Abstract
Using Affymetrix microarrays, we identified the expression of the CD200 gene in multiple myeloma cells (MMCs) of 112 patients with newly diagnosed multiple myeloma (MM). The CD200 gene was either absent or present (Affymetrix call) in 22% and 78% of MMCs, respectively. The CD200 gene is not expressed in cells of the patients' bone marrow (BM). CD200 is a membrane glycoprotein that imparts an immunoregulatory signal through CD200R, leading to the suppression of T-cell–mediated immune responses. Patients with CD200absent MMCs have an increased event-free survival (EFS; 24 months) compared with patients with CD200present MMCs (14 months), after high-dose therapy and stem cell transplantation. In a Cox proportional-hazard model, the absence or presence of CD200 expression in MMCs is predictive for EFS for patients independently of ISS stage or β2M serum levels. Thus, CD200 is an independent prognosis factor for patients with MM that could represent a new therapeutic target in MM.
Introduction
Multiple myeloma (MM) is a plasma cell neoplasm characterized by the accumulation of malignant plasma cells within the bone marrow (BM). Several autocrine or paracrine factors can promote multiple myeloma cell (MMC) survival and proliferation.1,2 We anticipate that in the future, the inhibition of MMC growth factors may have clinical applications in combination with other drugs.3-6
In order to identify new molecules involved in the communication between MMCs and the BM environment, we compared gene expression profiles (GEPs) of MMCs with those of normal plasma cells, normal plasmablasts, and normal peripheral blood B cells. We identified that CD200 was expressed in malignant plasma cells in 78% of newly diagnosed patients with MM. CD200, formerly known as OX-2, is a highly conserved type I transmembrane glycoprotein that is expressed by thymocytes, activated T cells, B cells, dendritic cells, endothelial cells, and neurons.7 The expression of the receptor for CD200 (CD200R1) is described as restricted to myeloid-derived antigen-presenting cells and certain populations of T cells.8 Three other genes, closely related to CD200R1 and termed CD200R2-4, have been identified, but the function of these encoded proteins, in particular their ability to bind CD200, is not fully elucidated.9 Several studies have shown that CD200 imparts an immunoregulatory signal through CD200R, leading to the suppression of T-cell–mediated immune responses. Increased survival of renal allografts following portal vein immunization with alloantigen correlates with an increase in CD200 expression in both hepatic and splenic dendritic cells (DCs) in a murine model.10 Tolerance in this setting is reversed with a monoclonal antibody to CD200.11 CD200-deficient mice have a compromised capacity to down-regulate the activation of antigenpresenting cells. This results in chronic central nervous system inflammation, which causes an exaggerated inflammatory response to trauma and an increased susceptibility to develop both experimental autoimmune encephalitis and collagen-induced arthritis.12 More recently, Gorczynski et al13 demonstrated that anti-CD200R2-4 monoclonal antibodies (MoAbs) promote the development of DCs and have the capacity to induce regulatory T cells (Tregs) and directly augment the production of Tregs in the thymus.
In this study, we demonstrate that MMCs of 78% of the patients with newly diagnosed MM express CD200. For patients included in protocols with high-dose chemotherapy (HDC) and autologous hematopoietic stem cell transplantation (ASCT), the presence or absence of CD200 expression is a prognostic factor independent of ISS stage or β2M.
Materials and methods
Cell samples
XG human myeloma cell lines (HMCLs) were obtained as described.14-16 SKMM, OPM2, LP1, and RPMI8226 HMCLs were purchased from ATCC (LGC Promochem, Molsheim, France). MMCs were purified from 112 patients with newly diagnosed MM after written informed consent was given. The institutional review board approval was given by the local ethics committee (Comité Consultatif de Protection des Personnes dans la recherche biomédicale; CPPRB). These 112 patients were treated with HDC and ASCT. Seven of these 112 patients received allogenic bone marrow transplantation after HDC and ASCT, and their event-free survival (EFS) was censured at the time of the allograft. In the series, according to the Durie-Salmon classification, 9 patients were of stage IA; 14, of stage IIA; 83, of stage IIIA; and 6, of stage IIIB. According to the International Staging System (ISS),17 44 patients were of stage I; 53, of stage II; and 15, of stage III. Seventeen patients had IgAκ MM; 6, IgAλ MM; 38, IgGκ MM; 26, IgGλ MM; 14, Bence-Jones κ MM; 7, Bence-Jones λ MM; and 4, nonsecreting MM. The obtainment and purification of MMCs, normal bone marrow (BM) plasma cells (BMPCs), memory B cells, polyclonal plasmablasts, osteoclasts, BM stromal cell lines, BM CD3 T cells, BM monocytes, and BM polymorphonuclear neutrophils were performed as previously described.18
Preparation of complementary RNA (cRNA) and microarray hybridization
RNA was extracted using the RNeasy Kit (Qiagen, Hilden, Germany). Biotinylated cRNA was amplified with a double in vitro transcription reaction and hybridized to the Affymetrix HG U133 set of Gene Chips, according to the manufacturer's instructions (Affymetrix, Santa Clara, CA).
CD200 expression by MMCs
CD200 expression by primary MMCs was determined using a double labeling of primary MMCs with an FITC-conjugated anti-CD138 MoAb and a PE-conjugated anti-CD200 MoAb (clone MRCOX-104; Becton Dickinson, San Jose, CA), or with FITC- or PE-conjugated isotype-matched control antibodies. The fluorescence intensity was determined with a FACScan device (Becton Dickinson), setting up the mean fluorescence intensity obtained with the control antibodies between 4 and 6.
Statistical analysis
Gene expression data were normalized with the MAS5 algorithm and analyzed with our bioinformatics platforms (RAGE, http://rage.montp.inserm.fr/demo/demo.shtml?submit=Demo and Amazonia, http://amazonia.montp.inserm.fr/). Statistical comparisons were done with R (http://www.r-project.org/) or SPSS10 (SPSS, Chicago, IL) software.
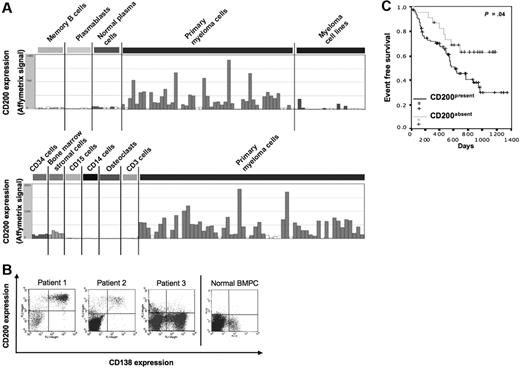
CD200expression and association with event-free survival in patients with MM. (A) Affymetrix CD200 gene expression in normal memory B cells; normal polyclonal plasmablasts; normal BM plasma cells (BMPCs); purified myeloma cells from patients with multiple myeloma (MM); human myeloma cell lines; BM stromal cells; BM CD34 cells; purified BM CD15, CD14, and CD3 cells; and osteoclasts. (B) CD200 expression by primary MMCs or normal BMPCs was determined using a double labeling of primary MMCs with an FITC-conjugated anti-CD138 MoAb and a PE-conjugated anti-CD200 MoAb, or with FITC- or PE-conjugated isotype-matched control antibodies. (C) Kaplan-Meier plot of the event-free survival in patients with CD200present and CD200absent MMCs.
CD200expression and association with event-free survival in patients with MM. (A) Affymetrix CD200 gene expression in normal memory B cells; normal polyclonal plasmablasts; normal BM plasma cells (BMPCs); purified myeloma cells from patients with multiple myeloma (MM); human myeloma cell lines; BM stromal cells; BM CD34 cells; purified BM CD15, CD14, and CD3 cells; and osteoclasts. (B) CD200 expression by primary MMCs or normal BMPCs was determined using a double labeling of primary MMCs with an FITC-conjugated anti-CD138 MoAb and a PE-conjugated anti-CD200 MoAb, or with FITC- or PE-conjugated isotype-matched control antibodies. (C) Kaplan-Meier plot of the event-free survival in patients with CD200present and CD200absent MMCs.
Results and discussion
Comparing the GEPs of MMCs in an initial series of 48 patients with newly diagnosed MM with the GEP of normal BM plasma cells, normal plasmablasts, and normal memory B cells using Affymetrix U133 A+B DNA microarrays, we found a clear-cut expression of CD200 in MMCs. In 9 of 48 patients, CD200 had an “absent” Affymetrix call (CD200absent) in MMCs. In the remaining 39 patients, CD200 had a “present” call (CD200present) in MMCs and was overexpressed in MMCs compared with normal BMPCs (ratio = 6.2 and P < .01), plasmablasts (ratio = 26.5 and P < .01), or memory B cells (ratio = 10 and P < .01) (Figure 1A). These data were confirmed with another independent series of MMCs from 64 newly diagnosed patients using Affymetrix U133 2.0 plus microarrays (Figure 1A). Combining the 2 sets of data, CD200 had a present call in MMCs in 87 (78%) of 112 patients. Microarray CD200 expression was validated by real-time reverse transcriptase–polymerase chain reaction (RT-PCR) for 20 HMCLs and 5 patients' MMCs (r = .81, P < .001) (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Using fluorescence-activated cell sorting (FACS) analysis, the CD200 protein was detected on MMCs in 13 (86%) of 15 consecutive newly diagnosed patients, confirming the frequency of CD200 expression determined by absent/present calls with Affymetrix microarrays (Table 1). If CD200 is expressed in MMCs, the mean fluorescence intensity ranged from 364 to 8038 (Table 1) (Figure 1B). On the panel of 20 HMCLs used, only HMCLs with detectable CD200 mRNA displayed a positive labeling with the anti-CD200 MoAb. Moreover, we found a very good correlation (r = .92; P < .001) between Affymetrix CD200 expression and protein expression (mean fluorescence intensity) at the surface of our HMCLs (Figure S2). Subsequently, we investigated the CD200 gene expression in the BM environment from patients with MM. CD200 was not expressed by CD14 monocytes, CD15 polynuclear cells, and CD3 T cells that were purified from the BM of 5 newly diagnosed patients. It is also not expressed in 7 osteoclast samples (Figure 1A). BM stromal cells from 5 patients with MM expressed CD200, but at a 3.9-fold lower median signal compared with that in CD200present MMCs (P = .04). CD34+ hematopoietic stem cells from 5 patients with MM expressed CD200, but at a 2.5-fold lower median signal compared with that in CD200present MMCs (P = .05).
CD200 expression at the surface of myeloma cells
. | CD200 labeling . | . | |
|---|---|---|---|
| Patient no. . | % . | MFI . | |
| 1 | 84.8 | 2273 | |
| 2 | 90.4 | 4347 | |
| 3 | 88.8 | 986 | |
| 4 | 97.7 | 2023 | |
| 5 | 80.0 | 1219 | |
| 6 | 98.1 | 1155 | |
| 7 | < 5.0 | — | |
| 8 | 99.6 | 8038 | |
| 9 | < 5.0 | — | |
| 10 | 95.9 | 804 | |
| 11 | 95.6 | 832 | |
| 12 | 30.7 | 364 | |
| 13 | 99.9 | 1259 | |
| 14 | 62.0 | 601 | |
| 15 | 97.8 | 442 | |
. | CD200 labeling . | . | |
|---|---|---|---|
| Patient no. . | % . | MFI . | |
| 1 | 84.8 | 2273 | |
| 2 | 90.4 | 4347 | |
| 3 | 88.8 | 986 | |
| 4 | 97.7 | 2023 | |
| 5 | 80.0 | 1219 | |
| 6 | 98.1 | 1155 | |
| 7 | < 5.0 | — | |
| 8 | 99.6 | 8038 | |
| 9 | < 5.0 | — | |
| 10 | 95.9 | 804 | |
| 11 | 95.6 | 832 | |
| 12 | 30.7 | 364 | |
| 13 | 99.9 | 1259 | |
| 14 | 62.0 | 601 | |
| 15 | 97.8 | 442 | |
CD200 expression by primary MMCs was determined using a double labeling of primary MMCs with an FITC-conjugated anti-CD138 MoAb and a PE-conjugated anti-CD200 MoAb, or with FITC- or PE-conjugated isotype-matched control antibodies. The fluorescence intensity was determined with a FACScan device, setting up the mean fluorescence intensity obtained with the control antibodies between 4 and 6.
— indicates no labeling of anti-CD20 antibody.
A significantly higher number of patients with CD200present had a monoclonal protein containing lambda light chains, whereas the characteristic of age of 65 years or older appeared at a significantly higher frequency in patients with CD200absent MMCs (Table S1). In our group of 112 newly diagnosed patients treated with HDT and ASCT, patients with CD200absent MMCs had a better event-free survival (24 months) compared with patients with CD200present MMCs (14 months) (Figure 1C). In a Cox proportional-hazard model monitoring for the absence or presence of CD200 (P = .04) and ISS stage (P = .01), both parameters are independently predictive for EFS (P = .01). If CD200 expression is tested together with classical prognostic factors (ie, serum albumin and serum β2M) CD200 expression (P = .04) and β2M (P = .015) remain independent prognostic factors, whereas serum albumin marginally fails to be significant (P = .058).
This better EFS of patients with MMCs lacking CD200 could be linked to the role of CD200 in suppression of T-cell–mediated immune responses and in the development of DCs with a capacity to induce Tregs.9-11,13 A recent study has demonstrated that B-cell chronic lymphocytic leukemia expresses CD200 that leads to inhibition of the Th1 response in mixed lymphocyte reactions.19 In conclusion, we have identified that CD200 expression by MMCs is an independent prognostic factor that could represent a new therapeutic target for patients with MM.
Authorship
Contribution: J.M. performed the experiments and participated in the writing of the paper; D.H., M.H., and H.G. collected bone marrow samples and clinical data and participated in the writing of the paper; T.R. and J.D.V. performed the bioinformatic studies and participated in the writing of the paper; E.J., E.L., J.F.R., and T.M. collected bone marrow samples and clinical data; P.M. provided technical assistance; M.M. participated in the writing of the paper; P.B. and J.C. collected bone marrow samples; B.K. participated in the design of the research and the writing of the paper.
Conflict of interest disclosure: The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 31, 2006; DOI 10.1182/blood-2006-06-029355.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants from the Ligue Nationale Contre Le Cancer (équipe labellisée), Paris, France. The study is part of a national program called Carte d'Identité des Tumeurs (CIT), or the Tumor Identity Card developed, and is funded by the Ligue Nationale Contre le Cancer.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal