Formation of inhibitory antibodies is a serious complication of protein or gene replacement therapy for hemophilias, congenital X-linked bleeding disorders. In hemophilia B (coagulation factor IX [F.IX] deficiency), lack of endogenous F.IX antigen expression and other genetic factors may increase the risk of antibody formation to functional F.IX. Here, we developed a protocol for reducing inhibitor formation in gene therapy by prior mucosal (intranasal) administration of a peptide representing a human F.IX-specific CD4+ T-cell epitope in hemophilia B mice. C3H/HeJ mice with a F.IX gene deletion produced inhibitory IgG to human F.IX after hepatic gene transfer with an adeno-associated viral vector. These animals subsequently lost systemic F.IX expression. In contrast, repeated intranasal administration of the specific peptide resulted in reduced inhibitor formation, sustained circulating F.IX levels, and sustained partial correction of coagulation following hepatic gene transfer. This was achieved through immune deviation to a T-helper–cell response with increased IL-10 and TGF-β production and activation of regulatory CD4+CD25+ T cells.
Introduction
In protein or gene replacement therapy for the X-linked bleeding disorders hemophilia A and B (deficiency in coagulation factor VIII or IX, respectively), the risk of production of antibodies that are inhibitory to clotting factor activity is a major concern. In conventional therapy, based on intravenous infusion of plasma-derived or recombinant protein, the incidence of inhibitors is 3% to 4% in hemophilia B and 20% to 30% in hemophilia A.1 Treatment of inhibitor patients is complicated and requires expensive bypass reagents such as protein complex concentrates and activated factor VII. Several protocols have been developed to eliminate inhibitors by frequent intravenous infusion of high doses of the clotting factor protein, often in combination with immunoglobulin infusion and immunosuppressive drug therapy.2 It is known that formation of inhibitors is dependent on activation of CD4+ T-helper cells and that the risk for this immune response is increased in subjects with large gene deletions and nonsense mutations compared with missense mutations (eg, with mutations leading to greater loss of coding information).3-7 The latter has been documented more clearly in hemophilia B. Presumably, CD4+ T cells with high-affinity T-cell receptor to factor IX (F.IX) would have been deleted or may have acquired a regulatory phenotype in thymic development in those patients who have most of the endogenous F.IX coding sequence intact. Limited data are available on strategies to block inhibitor formation a priori, which could be particularly important for subjects with “high-risk” mutations.
Adeno-associated virus (AAV)–mediated gene transfer of a functional F.IX gene to adult patients with severe hemophilia B (F.IX activity < 1% of normal) has recently been evaluated in phase 1/2 clinical trials.8,9 AAV vectors are derived from a replication-deficient, nonpathogenic parvovirus with a 4.7-kb single-stranded DNA genome. The vector genome does not contain viral coding sequences, with the result that F.IX is the only gene product expressed after gene transfer. In preclinical studies, sustained therapeutic (and even curative) expression of F.IX has been achieved after in vivo hepatic gene transfer with AAV vectors in mice and dogs with null mutations of the factor IX gene, F9 (gene deletion and early stop codon, respectively).10-12 Similarly, sustained expression of a human F.IX (hF.IX) transgene has been demonstrated in several strains of mice with this route of vector administration.13,14 Moreover, hepatocyte-derived expression can lead to induction of immune tolerance to F.IX and other secreted transgene products, which involves anergy and deletion of CD4+ T cells with receptors for the transgene product.13,15-17 Additionally, we had evidence for activation of regulatory CD4+ T cells capable of suppressing antibody formation to F.IX.13 The result is hyporesponsiveness of CD4+ T cells to F.IX and a substantially reduced ability to form inhibitors, even after injection of F.IX in adjuvant.
Nonetheless, genetic factors may reduce the ability to induce tolerance by hepatic gene transfer. Using lines of inbred mice, we found that hemophilia B mice with an F9 gene deletion on C3H/HeJ genetic background (in contrast to C57BL/6 or BALB/c) form inhibitors to F.IX after AAV gene transfer to the liver.13 Here, we used this strain of mice to determine whether the risk of inhibitor formation in hepatic gene transfer can be further reduced by mucosal antigen presentation to F.IX-specific CD4+ T cells. Antigen presentation following administration to mucosal surfaces (eg, nasal or oral) is often tolerogenic.18 A local immune response in mucosa-associated lymphoid tissues can cause activation of CD4+ T-helper cells promoting formation of IgA, which is secreted into the mucosal surface. However, because of their cytokine-expression pattern (secretion of high levels of TGF-β), these T-helper 3 (Th3) cells have immune suppressive properties elsewhere.19
In this study, we have identified a peptide containing the immunodominant CD4+ T-cell epitope of hF.IX in hemophilia B C3H/HeJ mice. This peptide was repeatedly administered by the intranasal route before hepatic AAV-hF.IX gene transfer. As a result, the incidence and titers of inhibitors formed in the context of gene transfer were reduced, and partial correction of coagulation was achieved. Cytokine release assays and adoptive transfer studies indicate that the mechanism of tolerance induction was an immune deviation, causing a shift from an adaptive Th1/Th2 to a suppressive Th2/Th3 response, which included activation of regulatory CD4+ T cells.
Materials and methods
Viral vectors
AAV vector expressing an hF.IX minigene from the apolipoprotein A enhancer linked to the human α1-antitrypsin promoter (ApoE/hAAT) was as described.13 AAV (serotype 2) vector was produced by triple transfection of HEK-293 cells and purified by repeated CsCl centrifugation as published.20 Vector titers were determined by quantitative slot blot hybridization.
Animal experiments
C3H/HeJ mice were purchased from Jackson Laboratories (Bar Harbor, ME). Hemophilia B mice without endogenous F.IX expression (due to a targeted deletion of the promoter and the first 3 exons of the F9 gene) were repeatedly backcrossed onto C3H/HeJ background (> 10 generations) prior to experiments.13,21 All experimental animals were 4- to 6-week-old males. For intranasal administration, 25 μL of PBS containing 100 μg of hF.IX-derived peptide 2A-54 or ova peptide (amino acids 323-339 of chicken ovalbumin; irrelevant peptide control) or no antigen (saline control) was instilled into each nostril of a hemophilia B C3H/HeJ mouse (while under anesthesia) using a micropipette. This procedure was carried out twice per week starting 2 weeks before and continuing until week 8 after AAV vector administration. AAV vector was infused into the portal vein of hemophilia B mice as described.22 Pooled normal C3H/HeJ mouse plasma (200 μL) was given by tail vein injection less than 30 minutes before and again less than 30 minutes after surgery in order to prevent bleeding. Blood samples from hemophilia B mice were collected in 0.38% sodium citrate buffer during bleed from the tail vein.13 Normal mice were bled from the retro-orbital plexus using heparinized capillary tubes. All studies were in accordance with protocols approved by Institutional Animal Care and Use Committees at The Children's Hospital of Philadelphia, PA, and the University of Florida, Gainesville.
T-cell epitope mapping
Hemophilia B mice (C3H/HeJ or C57BL/6 background) were immunized by subcutaneous injection on the back using 50 μg hF.IX protein emulsified in complete Freund adjuvant (cFA; GIBCO/BRL, Rockville, MD). Ten days later, splenocytes were isolated and cultured in 2-MLC medium (DMEM, 2% heat-inactivated fetal calf serum, 1 mM sodium pyruvate, 10 mM HEPES, 0.1 mM nonessential amino acids, 10–6 M 2-mercaptoethanol, and antibiotics) in the absence (mock) or presence of hF.IX (10 μg/mL) or in the presence of hF.IX-derived peptide (1 μg/mL). Peptide antigens were from a chemically synthesized library composed of 44 different 20mers overlapping by 10 amino acids at each end and spanning the entire mature hF.IX protein. Source of hF.IX protein for immunization and in vitro stimulation was high-purity plasma-derived hF.IX (Mononine; Armour Pharmaceutical, Kankakee, IL). Lymphocyte proliferation was measured by scintillation count of 3H-thymidine incorporation (triplicate measurements, 48 hours of in vitro stimulation followed by 8 hours of thymidine pulse).13
F.IX and antibody assays
Plasma levels of hF.IX antigen were measured by hF.IX-specific enzyme-linked immunosorbent assay (ELISA).3,13 Coagulation activity of plasma samples obtained from hemophilia B mice was determined by measurement of activated partial thromboplastin time (aPTT) using a fibrometer (Fibrosystem; BBL, Cockeysville, MD) and reagents from Organon Technika (Durham, NC).23 Murine plasma samples (50 μL) were added to an equal volume of F.IX-deficient human plasma prior to aPTT assay. Immunocapture assay for determination and quantitation of anti-hF.IX or anti–murine F.IX (anti-mF.IX) subclasses (IgG1, IgG2a, IgG2b, IgG3, IgA) was performed as published using antibodies from Roche Molecular Biochemicals (Indianapolis, IN) and immunoglobulin standards from Sigma (St Louis, MO).3,13,23 Inhibitory antibodies to hF.IX were measured by Bethesda assay.23 Briefly, samples were serially diluted in imidazole buffer and mixed with an equal volume of normal human plasma (Verify 1; Organon Technika) and incubated at 37°C for 2 hours. Residual coagulation activity was determined by aPTT. One Bethesda unit (BU) is equivalent to 50% residual activity. Statistical differences between experimental groups for hF.IX levels, Bethesda titers, and aPTTs were determined by unpaired Student t test and reported as significant for P less than .05.
Cytokine release assay
Lymphocytes extracted from spleens or superficial cervical lymph nodes were isolated for in vitro T-cell assays (3 animals/experimental cohort). Lymphocytes from individual animals were cultured in 2-MLC medium in the absence (mock) or presence of hF.IX (10 μg/mL).24 Conditioned media (triplicate cultures) were analyzed for cytokine secretion by ELISA using reagents from Pharmingen (San Diego, CA) or Promega (Madison, WI). IFN-γ and IL-10 were measured after 3 days of in vitro stimulation and TGF-β after 5 days.
Adoptive T-cell transfer
Splenocytes were isolated from hemophilia B C3H/HeJ mice that had received nasal administration of peptide 2A-54 and hepatic AAV-hF.IX gene transfer (3 months after gene transfer). CD4+ T cells were purified from splenocytes (pooled from 4 animals) by magnetic cell sorting (MACS) using a column for positive selection with anti–murine CD4 (Miltenyi Biotech, Auburn, CA) according to manufacturer's instructions.13 Similarly, CD4+CD25+ and CD4+CD25– cells were isolated by magnetic cell sorting using the Miltenyi CD4CD25 Isolation Kit. Here, CD4+ cells are negatively selected followed by CD25+ positive selection. Analysis by flow cytometry showed greater than 85% purity of CD4+ T cells with less than 1% CD8+ T cells and less than 7% B cells. CD4+CD25+ cells were greater than 80% pure (< 15% CD4+CD25–). CD4-depleted cells (5 × 107/recipient mouse), CD4+ cells (1 × 107/recipient mouse), CD4+CD25– cells (1 × 107/recipient mouse), or CD4+CD25+ cells (1 × 106/recipient mouse) were adoptively transferred to naive wild-type C3H/HeJ mice via tail vein injection. Control animals received naive wild-type C3H/HeJ cells. Recipient mice (n = 4-5 per experimental cohort) were subcutaneously injected with 5 μg hF.IX in cFA 36 hours after adoptive transfer.13 Anti–hF.IX IgG titers in plasma were measured 2.5 weeks after immunization.
Results
Previously, we found that hepatic AAV-mediated gene transfer to adult mice often, but not always, induced immune tolerance to the F.IX transgene product.13 Hemophilia B mice (lacking endogenous F.IX expression due to a targeted deletion of the F9 gene) on a C3H/HeJ background developed inhibitors after hepatic gene transfer. In an attempt to induce antigen-specific immune tolerance to F.IX in order to block inhibitor formation upon gene transfer, we sought to target F.IX-specific CD4+ T cells, activation of which is required for inhibitor formation.
Mapping of the immunodominant T-cell epitope in C3H/HeJ mice
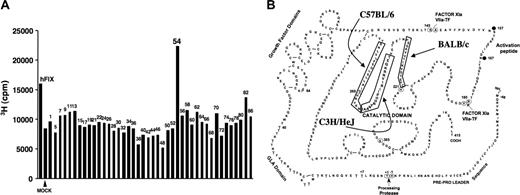
In an effort to map CD4+ T-cell epitopes of hF.IX in mice, we immunized hemophilia B mice on C3H/HeJ background (> 10 generations backcross) by subcutaneous administration of hF.IX protein in cFA. At day 10 after immunization, splenocytes were isolated and cultured in vitro in the presence of hF.IX (10 μg/mL media), a single hF.IX-derived peptide (1 μg/mL), or in mock media (no hF.IX antigen). Peptide antigens were from a hF.IX peptide library composed of 44 20mers overlapping by 10 amino acids at each end and spanning the entire mature hF.IX protein. Lymphocyte proliferation assays were carried out for each individual peptide (triplicate measurements). In 3 independent experiments, only peptide 2A-54 (TEQKRNVIRIIPHHNYNAAI) and full-length hF.IX protein yielded proliferation as determined by increase in 3H-thymidine incorporation compared with mock-stimulated cells. Proliferation was higher for peptide 2A-54 (stimulation index of 2-3) compared with hF.IX protein. A representative example is shown in Figure 1A. Splenocytes from hemophilia B mice on a C57BL/6 background proliferated upon stimulation with peptide 2A-58 (NKYNNHDIALLELDEPLVLNS), which is consistent with data published by Greenwood et al,25 who identified immunodominant CD4+ T-cell epitopes for hF.IX in C57BL/6 and BALB/c mice. Combined data from our study and the Greenwood et al25 study show that peptides containing immunodominant CD4+ T-cell epitopes in C57BL/6 (H-2b), BALB/c (H-2d), and C3H/HeJ (H-2k) mice are clustered in a sequence of hF.IX that is part of the catalytic domain of the serine protease (Figure 1B).25 There is no overlap between the epitopes in these 3 strains.
Effect of nasal peptide administration on immune responses and efficacy gene transfer
The hF.IX peptide described in the previous paragraph (100 μg 2A-54 peptide in 25 μL of PBS per nostril) or saline control was administered into both nostrils of hemophilia B C3H/HeJ mice twice a week, starting 2 weeks before and continuing until 8 weeks after hepatic gene delivery of AAV-hFIX vector (1 × 1011 vector genomes [vg]/mouse).26 Control mice formed inhibitors within 4 weeks after gene transfer (Figure 2A). Ten of 15 mice treated with saline had Bethesda titers of between 1 and 6 BU, and 6 of 7 mice treated with an irrelevant peptide (ova) had between 1 and 5 BU (Figure 2A). Mice treated with peptide 2A-54 had undetectable to less than 1 BU (6 of 6 animals; Figure 2A), indicating that hF.IX-specific peptide administration had suppressed gene transfer–induced inhibitor formation. Titers of inhibitors to hF.IX were consistently reduced compared with controls when analyzed 4, 12, and 20 weeks after gene transfer, reaching statistical significance at 4 and 12 weeks (Figure 2A-C). These results also show that the epitope contained in peptide 2A-54 (initially identified by immunization with F.IX in adjuvant) is presented to T cells in the gene transfer protocol. Administration of peptide 2A-54 largely eliminated the subset of hemophilia B mice that gave a higher inhibitor response. At all 3 time points, 50% to 80% of control mice produced at least 2 BU, whereas 0% to 20% of 2A-54–treated mice had at least 2 BU (Figure 2A-C).
Mapping of dominant hF.IX CD4+ T-cell epitopes. (A) Representative result of T-cell proliferation following in vitro stimulation with individual peptides of a hF.IX peptide library. Splenocytes were isolated from hemophilia B C3H/HeJ mice after immunization with hF.IX in cFA. Incorporation of 3H thymidine was measured and compared with mock- and hF.IX protein–stimulated cells. cpm indicates counts per minute. (B) Diagram of hF.IX primary amino acid sequence. Boxes indicate peptides that contain dominant CD4+ T-cell epitopes in hemophilia B mice on C57BL/6, C3H/HeJ (this study), or BALB/c (Greenwood et al25 ) genetic background. Also indicated are domains and protease cleavage sites and numbering of some reference amino acids.
Mapping of dominant hF.IX CD4+ T-cell epitopes. (A) Representative result of T-cell proliferation following in vitro stimulation with individual peptides of a hF.IX peptide library. Splenocytes were isolated from hemophilia B C3H/HeJ mice after immunization with hF.IX in cFA. Incorporation of 3H thymidine was measured and compared with mock- and hF.IX protein–stimulated cells. cpm indicates counts per minute. (B) Diagram of hF.IX primary amino acid sequence. Boxes indicate peptides that contain dominant CD4+ T-cell epitopes in hemophilia B mice on C57BL/6, C3H/HeJ (this study), or BALB/c (Greenwood et al25 ) genetic background. Also indicated are domains and protease cleavage sites and numbering of some reference amino acids.
Inhibitory antibodies to hF.IX (measured as Bethesda units [BUs]) as a function of time after hepatic AAV-hF.IX gene transfer in hemophilia B C3H/HeJ mice. (A) Four weeks. (B) Twelve weeks. (C) Twenty weeks. Mice had received repeated intranasal administration of ova peptide (♦), hF.IX-derived peptide 2A-54 (▴), or saline control (•). Each data point represents an individual animal. Horizontal bars show average antibody titers. P values are indicated. Numbers of mice are indicated for each cohort and time point.
Inhibitory antibodies to hF.IX (measured as Bethesda units [BUs]) as a function of time after hepatic AAV-hF.IX gene transfer in hemophilia B C3H/HeJ mice. (A) Four weeks. (B) Twelve weeks. (C) Twenty weeks. Mice had received repeated intranasal administration of ova peptide (♦), hF.IX-derived peptide 2A-54 (▴), or saline control (•). Each data point represents an individual animal. Horizontal bars show average antibody titers. P values are indicated. Numbers of mice are indicated for each cohort and time point.
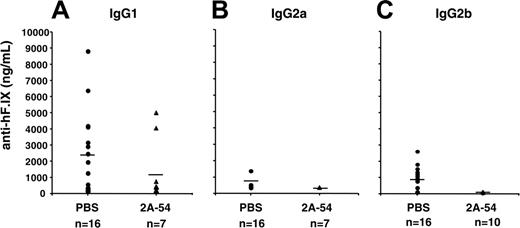
Analysis of plasma samples by immunoglobulin-specific ELISA showed that antibodies to hF.IX were predominantly IgG1, but also included IgG2b, whereas IgG2a responses were infrequent (Figure 3). These results indicate that Th2 responses were primarily driving antibody formation. No IgA or IgG3 anti-hF.IX was detected in the plasma (data not shown). Circulating antibodies to peptide 2A-54 were found to be at or below the limit of detection of this assay (< 200 ng/mL; data not shown). Nasal administration of peptide 2A-54 by itself (ie, in the absence of gene transfer) did not result in antibody formation to hF.IX protein (data not shown).
Anti-hF.IX titers for C3H/HeJ hemophilia B mice treated with hF.IX-derived peptide 2A-54 (▴) or saline control (•) 2 weeks after hepatic AAV-hF.IX gene transfer. Shown are IgG1 (A), IgG2a (B), and IgG2b (C) titers. Each data point represents an individual animal. Horizontal bars show average antibody titers. Numbers of mice are indicated for each cohort.
Anti-hF.IX titers for C3H/HeJ hemophilia B mice treated with hF.IX-derived peptide 2A-54 (▴) or saline control (•) 2 weeks after hepatic AAV-hF.IX gene transfer. Shown are IgG1 (A), IgG2a (B), and IgG2b (C) titers. Each data point represents an individual animal. Horizontal bars show average antibody titers. Numbers of mice are indicated for each cohort.
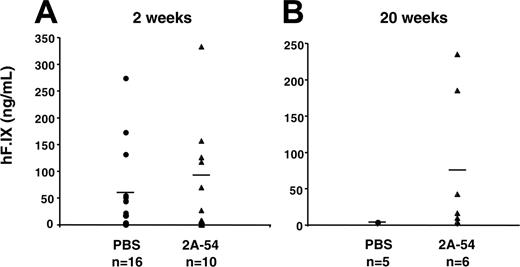
While systemic levels of hF.IX antigen were similar for peptide and control groups at 2 weeks after gene transfer (Figure 4A), systemic expression was subsequently lost in the control group, whereas 2A-54–treated mice continued to express hF.IX for at least 20 weeks (5/6 mice analyzed had detectable hF.IX levels ranging from 10-235 ng/mL; Figure 4B; note that hepatic gene transfer with AAV or other viral vectors is typically less efficient in C3H/HeJ mice compared with other strains such as the commonly used C57BL/6).
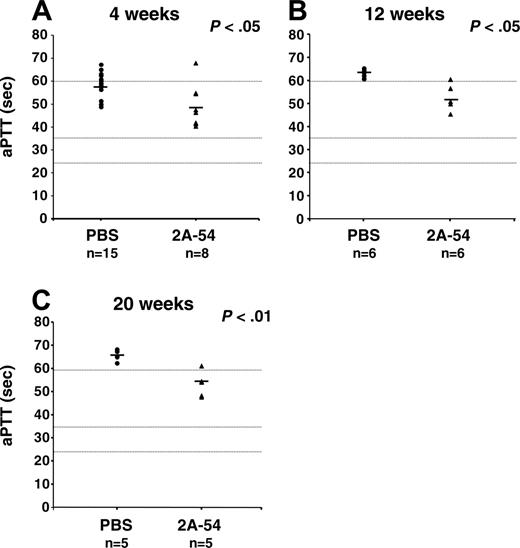
Average coagulation times (aPTTs) of plasma samples were consistently approximately 10 seconds faster for 2A-54–treated mice compared with controls, reaching statistical significance at all time points tested (4, 12, and 20 weeks; Figure 5A-C). Average hF.IX antigen levels in 2A-54–treated mice were approximately 2% of normal hF.IX plasma levels (∼100 ng/mL, weeks 2 and 20; Figure 4) and therefore in good agreement with average coagulation times. Average aPTTs represented hF.IX activities of approximately 3% (week 4), approximately 2% (week 12), and approximately 2% (week 20; Figure 5 and data not shown). Controls had approximately 1% average hF.IX activity at 4 weeks and less than 1% at later time points. The most informative time point to evaluate the effect of hF.IX-specific peptide administration on efficacy of gene transfer is week 20 (data from 3 assays are available for these plasma samples). Five months after hepatic gene transfer, only mice from the cohort treated with peptide 2A-54 had circulating hF.IX antigen and partially corrected coagulation times, and inhibitor titers were reduced (Figures 2C, 4B, and 5C).
Systemic hF.IX levels in for C3H/HeJ hemophilia B mice treated with hF.IX-derived peptide 2A-54 (▴) or saline control (•) after hepatic AAV-hF.IX gene transfer. (A) Two weeks. (B) Twenty weeks. Each data point represents an individual animal. Horizontal bars show average hF.IX levels in plasma. Numbers of mice are indicated for each cohort and time point.
Systemic hF.IX levels in for C3H/HeJ hemophilia B mice treated with hF.IX-derived peptide 2A-54 (▴) or saline control (•) after hepatic AAV-hF.IX gene transfer. (A) Two weeks. (B) Twenty weeks. Each data point represents an individual animal. Horizontal bars show average hF.IX levels in plasma. Numbers of mice are indicated for each cohort and time point.
Activated partial thromboplastin times (aPTTs in s) of C3H/HeJ hemophilia B mice treated with hF.IX-derived peptide 2A-54 (▴) or saline control (•) after hepatic AAV-hF.IX gene transfer. (A) Four weeks. (B) Twelve weeks. (C) Twenty weeks. Each data point represents an individual animal. Horizontal bars show average antibody titers. Numbers of mice are indicated for each cohort and time point.P values are indicated. Coagulation times were greater than 60 seconds for pooled plasma from untreated C3H/HeJ hemophilia B mice and were 25 to 35 seconds for pooled wild-type C3H/HeJ plasma. These parameters are indicated as dotted horizontal lines.
Activated partial thromboplastin times (aPTTs in s) of C3H/HeJ hemophilia B mice treated with hF.IX-derived peptide 2A-54 (▴) or saline control (•) after hepatic AAV-hF.IX gene transfer. (A) Four weeks. (B) Twelve weeks. (C) Twenty weeks. Each data point represents an individual animal. Horizontal bars show average antibody titers. Numbers of mice are indicated for each cohort and time point.P values are indicated. Coagulation times were greater than 60 seconds for pooled plasma from untreated C3H/HeJ hemophilia B mice and were 25 to 35 seconds for pooled wild-type C3H/HeJ plasma. These parameters are indicated as dotted horizontal lines.
Nasal administration of hF.IX-specific peptide causes a deviation in T-cell responses
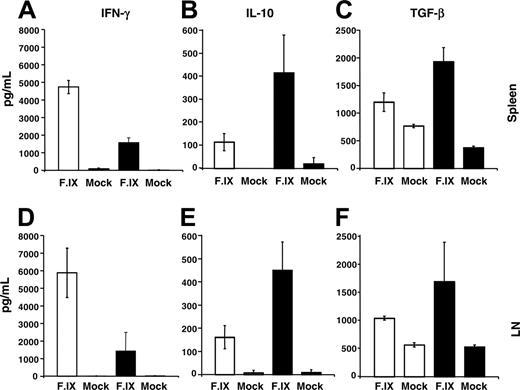
Four months after gene transfer, splenocytes and cells from the superficial cervical lymph node (draining the respiratory tract) were isolated and restimulated in vitro with hF.IX followed by measurement of cytokine release. Compared with saline controls, cells from 2A-54 peptide–treated mice produced less IFN-γ and more IL-10 and TGF-β (results were similar for both lymphoid organs; Figure 6), suggesting a shift from a Th1/2 response to a Th2/3 response.
Cytokine release upon in vitro stimulation of splenocytes and superficial cervical lymph node (LN) cells with hF.IX-containing or mock media. Average numbers ± SD are for 3 individual animals/experimental group of cells isolated from C3H/HeJ hemophilia B mice treated with hF.IX-derived peptide 2A-54 (▴) or saline control (□). Cells were isolated 4 months after hepatic AAV-hF.IX gene transfer. (A-C) Splenocytes. (D-F) LN cells. (A,D) IFN-γ. (B,E) IL-10. (C,F) TGF-β.
Cytokine release upon in vitro stimulation of splenocytes and superficial cervical lymph node (LN) cells with hF.IX-containing or mock media. Average numbers ± SD are for 3 individual animals/experimental group of cells isolated from C3H/HeJ hemophilia B mice treated with hF.IX-derived peptide 2A-54 (▴) or saline control (□). Cells were isolated 4 months after hepatic AAV-hF.IX gene transfer. (A-C) Splenocytes. (D-F) LN cells. (A,D) IFN-γ. (B,E) IL-10. (C,F) TGF-β.
Activation of regulatory CD4+CD25+ T cells
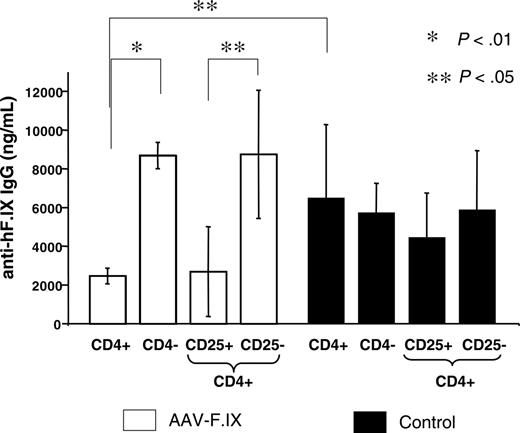
In order to test for active suppression of antibody formation in mice treated with the nasal tolerance protocol, we adoptively transferred splenocytes from 2A-54–treated hemophilia B mice (3 months after hepatic gene transfer) to naive wild-type C3H/HeJ mice. Recipient mice were immunized 36 hours later by subcutaneous administration of hF.IX in cFA. Compared with controls, which received CD4+ splenocytes from naive C3H/HeJ mice, anti–hF.IX IgG formation was significantly reduced when CD4+ cells, but not CD4-depleted splenocytes, were transferred, indicating activation of regulatory CD4+ T cells in our combination peptide/gene transfer protocol (Figure 7).
In immune competent animals, a subset of CD4+ T cells (5%-10% of total CD4+ T cells) constitutively expresses CD25 (the α chain of the IL-2 receptor) and represents naturally occurring regulatory cells that suppress CD4+CD25– effector cells through a contact-dependent mechanism.27,28 These cells are required to avoid autoimmunity. Alternatively, CD4+CD25– regulatory cells (that function through secretion of suppressive cytokines) may have been induced.29 In our study, suppression of antibody formation was lost if CD4+ cells were depleted of CD25+ cells prior to adoptive transfer (CD4+CD25– cells in Figure 7). In contrast, CD4+CD25+ cells from peptide/vector-treated mice were as effective in suppressing anti-hF.IX formation as total CD4+ cells (Figure 7). Mice that received CD4+CD25+ cells from controls showed only a minor reduction in anti-hF.IX titers (not significant) compared with mice that received total CD4+, CD4+CD25–, or CD4– control cells and on average did not achieve the level of suppression obtained with CD4+CD25+ cells from peptide-treated mice (Figure 7).
Discussion
Antigen-specific CD4+ T cells have been targeted extensively in studies on autoimmune diseases in an effort to establish a state of hyporesponsiveness to the causal protein antigen. Because inhibitor formation is dependent on T help, CD4+ T cells should also be ideal targets to prevent detrimental immune responses in treatment of hemophilia.30 The outcome of T-cell activation is substantially influenced by the location of antigen presentation. For example, in AAV-mediated gene transfer of a neo-antigen, a local immune response limits this approach in skeletal muscle, whereas hepatic gene transfer often promotes immune tolerance.13,16,24,31 Nevertheless, similar to protein-based therapies, a particular combination of the underlying mutation in the F9 gene and other genetic factors may still result in inhibitor formation after hepatic gene transfer.32 Treatment under these circumstances will therefore only be successful if protocols are developed that limit activation of pathogenic T cells. We chose to test mucosal antigen administration for specific targeting of CD4+ T cells reactive to hF.IX.
Adoptive transfer of splenocytes from hemophilia B C3H/HeJ mice, which had received intranasal administration of peptide 2A-54 and hepatic AAV-hF.IX gene transfer (□). Recipients were naive wild-type C3H/HeJ mice, and controls (▴) received splenocytes from other naive wild-type C3H/HeJ mice. All recipients were immunized with hF.IX in cFA 36 hours after adoptive transfer. Shown are average anti-hF.IX IgG titers ± SD for cohorts of mice that had received CD4–, CD4+, CD4+CD25+, or CD4+CD25– cells (2.5 weeks after immunization, n = 4-5 per experimental cohort).P values are indicated.
Adoptive transfer of splenocytes from hemophilia B C3H/HeJ mice, which had received intranasal administration of peptide 2A-54 and hepatic AAV-hF.IX gene transfer (□). Recipients were naive wild-type C3H/HeJ mice, and controls (▴) received splenocytes from other naive wild-type C3H/HeJ mice. All recipients were immunized with hF.IX in cFA 36 hours after adoptive transfer. Shown are average anti-hF.IX IgG titers ± SD for cohorts of mice that had received CD4–, CD4+, CD4+CD25+, or CD4+CD25– cells (2.5 weeks after immunization, n = 4-5 per experimental cohort).P values are indicated.
Because of proteolytic barriers in the digestive tract, nasal administration is likely to result in tolerance induction with lower antigen doses compared with oral administration. Use of small peptides instead of the native protein has the additional safety feature of a reduced risk of accidental induction of an antibody following mucosal antigen administration. If an antibody was formed against the peptide, the immunoglobulin is unlikely to cross-react or have high affinity to the cognate native protein. Furthermore, peptides cross epithelia more easily than large proteins and may therefore be more efficacious.18,26,33
Our data illustrate the feasibility of the approach of nasal peptide administration toward blocking inhibitor formation to hF.IX. While most inhibitor titers in these control groups would be considered low in a clinical setting (< 5 BU), these titers are nonetheless sufficient to eliminate therapeutic effects of transgene expression in gene therapy or of usual doses in protein therapy.32 Moreover, such titers would likely increase upon injection of exogenous hF.IX. The functional consequence of suppressing inhibitor formation by nasal peptide administration is obvious from partial correction of coagulation from hepatocyte-derived hF.IX in this cohort of hemophilia B mice (Figure 5).
While the 20-week time point showed the most significant difference in coagulation times between A2-54–treated and control groups (consistent with the difference in circulating hF.IX levels), low-titer inhibitors (1-2 BU) were also measured in A2-54–treated mice (Figure 2C), and average aPTTs were slightly increased compared with earlier time points (Figure 5). This may mean that hyporesponsiveness is less robust at late time points or, alternatively, may reflect variation in detection of very low-titer antibodies. It will be important to carry out longer-term studies and to test whether low-titer antibodies can be further reduced if peptide administration was periodically repeated at late time points. Furthermore, it is possible that subdominant epitopes may have contributed to a weak immune response that emerged over time. Administration of F.IX protein instead of an individual peptide may shed light on this question. Immunoglobulin subtyping suggests that activation of Th2 cells was largely responsible for inhibitor formation. However, control mice also showed activation of Th1 cells as judged from production of the proinflammatory cytokine IFN-γ upon stimulation with hF.IX. The data suggest that a mixed Th1/Th2 response was prone to result in inhibitor formation, similar to our published data on inhibitor formation in muscle gene transfer.24 Mucosal peptide administration likely caused a shift toward a Th2/Th3 response, in which production of IFN-γ was decreased and production of anti-inflammatory and immunosuppressive cytokines IL-10 and TGF-β was increased. This process is distinct from our previous findings on tolerance induction by hepatic gene transfer.24 We do not know whether cytotoxic T lymphocytes (CTLs) are activated in hemophilia B C3H/HeJ mice upon hepatic AAV gene transfer. We can therefore not rule out that peptide treatment suppressed CTLs in addition to antibody responses.
Experimental animals that can be tolerized to hF.IX by hepatic gene transfer alone show a decrease in Th1- and Th2-type cytokines. Increases in cytokine production documented here indicate that T-helper cells were activated and that we had achieved decreased antibody formation through an immune deviation mechanism rather than T-cell tolerance.
It is possible that the deviation from an adaptive to a suppressive response facilitated activation of regulatory T cells capable of suppressing antibody formation. Adoptive transfer studies demonstrate activation of CD4+CD25+ regulatory T cells, which were required to achieve CD4+ T-cell–mediated suppression of antibody formation. Based on data from the literature, it is likely that both routes (nasal and hepatic) of antigen administration/expression contributed to generation of regulatory T cells.13,33-35 Additional studies are necessary to assess whether these cells are naturally occurring or induced regulatory CD4+CD25+ T cells, whether they express molecules typically associated with this type of regulatory cell such as FoxP3 or GITR, and what their mechanism of action is. Other studies provide evidence that TGF-β can mediate in vivo expansion of CD4+CD25+ regulatory T cells regulated, as well as generation of CD4+CD25+ regulatory T cells from CD4+CD25– cells.36,37 These secondary regulatory T cells generated by “infectious tolerance” may act through IL-10 and TGF-β production.37 IL-10 has also been implemented in generation of CD4+CD25+ regulatory T cells.38 On the other hand, CD4+CD25– cells (such as Th3 or Tr1 cells), which may contribute to the hyporesponsiveness through secretion of anti-inflammatory cytokines, were not capable of actively suppressing antibody formation to hF.IX after adoptive transfer, unless these cells were mostly present in an activated state, which leads to transient expression of CD25. Interestingly, those animals with the highest anti-hF.IX response following immunization were within the cohort that had received CD4+CD25– cells from peptide/gene transfer–treated mice (Figure 7), which may suggest that this population contained effector T cells that enhanced antibody formation in the absence of regulatory CD4+CD25+ cells.
There are several alternative strategies to induce a state of hypo-responsiveness to hF.IX. Zhang et al39 described retroviral hepatic gene transfer to neonatal animals, which resulted in immune tolerance in hemophilia B mice. Success of this protocol in humans will depend on whether the human immune system is immature enough at birth to allow tolerance induction. Additionally, risks associated with insertional mutagenesis of an integrating vector remain to be evaluated. Data from our laboratory and Dr Ponder's laboratory (Zhang et al39 ) illustrate the usefulness of hemophilia B C3H/HeJ mice as a general model for development of tolerance induction protocols. While it is likely that viral vector particles provided activation signals to the immune system in our study, the inhibitor response to F.IX in adult hemophilia B C3H/HeJ mice is not specific to the AAV vector but is also seen in hepatic retroviral gene transfer and in protein therapy.39 Another alternative is expression of IgG fusion proteins after gene transfer to B cells, as pioneered by Lei and Scott.40 Expression of human F.VIII–derived domains (fused to the IgG scaffold) in B cells reduced inhibitor formation in hemophilia A mice after injection of hF.VIII protein.40 Administration of certain immune suppressive drugs has been shown to block inhibitor formation in hemophilia B mice and dogs in the context of AAV gene transfer.23,41 These substances are not antigen specific and therefore cause risks associated with general immune suppression. Blockers of costimulatory signals such as CTLA4-IgG or anti-CD40L have been successfully applied to limit inhibitor titers to hF.VIII in hemophilia A mice.6,42 Whereas inhibitor titers increased if coadministration with CTLA4-IgG was stopped for subsequent hF.VIII injections, more prolonged hypo-responsiveness was seen with antibody to CD40L.42
While the data presented here are encouraging, additional work will be necessary to translate the approach to human application. Inhibitor formation was suppressed but not completely abolished, which may be achieved through further optimization of dose and schedule of peptide administration. Most importantly, knowledge about CD4+ T-cell epitopes of hF.IX in humans has to be obtained. While one would expect a diversity of epitopes in the outbred human population, it is conceivable that a cocktail of peptides containing prevalent epitopes could be administered. One should not have to tolerize every T-cell with receptor to hF.IX, as long as activation of regulatory T cells is sufficient to keep pathogenic T cells in check. This may be facilitated in the context of suppressive cytokines described above. Data by others show that the mucosal route is also useful in suppressing immune responses to in the context of F.VIII or F.IX protein therapy.43,44
Prepublished online as Blood First Edition Paper, March 16, 2006; DOI 10.1182/blood-2005-11-4668.
Supported by National Institutes of Health (NIH) grant R01 AI/HL51390-01 (R.W.H.) and by the Howard Hughes Medical Institute (K.A.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 2. Inhibitory antibodies to hF.IX (measured as Bethesda units [BUs]) as a function of time after hepatic AAV-hF.IX gene transfer in hemophilia B C3H/HeJ mice. (A) Four weeks. (B) Twelve weeks. (C) Twenty weeks. Mice had received repeated intranasal administration of ova peptide (♦), hF.IX-derived peptide 2A-54 (▴), or saline control (•). Each data point represents an individual animal. Horizontal bars show average antibody titers. P values are indicated. Numbers of mice are indicated for each cohort and time point.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/2/10.1182_blood-2005-11-4668/2/m_zh80140698280002.jpeg?Expires=1769122277&Signature=vVuI1liy32m7a6IN~VF4a2vitL0kVen0ke62e~IvbrkWOLlK91y38hMUod6cLTlpNlrwBm4GgUN7S3Zotm7jIC1GKnmTIvYziyUSgU4qbJvcjLPQrNXNA~lqgMZM~CiffFpcNPMZC62GYSe3mCAnPQ7PTf5edwsg6JdKO1yIdEm7ZrVmSk2iLYZe-2U6F9SOm-mm6bMRzNHIokhfaQprdywHlYiTGjzGyYrsqUbzuOtGN9SNNSY7wbiHlLPT9amdoWQ9xNpu816TTY6mZ7n-3p5oup77CNLUBTalT1RcCfGGpfhIxijr6jTb4bHMpaxRoO6XexvyIRdlZ8VKl0vvxg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal