Abstract
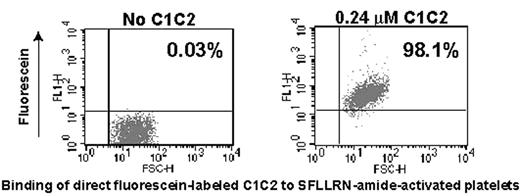
The C domains of factor VIII contain the primary binding site for the cofactor, activated factor VIII, to interact with the phospholipid membranes, including those on the platelet surface. Isolated C2 domain has been shown to bind to phosphotidyl-L-serine-rich lipids and platelets; under flow cytometry, binding to activated platelets was confirmed. For comparison, C1C2, expressed in E.coli, was prepared with up to mg quantities isolated. Fresh, gel-filtered platelets were then studied in a flow cytometer either with or without activation by the thrombin receptor peptide, SFLLRN-amide. Depending upon the conditions, up to 80% of the platelets could be stained with a monoclonal antibody to C2 (ESH8) that is known not to compete with lipid or von Willebrand factor binding. The results were confirmed using a S2296C mutant C1C2 where the free suflhydryl group was either biotinylated and detected by fluorescein labeled streptavidin or directly labeled with fluorescein. As shown in the figure, essentially all platelets bound directly fluorescein labeled C1C2. Using standardized, labeled microbeads, it was estimated that there are 7000–10,000 binding sites per platelet. After platelet activation, the number of platelets binding C1C2 increased with all three detecting systems but only by 15–30%. In contrast, binding of isolated C2, as determined either by ESH8 or as a C2296 biotinylated species, was much lower when the same molar amounts were added, and was primarily detectable following platelet activation. C1C2 binding appeared independent of von Willebrand factor as platelets from two unrelated subjects with severe, type 3 von Willebrand disease gave the same patterns on flow cytometry as seen in platelets from normal subjects. ESH4, a monoclonal antibody known to inhibit binding of C2 to lipid membranes effectively competed C1C2 binding to platelets. Although an indirect alteration the C2 domain conformation cannot be excluded, results support a direct role of C1 in enhancing platelet binding.
Binding of direct florescein-labeled C1C2 to SFLLRN-amide-activated platelets
Binding of direct florescein-labeled C1C2 to SFLLRN-amide-activated platelets
Disclosures: National Institutes of Health Grant-Principal Investigator, A.R. Thompson.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal