In this prospective randomized trial, we compared the efficacy and toxicity of cladribine (2-CdA) alone to 2-CdA combined with cyclophosphamide (CC) or cyclophosphamide and mitoxantrone (CMC) in untreated progressive chronic lymphocytic leukemia (CLL). Study end points were complete response (CR), overall response, minimal residual disease (MRD), progression-free survival, overall survival, and toxicity. From January 1, 1998 to December 31, 2003, 508 patients from 15 hematology departments were randomized. Compared with 2-CdA, CMC induced higher CR rate (36% vs 21%, P = .004), and a trend for higher CR rate with CC was observed (29% vs 21%, P = .08). Furthermore, the percentage of patients who were in CR and were MRD negative was higher in CMC compared with 2-CdA (23% vs 14%, P = .042). There were no differences in overall response, progression-free survival, and overall survival among treatment groups. Grade 3/4 neutropenia occurred more frequently in CC (32%) and CMC (38%) than in 2-CdA (20%) (P = .01 and P = .004, respectively). Infections were more frequent in CMC compared with 2-CdA (40% vs 27%, P = .02). In conclusion, CMC used in first-line treatment of CLL results in a higher CR rate and suppresses MRD more efficiently than 2-CdA monotherapy, although associates with increased toxicity. No important differences in efficacy and toxicity were found between CC and 2-CdA regimens.
Introduction
Chronic lymphocytic leukemia (CLL) is a malignant disease characterized by an accumulation of mature monoclonal B cells in the blood, bone marrow, spleen, and liver.1,2 The disease most commonly is diagnosed in the elderly (median age, 65 years), with only 10% to 15% of patients younger than 55 years.3 Management is determined by the stage and activity of the disease, with chemotherapy not normally indicated in early and stable disease.4 Moreover, one third of patients never require treatment and die from causes unrelated to CLL.5 The remaining patients, with more advanced disease, required a therapeutic intervention.
For many years, chlorambucil (Chl) has been considered the drug of choice for first-line therapy of CLL. Chl gives initial overall response rates (ORRs) of 60% to 90%, with a complete response (CR) in up to 20% of all patients.6,7 More recently, treatment approaches have included purine nucleoside analogues (PNAs), fludarabine (FA), cladribine (2-CdA, 2-chlorodeoxyadenosine), and pentostatin (2′-deoxycoformycin, DCF); 2-CdA and FA seem to be the treatment of choice for patients failing Chl therapy.7 Moreover, the role of PNAmonotherapy as a first-line treatment of CLL has been investigated in randomized studies, and the results recently have been published.8-11 These studies, including our randomized comparison of 2-CdA to Chl,11 have shown a higher ORR and CR rate in patients treated with PNA monotherapy compared with alkylating agents monotherapy, but the overall survival (OS) rates were similar.
Some clinical reports suggest that the combination of PNA with other cytotoxic drugs may increase the CR and ORR and possibly suppress minimal residual disease (MRD) and prolong survival.12-15 Among cytotoxic agents, alkylating drugs and anthracyclines (eg, mitoxantrone [MIT]) were the primary candidates for use in combination with PNA. In our previous phase 2 studies, we showed that combined treatment with 2-CdA and CY (CC regimen) or with 2-CdA, CY, and MIT (CMC regimen) gave high ORRs and CRs and acceptable toxicity in previously untreated patients with CLL.16,17
In this report, we present the results of a prospective, randomized multicenter trial comparing the efficacy and toxicity of CC and CMC combination regimens to 2-CdA alone.
Patients, materials, and methods
Criteria for eligibility
Patients with previously untreated progressive CLL, older than 18 years, were enrolled in the study. Diagnosis of CLL was established according to the National Cancer Institute–Sponsored Working Group (NCI-WG) recommended criteria.18 The clinical stage of the disease was determined according to the modified Rai classification.19,20 Progressive CLL was defined as CLL in all patients with Rai stage III and IV or CLL in those patients with stage 0, I, and II who fulfilled at least one of the following criteria: progressive lymphocytosis (doubling time shorter than 6 months), massive splenomegaly or bulky lymphadenopathy, recurrent disease-related infections, weight loss more than 10% over a 6-month period, fever of 38°C or higher related to disease, or extreme fatigue. Negative direct antiglobulin (Coombs) test was not required. Patients with poor performance status (World Health Organization [WHO] scale grade 4), hemolytic anemia, active infections, abnormal liver or renal function, Richter syndrome, and secondary neoplasm were not considered eligible. The study was approved by the Ethics Committee of the Medical University of Lodz, Poland, and all patients signed informed consent forms.
Randomization procedure and treatment modality
Central randomization was performed in the Department of Hematology, Medical University of Lodz, and the participating centers were informed about treatment assignment by phone, fax, or e-mail. Eligible patients were randomly assigned to 1 of the 3 following treatments: (1) cladribine (2-CdA) administered at a dose of 0.12 mg/kg in 2-hour intravenous infusions for 5 days; (2) CC protocol (2-CdA at a dose of 0.12 mg/kg in 2-hour intravenous infusion for 3 days + CY 650 mg/m2 intravenously on day 1); (3) CMC protocol (2-CdA at a dose of 0.12 mg/kg in 2-hour intravenous infusion for 3 days + CY 650 mg/m2 on day 1 + MIT 10 mg/m2 intravenously on day 1.
2-CdA (Biodrybin) was synthesized according to the method of Kazimierczuk et al21 and was commercially available from the Institute of Biotechnology and Antibiotics–Bioton (Warsaw, Poland). The doses and schedule of treatment were based on previous studies in CLL or low-grade lymphoma patients, performed earlier by our group.11,16,17 Regarding CMC, 3-day protocol was selected for this study based on our previous results.17 We showed that a 3-day schedule (previously referenced as CMC3) has similar efficacy and lower toxicity than a 5-day CMC (CMC5).17
The cycles were repeated every 28 days, for up to 6 courses. The response according to NCI-WG criteria18 was initially assessed after administration of 3 courses of the assigned treatment. In patients who achieved CR after 3 cycles, the treatment was stopped. In patients with partial response (PR), the treatment was continued until maximal response for up to 3 additional courses. If no response or progression of the disease was observed after the first 3 cycles, the treatment was discontinued.
If hematological complications (thrombocytopenia count < 50 × 109/L, granulocytopenia count < 0.5 × 109/L) or severe infection developed, the cycles were delayed until normalization of hematological parameters or recovery from infection. Patients who developed autoimmune hemolytic anemia (AIHA) or immune thrombocytopenia (IT) or nonhematological grade 3/4 toxicity during chemotherapy were excluded from further treatment with 2-CdA–based regimens.
No routine prophylactic antibiotics, antiviral agents, or growth factor administration was planned. Second-line treatment was at the discretion of local physicians.
Study end points, response, and toxicity criteria
The primary end point of the study was CR achieved after administration of at least 3 initial courses of one of the assigned treatments. The secondary end points were rate of CR with MRD negativity, ORR, progression-free survival (PFS), OS, and treatment-related toxicity.
Treatment effects and toxicity were monitored by physical examination, blood count evaluation, bone marrow aspiration, and biopsy. Guidelines for response were those developed by the NCI-WG.18 CR required the absence of symptoms and organomegaly, a completely normal blood cell count (absolute lymphocyte count < 4 × 109/L, absolute neutrophil count > 1.5 × 109/L, hemoglobin concentration > 110 g/L [11.0 g/dL], platelet count > 100 × 109/L), bone marrow containing fewer than 30% lymphocytes for at least 2 months, and no lymphoid infiltrate in bone marrow biopsy performed 2 months after clinical evidence of a CR. Partial response (PR) was considered in the case of a 50% or greater decrease in the size of lymph nodes, spleen, and liver on physical examination, if they were enlarged before therapy, and peripheral blood findings either identical to those of CR or improved over pretherapy values by at least 50%. Patients with nodular partial response (nPR); for instance, patients meeting criteria of CR with the exception of presence of lymphoid nodules in bone marrow biopsy, were analyzed as having PR. The patients who had not achieved CR or PR were classified as nonresponders (NRs).
PFS was defined as the time from the end of first-line therapy to disease progression or death from any cause. Disease progression was considered if at least one of the following occurred: an increase in the absolute lymphocyte count above 10 × 109/L, more than a 50% increase in the new lymph nodes, more than a 50% increase in the liver or spleen below the costal margin, the new appearance of palpable hepatosplenomegaly, or development of an aggressive lymphoma. OS was measured from the time of randomization to death or last contact.
Hematological toxicity was evaluated according to the criteria developed by the NCI-WG.18 Treatment-induced anemia, neutropenia, and thrombocytopenia were diagnosed if a further decrease of hemoglobin, neutrophils, or platelet levels was observed after chemotherapy. Only WHO grade 3 and 4 infections were recorded.22 Fever of unknown origin (FUO) requiring parenteral antibiotic therapy also was recorded as an infectious event. Infections were reported as treatment related if they developed on therapy or within 4 months of the completion of chemotherapy.
Flow cytometry assessment of residual disease
Immunophenotyping of peripheral blood and bone marrow was performed initially in all patients, and after treatment in patients with CR by dual-color flow cytometry as described by Brugiatelli et al.23 Presence of MRD in patients who achieved CR was defined as detection of more than 10% cells co-expressing CD5/CD19 and CD5/CD20 with monotypic light-chain expression of the total B-lymphocyte population according to the criteria developed by Robertson et al.24
Statistical analysis
Preliminary data have suggested that a 25% CR might be expected with 2-CdA and, thus, sample size estimation was calculated on the assumption of a treatment benefit of 15% in the CR rate with either the CMC or CC regimen. Based on error levels of α= 0.05 and β= 0.01 for a 2-sided test, 140 patients were required to be assigned to each of the treatment groups. Taking into account possible drop-outs, accrual was increased by 20%, resulting in a requirement of 168 patients per randomization arm.
Treatment groups were compared using either the Kruskal-Wallis or Mann-Whitney test for continuous data, and the chi-squared or Fisher exact test for categorical data. The influence of different variables, including type of chemotherapy, on the probability of achieving a response was analyzed initially by a chi-squared test, and then by logistic regression analysis to find independent factors. PFS and OS curves were plotted using the Kaplan-Meier method.25 Probabilities of PFS and OS were compared between groups by the log-rank test.26 A multivariate analysis of potential prognostic factors influencing PFS and OS was performed using a Cox regression model. For each analysis, a P value below .05 was considered statistically significant.
Results
Patient characteristics
Between January 1, 1998, and December 31, 2003, 508 patients from 15 hematology departments were enrolled in the trial. One hundred seventy-four patients were randomized to receive 2-CdA, 171 to receive CC, and 163 to receive CMC. Twenty-nine patients were lost from observation. Survival data were collected for 479 patients. Response data were available in 441 patients who received at least 3 courses of chemotherapy, and toxicity data were available in 479 patients. The characteristics of the patients are presented in Table 1. The 3 groups were similar with respect to age, sex distribution, disease stage, and peripheral blood count parameters.
Characteristics of 479 study patients available for analysis
Characteristics . | 2-CdA . | CC . | CMC . | P . |
|---|---|---|---|---|
| No. patients | 166 | 162 | 151 | |
| Sex, no. (%) | .52 | |||
| Male | 106 (64) | 97 (60) | 101 (69) | |
| Female | 60 (36) | 65 (40) | 50 (31) | |
| Age, median (range) | 61 (28-81) | 62 (28-80) | 59 (33-79) | .41 |
| Age groups, no. (%) | .11 | |||
| Younger than 65 y | 105 (63) | 103 (64) | 110 (73) | |
| 65 y or older | 61 (37) | 59 (36) | 41 (27) | |
| Rai stage, no. (%) | .71 | |||
| 0 | 2 (1) | 6 (4) | 3 (2) | |
| I and II | 92 (55) | 92 (57) | 80 (53) | |
| III and IV | 72 (44) | 64 (39) | 68 (45) | |
| Median white cell count, × 109/L (range) | 75 (6-820) | 79 (5-555) | 81 (5-961) | .88 |
| Median hemoglobin, g/L (range) | 120 (40-160) | 120 (50-160) | 120 (30-170) | .53 |
| Median platelet count, × 109/L (range) | 156 (11-351) | 148 (30-370) | 153 (7-378) | .60 |
Characteristics . | 2-CdA . | CC . | CMC . | P . |
|---|---|---|---|---|
| No. patients | 166 | 162 | 151 | |
| Sex, no. (%) | .52 | |||
| Male | 106 (64) | 97 (60) | 101 (69) | |
| Female | 60 (36) | 65 (40) | 50 (31) | |
| Age, median (range) | 61 (28-81) | 62 (28-80) | 59 (33-79) | .41 |
| Age groups, no. (%) | .11 | |||
| Younger than 65 y | 105 (63) | 103 (64) | 110 (73) | |
| 65 y or older | 61 (37) | 59 (36) | 41 (27) | |
| Rai stage, no. (%) | .71 | |||
| 0 | 2 (1) | 6 (4) | 3 (2) | |
| I and II | 92 (55) | 92 (57) | 80 (53) | |
| III and IV | 72 (44) | 64 (39) | 68 (45) | |
| Median white cell count, × 109/L (range) | 75 (6-820) | 79 (5-555) | 81 (5-961) | .88 |
| Median hemoglobin, g/L (range) | 120 (40-160) | 120 (50-160) | 120 (30-170) | .53 |
| Median platelet count, × 109/L (range) | 156 (11-351) | 148 (30-370) | 153 (7-378) | .60 |
Clinical response
Overall, 355 (81%) of 441 of the included subjects responded to the therapy, with 127 (29%) of 441 achieving CR and 228 (52%) of 441 achieving PR. The ORRs for the 3 treatment arms were 78% for 2-CdA, 83% for CC, and 80% for CMC (P = .46). The proportions of response according to treatment group in the whole cohort as well in subgroups regarding Rai clinical stage and age are shown in Table 2.
Comparison of response rates in the 3 treatment arms stratified by clinical stage and age
Response . | 2-CdA, % . | CC, % . | CMC, % . | P . |
|---|---|---|---|---|
| All patients | .058 | |||
| CR | 21 | 29 | 36 | |
| PR | 56 | 54 | 44 | |
| NR | 22 | 17 | 20 | |
| Patients with Rai 0-II | .10 | |||
| CR | 26 | 32 | 46 | |
| PR | 56 | 53 | 40 | |
| NR | 17 | 15 | 20 | |
| Patients with Rai III-IV | .53 | |||
| CR | 14 | 24 | 23 | |
| PR | 56 | 56 | 49 | |
| NR | 29 | 20 | 28 | |
| Patients younger than 65 y | .04 | |||
| CR | 23 | 27 | 39 | |
| PR | 57 | 61 | 43 | |
| NR | 20 | 12 | 18 | |
| Patients 65 y or older | .49 | |||
| CR | 18 | 33 | 28 | |
| PR | 56 | 43 | 47 | |
| NR | 26 | 24 | 25 |
Response . | 2-CdA, % . | CC, % . | CMC, % . | P . |
|---|---|---|---|---|
| All patients | .058 | |||
| CR | 21 | 29 | 36 | |
| PR | 56 | 54 | 44 | |
| NR | 22 | 17 | 20 | |
| Patients with Rai 0-II | .10 | |||
| CR | 26 | 32 | 46 | |
| PR | 56 | 53 | 40 | |
| NR | 17 | 15 | 20 | |
| Patients with Rai III-IV | .53 | |||
| CR | 14 | 24 | 23 | |
| PR | 56 | 56 | 49 | |
| NR | 29 | 20 | 28 | |
| Patients younger than 65 y | .04 | |||
| CR | 23 | 27 | 39 | |
| PR | 57 | 61 | 43 | |
| NR | 20 | 12 | 18 | |
| Patients 65 y or older | .49 | |||
| CR | 18 | 33 | 28 | |
| PR | 56 | 43 | 47 | |
| NR | 26 | 24 | 25 |
CR indicates complete response; NR, no response; PR, partial response.
* P value derived from chi-squared test counted for 3 × 3 cell table; for instance, table containing 3 types of responses (CR, PR, NR) in rows versus all 3 types of treatment (2-CdA, CC, CMC) in columns.
The median number of courses administered in the 2-CdA arm was 5, while in CC and CMC arms the median number of courses administered was 3. The treatment was stopped because of CR achievement after 3 cycles, as planned in the design of the study, in 10%, 13%, and 26% of patients assigned to 2-CdA, CC, and CMC arms, respectively. The treatment was discontinued due to NR or progressive disease after 3 courses in 14% of patients in the 2-CdA arm, 12% of patients in the CC arm, and in 14% of patients in the CMC arm. Treatment-related toxicity was the reason for earlier treatment withdrawal in 4%, 6%, and 2% of all patients randomized to 2-CdA, CC, and CMC arms, respectively. The median time between courses was 31 days in all treatment groups. Six courses of treatment were given in 49% of patients in the 2-CdAarm, 35% of patients in the CC arm, and in 26% of subjects assigned to the CMC arm.
Patients in the CMC group obtained the highest CR rate, 51 (36%) of 141 compared with 44 (29%) of 151 in the CC arm, and 32 (21%) of 149 in the 2-CdA arm (P = .021). Paired comparison revealed a significantly higher CR rate in the CMC arm than in the 2-CdA arm (odds ratio [OR] = 2.1 with 95% confidence interval [CI] = 1.2-3.5, P = .004) (Table 3). Of the pretreatment variables included in univariate analyses, the following factors associated with CR attainment: less advanced disease (OR = 2.1, 95% CI = 1.3-3.3, P = .001), peripheral blood lymphocyte count lower than 100 × 109/L (OR = 2.1, 95% CI = 1.3-3.5, P = .003), and female sex (OR = 1.8, 95% CI = 1.2-2.7, P = .006).
Paired comparison of efficacy 2-CdA monotherapy with CC and CMC regimens
Response . | 2-CdA vs CC, % (P) . | 2-CdA vs CMC, % (P) . |
|---|---|---|
| CR | 21/29 (.08) | 21/36 (.004) |
| CR with negative MRD | 14/20 (.18) | 14/23 (.042) |
| ORR | 78/83 (.1) | 79/80 (.4) |
Response . | 2-CdA vs CC, % (P) . | 2-CdA vs CMC, % (P) . |
|---|---|---|
| CR | 21/29 (.08) | 21/36 (.004) |
| CR with negative MRD | 14/20 (.18) | 14/23 (.042) |
| ORR | 78/83 (.1) | 79/80 (.4) |
CR indicates complete response; MRD, minimal residual disease; ORR, overall response rate.
In multivariate logistic regression analysis, the CMC regimen retained superiority over 2-CdAin terms of CR achievement (OR = 2.16, 95% CI = 1.22-2.82, P = .008), even when adjusted by the other prognostic factors, female sex (OR = 1.85, 95% CI = 1.17-2.92, P = .008), Rai stage III/IV (OR = 0.48, 95% CI = 0.29-0.78, P = .003), and initial lymphocytosis count higher than 100 × 109/L (OR = 0.52, CI = 0.31-0.87, P = .014) (Tables 4, 5).
Multivariate analyses of prognostic factors for complete response attainment (logistic regression)
Variable . | No. patients . | Risk, OR . | 95% CI . | P . |
|---|---|---|---|---|
| Treatment | ||||
| 2-CdA | 149 | 1.00 | NA | NA |
| CC | 151 | 1.55 | 0.91-0.64 | .107 |
| CMC | 141 | 2.16 | 1.22-0.82 | .008 |
| Age | ||||
| Younger than 65 y | 301 | 1.00 | NA | NA |
| 65 y or older | 140 | 0.97 | 0.60-1.58 | .903 |
| Sex | ||||
| Male | 280 | 1.00 | NA | NA |
| Female | 161 | 1.85 | 1.17-2.92 | .008 |
| Lymphocyte count | ||||
| Less than 100 × 109/L | 272 | 1.00 | NA | NA |
| Greater than or equal to 100 × 109/L | 129 | 0.52 | 0.31-0.87 | .014 |
| Stage | ||||
| Rai 0/I/II | 263 | 1.00 | NA | NA |
| Rai III/IV | 178 | 0.48 | 0.29-0.78 | .003 |
Variable . | No. patients . | Risk, OR . | 95% CI . | P . |
|---|---|---|---|---|
| Treatment | ||||
| 2-CdA | 149 | 1.00 | NA | NA |
| CC | 151 | 1.55 | 0.91-0.64 | .107 |
| CMC | 141 | 2.16 | 1.22-0.82 | .008 |
| Age | ||||
| Younger than 65 y | 301 | 1.00 | NA | NA |
| 65 y or older | 140 | 0.97 | 0.60-1.58 | .903 |
| Sex | ||||
| Male | 280 | 1.00 | NA | NA |
| Female | 161 | 1.85 | 1.17-2.92 | .008 |
| Lymphocyte count | ||||
| Less than 100 × 109/L | 272 | 1.00 | NA | NA |
| Greater than or equal to 100 × 109/L | 129 | 0.52 | 0.31-0.87 | .014 |
| Stage | ||||
| Rai 0/I/II | 263 | 1.00 | NA | NA |
| Rai III/IV | 178 | 0.48 | 0.29-0.78 | .003 |
OR indicates odds ratio; CI, confidence interval; NA, not applicable.
Multivariate analyses of prognostic factors for overall survival (proportional-hazard model)
Variable . | No. patients . | Risk, HR . | 95% CI . | P . |
|---|---|---|---|---|
| Treatment | ||||
| 2-CdA | 166 | 1.00 | NA | NA |
| CC | 162 | 0.82 | 0.55-1.21 | .308 |
| CMC | 151 | 0.98 | 0.67-1.45 | .931 |
| Age | ||||
| Younger than 65 y | 318 | 1.00 | NA | NA |
| 65 y or older | 161 | 1.49 | 1.08-2.06 | .016 |
| Sex | ||||
| Male | 304 | 1.00 | NA | NA |
| Female | 175 | 0.73 | 0.52-1.42 | .079 |
| Lymphocyte count | ||||
| Less than 100 × 109/L | 297 | 1.00 | NA | NA |
| Greater than or equal to 100 × 109/L | 182 | 1.44 | 1.03-2.00 | .032 |
| Stage | ||||
| Rai 0/II/II | 275 | 1.00 | NA | NA |
| Rai III/IV | 204 | 1.37 | 0.99-1.91 | .058 |
Variable . | No. patients . | Risk, HR . | 95% CI . | P . |
|---|---|---|---|---|
| Treatment | ||||
| 2-CdA | 166 | 1.00 | NA | NA |
| CC | 162 | 0.82 | 0.55-1.21 | .308 |
| CMC | 151 | 0.98 | 0.67-1.45 | .931 |
| Age | ||||
| Younger than 65 y | 318 | 1.00 | NA | NA |
| 65 y or older | 161 | 1.49 | 1.08-2.06 | .016 |
| Sex | ||||
| Male | 304 | 1.00 | NA | NA |
| Female | 175 | 0.73 | 0.52-1.42 | .079 |
| Lymphocyte count | ||||
| Less than 100 × 109/L | 297 | 1.00 | NA | NA |
| Greater than or equal to 100 × 109/L | 182 | 1.44 | 1.03-2.00 | .032 |
| Stage | ||||
| Rai 0/II/II | 275 | 1.00 | NA | NA |
| Rai III/IV | 204 | 1.37 | 0.99-1.91 | .058 |
HR indicates hazard ratio; CI, confidence interval; NA, not applicable.
In the group of 127 patients who achieved CR, MRD negativity was demonstrated in 17 (53%) of 32 patients in the 2-CdA arm, 22 (50%) of 44 in the CC arm, and 31 (61%) of 51 in the CMC arm (P = .6). However, regarding all 441 patients evaluated for response, paired comparisons showed that the percentage of patients who were in CR and were MRD negative was significantly higher in the CMC arm compared with the 2-CdA arm (23% vs 14%, P = .042), but not different from the CC arm (20%, P = .18) (Table 3).
Progression-free survival
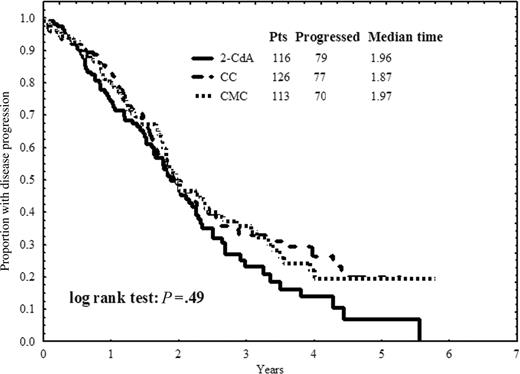
The median PFS in the whole group was 23.5 months (95% CI = 21.1-26.0). In the separate treatment groups, the median PFS was 23.5 months (95% CI = 16.0-26.8) in the 2-CdA arm, 22.4 months (95% CI = 18.0-27.0) in the CC arm, and 23.6 months (95% CI = 18.2-29.0) in the CMC arm (P = .49). The estimated PFS curves according to treatment arm are plotted in Figure 1. In univariate analyses, peripheral blood lymphocytosis counts less than 100 × 109/L emerged as the only pretreatment prognostic factor for longer PFS (P = .001).
Overall survival
The median time of OS in all patients was 56 months (95% CI = 49.2-62.8). Of the 479 patients analyzed, 173 (39%) have died, with 64 (39%), 51 (32%), and 57 (38%) in the 2-CdA, CC, and CMC groups, respectively. The most frequent causes of death were disease progression and infectious complications (Table 6).
Cause of death
Cause of death . | Total . | 2-CdA . | CC . | CMC . |
|---|---|---|---|---|
| No. of patients | 479 | 166 | 162 | 151 |
| Total deaths, no. (%) | 173 (36) | 64 (38) | 52 (32) | 57 (38) |
| Pneumonia | 38 | 14 | 13 | 11 |
| Septic shock | 33 | 9 | 7 | 17 |
| Hemorrhage | 11 | 6 | 2 | 3 |
| Second neoplasm | 8 | 1 | 3 | 4 |
| AIHA | 4 | 3 | 1 | 0 |
| CLL progression | 27 | 11 | 10 | 6 |
| Cardiac | 30 | 12 | 9 | 9 |
| Other reasons* | 22 | 8 | 7 | 7 |
Cause of death . | Total . | 2-CdA . | CC . | CMC . |
|---|---|---|---|---|
| No. of patients | 479 | 166 | 162 | 151 |
| Total deaths, no. (%) | 173 (36) | 64 (38) | 52 (32) | 57 (38) |
| Pneumonia | 38 | 14 | 13 | 11 |
| Septic shock | 33 | 9 | 7 | 17 |
| Hemorrhage | 11 | 6 | 2 | 3 |
| Second neoplasm | 8 | 1 | 3 | 4 |
| AIHA | 4 | 3 | 1 | 0 |
| CLL progression | 27 | 11 | 10 | 6 |
| Cardiac | 30 | 12 | 9 | 9 |
| Other reasons* | 22 | 8 | 7 | 7 |
AIHA indicates autoimmune hemolytic anemia.
Chronic obstructive airways disease, stroke, pulmonary embolism, accident.
There were no significant differences in survival probabilities between the 3 treatment arms (P = .73). The median OS time for the 2-CdA group was 51.2 months (95% CI = 40.7-61.8), while for the CC and CMC groups the medians have not yet been reached (Figure 2). There also were no differences in OS between the treatment groups for patients in early Rai stages of CLL (P = .6) and advanced stages of the disease (P = .23) (data not shown). We also have not shown any statistically significant difference in the OS in patients treated with 2-CdA, CC, or CMC younger than 65 years (P = .67) and patients 65 years or older (P = .86) (data not shown). The prognostic factors associated with longer OS in univariate analysis were older than 65 years (P < .001), Rai stage II/IV (P = .003), lymphocyte count higher than 100 × 109/L (P = .004), and female sex (P = .035).
The multivariate Cox regression survival analysis demonstrated that older than 65 years (OR = 1.49, 95% CI = 1.08-2.06, P = .016) and lymphocytosis count more than 100 × 109/L (OR = 1.44, 95% CI = 1.03-2.00, P = .032) retained their independent impact on OS, while only a trend was noted for more advanced disease (OR = 1.37, 95% CI = 0.99-1.91, P = .058) and female sex (OR = 0.73, 95% CI = 0.52-1.42, P = .079) (Table 4). Confirming the results of the univariate analysis, the type of randomly assigned therapy (CC and CMC compared to 2-CDA) had no influence on duration of OS.
Progression-free survival. Progression-free survival defined as time from the end of first-line therapy to disease progression or death for patients in CR or PR after treatment with 2-CdA, CC, or CMC.
Progression-free survival. Progression-free survival defined as time from the end of first-line therapy to disease progression or death for patients in CR or PR after treatment with 2-CdA, CC, or CMC.
Toxicity
Percentages of patients with grade 3/4 hematological and nonhematological toxicities according to randomization group are reported in Table 7. In paired comparisons, neutropenia was more frequent in the CC and CMC arms (32% and 38%, respectively) compared with 2-CdA–treated patients (19%) (P = .01 and P = .004, respectively). The percentages of thrombocytopenia were comparable between CMC and 2-CdA (24% vs 19%, P = .2) and CC and 2CdA (17% vs 19%, P = .4). Interestingly, there was an increase in anemia rates in the 2-CdA group compared with the CMC group (13% and 9%, respectively, P = .02). The distribution of AIHA was similar in all 3 treatment groups: 11 patients (7%) in the 2-CdA group, 13 (8%) in the CC, and 9 (6%) in the CMC group (P = .44).
Grade 3/4 hematological and nonhematological treatment side-effects stratified according to randomization group
Side effects . | 2-CdA, no. (%) . | CC, no. (%) . | CMC, no. (%) . | P . |
|---|---|---|---|---|
| Neutropenia | 32 (19) | 50 (32) | 57 (38) | .001 |
| Thrombocytopenia | 31 (19) | 28 (17) | 36 (24) | .47 |
| Anemia (including AIHA) | 21 (13) | 19 (12) | 13 (9) | .27 |
| Infections and FUO | 45 (27) | 55 (34) | 60 (40) | .057 |
| Vomiting | 0 (0) | 3 (2) | 4 (3) | .11 |
| Alopecia | 0 (0) | 0 (0) | 1 (1) | .96 |
| Aminotransferases increased | 0 (0) | 0 (0) | 1 (1) | .96 |
| Richter syndrome | 1 (1) | 2 (1) | 1 (1) | .52 |
| Secondary neoplasms | 2 (1) | 6 (4) | 7 (4) | .57 |
Side effects . | 2-CdA, no. (%) . | CC, no. (%) . | CMC, no. (%) . | P . |
|---|---|---|---|---|
| Neutropenia | 32 (19) | 50 (32) | 57 (38) | .001 |
| Thrombocytopenia | 31 (19) | 28 (17) | 36 (24) | .47 |
| Anemia (including AIHA) | 21 (13) | 19 (12) | 13 (9) | .27 |
| Infections and FUO | 45 (27) | 55 (34) | 60 (40) | .057 |
| Vomiting | 0 (0) | 3 (2) | 4 (3) | .11 |
| Alopecia | 0 (0) | 0 (0) | 1 (1) | .96 |
| Aminotransferases increased | 0 (0) | 0 (0) | 1 (1) | .96 |
| Richter syndrome | 1 (1) | 2 (1) | 1 (1) | .52 |
| Secondary neoplasms | 2 (1) | 6 (4) | 7 (4) | .57 |
There were 166 patients in the 2-CdA group, 162 in the CC group, and 151 in the CMC group.
AIHA indicates autoimmune hemolytic anemia; FUO, fever of unknown origin.
Infections occurred more frequently in patients treated with CMC (40%) than with 2-CdA (27%) (P = .02), while the percentage of infections in the 2-CdA and CC arms was similar (27% and 34%, respectively, P = .2). Other side effects, including Richter syndrome, were observed sporadically, with similar incidences in all 3 groups. The difference in incidence of secondary neoplasm was not significant, although there was a trend to increased occurrence in combination treatment groups (P = .32). We observed 2 secondary cancers in the 2-CdA group (2 lung cancers), 6 secondary neoplasms in the CC group (2 breast cancers, 2 lung cancers, 1 gastric carcinoma, and 1 clear cell carcinoma of the kidney), and 7 secondary cancers in the CMC group (3 lung cancers, 2 gastric carcinomas, 1 clear cell carcinoma, and 1 colon cancer) (Table 7).
Overall survival time. Overall survival time calculated from the first day of treatment to the last day of follow-up or death for patients treated with 2-CdA, CC, or CMC as first-line therapy.
Overall survival time. Overall survival time calculated from the first day of treatment to the last day of follow-up or death for patients treated with 2-CdA, CC, or CMC as first-line therapy.
Discussion
In this multicenter prospective randomized study, we compared CC and CMC combination chemotherapy to 2-CdA alone in previously untreated CLL. We found that the CMC regimen was more effective than 2-CdA alone in terms of CR achievement (36% vs 21%, P = .004) and achievement of CR with eradication of MRD (23.4% vs 14.0%, P = .042), but it did not increase ORR or prolonged response duration or overall survival of the CLL patients. Regarding the CC regimen, no increased response or survival advantage compared to 2-CdA could be detected.
Besides increased number of CRs in CMC compared with the 2-CdA arm, the higher proportion of patients who were CR and were MRD negative also is of interest. It should be noted that at the time of the study initiation there was no consensus regarding assessment of MRD in CLL, and the 2-color flow-cytometry method developed by Brugiatelli et al23 and applied by us is certainly less sensitive than the recent 4-color flow cytometry or molecular tests.27 Moreover, we did not observe a significant difference in the number of patients MRD negative between CMC and 2-CdA when only CR patients were considered, which may mean that the tendency to achieve MRD status depends as well on the biology of CLL in an individual patient. Nevertheless, data considering the level of MRD suppression in CLL remission required for clinical benefit are limited.15,28,29 Recently, Moreton et al showed that MRD-negative remission detected by sensitive 4-color flow cytometry assay is achievable with alemtuzumab in a substantial proportion of patients, leading to an improved overall and treatment-free survival.28 Also, Moreno et al29 revealed that MRD status, measured both by quantitative polymerase chain reaction and flow cytometry, correlates with PFS and OS in CLL patients treated with autologous transplantation.
Of the pretreatment variables included in univariate analyses, Rai 0-II, peripheral blood lymphocyte counts less than 100 × 109/L, and female sex were the factors associated with CR attainment. Similar, better response in females has been observed by Catovsky et al30 in MRC trials. These results suggest a major biologic difference between the sexes, which has not been explained yet.
To the best of our knowledge, our study is the first phase 3 randomized comparison of 2-CdA–based combination chemotherapy with CY and MIT to 2-CdA alone. However, FA, another PNA used in the treatment of CLL, also has been shown to exert high activity when combined with CY, MIT, or both of these drugs.13-15,31-33 Significant activity of FA combined with CY in 128 chemotherapy-naive and previously treated patients has been demonstrated by O'Brien et al.13 Recently, Eichhorst et al reported the results of a phase 3 trial that confirmed higher efficacy of a fludarabine with cyclophosphamide (FC) protocol than FA alone in the treatment-naive advanced CLL.34 In total, 375 patients up to age 65 years were randomized to either FA alone (25 mg/m2 for 5 days, intravenously, every 28 days) or FA + CY (FA 30 mg/m2 and CY 250 mg/m2/d, both for 3 days, intravenously, every 28 days). The ORR and CR rates were significantly higher in the FC arm (94% and 24%, respectively) compared with the FA arm (83% and 7%, respectively). Furthermore, the most important impact of this study was associated with the observation that PFS of FC-treated patients was significantly longer compared with patients receiving FA. Compared with our results, FC seemed to induce a lower CR rate than the CC and CMC regimen (24% vs 29% and 36%, respectively) but higher ORR (94% vs 83% and 80%, respectively). Interestingly, some part of the difference in efficacy of PNA combination treatments assessed in these 2 trials may be a consequence of different modes of administration and higher total doses of CY in the FC regimen (250 mg/m2/d for 3 days compared to 650 mg/m2/d on day 1 in our trial). Of note, a preliminary analysis of phase 3 comparison of CC and FC with identical dosing of CY (250 mg/m2/d for 3 days) produced similar CR, ORR, and overall survival.35 It should be emphasized that in contrast to the Eichhorst et al study,34 we were not able to detect any prolongation of PFS using combination regimens. However, neither study showed differences in OS, which is the major goal of therapy of indolent lymphoma. The comparison of both trials with regard to survival and toxicity is complicated by differences in the target patient population. The study of Eichhorst et al34 included only patients younger than 65 years who are expected to have better survival and be less vulnerable to treatment toxicity than older patients, who constituted one third of the population treated in our trial. Fludarabine combined with CY and MIT (FCM) also seems to be a highly effective regimen in CLL. Bosch et al15 observed a 50% CR rate after administration of the FCM regimen in 60 patients with resistant or relapsed CLL. Interestingly, 17% of all patients achieved MRD-negative status as confirmed by cytofluorometric and molecular techniques. However, the results of randomized comparisons of FCM combination with other anti-CLL treatments are not currently available.
Despite significant differences in CR rates, the median OS did not differ in patients treated with CMC as compared with the 2-CdA–treated group. This is in agreement with the results of other randomized trials comparing PNA with alkylators or with PNA-based combinations, in which higher response rate, and even higher response duration, did not translate into longer OS.8-11,34 It is likely that significant improvements in prognosis in CLL patients may be achieved when PNAs are combined with monoclonal antibodies, rituximab, and, potentially, alemtuzumab.28,36-38 The recent report of a 2-phase study (CALGB 9712) comparing concurrent and sequential combination of FA and rituximab (FR) in 104 untreated CLL patients showed ORR of 90% (47% CR, 43% PR) in concurrent treatment arms compared with 77% (28% CR, 49% PR) with the sequential regimen.36 Interestingly, comparison of all 104 subjects treated with FR within this study, with historical controls receiving only FA (CALGB 9011 protocol), revealed significantly longer PFS and OS.37 Although the results of this retrospective analysis strongly suggest that addition of rituximab to FA may prolong survival of CLL patients, this needs to be confirmed in a direct phase 3 prospective trial because of potential confounding factors; for example, differences in supportive care. Furthermore, a single-institution study on FA, CY, and rituximab combination (FCR) produced an exceptionally high CR rate of 70%, with a projected PFS of 69% at 4 years in untreated CLL patients; thus, inclusion of this regimen into multicenter phase 3 studies is warranted.38 In the context of these promising results of rituximab and FA combinations, it seems possible that incorporating rituximab into other PNA-based regimens; for example, CMC or CC, also may be of clinical benefit for patients with CLL.
In our study, CMC and CC regimens caused neutropenia more frequently than 2-CdA alone. Higher myelotoxicity of PNA in combination with other cytotoxic drugs also was observed in other studies.34,39,40 In a previous phase 2 trial, we observed high myelotoxicity of CMC, which was the reason for reduction of 2-CdA administration from 5 to 3 days in combined regimens in this study.17 Similar reductions of PNA administration from 5 to 3 days when used in combination with CY or MIT were introduced in other studies.13-15,41 We also found an increase in grade 3/4 infections in patients treated with the CMC regimen (40%) than in patients treated with 2-CdA alone (27%, P = .02), but no difference was found for the CC group (34%). It is likely that routine prophylactic administration of growth factor (G-CSF) would reduce the infection rate in CMC and CC programs.42,43 Further decrease in treatment toxicity might be achieved by reduction of combination drug doses, but this approach also could compromise response.44,45
In conclusion, we found that the CMC regimen used for the treatment of progressive CLL gives a higher complete response rate and higher complete response rate with negative MRD than 2-CdA monotherapy, although it does not prolong survival. Based on the results of our study, we believe that 2-CdA monotherapy currently has a limited role in CLL treatment. High efficacy and acceptable toxicity of CMC necessitates its serious consideration in future clinical trials, especially in combination with monoclonal antibodies.
Appendix
Investigators, in alphabetical order of cities: Bialystok, Department of Hematology, Medical University: Janusz Kloczko, Jaroslaw Piszcz; Gorzow Wielkopolski, City Hospital: Barbara Moskwa-Sroka; Katowice, Department of Hematology and Bone Marrow Transplantation, Medical University: Beata Stella-Holowiecka, Jacek Najda; Krakow, City Hospital: Jacek Dybowicz, Andrzej Zdunczyk; Krakow, Department of Hematology, Jagiellonian University: Aleksander B. Skotnicki, Wieslaw Nowak; Lodz, Department of Hematology Medical University of Lodz: Tadeusz Robak, Jerzy Z. Blonski, Joanna Gora-Tybor, Krzysztof Jamroziak, Marek Kasznicki; Lublin, Department of Hematology and Bone Marrow Transplantation, Medical University of Lublin: Anna Dmoszynska, Malgorzata Kowal; Poznan, Department of Hematology Medical University of Poznan: Krystyna Zawilska, Alina Grzywacz; Szczecin, Department of Hematology, Pomeranian Medical University: Barbara Zdziarska; Torun, City Hospital: Malgorzata Calbecka, Aleksandra Kostyra; Warsaw, Department of Hematology and Oncology, Medical University of Warsaw: Jadwiga Dwilewicz-Trojaczek, Agnieszka Tomaszewska; Warsaw, Department of Internal Medicine, Military Institute of Medicine: Kazimierz Sulek, Krzysztof Gawronski; Warsaw, Institute of Hematology and Transfusiology: Krzysztof Warzocha, Lech Konopka, Bernadetta Ceglarek, Ilona Seferynska; Wroclaw, Department of Hematology, Medical University: Kazimierz Kuliczkowski, Dariusz Wolowiec, Stanislaw Potoczek.
Prepublished online as Blood First Edition Paper, March 21, 2006; DOI 10.1182/blood-2005-12-4828.
Supported in part by grants 4P05B0619 and 2P05B01828 from the Ministry of Science, Warsaw, Poland.
Preliminary results of this study were presented at the 44th Annual Meeting of the American Society of Hematology, Philadelphia, PA, December 6-10, 2002;46 and at the 10th International Workshop on CLL, Stresa, Lake Maggiore, Italy, October 10-12, 2003;47 and at the 46th Annual Meeting of the American Society of Hematology, San Diego, CA, December 4-7, 2004;48 and at the 11th International Workshop on CLL, New York, NY, September 16-18, 2005.49
A complete list of the members of the Polish Leukemia Group appears in “Appendix.”
T.R. designed and supervised the trial and wrote the report; J.Z.B., J.G.-T., and M. Kasznicki were responsible for patient's accrual and made analysis of the trial; K.J. monitored the trial and performed statistical analysis of the data; J.D.-T., A.T., B.C., J.K., A.D., M. Kowal, K.Z., B.S.-H., K.S., K.K., M.C., A.B.S., and K.W. were responsible for patient's accrual, monitoring, and management of the clinical data at their referring centers.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Prof Gunnar Juliusson (University Hospital, Lund, Sweden) for his critical reading of the manuscript. We thank Dr Richard Szydlo (Imperial College and Hammersmith Hospital, London, United Kingdom) for statistical advice.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal