Abstract
Umbilical cord blood (CB) is a valuable source of stem cells for transplantation, but CB transplantations are frequently complicated by delayed platelet engraftment. The reasons underlying this are unclear. We hypothesized that CB- and peripheral-blood (PB)–derived megakaryocytes (MKs) respond differently to the adult hematopoietic microenvironment and to thrombopoietin (Tpo). To test this, we cultured CB- and PB-CD34+ cells in adult bone marrow stromal conditioned media (CM) or unconditioned media (UCM) with increasing concentrations of recombinant Tpo and compared the effects of these conditions on CB-versus PB-MKs. PB-MKs reached highest ploidy in response to UCM + 100 ng/mL rTpo, and the addition of CM inhibited their maturation. In contrast, CB-MKs reached highest ploidy in CM without rTpo, and high rTpo concentrations (> 0.1 ng/mL) inhibited their maturation. This is the first evidence that human neonatal and adult MKs have substantially different biologic responses to Tpo and potentially to other cytokines.
Introduction
Umbilical cord blood (CB) contains stem cells that can be used for transplantation. However, a major drawback of CB transplantation is slow engraftment,1 particularly platelet engraftment, which takes approximately 70 days for CB versus 20 days for mobilized peripheral blood (PB).2,3
The delayed platelet engraftment following CB transplantation has been attributed mainly to decreased numbers of stem cells compared with other sources,1,4 but developmental differences between neonatal and adult megakaryocytes (MKs) might contribute to its pathogenesis. Specifically, MKs of neonates are smaller and have lower DNA content than adult MKs,5-8 features associated with a decreased ability to produce platelets.9 However, it is unclear how MKs derived from neonatal stem and progenitor cells respond to the adult hematopoietic microenvironment, and how this environment modifies their response to thrombopoietic stimuli.
To answer these questions, we cultured CD34+ cells isolated from CB and PB in adult bone marrow stromal conditioned media (CM) and in unconditioned media (UCM), both supplemented with recombinant thrombopoietin (rTpo) at different concentrations. The proliferation and maturation of CB-derived MKs (CB-MKs) were then compared with those of PB-derived MKs (PB-MKs).
Study design
Bone marrow stromal CM
Light-density mononuclear cells from adult bone marrow (n = 4) were purchased from Cambrex (East Rutherford, NJ) and cultured in Myelocult media with 1 μM hydrocortisone (StemCell Technologies, Vancouver, Canada). The adherent layer was maintained for 4 weeks, after which confluent stroma were irradiated (1500 cGy). The following day the irradiated cells were washed, and MK medium was added (IMDM with 1% BSA, 740 μg/mL partially saturated human transferrin, 10 μg/mL insulin, and 40 μg/mL low-density lipoproteins). After 48 hours of culture, CM was collected, filtered, and stored at –20°C. Unconditioned media (UCM) subjected to the same conditions but without stromal cells was prepared as control.
Cytokine levels in CM
Tpo concentrations in CM were determined by using a commercially available Tpo enzyme-linked immunoabsorbent assay (ELISA; R&D Systems, Minneapolis, MN). Levels of other cytokines were measured by using a commercially available cytokine antibody array (RayBio Human Cytokine Antibody Array V; RayBiotech, Norcross, GA).
Cell cultures
CB- and mobilized PB-CD34+ cells were purchased from Cambrex and plated in 24-well plates at 4 × 104 cells/mL in UCM and CM. Cultures were supplemented with rTpo (PeproTech, Rocky Hill, NJ) at 0, 0.1, 1, 10, and 100 ng/mL. Cells were maintained at less than 1 × 105/mL with biweekly media changes. On day 14 of culture, cells were collected, counted, and analyzed for ploidy.
MK number and ploidy
After labeling with anti-CD41 FITC (Beckman Coulter, Fullerton, CA), cells were fixed with 1% paraformaldehyde, treated with RNAse, stained with propidium iodide, and analyzed by flow cytometry (FACSort; Becton Dickinson, Franklin Lakes, NJ). The number of cultured MKs was calculated by multiplying the percentage of CD41+ cells by the total number of cells in the culture. Each experiment was repeated 4 times, using a different source of cells and CM each time.
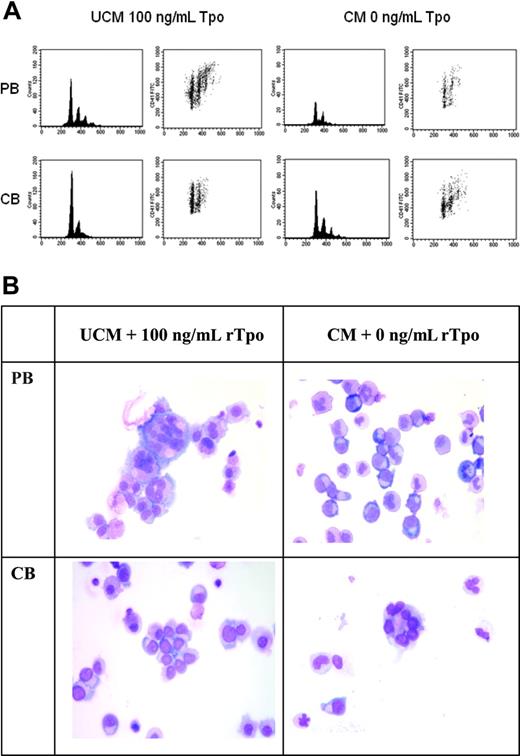
Recombinant thrombopoietin increases DNA content of PB-derived MKs but reduces DNA content of CB-derived MKs. PB- and CB-CD34+ cells cultured in UCM with 100 ng/mL rTpo or in CM without Tpo for 14 days were analyzed by flow cytometry and light microscopy. (A) Representative density plots and histograms from one experiment are displayed to show the ploidy distribution of PB- and CB-MKs. MKs were first selected by gating on CD41-FITC versus forward scatter. Propidium iodide versus CD41-FITC density plots and histograms were then generated to assess the ploidy distribution. The results were consistent in all 4 experiments performed. (B) Cytospin preparations of the cultured cells show that PB CD34+ cells generated large, multinucleated MKs in UCM cultures + 100 ng/mL rTpo and small immature MKs in CM without rTpo. In contrast, CB CD34+ cells generated large, multinucleated MKs in CM cultures without rTpo and immature MKs in UCM cultures + 100 ng/mL rTpo. Photomicrographs of Wright-Giemsa–stained cytospin preparations were taken on a Labophot-2 microscope using a 10× eyepiece and a 40×/0.65 objective lens (Nikon, Melville, NY). A SPOT-RT 2.2.0 color camera and SPOT Advanced 4.0.9 software (Diagnostic Instruments, Sterling Heights, MI) were used to capture and digitally acquire images, which were then inserted into PowerPoint 10 (Microsoft, Redmond, WA) for processing.
Recombinant thrombopoietin increases DNA content of PB-derived MKs but reduces DNA content of CB-derived MKs. PB- and CB-CD34+ cells cultured in UCM with 100 ng/mL rTpo or in CM without Tpo for 14 days were analyzed by flow cytometry and light microscopy. (A) Representative density plots and histograms from one experiment are displayed to show the ploidy distribution of PB- and CB-MKs. MKs were first selected by gating on CD41-FITC versus forward scatter. Propidium iodide versus CD41-FITC density plots and histograms were then generated to assess the ploidy distribution. The results were consistent in all 4 experiments performed. (B) Cytospin preparations of the cultured cells show that PB CD34+ cells generated large, multinucleated MKs in UCM cultures + 100 ng/mL rTpo and small immature MKs in CM without rTpo. In contrast, CB CD34+ cells generated large, multinucleated MKs in CM cultures without rTpo and immature MKs in UCM cultures + 100 ng/mL rTpo. Photomicrographs of Wright-Giemsa–stained cytospin preparations were taken on a Labophot-2 microscope using a 10× eyepiece and a 40×/0.65 objective lens (Nikon, Melville, NY). A SPOT-RT 2.2.0 color camera and SPOT Advanced 4.0.9 software (Diagnostic Instruments, Sterling Heights, MI) were used to capture and digitally acquire images, which were then inserted into PowerPoint 10 (Microsoft, Redmond, WA) for processing.
Statistical analysis
A mixed model analysis was carried out using PROC MIXED in SAS 9.2 (SAS Institute, Cary, NC). The experiment was analyzed as a split-unit, using time points as blocks and a completely randomized design for the main units (source of CD34+ cells, PB and CB). The subunits were the types of culture media (CM and UCM) and the Tpo concentrations. Significance was set at P less than .05. Results are expressed as mean plus or minus SEM.
Results and discussion
In UCM, both PB- and CB-CD34+ cells required rTpo to survive. As little as 1 ng/mL rTpo supported CB progenitor proliferation, whereas PB progenitors required higher rTpo concentrations (10 or 100 ng/mL). In contrast, CM without rTpo supported the proliferation of multilineage progenitors from both CB and PB, although only 6% to 7% of the cells were CD41+. This effect was not accounted for by endogenous Tpo, because CM contained no detectable Tpo (detection limit = 10 pg/mL).
In both CM and UCM cultures, an rTpo dose-dependent increase in MK number was observed. However, MK number was also dependent on the type of media and cells cultured. Specifically, CM cultures yielded significantly more MKs than UCM cultures at the same rTpo concentrations. Similarly, CB cells generated more MKs than PB cells cultured in the same media and with rTpo concentrations of 1 ng/mL or greater (P < .01). These findings are consistent with previous reports that CM synergizes with thrombopoietin to stimulate MK production10,11 and reflect the known increased proliferative potential of neonatal progenitors compared with adult progenitors.12-14
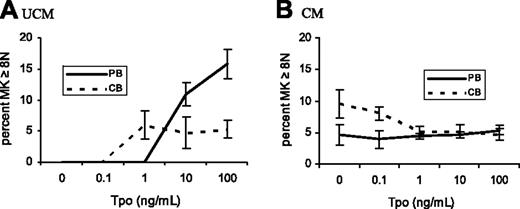
To establish the effects of marrow stromal factors and rTpo on the maturation of PB- and CB-MKs, CD41+ cells were analyzed for ploidy. When cultured in UCM, PB-MKs exhibited a rTpo dose-dependent increase in ploidy, reaching highest levels at 100 ng/mL rTpo (15.8% ± 2.3% MKs ≥ 8N; Figures 1 and 2A), a response that was inhibited by CM (5.3% ± 0.73% MKs ≥ 8N in CM + 100 ng/mL rTpo; Figure 2B).
In contrast to PB, CB-MKs cultured in UCM did not increase their ploidy in response to 100 ng/mL rTpo (5.3% ± 1.3% CB-MKs ≥ 8N; P < .001 versus PB-MKs; Figures 1 and 2A). Also opposite to PB-MKs, CM stimulated the maturation of CB-MKs, an effect that was inhibited by rTpo concentrations greater than 0.1 ng/mL. In fact, when cultured in CM without rTpo, CB-MKs achieved higher ploidy levels than PB-MKs (9.5% ± 2.2% versus 4.6% ± 1.7% MKs ≥ 8N; P = .009). CM cultures containing 0.1 ng/mL rTpo yielded similar results (P = .03). At higher rTpo concentrations CB-and PB-MKs had indistinguishable ploidy levels (Figure 2B).
Our finding of an rTpo dose-dependent increase in the maturation of PB-MKs cultured in UCM is consistent with Tpo's role as a potent stimulator of MK maturation.9,15 Although an inhibitory effect of CM on MK maturation has not been previously described, this might reflect the presence of cytokines in the CM that stimulate MK proliferation while decreasing maturation.16
CB-MKs remain as 2N and 4N cells under most culture conditions, which has been attributed to a developmental limitation of these cells, compared with adult MKs. In this study, however, we found that neonatal MKs responded in the opposite manner than adult MKs to the conditions tested, suggesting that the reported differences might be partly explained by different responses to thrombopoietic cytokines. The factor in CM that stimulates the maturation of neonatal MKs is unknown, but it might involve a thrombopoietic cytokine present in high concentrations in adult marrow stromal conditioned media (Table S1, available on the Blood website; see the Supplemental Table link at the top of the online article). Studies to identify the precise factor(s) are currently under way. In addition, we recognize that our CM does not fully reflect the complexities of the in vivo hematopoietic microenvironment. However, our findings are consistent with our observations in a murine transplantation model, which showed that MKs derived from neonatal liver cells were larger and more polyploid in an adult environment (after transplantation) than in the fetal/neonatal microenvironment.17
The percentage of MKs equal to or greater than 8N in PB and CB cultures differed depending on media source and rTpo concentration. PB- and CB-CD34+ cells were cultured for 14 days in UCM (A) and CM (B), with varying rTpo concentrations. PB-derived MKs (solid lines) cultured in UCM (A) exhibited a rTpo dose-dependent increase in ploidy levels, an effect inhibited by the presence of CM (B). CB-derived MKs (dashed lines) reached highest ploidy levels when cultured in CM with no rTpo, an effect that was reversed by rTpo concentrations of 1 ng/mL or more (B). Data shown represent the means and standard error of the mean (SEM) of 4 separate experiments.
The percentage of MKs equal to or greater than 8N in PB and CB cultures differed depending on media source and rTpo concentration. PB- and CB-CD34+ cells were cultured for 14 days in UCM (A) and CM (B), with varying rTpo concentrations. PB-derived MKs (solid lines) cultured in UCM (A) exhibited a rTpo dose-dependent increase in ploidy levels, an effect inhibited by the presence of CM (B). CB-derived MKs (dashed lines) reached highest ploidy levels when cultured in CM with no rTpo, an effect that was reversed by rTpo concentrations of 1 ng/mL or more (B). Data shown represent the means and standard error of the mean (SEM) of 4 separate experiments.
Our finding that rTpo inhibits the maturation of CB-MKs is concordant with a previous report by Schipper et al,18 although PB-MKs were not evaluated in that study. Indeed, the concept of developmental stage-specific responses is relatively new. A previous study showed that, in the presence of other growth factors, rTpo caused a reduction in the number of erythroid CFUs cultured from early murine embryos or yolk-sac cells,19 in contrast with its stimulatory effects on erythroid progenitors from adults.20,21 Similarly, GATA1s mutations were recently shown to cause hyperproliferation of a unique murine yolk sac and fetal liver megakaryocytic progenitor.22
Although those studies evidenced significant biologic differences between murine fetal and adult MKs, here we provide evidence that human neonatal MKs also have biologic features distinct from their adult counterparts. Understanding these differences may lead to better interventions for thrombocytopenia in neonates or following CB transplantation.
Prepublished online as Blood First Edition Paper, August 3, 2006; DOI 10.1182/blood-2006-04-018036.
Supported by the National Institutes of Health (grant HL69990) (M.C.S.-V. and L.M.R.).
The authors declare no competing financial interests.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal