Outcome of patients with acute myeloid leukemia (AML) who are older than 60 years of age remains unsatisfactory, with low remission rates and poor overall survival. We have previously established the activity of clofarabine plus cytarabine in AML relapse. We have now conducted a phase 2 study of clofarabine plus cytarabine in patients aged 50 years or older with previously untreated AML. Clofarabine was given at 40 mg/m2 as a 1-hour intravenous infusion for 5 days (days 2 to 6) followed 4 hours later by cytarabine at 1 g/m2/d as a 2-hour intravenous infusion for 5 days (days 1 to 5). Of 60 patients, 29 (48%) had secondary AML, 30 (50%) had abnormal karyotypes (monosomy 5 and/or 7 in 15 [25%]), and 11 (21%) showed FLT3 abnormalities. The overall response (OR) rate was 60% (52% CR, 8% CRp). Four patients (7%) died during induction. Adverse events were mainly grade 2 or lower and included diarrhea, nausea, vomiting, mucositis, skin reactions, liver test abnormalities, and infusion-related facial flushing and headaches. Myelosuppression was common. Clofarabine plus cytarabine has activity in adult AML, achieving a good CR rate. However, survival does not appear to be improved compared with other regimens. Modifications of this combination in AML therapy of older patients warrant further evaluation. (Blood. 2006;108:45-51)
Introduction
Acute myeloid leukemia (AML) is a clonal hematopoietic stem cell disorder leading to proliferation and accumulation of leukemic blasts in blood, marrow, and other organs. The backbone of AML therapy remains centered around cytarabine and is typically given in combination with an anthracycline such as idarubicin or daunorubicin. Whereas on average 50% to 70% of patients will achieve a complete remission (CR), most will relapse and succumb to their disease or associated complications, so that the search for new and more active agents and regimens continues.
Older patients with AML pose a particular challenge. Outcome of AML worsens continuously as patient age increases. Using a standard induction combination such as the “3 + 7” regimen (cytarabine plus an anthracycline), CR rates may be as low as 30% in patients over age 70. Remissions are usually transient and rarely last more than 12 months. The median time from treatment to death is 5 to 10 months; less than 10% of patients remain in remission at 3 years.1-4 The reasons for this discrepancy in outcome compared with younger patients are not completely understood but are related to differences in disease biology, tolerance to intensive chemotherapy, and presence of comorbid conditions. That there are differences in the biology of AML in older patients is suggested by a higher incidence of unfavorable karyotypes, a higher rate of primary drug resistance associated with overexpression of P-glycoprotein (P-gp), and an increased frequency of disease evolution from a preexisting and, at the time, probably unrecognized myelodysplastic syndrome (MDS).5-9 The development of safe and effective anti-AML agents therefore remains a cornerstone of the continued efforts to improve the outcome of poor-prognosis patients with AML.
Clofarabine (2-chloro-2′-fluoro-deoxy-9-β-D-arabinofuranosyladenine) is a next-generation nucleoside analog that was synthesized with the goal to combine the most favorable pharmacokinetic properties of fludarabine and cladribine and at the same time to avoid the doselimiting neurotoxicity of the other deoxynucleoside analogs.10 Minor chemical modifications in the structure of clofarabine (halogenation at the 2-position of adenine and substitution of a fluorine group at the C-2′ position of the arabinofuranosyl moiety) resulted in many of its characteristic features: (1) high affinity to deoxycytidine kinase (dCyd); (2) prolonged retention of clofarabine triphosphate in leukemic blasts; and (3) potent inhibition of DNA synthesis and of ribonucleotide reductase (RNR), an enzyme involved in maintaining intracellular nucleoside pools.11-14 Clofarabine was found to be active in phase 1 and 2 studies in patients with relapsed and refractory acute leukemias in adults and children.15-18 Based on a response rate of 31% (including 12% CRs) in relapsed/refractory children with acute lymphoblastic leukemia (ALL), clofarabine has recently received Food and Drug Administration (FDA) approval for salvage therapy of pediatricALL. In addition to its antileukemic activity as a single agent, in vitro studies supported a possible role for clofarabine in biochemical modulation strategies to enhance the efficacy of other nucleoside analogs such as ara-C.13,14,19-21 In a previous phase 2 study of clofarabine with cytarabine in AML salvage, we demonstrated the feasibility of this combination and showed that accumulation of clofarabine triphosphate can increase levels of intracellular ara-CTP in some patients.22
The goal of the current study was to expand our experience with clofarabine and cytarabine in the therapy of patients 50 years or older with newly diagnosed AML.
Patients, materials, and methods
Study group
Previously untreated adults with AML or high-risk MDS (more than 10% blasts) 50 years or older were eligible after informed consent was obtained according to institutional guidelines. Prior therapy with hydroxyurea, single-agent chemotherapy, hematopoietic growth factors, and biologic or “targeted” therapies was allowed. Additional eligibility criteria included the following: (1) performance status 2 or below (Eastern Cooperative Oncology Group [ECOG]); (2) adequate liver function (serum bilirubin 2 mg/dL [34.2 μM/L] or below, alanine aminotransferase [ALT] or aspartate aminotransferase up to 4 times the upper limit of normal) and renal function (serum creatinine 2 mg/dL [176.8 μM/L] or below); (3) absence of the following cytogenetic abnormalities: t(15;17), t(8;21), and inv(16); (4) cardiac ejection fraction (as assessed by either echocardiography or multigated acquisition scan [MUGA]) 30% or more; and (5) absence of any active and uncontrolled infection or any other severe concurrent disease. Approval for the study was granted by the Institutional Review Board (IRB) of the University of Texas MD Anderson Cancer Center. The study was conducted in accordance with the basic principles of the Declaration of Helsinki.
Treatment schedule
The treatment schedule was identical to what has been reported previously.22 Clofarabine was administered as a 1-hour intravenous infusion at a dose of 40 mg/m2 daily for 5 consecutive days on days 2 through 6 followed 4 hours later by cytarabine at a dose of 1 g/m2 as a 2-hour intravenous infusion daily for 5 consecutive days on days 1 through 5. On day 1, only cytarabine was administered, and on day 6, only clofarabine was given. Cycles were repeated every 4 to 6 weeks depending on leukemia response, recovery of normal hematopoiesis, or occurrence of treatment-related toxicities. Patients were allowed to receive a maximum of 3 induction cycles or until a CR, CR with incomplete platelet recovery (CRp), or partial response (PR) was achieved. For patients who received a second or third induction course, both clofarabine and cytarabine were given on days 1 through 5. Patients who did not achieve at least a PR after 3 induction courses were taken off study. Responding patients could receive up to 6 additional courses of maintenance therapy, where clofarabine was given at 40 mg/m2 daily for 3 consecutive days (days 1 through 3) followed 4 hours later by cytarabine at 1 g/m2 daily for 3 consecutive days (days 1 through 3). Sufficient recovery of normal hematopoiesis (absolute neutrophil count [ANC], 1 × 109/L or more; platelet count, 60 × 109/L or more) was required prior to each maintenance course, except for patients in CRp, in whom a platelet count of less than 60 × 109/L was permissible for continuation. Dose-level adjustments were implemented for grade 2 (25% dose reduction) and grade 3 and higher (50% dose reduction) study-drugrelated extramedullary toxicities, including life-threatening infections. Dose reductions due to toxicities could be modified at the discretion of the treating physician as judged to be in the best interest of the patient.
The pretreatment evaluation included history, physical examination, complete blood counts (CBCs) with differentials and platelet count, a complete chemistry survey, and marrow aspiration (with or without biopsy) with cytogenetic analysis. No marrow aspirate was required if the diagnosis of AML could be made unequivocally from peripheral-blood specimens (including immunophenotyping by flow cytometry and chromosome analysis). An echocardiogram or MUGA scan to evaluate left ventricular ejection fraction was required. Follow-up studies included CBC, differential, and platelet count as well as a chemistry profile at least weekly until remission. Marrow aspiration was performed starting at day 21 and then every 2 weeks until remission or nonresponse. Marrow tests were repeated more frequently if considered indicated by the treating physician. Patients with marrow studies showing no evidence of leukemia, but with persistent cytopenia, were allowed time to recover their counts to document the best response. Cardiac assessments (echocardiogram or MUGA scan) were repeated at the end of therapy. Supportive measures for optimal medical care were provided throughout the study and included admission to a protective environment for the duration of the induction, use of prophylactic antibiotics (levofloxacin, valacyclovir, and itraconazole, whereby itraconazole was started the day after the completion of the clofarabine infusion), and irradiated blood-product support as indicated. Use of hematopoietic growth factors was not mandatory and was determined by the treating physician to meet the patients' medical need. Overall, 25 patients received either granulocyte colony-stimulating factor (G-CSF) or granulocytemacrophage colony-stimulating factor (GM-CSF) at some point during the induction course.
Response criteria
A CR required normalization of the marrow blasts (up to 5%) and recovery of normal hematopoiesis with an ANC of 1 × 109/L or more and platelet count of 100 × 109/L or more in addition to disappearance of all clinical or radiologic evidence of disease. CRp had similar criteria to a CR but with platelet counts from 20 × 109/L to less than 100 × 109/L. CRp was defined after a period of observation during which neutrophil counts recovered, marrow blasts were up to 5%, and no further platelet recovery occurred. PR required blood recovery as for CR but with both a decrease in marrow blasts of at least 50% and not more than 25% abnormal cells in the marrow.
Statistical analysis
This is a phase 2 study of the combination of clofarabine plus cytarabine in patients age 50 or older with previously untreated AML and high-risk MDS. The primary objective was to estimate the CR rate. Patients who were eligible for this trial and received any dose of clofarabine were included in estimating the CR rate and were counted as treatment failures if response could not be assessed for any reason. The trial was conducted in 2 groups, with 30 patients entered into each group: (1) patients with diploid cytogenetics and (2) patients with abnormal cytogenetics (excluding inv(16) and translocation t(8;21)). The trial would be regarded as successful if the CR rate was at least as good as that obtained with the induction regimens used at MD Anderson Cancer Center since 1990 (64% CR in 378 patients in group 1 and 48% CR in 495 patients in group 2). The sample size of 60 patients was calculated to provide sufficient power to assess differences in CR rates between both groups (1 and 2). Duration of CR (date of initial response until date of disease recurrence) and overall survival (date of start of therapy until death), which was estimated according to Kaplan-Meier method, established secondary objectives. Interim analyses were performed in both groups after every 10th patient had been evaluated for response and criteria for early study termination were established based on the CR rate in each patient cohort. Cohort sizes of 10 patients for implementation of early stopping rules were considered sufficient to provide adequate protection against continuing to accrue patients in the face of inferior response rates. In the diploid group, the trial would thus have been terminated after the first 10 patients for 3 or fewer CRs or after 20 patients for 8 or fewer CRs. Given the operating characteristics, the probability of an early stop would have been 64% for a CR rate of 40%, 31% for a CR rate of 50%, 4% for a CR rate of 64%, and less than 1% for a CR rate of 80%. Similar stopping rules applied for the group of patients with abnormal karyotypes. The trial would have stopped after 10 patients for a CR rate of 1 or below and after 20 patients for a CR rate of 5 or fewer patients The probability of an early stop was 64% for a CR rate of 25%, 27% for a CR rate of 35%, 4% for a CR rate of 48%, and 1% or less for a CR rate of 60%.
Results
Study population
A total of 60 patients with previously untreated AML and age 50 years or older were enrolled in the study and treated with the combination of clofarabine and cytarabine. Only one patient received treatment for AML with the FLT3 inhibitor PKC412 (no response) prior to enrollment. The patient characteristics are summarized in Table 1. The median age of the study group was 61 years (range, 50 to 74 years). Thirty-five patients (58%) were older than 60 years. There were 54 patients with AML as defined by the World Health Organization (WHO) classification defining 20% as the cutoff between AML and high-risk MDS (at least 10% marrow blasts). Among the remaining 6 patients, 5 had a diagnosis of refractory anemia with excess blasts-2 (RAEB-2) and 1 of chronic myelomonocytic leukemia-2 (CMML-2) according to WHO criteria. Twenty-nine patients (48%) had a diagnosis of secondary AML indicating a diagnosis of MDS (n = 18, 30%) or another malignancy such as non-Hodgkin lymphoma or carcinoma of the breast (n = 11, 18%) prior to the AML diagnosis. Half of the patients had abnormal karyotypes, which included abnormalities of chromosomes 5 and/or 7 in 15 patients (25%), trisomy 8 in 10 (16%), and deletions of 11q23 in 3 (5%). Of 53 patients whose marrow samples have been tested for FLT3 abnormalities, 8 showed internal tandem duplications (ITDs) and 3 mutations in aspartic acid residue 835 of the tyrosine kinase loop (21%).
Patient characteristics; n = 60
Median age, y (range) | 61 (50-74) |
| Diagnosis, no. (%) | |
| AML* | 54 (90) |
| RAEB-2/CMML-2 | 6 (10) |
| Secondary AML/MDS, no. (%) | |
| Prior MDS | 18 (30) |
| Other malignancy† | 11 (18) |
| Karyotype, no. (%) | |
| Diploid | 30 (50) |
| Abnormal | 30 (50) |
| −5/−7 | 15 (25) |
| +8 | 10 (16) |
| del(11)(q23) | 3 (5) |
| FLT3 abnormalities, no. (%) | |
| ITD, D835 | 11 (21) |
| Not assessed | 7 (12) |
Median age, y (range) | 61 (50-74) |
| Diagnosis, no. (%) | |
| AML* | 54 (90) |
| RAEB-2/CMML-2 | 6 (10) |
| Secondary AML/MDS, no. (%) | |
| Prior MDS | 18 (30) |
| Other malignancy† | 11 (18) |
| Karyotype, no. (%) | |
| Diploid | 30 (50) |
| Abnormal | 30 (50) |
| −5/−7 | 15 (25) |
| +8 | 10 (16) |
| del(11)(q23) | 3 (5) |
| FLT3 abnormalities, no. (%) | |
| ITD, D835 | 11 (21) |
| Not assessed | 7 (12) |
RAEB-2 indicates refractory anemia with excess blasts-2 (more than 10%); D835, FLT3 mutation of aspartate residue 835 of tyrosine kinase loop.
Distribution by French-American-British (FAB) classification: MO (2 patients), M1 (2), M2 (4), M4 (8), M5 (4), M6 (2), not available (32)
Includes breast cancer (3 patients), non-Hodgkin lymphoma (3), chronic myeloproliferative disorder (2), multiple myeloma (1), ALL (1), and chronic lymphocytic leukemia (1).
Response and outcome
Thirty-one patients (52%) achieved CR and 5 (8%) CRp, for an overall response (OR) rate of 60%. Twenty patients (33%) had resistant disease, and 4 (7%) died during induction (ie, the first course of therapy), for a failure rate of 40%. Among 35 patients older than 60 years of age, 20 patients (57%) achieved CR and 2 (6%) CRp, resulting in an OR of 63%.
Response by karyotype is shown in Table 2. Of 30 patients with diploid cytogenetics, 18 (60%) had CR and 2 (7%) CRp (OR, 67%). Among 30 patients with abnormal karyotypes, the CR rate was 43%, CRp 10%, and OR 53%. The CR rate was 33% (5 of 15) in patients with abnormalities of chromosomes 5 and/or 7 with or without additional karyotypes and 60% (6 of 10) in those whose karyotype analysis included trisomy 8. Of 18 patients with secondary AML and preceding MDS, 6 patients (33%) achieved CR and 3 (17%) CRp, for an OR rate of 50%. Cytogenetic remission in addition to morphologic CR was achieved in 8 patients (62%). In 4 patients, cytogenetic abnormalities persisted during morphologic CR, and analysis in 1 patient yielded only insufficient metaphases. Eleven patients had abnormalities of FLT3 in the form of ITDs or amino acid mutations of the tyrosine kinase loop. CR rates were 55% (23 or 42) in FLT3-negative patients versus 45% (5 of 11) in those who were FLT3 positive. Characteristics of all 31 complete responders according to age, sex, karyotype, and FLT3 status are summarized in Table 3. Eleven patients (18%; 5 diploid and 6 abnormal cytogenetics) who failed to achieve CR following the first induction course received a second induction with identical doses and schedules of clofarabine and cytarabine. Of these patients, 2 (18%) achieved CR and 2 (18%) CRp. The CR rate following a second induction is similar to historical experience with other induction regimens. Median time to CR was 29 days (range, 20 to 98 days) for all patients, 28 days (range, 20 to 52 days) in the diploid, and 29 days (range, 20 to 98 days) in the abnormal cytogenetic group. Median time to CRp was 34 days (range, 24 to 75 days).
Response by karyotype
Karyotype . | CR (%) . | CRp (%) . | Fail (%) . | Total . |
|---|---|---|---|---|
| Diploid | 18 (60) | 2 (7) | 10 (17) | 30 |
| Abnormal | 13 (43) | 3 (10) | 14 (23) | 30 |
| −5/−7 | 5 (33) | 1 (7) | 9 (60) | 15 |
| +8 | 6 (60) | 0 (0) | 4 (40) | 10 |
| Total | 31 (52) | 5 (8) | 24 (40) | 60 |
Karyotype . | CR (%) . | CRp (%) . | Fail (%) . | Total . |
|---|---|---|---|---|
| Diploid | 18 (60) | 2 (7) | 10 (17) | 30 |
| Abnormal | 13 (43) | 3 (10) | 14 (23) | 30 |
| −5/−7 | 5 (33) | 1 (7) | 9 (60) | 15 |
| +8 | 6 (60) | 0 (0) | 4 (40) | 10 |
| Total | 31 (52) | 5 (8) | 24 (40) | 60 |
Characteristics of complete responders; n = 31
Patient . | Age/sex . | Karyotype . | FLT3 abnormality . | History of secondary AML . | No. of cycles to CR . | CRD, mo . |
|---|---|---|---|---|---|---|
| 1 | 67/M | Diploid | — | — | 1 | 25.4+ |
| 2 | 54/M | Diploid | D835 | — | 1 | 22.2+ |
| 3 | 72/F | Diploid | — | — | 1 | 18.2+ |
| 4 | 62/M* | Diploid | — | NA | 1 | 17.8+ |
| 5 | 71/M | Diploid | — | + | 1 | 17.3+ |
| 6 | 66/F | Diploid | — | — | 1 | 15.5+ |
| 7 | 69/M | Diploid | — | — | 1 | 12.2+ |
| 8 | 53/M* | Diploid | — | NA | 1 | 9 |
| 9 | 70/F | Diploid | D835 | — | 1 | 7.6+ |
| 10 | 74/M | Diploid | — | + | 1 | 5.3 |
| 11 | 68/M | −Y | ITD | + | 1 | 5.1 |
| 12 | 72/M | Diploid | — | — | 1 | 4† |
| 13 | 74/F | Diploid | — | + | 1 | 3.9 |
| 14 | 61/F | inv(9) (constitutional) | ITD | — | 1 | 3.5 |
| 15 | 62/M | Diploid | — | — | 1 | 2.8 |
| 16 | 69/M | Diploid | — | — | 1 | 2† |
| 17 | 67/F | Diploid | — | — | 1 | 1‡ |
| 18 | 68/F | Diploid | ITD | — | 1 | 1.2 |
| 19 | 53/F | Complex, +8, +21 | — | — | 2 | 15.9+ |
| 20 | 59/M | −7 | ND | + | 1 | 15+ |
| 21 | 59/M* | +8 | ND | NA | 1 | 8.1 |
| 22 | 65/M | +8 | — | + | 1 | 6.5 |
| 23 | 65/M | Complex, del(5), +8 | — | + | 1 | 6.2 |
| 24 | 54/M* | Complex, −7 | — | NA | 1 | 6 |
| 25 | 55/F | +19 | — | + | 2 | 4.8 |
| 26 | 56/F | Complex, −5, −7 | ND | + | 1 | 3.2 |
| 27 | 66/M | t(1;6), del(3) | — | + | 1 | 2.5† |
| 28 | 57/F | Complex, −5, −7 | — | — | 1 | 2.3 |
| 29 | 67/M | i(14)(q10) | — | + | 1 | 1.6 |
| 30 | 56/M | Dup(1), +8 | — | — | 1 | 0.9 |
| 31 | 52/M | Complex, +8, del(11)(q23) | — | — | 1 | 0.5 |
Patient . | Age/sex . | Karyotype . | FLT3 abnormality . | History of secondary AML . | No. of cycles to CR . | CRD, mo . |
|---|---|---|---|---|---|---|
| 1 | 67/M | Diploid | — | — | 1 | 25.4+ |
| 2 | 54/M | Diploid | D835 | — | 1 | 22.2+ |
| 3 | 72/F | Diploid | — | — | 1 | 18.2+ |
| 4 | 62/M* | Diploid | — | NA | 1 | 17.8+ |
| 5 | 71/M | Diploid | — | + | 1 | 17.3+ |
| 6 | 66/F | Diploid | — | — | 1 | 15.5+ |
| 7 | 69/M | Diploid | — | — | 1 | 12.2+ |
| 8 | 53/M* | Diploid | — | NA | 1 | 9 |
| 9 | 70/F | Diploid | D835 | — | 1 | 7.6+ |
| 10 | 74/M | Diploid | — | + | 1 | 5.3 |
| 11 | 68/M | −Y | ITD | + | 1 | 5.1 |
| 12 | 72/M | Diploid | — | — | 1 | 4† |
| 13 | 74/F | Diploid | — | + | 1 | 3.9 |
| 14 | 61/F | inv(9) (constitutional) | ITD | — | 1 | 3.5 |
| 15 | 62/M | Diploid | — | — | 1 | 2.8 |
| 16 | 69/M | Diploid | — | — | 1 | 2† |
| 17 | 67/F | Diploid | — | — | 1 | 1‡ |
| 18 | 68/F | Diploid | ITD | — | 1 | 1.2 |
| 19 | 53/F | Complex, +8, +21 | — | — | 2 | 15.9+ |
| 20 | 59/M | −7 | ND | + | 1 | 15+ |
| 21 | 59/M* | +8 | ND | NA | 1 | 8.1 |
| 22 | 65/M | +8 | — | + | 1 | 6.5 |
| 23 | 65/M | Complex, del(5), +8 | — | + | 1 | 6.2 |
| 24 | 54/M* | Complex, −7 | — | NA | 1 | 6 |
| 25 | 55/F | +19 | — | + | 2 | 4.8 |
| 26 | 56/F | Complex, −5, −7 | ND | + | 1 | 3.2 |
| 27 | 66/M | t(1;6), del(3) | — | + | 1 | 2.5† |
| 28 | 57/F | Complex, −5, −7 | — | — | 1 | 2.3 |
| 29 | 67/M | i(14)(q10) | — | + | 1 | 1.6 |
| 30 | 56/M | Dup(1), +8 | — | — | 1 | 0.9 |
| 31 | 52/M | Complex, +8, del(11)(q23) | — | — | 1 | 0.5 |
— indicates absent; +, present; CRD, complete remission duration; D835, FLT3 mutation; NA, not applicable; ITD, internal tandem duplication; and ND, not done.
RAEB-2.
Taken off study in CR because of patient request (remission duration of patient no. 12: 16.2+ months; patient no. 16: 9 months; and patient no. 27: 20.3 months).
The patient died in CR.
Four patients (7%) died during the first induction course on days 10 (acute renal failure, bullous skin rash), 20 (Stevens-Johnson syndrome and sepsis), 35 (bacterial sepsis with multisystem organ failure), and 42 (fungal sepsis). All induction deaths occurred in patients who were older than 60 years of age. One patient (67-year-old female) died in CR on day 12 of the first consolidation course from complications of neutropenia and sepsis. Four more patients died during the second course from sepsis-related complications that, in 2 patients, led to multisystem organ failure. Three of the 4 patients failed to respond to the first induction course and were undergoing a second induction. The fourth patient achieved a partial response following induction and was receiving a first consolidation course. Overall, 9 patients (16%) died while on study.
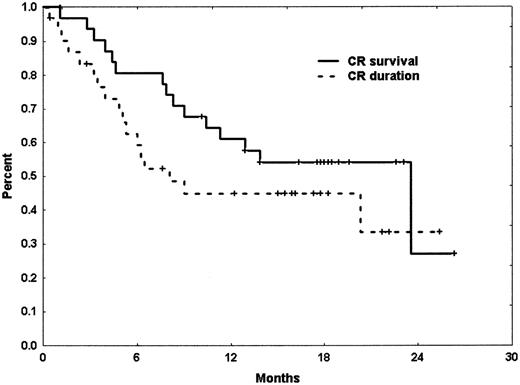
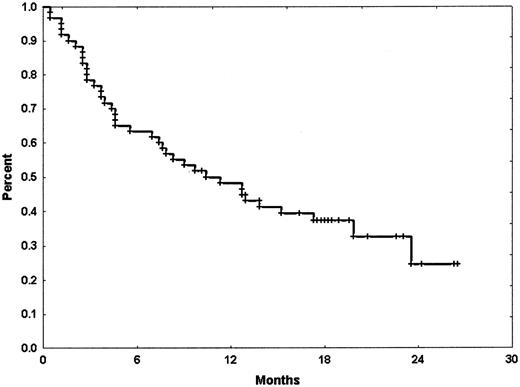
With a median follow-up of 18.2 months (range, 10.2 to 26.5 months), the median remission duration for CR patients has been 8.1 months (range, 0.5 to 25.4+ months). By cytogenetic group, median CR duration of patients with diploid cytogenetics was 8.1 months (range, 0.5 to 25.4+ months) whereas it was 6 months (range, 0.5 to 20.3+ months) in those with abnormal karyotype. Patients who achieved CRp had a median CR duration of only 1.8 months (range, 0.9 to 4.2 months). Median overall survival for the intent-to-treat population was 10.3 months (range, 0.5 to 26.5+ months). Median overall survival of those patients who have achieved CR was 23.5 months (range, 1.2 to 26.3+ months) (Figures 1-2).
Side effects
Common side effects of the combination during induction are summarized in Table 4. The most frequently observed side effects were diarrhea, nausea, vomiting, headaches, skin rashes (including palmoplantar erythrodysesthesia), facial flushing, and liver function abnormalities including hyperbilirubinemia and elevations of the ALT and/or AST. Facial flushing and headaches occurred mostly during chemotherapy administration and were transient, typically resolving upon completion of the infusion. Abnormalities of liver function tests were common. Hyperbilirubinemia of below grade 3 was observed in 35 (58%) patients and elevations of ALT and/or AST in 28 (47%). In most cases, these were transient and would resolve after a few days. Hyperbilirubinemia below grade 3 was seen at a median of 8 days from the start of induction (range, 2 to 22 days) and lasted for a median of 4 days (range, 1 to 35 days). More severe hyperbilirubinemia (grade 3 or higher) began at a median of 15 days on therapy (range, 5 to 20 days) and lasted for a median of 3 days (range, 1 to 7 days). Elevation of transaminases occurred at around days 5 to 6 (range, days 3 to 21) and lasted 3 to 6 days (range, 1 to 26 days) until resolution. No deaths occurred that were considered due to liver function abnormalities. Frequently, however, abnormalities of liver tests accompanied complex medical problems in the context of neutropenia, infections, and exposure to multiple medications including polyantimicrobial therapy.
Nonhematologic side effects; n = 60
Side effect . | Grade 2 or lower (%) . | Grade 3 or higher (%) . |
|---|---|---|
| Diarrhea | 40 (67) | 3 (5) |
| Nausea | 40 (67) | 1 (2) |
| Headache | 40 (67) | — |
| Rash | 38 (63) | 11 (18)‡ |
| Hyperbilirubinemia | 35 (58) | 9 (15) |
| Flushing* | 35 (58) | 1 (2) |
| Vomiting | 31 (52) | — |
| Mucositis | 30 (50) | 2 (3) |
| ALT/AST elevation | 28 (47) | 10 (17) |
| Palmoplantar erythrodysesthesia† | 22 (37) | 2 (3) |
| Elevations of alkaline phosphatase | 10 (17) | 1 (2) |
| Extremity edema | 9 (15) | 1 (2) |
| Fatigue | 9 (15) | — |
| Pruritus | 7 (12) | — |
| Elevation of creatinine | 6 (10) | — |
| Ageusia | 6 (10) | — |
Side effect . | Grade 2 or lower (%) . | Grade 3 or higher (%) . |
|---|---|---|
| Diarrhea | 40 (67) | 3 (5) |
| Nausea | 40 (67) | 1 (2) |
| Headache | 40 (67) | — |
| Rash | 38 (63) | 11 (18)‡ |
| Hyperbilirubinemia | 35 (58) | 9 (15) |
| Flushing* | 35 (58) | 1 (2) |
| Vomiting | 31 (52) | — |
| Mucositis | 30 (50) | 2 (3) |
| ALT/AST elevation | 28 (47) | 10 (17) |
| Palmoplantar erythrodysesthesia† | 22 (37) | 2 (3) |
| Elevations of alkaline phosphatase | 10 (17) | 1 (2) |
| Extremity edema | 9 (15) | 1 (2) |
| Fatigue | 9 (15) | — |
| Pruritus | 7 (12) | — |
| Elevation of creatinine | 6 (10) | — |
| Ageusia | 6 (10) | — |
—indicates not applicable.
Infusion related.
Hand-foot syndrome.
Including one patient with Stevens-Johnson syndrome.
One patient (68-year-old male with a diagnosis of AML FABM4) developed pancreatitis in the setting of sepsis and multisystem organ failure and died on day 35 of therapy. Pancreatitis is not an expected adverse event with clofarabine or cytarabine, and its relation to the study drugs is not clear. Another patient (61-year-old male with secondary AML) developed a bullous and blistering skin rash and renal failure and died on day 10 of therapy. The patient was not at risk for tumor lysis syndrome, presenting with a white blood cell count of 1.3 × 109/L. Although the renal failure could be interpreted in context with the skin rash, infections, and exposure to nephrotoxic medications (amphotericin B), the early onset shortly after completion of therapy raises the possibility of a relationship to clofarabine.
Occurrence of myelosuppression was almost ubiquitous. Myelosuppression-associated complications are summarized in Table 5. About two thirds of the patients developed at least one episode of fever of unknown origin (FUO). Among documented infections were pneumonias (38%), septicemia (33%), skin infections (13%), and infection of the urinary tract (8%), as well as sinuses (3%) and stool infections (Clostridium difficile) (3%). Most pneumonias were diagnosed based on the occurrence of fever and infiltrates on chest x-ray or computed tomography scans. Fungal pneumonias (based on positive cultures from bronchoalveolar lavage) and bacterial infections were documented in 2 patients. The most common organisms found in cases of septicemia were coagulase-negative staphylococcus (6 patients), α-hemolytic streptococcus (3), stomatococcus (2), and Escherichia coli (2). Additional organisms included micrococcus, Streptococcus viridans, and vancomycin-resistant enterococcus (VRE). Skin infections were fungal in 3 patients (Aspergillus, Fusarium, Torulopsis glabrata).
Myelosuppression-associated side effects; n = 60
Adverse event . | No. . | % . |
|---|---|---|
| Prolonged myelosuppression*; grade 3 or higher | ||
| Anemia | 19 | 32 |
| Neutropenia | 26 | 43 |
| Thrombocytopenia | 25 | 42 |
| Infections | ||
| FUO | 38 | 63 |
| Documented infections | ||
| Pneumonia | 23 | 38 |
| Septicemia | 20 | 33 |
| Skin | 8 | 13 |
| Urine | 5 | 8 |
| Sinus | 2 | 3 |
| Stool; C difficile | 2 | 3 |
Adverse event . | No. . | % . |
|---|---|---|
| Prolonged myelosuppression*; grade 3 or higher | ||
| Anemia | 19 | 32 |
| Neutropenia | 26 | 43 |
| Thrombocytopenia | 25 | 42 |
| Infections | ||
| FUO | 38 | 63 |
| Documented infections | ||
| Pneumonia | 23 | 38 |
| Septicemia | 20 | 33 |
| Skin | 8 | 13 |
| Urine | 5 | 8 |
| Sinus | 2 | 3 |
| Stool; C difficile | 2 | 3 |
Blood count recovery exceeding 42 days from start of therapy.
Consolidation therapy
Overall, 44 patients received consolidation courses. This number is slightly higher than the number of patients achieving CR or CRp, because in some cases consolidation was preferred over reinduction for patients achieving PR. The median number of total treatment courses was 2 (range, 1 to 7). Of the 44 patients, 98% experienced at least one episode of grade 3 or higher myelosuppression, whereas 84% experienced grade 3 or higher myelosuppression with each course. Documented infections occurred in 52% of patients during consolidation at least once and in 48% of the patients with each consolidation course that they received. Dose reductions during consolidation courses were necessary in 36% of the patients.
Discussion
Since their introduction in the 1960s, nucleoside analogs have been a major source of new and active anticancer drugs.23 Clofarabine is a next-generation nucleoside analog that has been developed as a rational extension of the experience with fludarabine and cladribine.
The development of clofarabine in adults has been focused on AML. The phase 1 study included 32 patients with acute leukemias who were treated with clofarabine at dose levels from 7.5 mg/m2 per dose up to 55 mg/m2 per dose.15 Five patients with AML (16% including 2 CR and 3 CRp) responded, and 40 mg/m2 per dose was defined as the phase 2 dose for clofarabine. A subsequent singlecenter phase 2 study of clofarabine in patients with relapsed and refractory acute leukemias confirmed the antileukemic activity in AML (42% CR) and MDS (25% CR).16 The development of clofarabine in adult AML proceeded to combination strategies. In a previous study of patients with relapsed and refractory AML, we gave clofarabine as a 1-hour intravenous infusion for 5 days followed approximately 4 hours later by cytarabine at 1 g/m2/d as a 2-hour intravenous infusion over 5 days.22 During the initial phase 1 part, the clofarabine doses were gradually increased from 15 mg/m2 through 22.5 mg/m2 and 30 mg/m2 to 40 mg/m2 as the final dose level. Of 32 patients (25 AML, 4 high-risk MDS, 2 ALL, and 1 chronic myelogenous leukemia [CML] blast phase), 12 (38%) responded, including 7 patients (22%) with CR and 5 (16%) with CRp. Induction mortality was low and adverse events predictable and manageable. Cellular pharmacology of ara-CTP and clofarabine triphosphate suggested that clofarabine triphosphate accumulation resulted in an increase of intracellular ara-CTP in the leukemic blasts in some patients.
The current study represents a further step in the development of clofarabine in adult acute leukemias by attempting to use the combination in previously untreated adults with AML. We focused on an older age group based on the poorer prognosis of older AML induction patients, the lack of a standardized approach to these patients, and subsequently a greater medical need for safe and effective treatment programs in this patient group. The age limits chosen for this trial were also dependent upon ongoing other clinical trials for those patients under age 50 and proposed programs for patients older than 75 years for whom chemotherapy options in general may be considered unsuitable or too toxic to tolerate. We defined expectations of response based on a historical experience of comparable patient populations treated at MD Anderson Cancer Center from 1990 until present. In the current study, we report CR rates of 60% for patients with diploid cytogenetics and 43% for those with abnormal karyotypes. Compared with the historical response rates of 64% and 48%, respectively, the combination of clofarabine with cytarabine has achieved comparable response rates but did not exceed the historical expectations from our previous experience. On the other hand, the study combination was associated with manageable toxicities in most patients, and induction mortality was low.
Induction therapy in elderly AML patients remains challenging, and various regimens have been investigated by other groups (Table 6).1,3,24 In the Medical Research Council (MRC) AML11 trial, 1314 patients were randomized to 1 of 3 induction arms: daunorubicin, cytarabine, thioguanine (DAT); daunorubicin, cytarabine, etoposide (ADE); and mitoxantrone and cytarabine (MAC).4 Cytarabine was administered at a standard dose of 100 mg/m2/d in all 3 regimens. Patients were eligible if they were 60 years of age or older or if they were younger and not considered suitable for alternative induction programs. In fact, 13% of patients in the AML11 trial were younger than 60 years old. Remission rates were highest with DAT (62%) compared with ADE (50%) or MAC (55%). No difference existed with respect to 5-year overall survival (8% to 12%) and whether or not G-CSF was used. Induction mortality ranged from 16% (DAT) to 26% (ADE). The Southwest Oncology Group (SWOG) randomized patients older than 55 years of age with untreated AML to receive either mitoxantrone plus etoposide (ME) or cytarabine (200 mg/m2/d for 7 days) plus daunorubicin (45 mg/m2/d for 3 days) (DA).25 Among 328 patients, 34% achieved remission with ME and 43% with DA. The 2-year overall survival was 11% and 19%, respectively. Fourteen percent of the patients died within 7 days after therapy. Juliusson et al randomized patients older than age 60 (median age, 71 years) to idarubicin with intermediate doses of cytarabine (1 g/m2 twice daily for 4 consecutive days) with or without cladribine.26 They report a remission rate after one course of 51% with the 3-drug combination versus 35% for idarubicin plus cytarabine. No difference with regard to treatment arm was demonstrated in the 2-year survival rate. Fourteen percent of patients treated with all 3 drugs had secondary AML. Early induction mortality was 11%. ECOG evaluated 3 induction regimens in 362 older adults (median age, 67-69 years; range, 56-86 years).27 Patients received either daunorubicin, idarubicin, or mitoxantrone with standard-dose cytarabine. Overall CR rate was 42%, ranging from 29% in patients older than 70 years to 51% in those patients younger than 70 years. Induction mortality was 17%, with a significant increase in those older than age 70 (22%). There was no difference in CR rate or survival as to which anthracycline has been used. Given the incongruities of patient and disease characteristics, it continues to be difficult to compare the outcome of separate studies with each other.
Induction regimens in older patients with AML
Study . | No. . | Median age, y (range) . | CR, % . | Survival . | Induction death, % . |
|---|---|---|---|---|---|
| Goldstone et al4 | |||||
| DAT | 328 | —* | 62 | 18† | 16 |
| ADE | 327 | —* | 50 | 15 | 26 |
| MAC | 656 | —* | 55 | 16 | 17 |
| Anderson et al25 | |||||
| AD | 161 | 68 (56-84) | 43 | 19‡ | 15 |
| ME | 167 | 67 (56-86) | 43 | 11 | 19 |
| Juliusson et al26 | |||||
| CCI | 43 | 70 (64-75) | 51 | > 30§ | 12 |
| CI | 20 | 73 (65-77) | 35 | — | 10 |
| Rowe et al27 ¶ | |||||
| DA | 116 | 67 (56-82) | 40 | 5.7∥ | —22 |
| IA | 118 | 68 (56-86) | 43 | 9.4 | — |
| MA | 114 | 69 (56-84) | 46 | 7.1 | — |
Study . | No. . | Median age, y (range) . | CR, % . | Survival . | Induction death, % . |
|---|---|---|---|---|---|
| Goldstone et al4 | |||||
| DAT | 328 | —* | 62 | 18† | 16 |
| ADE | 327 | —* | 50 | 15 | 26 |
| MAC | 656 | —* | 55 | 16 | 17 |
| Anderson et al25 | |||||
| AD | 161 | 68 (56-84) | 43 | 19‡ | 15 |
| ME | 167 | 67 (56-86) | 43 | 11 | 19 |
| Juliusson et al26 | |||||
| CCI | 43 | 70 (64-75) | 51 | > 30§ | 12 |
| CI | 20 | 73 (65-77) | 35 | — | 10 |
| Rowe et al27 ¶ | |||||
| DA | 116 | 67 (56-82) | 40 | 5.7∥ | —22 |
| IA | 118 | 68 (56-86) | 43 | 9.4 | — |
| MA | 114 | 69 (56-84) | 46 | 7.1 | — |
DAT indicates daunorubicin, cytarabine, thioguanine; ADE, daunorubicin, cytarabine, etoposide; MAC, mitoxantrone, cytarabine; AD, cytarabine, daunorubicin; ME, mitoxantrone, etoposide; CCI, cladribine, cytarabine, idarubicin; CI, cytarabine, idarubicin; DA, daunorubicin, cytarabine; IA, idarubicin, cytarabine; MA, mitoxantrone, cytarabine; and—, not available.
The median age for the entire group was 66 years (range, younger than 56 to 80).
Five-year disease-free survival (DFS) (%).
2-year overall survival (%).
2-year overall survival (%) for the whole group of patients.
Median DFS in months.
As much as uncertainties exist as to the role of the clofarbine-cytarabine combination in comparison with other induction regimens, questions remain also about the optimal dose and schedule of cytarabine and clofarabine. The pharmacokinetic properties of clofarabine not only serve to increase intracellular levels of ara-CTP but also lead to prolonged retention of clofarabine triphosphate in leukemic blasts. This property suggests the possibility of activity at lower doses than the established phase 2 dose of 40 mg/m2 per dose or even a less intense schedule of administration.
Experience with single-agent clofarabine at lower doses has recently been presented. Burnett et al used clofarabine at 30 mg/m2 intravenously daily for 5 days for the treatment of newly diagnosed older patients (older than 70 years or older than 60 years if considered unfit for chemotherapy) with AML.28 Sixteen of 27 patients (59%) have achieved a CR. Although patient characteristics are important to consider and no comparison with a matched control population of patients has been presented, a CR rate of almost 60% in this patient group may be considered encouraging enough to further investigate some of the alternate doses and schedules further. Given the outcome with clofarabine at 30 mg/m2 in the study by Burnett et al, results of a study randomizing older AML induction patients to low-dose cytarabine versus hydroxyurea favoring the cytarabine arm,29 and our own experience with the combination regimens involving clofarabine, we are currently conducting a randomized phase 2 study of clofarabine at the 30 mg/m2 dose versus clofarabine plus low-dose cytarabine. This study, together with the clofarabine experience so far, should help to assess the position of clofarabine in the therapy of AML, especially in older patients where both more active drugs and less toxic regimens continue to be urgently needed.
The current study provides the first experience of a clofarabine combination in older patients with previously untreated AML. The combination has shown activity with a good CR rate and an acceptable safety profile, but remission duration and overall survival do not appear to be improved compared with the experience of other induction regimens. Modifications to investigate the most optimal dose and schedule of clofarabine combinations in adult AML with regard to response, duration of response and survival, and toxicities continue to be tested in clinical trials.
Prepublished online as Blood First Edition Paper, January 10, 2006; DOI 10.1182/blood-2005-08-3294
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal