH rearrangements on the Ig heavy-chain (IgH) locus.11 Second, progenitor pools enriched for lymphoid precursors, including cells designated common lymphoid progenitors (CLPs) or more HSC-proximal early lymphoid progenitors (ELPs) described by Kincade and colleagues, yield PDCs following transfer into adoptive hosts.10,12-15 In addition, several reports demonstrate that myeloid progenitors can also generate PDCs.16 Moreover, recent data suggest that common myeloid progenitor (CMP)–derived PDCs express the lymphoid-associated genes pre-Tα and RAG1.10,12 A dual origin for PDCs could also be the source of the heterogeneity of an array of lymphoid-associated genes described in a recent report,14 although this relationship has not been firmly demonstrated. Thus, current evidence suggests that both myeloid and lymphoid progenitors may function as PDC progenitors.
Currently there are 2 opposing models describing precursor-product relationships for early lymphoid and myeloid progenitors. The first predicts that myeloid- and lymphoid-restricted progenitors can be cleanly identified and separated based on distinct cell surface phenotypes. Data supporting this viewpoint include descriptions of CMP and CLP populations defined by their restricted differentiation potentials for myeloid and lymphoid lineages, respectively.17,18 However, certain observations appear to conflict with this model. For instance, CMPs generate a small but persistent number of B cells,12 and a recently described progenitor thought to be downstream of the CLP retains macrophage potential in vitro.19 The requirement that all lymphoid cells must pass through a CLP intermediate during steady-state lymphopoiesis has also not been determined conclusively. Indeed, early T-lineage progenitors (ETPs) in the thymus can arise independent of CLPs,20 and initiation of recombinase activity among CLPs requires the B lineage–restricted enhancer element eRAG.21 These observations suggest an alternative model in which the CLP population consists primarily of early B-lineage progenitors (EBPs) characterized by residual T-lineage potential. Thus, the ability of EBPs/CLPs to generate PDCs may also reflect the natural differentiation plasticity of early hematopoietic progenitors.
We used a combination of experimental strategies to determine whether PDCs arise from early lymphoid versus early myeloid progenitors. These strategies included single and competitive bone marrow (BM) chimeras established by transfer of well-defined multipotent lymphoid and myeloid progenitor populations, evaluation of the impact of the age-associated loss and the estrogen-mediated depletion of ELP pools on PDC development, and an assessment of whether lymphoid-associated genes expressed by BM PDCs are also expressed by PDC progenitors within the CMP pool. Our data indicate that PDCs arise predominantly from a subset of estrogen-resistant Flt3/Flk2+ CMPs that are further characterized by low-level expression of a RAG2/GFP reporter transgene and the additional lymphoid-associated genes terminal deoxynucleotidyl transferase (TdT) and sterile IgH (μ0) transcripts.
Materials and methods
Mice
Six- to 10-week-old C57BL/6 mice and B6.Ly5.2 (referred to herein as B6.Ly5SJL) mice were purchased from the National Cancer Institute animal facility (Frederick, MD) or the Jackson Laboratory (Bar Harbor, ME). (C57BL/6 × B6.Ly5SJL) F1s and aged C57BL/6 mice were generated and maintained in our animal colony. NG-BAC (RAG2/GFP) transgenic reporter mice22 were kindly provided by Dr Michel Nussenzweig (Rockefeller University, New York, NY). H2-SVEX21 transgenic mice were maintained in our animal colonies.
Antibodies and analytical flow cytometry
For flow cytometric analyses BM, spleen, and thymus suspensions were prepared and stained with optimal dilutions of directly conjugated fluorescent antibodies as previously described,23 then analyzed on a 10-color LSR II flow cytometer (Becton Dickinson, San Jose, CA) equipped with 4 lasers for excitation of UV-, violet-, blue-, and red-excited dyes. Antibodies used included fluorescein (FL), phycoerythrin (PE), PE-Cy5.5, PE-Cy7, allophycocyanin (APC), APC-Cy7, or biotin (BI)–conjugated versions of purified antibodies to the following cell surface antigens: B220 (RA3-6B2), CD11b (M1/70), Gr-1 (8C5), Ter-119, CD3 (2C11), CD127/IL-7Rα (A7R3424 ), CD135/Flt3 (A2F10), CD117/c-kit (2B8), C1qR/AA4 (AA4.1),25 Sca-1/Ly6 A/E (E13-161.7), CD19 (1D3), CD16/32 (93), CD34 (RAM34), Ly6C (AL-21), CD11c (HL3), NK1.1 (PK136). FL-conjugated Fab fragments of polyclonal goat anti–mouse IgM antibodies were purchased from Jackson ImmunoResearch (West Grove, PA). BI-conjugated antibodies were revealed by secondary staining with streptavidin (SA) coupled to Pacific blue (Molecular Probes, Eugene, OR) or SA-PE-Cy5.5, SA-PE-Cy7, or SA-APC-Cy7 (Caltag, Burlingame, CA). All directly conjugated antibodies were purchased from eBiosciences (San Diego, CA) except for CD11c and Ly6C, which were purchased from PharMingen (San Diego, CA), and AA4, CD19, B220, and TER-119, which were purified and conjugated by standard methods in our laboratory. Nonviable cells were excluded from all analyses by staining with the UV-excited DNA dye DAPI. All flow cytometric data were analyzed by uploading files into FlowJo 4.6 (Tree Star, San Carlos, CA).
Cell sorting
Cell suspensions were applied to an 11-parameter MoFlo (DakoCytomation, Fort Collins, CO) or one of two 12-parameter FACSDiva high-speed cell sorters. The MoFlo and one of the two FACSDivas are equipped with 2 Coherent Innova argon lasers tuned for blue (488 nm) or UV (351/363) excitation and a Coherent Spectrum argon/krypton laser tuned to 647 nm for excitation of APC and its derivatives. The alternative FACSDiva is equipped with argon and argon/krypton lasers for excitation in the blue and red wavelengths as described and a Coherent Innova 302C krypton laser tuned to 407 nm for violet excitation. For the MoFlo, stained cell suspensions were applied at a sheath pressure of 60 psi with a drop delay of approximately 98 000 drops/s. This resulted in sorting rates of 28 000 to 30 000 cells/s with abort rates of 10% to 12%. For the FACSDiva, cells were applied at a sheath pressure of 40 psi and a drop delay frequency of approximately 70 000 drops/s resulting in sort trigger rates of 20 000 to 25 000 cells/s.
Intravenous transfers
Hosts were maintained on water containing a Bactrim suspension (400 mg sulfamethoxazole and 80 mg trimethoprim/500 mL water) for 1 week prior to, and at least 3 weeks following, lethal (900 R) irradiation. Hosts were irradiated 1 day before intravenous transfer of 500 to 1000 sorted progenitor populations mixed with either additional sorted precursors or 1 to 2 × 105 unfractionated host-type BM cells as indicated. In mice receiving CMPs subdivided based on Flt3/Flk2 expression, 2000 donor cells were given per recipient.
Intrathymic transfers
Intrathymic transfers were performed as previously described.23 Briefly, 500 freshly sorted BM progenitors from female C57BL/6 (Ly5B6) adults were injected into thymi of anesthetized female B6.Ly5SJL mice given 500 R 6 hours previously.
Estrogen administration
17β-estradiol pellets or their placebo controls (21-day release; Innovative Research of America, Sarasota, FL) were implanted under the posterior skin of the neck of female C57BL/6 or NG-BAC mice using a trochar as described by Medina et al.26 The spleens and BM of recipient mice were analyzed 7 and 14 days afterward with no appreciable differences. Data presented represent 14 days following the beginning of treatment unless stated.
In vivo BrdU labeling
Continuous in vivo BrdU labeling was performed as previously described27 with the addition of the appropriately conjugated antibodies. Briefly, adult estrogen-treated and control C57BL/6 mice were inoculated with 0.5 mg BrdU (Sigma, St Louis, MO) in PBS every 12 hours for 0.5 to 7 days. BM and spleen cells were stained with PE-CD19, PE-CD3, PE-NK1.1, APC-Cy7 B220, APC-CD11c, and biotinylated Ly6C followed by streptavidin-Pacific blue in standard fluorescence-activated cell sorting (FACS) buffer, washed twice with protein-free PBS, then permeabilized using “Fix and Perm” (Caltag). Subsequently, cells were washed, incubated with DNaseI, washed, and then stained with FL-anti-BrdU antibodies (Becton Dickinson) before analysis.
RT-PCR
Cells were sorted directly into RNA-lysis buffer consisting of 4.23 mM guanidium isothiocyanate, 0.67% Sarcosyl, 33.3 μM citrate buffer, and 134 mM 2-ME and extracted and RNA precipitated as described.28 cDNA for each sample was synthesized using the First Strand cDNA synthesis kit (Roche Applied Science, Indianapolis, IN) before loading normalization by polymerase chain reaction (PCR) using β-actin oligos. cDNA was then subjected to PCR using Taq DNA polymerase (Invitrogen, Carlsbad, CA) and previously published oligonucleotides and conditions.28,29 Amplification of gDNA was controlled for by amplification of reverse transcriptase (RT)–negative controls from each population and by using oligonucleotides that span intronic sequences.
Methylcellulose cultures
Methylcellulose culture conditions were as previously described.17 Triplicate cultures were established by plating sorted cells at 500 cells/plate according to the manufacturer's instructions. On day 12, discrete colonies were counted and typed by morphology: M, colony-forming unit (CFU) macrophage; G, CFU granulocyte; GM, CFU granulocyte/macrophage, E, burst-forming unit (BFU) erythroid; GEM, granulocytes, erythrocytes, and macrophages; Mix, GEM plus megakaryocytes.
ELISA
BM PDCs (CD19–CD3–NK1.1–CD11c+PDCA+CD11b–B220+Ly6c+) were sorted as described (see “Cell sorting”). Triplicate cultures, each containing 3 × 104 sorted cells, were stimulated in vitro with influenza virus at 300 U/mL overnight in triplicate. Supernatant was collected and analyzed using an enzyme-linked immunosorbent assay (ELISA) kit specific for IFN-α (PBL Laboratories, Piscataway, NJ).
Statistical analyses
Statistical analyses were performed by calculating the mean and the standard error of the mean (SEM) of data generated from a minimum of 3 mice per group or an unpaired Student t test.
Results
Age-associated loss of ELPs but not PDCs
Current data suggest that potential PDC precursors are enriched among Flt3/Flk2+ early lymphoid and myeloid precursors.10,12,13 Candidate Flt3/Flk2+ lymphoid progenitors include IL-7Rα+ EBPs/CLPs and more HSC-proximal IL-7Rα– ELPs defined by RAG-1/2 expression and the surface phenotype lineage– c-kithighSca-1+ Flt3/Flk2+ (LSK-Flt3+) (see Figure 3A).20,30,31 Recent evidence indicates that very early lymphoid progenitors including the BM EBP/CLP population begin to decline in middle-aged (9-14 months) C57BL/6 mice.32,43 We reasoned that if significant numbers of PDCs derive from the EBP/CLP pool, then frequencies of developing PDCs in the BM might also decline with age. Accordingly, we compared frequencies and absolute numbers of EBPs/CLPs and BM PDCs in 3-month-old and 12-month-old C57BL/6 females. As shown, whereas frequencies and absolute numbers of EBPs/CLPs declined appreciably by 12 months of age, frequencies and absolute numbers of BM PDCs remained unperturbed (Figure 1). Indeed, frequencies of PDCs remained unchanged with age in C57BL/6 mice that were as old as 20 months of age (not shown), despite our additional observation that frequencies of HSC-proximal Flt3+ LSKs also declined significantly and consistently by 12 months of age (Figure 1).
These data raise questions about whether Flt-3+ ELPs are obligate precursors for BM PDCs. Alternatively, the source of BM PDCs and the resulting cellular dynamics associated with BM PDC development, including their cellular half-life, may be altered in the aged environment. Accordingly, we initiated a series of experiments to test the degree to which both early lymphoid and early myeloid progenitors generate PDCs. These experiments included the use of single and double BM chimeras to test the PDC differentiation potential of well-defined Flt3/Flk2+ lymphoid progenitors, and estrogen-depletion studies designed to test the impact of selective depletion of early lymphoid progenitors on PDC differentiation.
EBPs/CLPs do not yield PDCs in vivo
Our first set of adoptive transfer experiments was designed to compare the relative capacity of Flt3/Flk2+ cells within the EBP/CLP and LSK-Flt3+ progenitor pools to generate splenic PDCs in vivo. Five hundred sorted LSK-Flt-3+ cells or EBPs/CLPs (Lin–IL-7Rα+AA4+Sca-1lowc-kitlowFlt3/Flk2+) from C57BL/6 donors were transferred intravenously together with 105 unfractionated host-type (Ly5SJL+) BM cells into irradiated Ly5 congenic (B6.Ly5SJL) hosts. Host splenocytes and BM cells were assessed at 14 to 35 days after transfer for donor-derived (Ly5B6+) B220+CD19+sIgM+ B cells and CD11c+ dendritic cells (DCs) that were further segregated into conventional DCs (CD11chighB220–CD11b+) and PDCs (CD11clowB220+ CD11b–). Significantly, we could not detect donor-derived CD11c+ cells including PDCs among splenocytes of recipients given EBPs/CLPs. Moreover, consistent with previous data, the vast majority of donor-derived splenocytes in these mice were B220+CD19+ B cells (Figure 2A), and parallel intrathymic transfer experiments performed with the identical donor population revealed the expected repopulation of host thymi (Figure 2B).18,20 In contrast, LSK-Flt3+ cells efficiently generated both conventional DCs and PDCs, and yielded B- and T-lineage cells following intravenous and intrathymic transfer with the expected kinetics18,20,30,31 (Figure 2A-C). In these experiments, the vast majority of donor-derived cells were found in the spleen rather than the BM. These data show that Flt3/Flk2+ EBPs/CLPs are not highly enriched for PDC precursors, and instead suggest that PDC differentiation potential is restricted to more HSC-proximal progenitors including LSK-Flt-3+ MPPs characterized by high surface expression of both Flt3/Flk2 and c-kit.
Differential impact of aging on ELPs and PDCs in the BM. (A) BM cells from 3-month-old and 12-month-old C57BL/6 mice were stained with the indicated antibodies before collection of 500 000 events on an LSRII flow cytometer. Lineage (Lin) cocktail included antibodies to B220, CD3ϵ, TER-119, Gr-1, and CD11b. Initial gating of Lin–IL-7Rα– and Lin–IL-7Rα+ was as previously described.20 (B) BM cells from the mice in panel A were also stained with the indicated antibodies for resolution of BM PDCs as shown. Nonviable cells were excluded from analyses as described in Figure 1. (C) Absolute numbers of cells within the indicated population were calculated by multiplying the frequency of cells within the gates indicated in panels A and B times the total number of viable BM cells harvested from each mouse. Data are the mean and the SEM with 3 mice per group. The age-related loss of EBPs/CLPs and Flt3 + LSKs was also judged to be statistically significant as determined by unpaired Student t test (P < .01).
Differential impact of aging on ELPs and PDCs in the BM. (A) BM cells from 3-month-old and 12-month-old C57BL/6 mice were stained with the indicated antibodies before collection of 500 000 events on an LSRII flow cytometer. Lineage (Lin) cocktail included antibodies to B220, CD3ϵ, TER-119, Gr-1, and CD11b. Initial gating of Lin–IL-7Rα– and Lin–IL-7Rα+ was as previously described.20 (B) BM cells from the mice in panel A were also stained with the indicated antibodies for resolution of BM PDCs as shown. Nonviable cells were excluded from analyses as described in Figure 1. (C) Absolute numbers of cells within the indicated population were calculated by multiplying the frequency of cells within the gates indicated in panels A and B times the total number of viable BM cells harvested from each mouse. Data are the mean and the SEM with 3 mice per group. The age-related loss of EBPs/CLPs and Flt3 + LSKs was also judged to be statistically significant as determined by unpaired Student t test (P < .01).
Lymphoid-specified ELPs are inefficient PDC progenitors
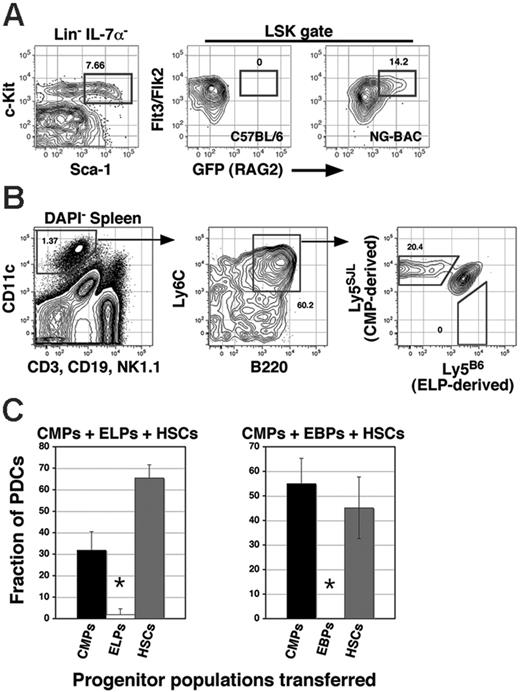
Given that c-kithigh LSK-Flt3+ precursors were more efficient than EBPs/CLPs at generating PDCs (Figure 2) and BM PDCs exhibit evidence of ongoing or past RAG2 expression (see Figure 6D), we hypothesized that PDCs arise primarily from the c-kithighFlt3/Flk2+RAG1/2+ ELP population described by Igarashi et al.31 However, because CMPs also generate PDCs following adoptive transfer, we established a competitive scenario to test the relative efficiency with which each population contributes to the PDC pool. Accordingly, we established mixed BM chimeras in which 1000 sorted CMPs and ELPs (or EBPs/CLPs) were coinjected into the same recipients. For these experiments, c-kithigh ELPs and EBPs/CLPs were identified and sorted from C57BL/6-backcrossed NG-BAC (Ly5B6+) transgenics (Figure 3A), CMPs were sorted from B6.Ly5SJL congenics, and 1000 cells from each population were mixed together with 250 host-type HSCs before transfer into (C57BL/6 × B6.Ly5SJL) F1s. With this strategy, EBP/CLP- and ELP-derived cells were identified as Ly5B6+Ly5SJL–, CMP-derived cells were identified as Ly5B6–Ly5SJL+, and host-type HSC-derived cells were Ly5B6+Ly5SJL+. Surprisingly, as shown in Figure 3B-C neither EBPs/CLPs nor ELPs generated significant numbers of PDCs in mixed BM chimeras. Indeed, analyses of multiple recipients within each group showed that whereas on average CMPs produced 40% of the total PDC pool in adoptive hosts, equal numbers of ELPs and EBPs/CLPs generated 3% and less than 1% of the PDC pool, respectively. These data suggest that PDCs arise primarily from within the CMP progenitor pool.
EBPs/CLPs fail to generate PDCs on adoptive transfer. (A) Five hundred sorted EBPs/CLPs (Lin–IL-7Rα+AA4+Sca-1lowc-kitlowFlt3/Flk2+) or LSK-Flt3+ (Lin–IL-7Rα–Sca-1+c-kithigh Flt3/Flk2+) progenitors from C57BL/6 (Ly5B6+) mice were transferred intravenously together with 105 unfractionated host-type BM cells into irradiated B6.Ly5SJL congenics. Host splenocytes were assessed for frequencies of donor-derived (Ly5B6+Ly5SJL–) B-lineage (CD19+B220+) and PDC-lineage (IgM–CD3–CD11c+B220+CD11b–) cells. Shown are analyses of recipients at day 14 after transfer. (B) Five hundred sorted cells from each population were also transferred via intrathymic injection into B6.Ly5SJL congenics, and host thymi assessed for donor-derived thymocytes 14 days later. (C) Absolute number of donor-derived splenocytes of the indicated surface phenotype at the indicated time points in mice given 500 EBPs/CLP versus LSK-Flt3+ progenitors as described. Data are the mean and SEM using 3 mice per group. Data are representative of 3 separate experiments.
EBPs/CLPs fail to generate PDCs on adoptive transfer. (A) Five hundred sorted EBPs/CLPs (Lin–IL-7Rα+AA4+Sca-1lowc-kitlowFlt3/Flk2+) or LSK-Flt3+ (Lin–IL-7Rα–Sca-1+c-kithigh Flt3/Flk2+) progenitors from C57BL/6 (Ly5B6+) mice were transferred intravenously together with 105 unfractionated host-type BM cells into irradiated B6.Ly5SJL congenics. Host splenocytes were assessed for frequencies of donor-derived (Ly5B6+Ly5SJL–) B-lineage (CD19+B220+) and PDC-lineage (IgM–CD3–CD11c+B220+CD11b–) cells. Shown are analyses of recipients at day 14 after transfer. (B) Five hundred sorted cells from each population were also transferred via intrathymic injection into B6.Ly5SJL congenics, and host thymi assessed for donor-derived thymocytes 14 days later. (C) Absolute number of donor-derived splenocytes of the indicated surface phenotype at the indicated time points in mice given 500 EBPs/CLP versus LSK-Flt3+ progenitors as described. Data are the mean and SEM using 3 mice per group. Data are representative of 3 separate experiments.
Lymphoid-related gene expression among Flk2+CMP
Because our adoptive transfer studies identified CMPs as the most efficient PDC progenitors, we next sought to better understand the cellular pathway underlying the activation of lymphoid-associated genes in BM PDCs. We began by assessing RAG2/GFP expression in CMPs and related myelo-erythroid BM progenitor fractions from NG-BAC mice using the flow cytometric strategy described by Akashi et al.17 Although evidence for RAG-1/2 expression has not been obtained through the analysis of RAG1/GFP knock-in mice,10 we reasoned that NG-BAC transgenic mice might provide a more sensitive assessment of low levels of RAG2 transcription due to the presence of multiple copies of the BAC transgene. Indeed, as shown in Figure 4A, 20% to 30% of CMPs from NG-BAC transgenic mice were RAG2/GFPlow, and GFP expression in these cells correlated with surface expression of Flt3/Flk2 and AA4. Further, though we did not reproducibly observe evidence for RAG1 or RAG2 transcripts by RT-PCR, we routinely were able to amplify TdT and μ0 transcripts from sorted RAG2/GFPlow CMPs (Figure 4B). Compared to their RAG2/GFP–Flt3/Flk2– counterparts, RAG2/GFPlowFlt3/Flk2+ CMPs also generated relatively few myeloid colonies in methylcellulose cultures optimized for myeloid lineage differentiation (Figure 4C), and consistent with previous data12,13 PDC differentiation potential among CMP subsets as determined by adoptive transfer of CMP subsets from NG-BAC transgenics (Ly5B6+) into B6.Ly5SJL congenics was restricted to RAG2/GFPlowFlt3/Flk2+ CMPs (Figure 5). Thus, PDC differentiation potential is enriched among a subset of CMPs further characterized by diminished myeloid differentiation potential and low but readily detectable μ0 and TdT expression.
Depletion of ELPs does not perturb PDC development
The data described, together with the previous observation that Flt3/Flk2+ CMPs are more efficient PDC progenitors than Flt3/Flk2– CMPs (Figure 5),12,13 suggests that RAG2/GFPlowFlt3/Flk2+ CMPs may serve as a primary PDC progenitor pool. To further test this notion and directly test whether early lymphoid progenitors including c-kithighFlt3/Flk2+ ELPs and EBP/CLPs are requisite for PDC differentiation, we treated normal adult NG-BAC transgenics with 17β-estradiol. In vivo treatment of adults with 17β-estradiol selectively depletes ELPs including ELPs residing among LSK-Flt3+ progenitors without affecting myelopoiesis.26,33 Accordingly, we implanted time-release 17β-estradiol capsules or placebo controls in adult NG-BAC mice as described in “Materials and methods.” As shown, consistent with previous work in vivo exposure to estrogen for 14 days led to significant depletion of BM lymphoid progenitors including c-kithighFlt3/Flk2+Sca-1+IL-7Rα–RAG2/GFP+ ELPs and IL-7Rα+AA4+Sca-1low Flt3/Flk2+RAG2/GFPhigh EBPs/CLPs (Figure 6). In sharp contrast, frequencies and absolute numbers of PDCs and RAG2/GFP+ CMPs were not reduced by this treatment. Interestingly, GFP levels intensified among RAG2/GFP+ cells within both the CMP and PDC pools, although the mechanism underlying this effect is unknown.
Relative PDC differentiation potential of early myeloid and lymphoid progenitors. (A) c-kithigh ELPs in a 10-week-old NG-BAC transgenic were identified by staining BM cells with the indicated antibodies before collection of 1 000 000 events on an LSRII flow cytometer. (B) A total of 1000 c-kithigh ELPs from NG-BAC transgenics and 1000 CMPs (Lin–IL-7Rα–Sca-1–c-kithighCD16/32intCD34+; see Figure 5A) from B6.Ly5SJL congenics were mixed with 250 host-type HSCs (Lin–IL-7Rα–Sca-1+c-kithighFlt3/Flk2–) before transfer into irradiated (C57BL/6 × B6.Ly5SJL) F1 hosts. Splenocytes from a representative recipient at day 14 after transfer were assessed for the relative contribution of each progenitor population to the PDC lineage by staining cells with the indicated antibodies and collection of 500 000 events. Recipients of host-type HSCs only did not contain detectable Ly5B6+Ly5SJL– or Ly5B6–Ly5SJL+ cells (not shown). (C) Summary data from 3 recipients per group given either ELPs or EBPs/CLPs mixed with CMPs. Data are the mean and SEM with 5 mice per group and are representative of 2 separate experiments with 5 recipients per group.
Relative PDC differentiation potential of early myeloid and lymphoid progenitors. (A) c-kithigh ELPs in a 10-week-old NG-BAC transgenic were identified by staining BM cells with the indicated antibodies before collection of 1 000 000 events on an LSRII flow cytometer. (B) A total of 1000 c-kithigh ELPs from NG-BAC transgenics and 1000 CMPs (Lin–IL-7Rα–Sca-1–c-kithighCD16/32intCD34+; see Figure 5A) from B6.Ly5SJL congenics were mixed with 250 host-type HSCs (Lin–IL-7Rα–Sca-1+c-kithighFlt3/Flk2–) before transfer into irradiated (C57BL/6 × B6.Ly5SJL) F1 hosts. Splenocytes from a representative recipient at day 14 after transfer were assessed for the relative contribution of each progenitor population to the PDC lineage by staining cells with the indicated antibodies and collection of 500 000 events. Recipients of host-type HSCs only did not contain detectable Ly5B6+Ly5SJL– or Ly5B6–Ly5SJL+ cells (not shown). (C) Summary data from 3 recipients per group given either ELPs or EBPs/CLPs mixed with CMPs. Data are the mean and SEM with 5 mice per group and are representative of 2 separate experiments with 5 recipients per group.
Lymphoid gene expression and diminished myeloid potential by a subset of CMPs. (A) BM cells from a 10-week-old NG-BAC transgenic mouse were stained with the indicated antibodies before collection of 500 000 events as described in Figure 1. (B) cDNA was prepared from the indicated populations from NG-BAC transgenics and subjected to PCR with the indicated oligonucleotides. Cell surface phenotypes used for cell sorting were HSCs, Lin–IL-7Rα–Sca-1+c-kithighFlt3/Flk2–; EBPs/CLPs, Lin–IL-7Rα+AA4+Sca-1lowc-kitlow Flt3/Flk2+RAG2/GFPhigh; pro-B, B220lowCD43+CD19+AA4+; CMPs, Lin–IL-7Rα–Sca-1–c-kithighCD16/32intCD34+Flt3/Flk2+/–RAG2/GFP+/–; PDCs, CD19–Ly6C+CD11c+B220+AA4lowRAG2/GFP+. (C) The indicated populations were cultured in methylcellulose medium and characterized as described in “Materials and methods.”
Lymphoid gene expression and diminished myeloid potential by a subset of CMPs. (A) BM cells from a 10-week-old NG-BAC transgenic mouse were stained with the indicated antibodies before collection of 500 000 events as described in Figure 1. (B) cDNA was prepared from the indicated populations from NG-BAC transgenics and subjected to PCR with the indicated oligonucleotides. Cell surface phenotypes used for cell sorting were HSCs, Lin–IL-7Rα–Sca-1+c-kithighFlt3/Flk2–; EBPs/CLPs, Lin–IL-7Rα+AA4+Sca-1lowc-kitlow Flt3/Flk2+RAG2/GFPhigh; pro-B, B220lowCD43+CD19+AA4+; CMPs, Lin–IL-7Rα–Sca-1–c-kithighCD16/32intCD34+Flt3/Flk2+/–RAG2/GFP+/–; PDCs, CD19–Ly6C+CD11c+B220+AA4lowRAG2/GFP+. (C) The indicated populations were cultured in methylcellulose medium and characterized as described in “Materials and methods.”
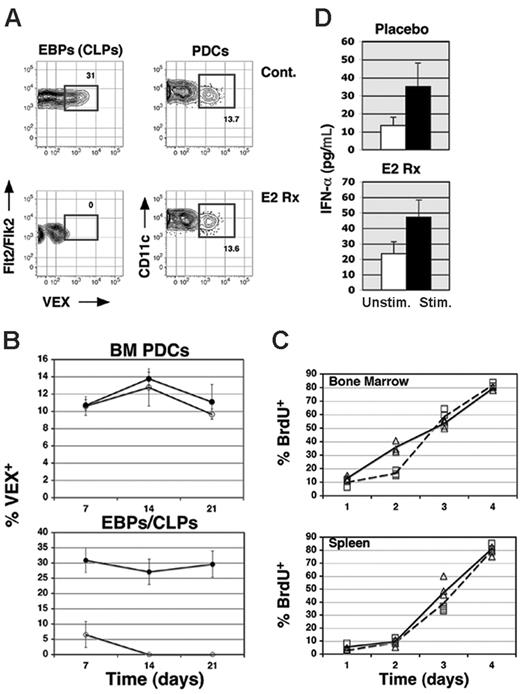
We also scrutinized the impact of 17β-estradiol treatment on PDC development and function. First we considered the possibility that despite our observations, at steady state a small fraction of BM PDCs nonetheless derive from lymphoid progenitors. In this regard, it should be noted that low frequencies of PDCs contain DH-JH rearrangements on the IgH locus.11 Consistent with this observation, we found that 10% to 15% of BM PDCs express the VEX-based RAG1/2-dependent V(D)J recombinase transgenic reporter substrate carried by H2-SVEX transgenic mice21 (Figure 7A). To test whether frequencies of recombinase-positive BM PDCs were diminished by in vivo 17β-estradiol treatment, H2-SVEX transgenics were exposed to 17β-estradiol for 1 to 3 weeks, then frequencies of VEX+ PDCs and EBPs/CLPs determined. As shown (Figure 7A-B), whereas frequencies of VEX+ PDCs were unaffected by 17β-estradiol treatment after even 3 weeks, VEX+ EBPs/CLPs were clearly and rapidly depleted via this strategy. Again, absolute numbers of BM PDCs were not affected by 17β-estradiol treatment (not shown).
Finally, we assessed the impact of 17β-estradiol treatment on BM PDC developmental kinetics and function. To test whether 17β-estradiol treatment affected the rate at which BM PDCs were generated, we determined PDC production rates in the BM and spleen via continuous in vivo BrdU labeling. The data show that cellular turnover and production rates for PDCs were unaltered in 17β-estradiol–treated mice compared with placebo controls (Figure 7C). To examine whether PDC function was affected, BM PDCs sorted from 2-week treated 17β-estradiol or placebo control mice were simulated with influenza virus, and IFN-α release in vitro assessed by ELISA. For these experiments we were able to assess IFN-α production from only 50 000 cultured PDCs. As shown, PDCs from both control and 17β-estradiol–treated mice produced IFN-α and similar levels (Figure 7D). Thus, depletion of early lymphoid progenitors via in vivo 17β-estradiol treatment did not affect the developmental kinetics or the function of BM PDCs. These data further support the notion that PDCs derive exclusively from estrogen-resistant progenitors within the CMP pool.
Discussion
Several studies suggest that PDCs develop from both lymphoid and myeloid sources.10,12,13,16,34 However, our data indicate that during steady-state hematopoiesis, PDCs arise mainly from a subset of PDC-biased CMPs further defined via low-level expression of genes associated with early lymphocyte development. Several lines of evidence support this conclusion. First, whereas aging leads to dramatic reductions in numbers of EBPs/CLPs and upstream LSK-Flk2+ progenitors, we did not observe a corresponding loss of BM PDCs. Second, CMPs were more effective than EBPs/CLPs and RAG2+ ELPs at generating PDCs in competitive BM chimeras and, consistent with previous observations, among CMPs differentiation into PDCs was restricted to Flt3/Flk2+RAG2/GFPlow cells. Third, depletion of EBPs/CLPs and RAG2+ ELPs with 17β-estradiol failed to affect the cellular dynamics, function, and absolute number of BM PDCs including numbers of recombinase-positive cells within the BM PDC pool. Finally, only Flt3/Flk2+RAG2/GFPlow cells within the CMP pool expressed low but detectable levels of TdT and μ0, and transcripts for each of these genes were also detected in BM PDCs.
Generation of PDCs by RAG2/GFPlow CMPs. Five hundred GFP/RAG2+Flt3/Flk2+ or GFP/RAG2–Flt3/Flk2– CMPs from NG-BAC (Ly5B6+) mice were mixed with 2 × 105 host-type BM cells before intravenous transfer into irradiated B6.Ly5SJL congenics. Splenocytes were stained with the indicated antibodies on day 14 after transfer. Data are representative of 2 experiments, both with at least 3 mice per group.
Generation of PDCs by RAG2/GFPlow CMPs. Five hundred GFP/RAG2+Flt3/Flk2+ or GFP/RAG2–Flt3/Flk2– CMPs from NG-BAC (Ly5B6+) mice were mixed with 2 × 105 host-type BM cells before intravenous transfer into irradiated B6.Ly5SJL congenics. Splenocytes were stained with the indicated antibodies on day 14 after transfer. Data are representative of 2 experiments, both with at least 3 mice per group.
β-Estradiol administration does not perturb PDCs or RAG2/GFPlow CMPs. Analyses of BM progenitor populations in 10-week-old NG-BAC transgenics given transplantations of placebo (cont.) or β-estradiol (E2 Rx) pellets 14 days previously as described in “Materials and methods.” BM populations analyzed included (A) ELPs, (B) EBPs/CLPs, (C) CMPs, and (D) PDCs. Note that in E2-treated mice GFP levels were elevated for both CMPs and PDCs. (E) Absolute number of cells within each indicated gate were calculated by multiplying total cells harvested by the percent viable cells within the indicated gate. Data are representative of 3 experiments. Three mice per group were used to calculate mean and SEM.
β-Estradiol administration does not perturb PDCs or RAG2/GFPlow CMPs. Analyses of BM progenitor populations in 10-week-old NG-BAC transgenics given transplantations of placebo (cont.) or β-estradiol (E2 Rx) pellets 14 days previously as described in “Materials and methods.” BM populations analyzed included (A) ELPs, (B) EBPs/CLPs, (C) CMPs, and (D) PDCs. Note that in E2-treated mice GFP levels were elevated for both CMPs and PDCs. (E) Absolute number of cells within each indicated gate were calculated by multiplying total cells harvested by the percent viable cells within the indicated gate. Data are representative of 3 experiments. Three mice per group were used to calculate mean and SEM.
The expression of μ0 and RAG-1/2 transcripts by BM PDCs and PDC-biased Flt3/Flk2+AA4low CMPs appears to represent an example of lineage promiscuity in gene expression. Previous studies have shown that myeloid and erythroid lineage genes are expressed in HSCs and, at lower levels, in early lymphoid and myeloid progenitors, respectively.35 Our data extend these observations by showing that certain lymphoid-associated genes are activated within the CMP pool. This observation suggests that transcription factors responsible for activation of early lymphoid genetic programs are transiently operative in CMPs. Although the identity of these transcriptional regulators will require further investigation, likely candidates include the Ikaros family of zinc-finger proteins, a possibility supported by the correlated expression of μ0 and RAG-1/2 with surface Flt3/Flk2 levels and previous data suggesting that Ikaros promotes Flt3/Flk2 expression.36 Other potentially relevant transcriptional regulators include the Ets family transcription factor Spi-B and the E2a-encoded E-box factors E12 and E47.37,38 Indeed, PDC differentiation from human progenitors is inhibited through overexpression of negative regulators of E-box proteins,37 and expression of RAG-1/2 is promoted in B-lineage precursors through the binding of E2a encoded proteins to the eRAG enhancer element within the RAG-1/2 locus.39
In contrast to other reports,10,12-15 we found that EBPs/CLPs are devoid of PDC potential as revealed by adoptive transfer into irradiated recipients. Moreover, more HSC-proximal lymphoid progenitors such as ELPs are also relatively inefficient PDC progenitors. Discrepancies between our adoptive transfer experiments and data reported by others may by due to one or more of several factors. First, one significant difference between our adoptive transfer protocol from other published experiments relates to the number of sorted cells transferred into each irradiated host. In our experiments, we routinely transfer no more than 2000 sorted progenitor cells per recipient, compared with 2 × 104 used in other studies.10,12,13,16 Thus, we are less likely to observe complications due to the cotransfer of small number of contaminating HSCs or CMPs. Second, we suggest that the simultaneous use of Flt3/Flk2, AA4, and RAG2 expression to define Lin–IL-7Rα+Sca-1lowc-Kitlow EBPs/CLPs is likely to provide a more rigorous scheme with which to identify and purify EBPs/CLPs. Importantly, we were able to control for EBP/CLP purity and engraftment in our adoptive transfer experiments by assessing EBP/CLP-generated B- and T-lineage development following intravenous and intrathymic transfer, respectively (Figure 2 and data not shown). Third, previous studies including work from our laboratory showed that EBPs/CLPs rapidly generate functional DCs when cultured with the appropriate cytokines.40,41 We suggest that these in vitro experiments reveal the natural plasticity of EBPs/CLPs to generate cell types other than B cells. It is conceivable that conditions in specific animal colonies or additional factors may create conditions more favorable to the generation of PDCs by cells with the EBP/CLP pool. In this regard, a recent study by Yang et al15 showed that intrasplenic transfer of 3 to 5 × 104 CLPs into irradiated hosts led to rapid PDC differentiation with kinetics that appear to closely mirror those observed on cytokine-induced DC differentiation of CLPs in vitro. It is tempting to speculate that the spleen of recently irradiated mice is highly enriched for cytokines that predispose EBPs/CLPs to generate PDCs rather than B cells. Further studies examining the relative generation of DCs and B cells following intrasplenic transfer might shed further light on this issue.
β-Estradiol treatment does not diminish VEX+PDCs, impair PDC function, or perturb their developmental kinetics. (A) Following 14 days of treatment, placebo and β-estradiol (E2 Rx) treated 10-week-old H2-VEX mice were analyzed for the presence of VEX+cells within the CLP and PDC fractions of the BM. The mean percentage of VEX+cells within either progenitor population is shown in panel B (○ indicates E2 Rx mice; •, placebo controls). (C) Placebo and β-estradiol (E2 Rx)–treated 10-week-old C57BL/6 mice were inoculated with BrdU at 12-hour intervals for 1 to 4 days as described in “Materials and methods” beginning 14 days after transplantation with placebo or β-estradiol pellets. On days 1 to 4 cohorts of 3 mice per group were examined for BrdU incorporation in BM (CD19–Ly6C+CD11c+B220+) and splenic (CD19–CD3ϵ–CD11c+CD11c+Ly6C+B220+) PDCs. ▵ indicates E2 Rx mice; □, placebo controls. (D) ELISA analysis of IFN-α production following stimulation was performed on PDCs isolated from placebo and β-estradiol–treated mice demonstrate no loss of functional activity following estrogen administration. Data from triplicate cultures were used to calculate the mean and SEM for each condition.
β-Estradiol treatment does not diminish VEX+PDCs, impair PDC function, or perturb their developmental kinetics. (A) Following 14 days of treatment, placebo and β-estradiol (E2 Rx) treated 10-week-old H2-VEX mice were analyzed for the presence of VEX+cells within the CLP and PDC fractions of the BM. The mean percentage of VEX+cells within either progenitor population is shown in panel B (○ indicates E2 Rx mice; •, placebo controls). (C) Placebo and β-estradiol (E2 Rx)–treated 10-week-old C57BL/6 mice were inoculated with BrdU at 12-hour intervals for 1 to 4 days as described in “Materials and methods” beginning 14 days after transplantation with placebo or β-estradiol pellets. On days 1 to 4 cohorts of 3 mice per group were examined for BrdU incorporation in BM (CD19–Ly6C+CD11c+B220+) and splenic (CD19–CD3ϵ–CD11c+CD11c+Ly6C+B220+) PDCs. ▵ indicates E2 Rx mice; □, placebo controls. (D) ELISA analysis of IFN-α production following stimulation was performed on PDCs isolated from placebo and β-estradiol–treated mice demonstrate no loss of functional activity following estrogen administration. Data from triplicate cultures were used to calculate the mean and SEM for each condition.
Our experiments with estrogen-treated and aged mice immediately suggest that ELPs are dispensable for PDC production. Whereas the lack of lymphoid progenitors in aged mice suggests that PDC development may not require lymphoid progenitors, these data do not rule out any changes in PDC longevity that may be associated with aging. In fact, it should be emphasized that aging leads to increased self-renewal activity for HSCs,42 and age-related changes in HSC function may result in alterations in the cellular dynamics of downstream populations such as BM PDCs.
The negative impact of sex steroids on early lymphoid progenitors has been reported33,43 ; however, little is known about their effect on DC subsets. It has been demonstrated that estrogen promotes the differentiation of CD11c+CD11bint cells from BM precursors and that this capacity was abrogated when such cells were grown ex vivo in hormone-deficient medium.44 In addition, 17β-estradiol treatment was suggested to suppress autoimmunity in an EAE model by attenuating DC function.45 Importantly, in our hands DC numbers and their function appeared to be comparable in estrogen-treated mice compared with controls. Less information is available on the consequences of aging on DC frequency and function, although there is a single report that provides evidence for a reduction in human PDC numbers as well as their capacity for IFN production.46 However, in this study only circulating blood PDCs were investigated. Whether this phenomenon is caused by a reduction in the release of PDCs from the BM or is indicative of a global reduction is not clear. However, we noted that older mice used in our studies exhibited normal PDC numbers compared with their younger counterparts.
Recent work suggests that initiation of TdT and RAG1 expression occur in nonoverlapping subsets of estrogen-sensitive Flt3/Flk2+ ELPs.31 This observation suggests that establishment of lymphoid-associated patterns of gene expression is asynchronous and likely reflective of the stochastic activation and implementation of discrete transcriptional regulatory circuits within HSC-proximal lymphoid progenitors. Our finding that PDC-biased CMPs are resistant to 17β-estradiol treatment adds further weight to this viewpoint by suggesting that estradiol receptor expression by hematopoietic progenitors does not always overlap with RAG-1/2, μ0, or Flt3/Flk2 expression.
The notion that PDC production is primarily directed via myeloid progenitors is congruent with data reported by Chicha et al34 who investigated PDC differentiation from human lymphoid and myeloid subsets in vitro. Interestingly, our data also suggest that the genotypic characteristics associated with PDCs are established in Flt3/Flk2+ CMPs. We provide evidence that expression of a number of lymphoid-associated genes, such as TdT, μ0, and Flt3/Flk2, is also detectable in these cells, suggestive of heterogeneity within the CMP pool. This phenomenon has been addressed previously. D'Amico and Wu identified the Flt3+ CMP and EBP/CLP fractions as being efficient progenitors for DCs and PDCs as compared with their Flt3/Flk2– cohorts.13 Flt3/Flk2 expression was also investigated in the CMP pool of PU.1 reporter mice,47 where these authors found that the Flt3/Flk2+ CMPs also expressed PU.1 at high levels, consistent with a myeloid phenotype, and generated granulocyte myeloid precursors (GMPs) after in vitro culture and myeloid progeny in vivo. Importantly, in agreement with previous data13 the Flt3/Flk2+PU.1hi CMPs were more efficient DC progenitors than Flt3/Flk2– subsets. Moreover, these data suggest that Flt3/Flk2 expression may be an important determinant for DC generation as Flt3/Flk2 PU.1hi CMPs demonstrate a lower capacity for DC generation.12 Why TdT and μ0 expression correlate with Flt3/Flk2 levels in CMPs is unclear. Nonetheless, our data illustrate that the CMP pool should be viewed as a heterogeneous mixture of progenitors undergoing specification toward the myeloid, erythroid, and PDC lineages. Further investigation into the mechanisms underpinning lineage specification and commitment for PDCs will greatly enhance our understanding of hematopoiesis as well as PDC differentiation and function.
Prepublished online as Blood First Edition Paper, February 28, 2006; DOI 10.1182/blood-2005-11-4545.
Supported by National Institutes of Health grants AI052861 and AI058066. D.A. is the recipient of a Career Development Award from the Leukemia and Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Avinash Bhandoola and Michael Cancro for helpful discussions and critically reviewing this manuscript. We also gratefully acknowledge the expert technical support in flow cytometry provided by the Abramson Cancer Center Flow Cytometry and Cell Sorting Shared Resource and in particular the efforts of Richard Schretzenmair, William Murphy, and William DeMuth.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal