Adenosine, released by cells in an injurious or hypoxic environment, possesses potent anti-inflammatory effects by inhibiting the production of proinflammatory cytokines and superoxide anions (O2-). We hypothesized that adenosine compounds also induced heterologous desensitization of chemokine receptors, which played a critical role in leukocyte trafficking. Our studies using adenosine receptor subtype-specific agonists revealed that pretreatment with adenosine compounds suppressed RANTES-induced chemotaxis and Ca2+ flux through activation of A2a adenosine receptor. Adenosine compounds also desensitized IL-8- and MCP-1-induced chemotaxis, but not that induced by fMLP. Activation of protein kinase A (PKA), a component of the signaling pathway induced by the A2a receptor, was sufficient to desensitize RANTES-induced chemotaxis. Inhibition of PKA reversed the desensitization effects of adenosine compounds, suggesting that PKA was necessary for A2a receptor-mediated heterologous desensitization. In a mouse model, prior activation of A2a receptors blocked RANTES-induced recruitment of leukocytes in an air pouch. Moreover, the A2a receptor-induced cross-desensitization also reduced the susceptibility of monocytes to infection by an R5 strain of HIV-1. Our results suggest that activation of A2a adenosine receptors suppresses chemokine receptor function, and such receptor cross-talk was based on the simple mechanism of PKA-mediated heterologous desensitization, thus contributing to the antiinflammatory activity of adenosine. (Blood. 2006;108:38-44)
Introduction
Chemoattractant receptors play an essential role in leukocyte trafficking, angiogenesis, inflammation, and brain development.1-5 In an inflammatory response, these 7 transmembrane receptors orchestrate the recruitment, infiltration, and activation of neutrophils, macrophages, and lymphocytes in a temporally and spatially specific manner. CCR5 is one of the key proinflammatory chemokine receptors expressed on granulocytes, macrophages, immature dendritic cells (DCs), CD8+ lymphocytes, and at a high level on TH1 lymphocytes.6 CCR5 was also found to be essential for the infection by M-tropic HIV.7 RANTES/CCL5, a major endogenous ligand of CCR5, induces chemotaxis of macrophages and lymphocytes at a sub-nanomolar concentration.8 Due to their critical function, the activity of chemokine receptors is tightly regulated at different levels of signaling cascades. At the receptor level, prolonged activation of one receptor typically results in desensitization of its own activity, termed homologous desensitization, or of other receptors in the same cell, called heterologous desensitization.9,10 Homologous desensitization is mainly based on the activation of a cascade of feedback inhibitors, including G proteincoupled receptor kinases (GRKs) and arrestins.10 Heterologous desensitization of chemokine receptors is typically dependent on target receptor phosphorylation by protein kinase C.9 Phosphorylation of the cytosolic portion of a chemokine receptor by these Ser/Thr kinases causes loss of effective coupling to downstream G proteins, and sometimes is followed by receptor internalization, resulting in the loss of receptor function.9-11 For example, CCR5 can be effectively homologously desensitized by treatment with RANTES, its own ligand, or by heterologous desensitization using fMLP, a potent ligand for the formal peptide receptor (FPR).11 Desensitization is a key safeguard feature of chemokine receptor regulation, which acts to prevent the damaging effects of prolonged or excessive activation.
Extracellular adenosine accumulates during inflammation or tissue injury and is a potent anti-inflammatory molecule.12,13 Four G protein-coupled receptors, namely, A1, A2a, A2b, and A3 receptors, have been identified to specifically respond to adenosine stimulation.14 Both high-affinity A1 and low-affinity A3 receptors couple to Gi/o proteins. Both high-affinity A2a and low-affinity A2b receptors couple and activate Gs proteins, and elevate cytosolic cAMP levels. Previous studies have suggested that A1 receptors function to enhance plasmacytoid DC or neutrophil chemotaxis.15,16 Nevertheless, the predominant effect of adenosine is anti-inflammatory, and this appears to be mediated nonredundantly by A2a receptors.13,17 In an in vitro system, A2a receptors suppress production of superoxide anion (O -2) and ligandstimulated adhesion of neutrophils.18,19 In Adora2a-/- mice, subthreshold doses of inflammatory stimuli induce prolonged inflammation and extensive tissue damage both in a liver damage model and in endotoxin-induced septic shock, due to enhanced production of proinflammatory cytokines, IFN-γ and TNF.17 In the present report we hypothesized that a portion of the anti-inflammatory activity of adenosine may involve the heterologous desensitization of proinflammatory chemokine receptors. We found that adenosine can effectively impair RANTES-induced chemotaxis of human monocytes. Moreover, adenosine also inhibits HIV infection, suggesting that CCR5 function is compromised. Furthermore, in an in vivo model, activation of A2a receptors effectively blocked RANTES-induced leukocyte recruitment into air pouches. Thus, our data from both in vitro and in vivo studies suggest that activation with adenosine induces a marked heterologous desensitization of RANTES receptors, and as a consequence, impairs leukocyte recruitment during inflammation. This observation provides a novel mechanistic basis for the anti-inflammatory activity of the A2a receptor.
Materials and methods
Chemicals, materials, and cells
RANTES (CCL5) was obtained from PeproTech (Rocky Hill, NJ); 125I-RANTES from Perkin Elmer Life Sciences (Wellesley, MA); adenosine, 5′-(N-ethylcarboxamino)-adenosine (NECA), CGS21680, N6-cyclopentyladenosine (CPA) from Sigma (St Louis, MO); N6-benzoyl-cAMP, cAMPS, Rp-8-Br, and cAMPS, Rp-isomer from Calbiochem (La Jolla, CA); cAMP assay kit from Amersham (Piscataway, NJ), and ZM241385 from Tocris (Ellisville, MO).
Human peripheral neutrophils, monocytes, and lymphocytes were obtained from healthy donor blood packs and isolated from buffy coat (Transfusion Medicine Department, National Institutes of Health Clinical Center, Bethesda, MD) by iso-osmotic Percoll gradient. The cells were more than 90% pure by nonspecific esterase staining or by morphologic analysis. Fresh monocytes were cultured in RPMI (Cambrex, Walkersvile, MD) supplemented with 10% fetal calf serum (Hyclone Laboratories, Logan, UT) at 37°C, 5% CO2 overnight before experiments.
The R5 JRFL strain of HIV-1 was obtained from the National Institute of Allergy and Infectious Diseases AIDS Research and Reference Reagent Program (Rockville, MD). Virus was propagated in cultures of peripheralblood mononuclear cells (PBMCs) from adult donors. Virus was concentrated from culture supernatants and purified by pelleting at 110 000g for 90 minutes. The pellets were gently washed and resuspended in medium.
RT-PCR
Total RNA from human neutrophils, monocytes, and lymphocytes were isolated by TriZol (Invitrogen, Carlsbad, CA) and reverse transcription-polymerase chain reaction (RT-PCR) was performed by using GeneAmp kit (Roche, Nutley, NJ) according to manufacturer's protocol. The 110- to 150-bp sequences at the 3′ end of each adenosine receptor gene were used to design RT-PCR primers.
Chemotaxis
Chemotaxis in micro-Boyden chambers was performed as described by the manufacturer (Neuroprobe, Gaithersburg, MD).20 In brief, monocytes were pretreated with adenosine or its analogs for 30 minutes at 37°C. The cells were then washed twice with the binding medium (RPMI supplemented with 1% bovine serum albumin and 20 mM HEPES) and loaded into the upper chemotaxis chambers. Chemokines diluted in binding medium were loaded into the lower chambers and covered with a piece of 5-μm polycarbonate filter membrane. The assays were performed in 37°C, 100% humidity, and 5% CO2 for 1 hour. The filter membrane was then washed, fixed, stained, and counted by microscopy. The total cell numbers in a defined area of high-power magnitude field (HPF) were recorded. The mean of 6 independent readings was used to plot each data point in the figure. Each figure is representative of at least 3 repetitive results. The statistic analysis was carried out by 2-way ANOVA analysis using PRIZM 3 software (GraphPad Software, San Diego, CA).
Calcium flux
Ligand-induced Ca2+ influx was measured as described.20 Briefly, cells at 107/mL were incubated in DMEM supplemented with 1 μM Fura-2 acetomethoxy ester (Invitrogen) at room temperature for 45 minutes, washed with DMEM once, Hanks balanced salt solution (HBSS) twice, and diluted to 2 × 106/mL. The cells were then loaded into a 2-mL cuvette at 37°C with a mini-magnetic stir-bar at the bottom, and put into a Perkin Elmer Life Science luminescence spectrometer. The relative ratio of fluorescent emission at 510 nm when excited by 340 and 380 nm was recorded as an indicator of intracellular concentration of Ca2+ ions.
cAMP assay
The cAMP assays were conducted as described.21 Briefly, monocytes were washed and resuspended in 0.1% BSA/DMEM at 1 × 107/mL. Aliquots of 200 μL cells were incubated with 2 μM CGS21680 at 30°C for the indicated time periods. The reactions were stopped by adding 200 μL 3.5% perchloric acid for 30 minutes and 100 μL of 50% KHCO3 for 10 minutes. The samples were centrifuged, and supernatants were used for cAMP assays according to the manufacturer's (Amersham) protocol. The concentration of cAMP at 0 minute was defined as 1.
Air pouch assay
All mice were obtained from the Animal Production Facility of the National Cancer Institute at Frederick, MD, and maintained under specific pathogenfree conditions in the Experimental Animal Facility at the National Cancer Institute at Frederick for several weeks to allow acclimation before the experiments. The animal studies were conducted in accordance with the principles and procedures outlined in the NIH Guide for the Care and Use of Animals. Air pouches were raised on the dorsum of 6- to 8-week-old Balb/c mice as described previously.22 Mice with a well-formed air pouch were randomized into groups. Each mouse was given an injection with 0.5 mL endotoxin-free PBS alone or PBS containing adenosine receptor agonists into the air pouches. Thirty minutes later, 1 mL RANTES or other chemoattractants in a 1-mL volume were injected into air pouches. In another 4 hours, the mice were humanely killed by CO2 asphyxiation, and cells in the air pouches were harvested with heparin and enumerated following trypan blue staining. Differential counting of leukocyte subpopulations migrating into the pouch space was performed after eosin-methylene blue staining of cells prepared by cytospin centrifugation.
Ligand-binding assay
The ligand-binding assay was performed as described with minor modification.20 The cells were washed and suspended in binding medium at 107/mL. The binding assays were carried out using 0.5 nM 125I-RANTES/CCL5 in the presence of an increasing concentration of competing unlabeled RANTES. The assay mixtures were incubated at room temperature for 30 minutes and unbound ligands were separated from the cells by centrifugation in a 10% sucrose/PBS gradient. The level of bound 125I-RANTES was detected by a γ-counter. Nonlinear regression analysis of the data was performed using PRIZM 3 (GraphPad Software) by fitting the following equation:
Total binding = Bmax * [Hot]/([Hot]+[Cold]+ Kd) + nonspecific binding
Flow cytometry
Human monocytes were harvested, washed, and cultured in a medium containing RPMI 1640, 25 mM HEPES, glutamine, human macrophage colony-stimulating-factor (50 ng/mL), and 10% fetal bovine serum for 24 hours. The cells were washed and suspended in binding medium at 107/mL. Cells were pretreated with RANTES (100 ng/mL), CGS21680 (2 μM), and PKAI (100 μM)/CGS21680 (2 μM) at 37°C for 30 minutes, then cooled down in ice. The following procedures were carried out at 4°C. Cells were immediately harvested, washed with cold HBSS (Life Technologies, Gaithersburg, MD) with 2% endotoxin-free FCS (HF; Hyclone Laboratories) twice, and resuspended in HBSS/FCS buffer. Cells were treated with FITC-conjugated anti-CCR5 (182F) antibody (R&D Research, San Diego, CA), incubated at 4°C for 60 minutes, washed, and analyzed in a Coulter Epics XL flow cytometer (Coulter, Hialeah, FL).
HIV infectivity by HIV p24 analysis
Infectivity analysis of HIV-1 strain JRFL was performed with fresh normal monocytes that were prepared by magnetic bead separation using anti-CD14 microbeads (Miltenyi Biotec, Auburn, CA) in RPMI 1640 medium supplemented with 10% heat-inactivated, low-endotoxin FCS (Hyclone Laboratories), 10 μg/mL gentamicin, and 1 mM L-glutamine The monocytes were washed and exposed to the designated concentrations of CGS21680, ZM241385, or both for 1 hour, followed by addition of HIV-1 (MOI, 0.1). The infected cells were washed after 1 hour to remove free virus and were allowed to incubate for 48 hours. The supernatants were then collected, and enzyme-linked immunosorbent assay (ELISA) was carried out to determine the level of HIV p24 (AIDS Vaccine Program, SAIC-Frederick, NCI-Frederick Cancer Research and Development Center, Frederick, MD).
Results
Chemotactic effects of adenosine
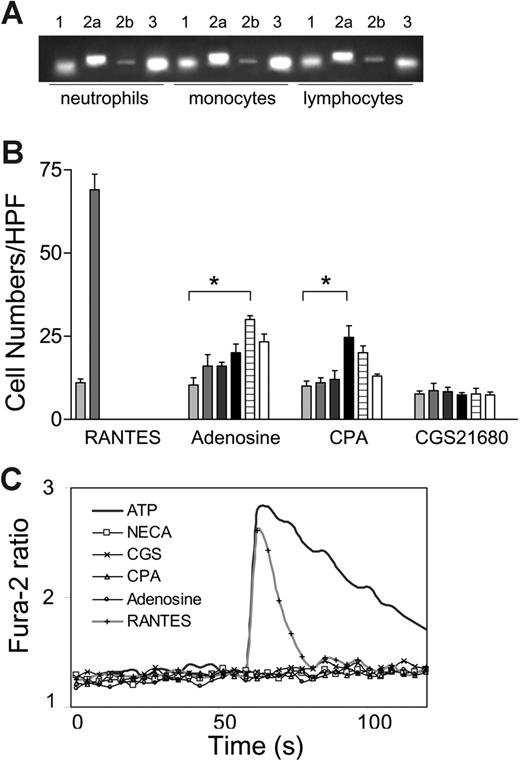
We used RT-PCR to assess the expression of 4 adenosine receptor subtypes in human neutrophils, monocytes, and lymphocytes. The results revealed that mRNA of each of the 4 adenosine receptors was detectable in all 3 types of human leukocyte population (Figure 1A). Gi-coupled low-affinity A3 receptors are abundant in neutrophil and monocytes. The level of Gi-coupled high-affinity A1 receptors was lower than that of A3 receptors, and the mRNA level of Gs-coupled high-affinity A2a receptors was significantly higher than that of low-affinity A2b receptors. These results are consistent with a previous report, which compared the expression pattern of adenosine receptors on mouse leukocytes.23
Chemotactic effects of adenosine. (A) Semiquantitative RT-PCR revealed the expression of A1, A2a, A2b, and A3 receptors on human neutrophils, monocytes, and lymphocytes. (B) Adenosine (from left, 0, 0.1, 1, 10, 100, 1000 μM) and CPA (from left, 0, 0.01, 0.1, 1, 10, 100 μM), but not CGS21680 (from left, 0, 0.1, 1, 10, 100, 1000 μM) induced chemotaxis by human monocytes. (*P < .05). (C) Adenosine and its analogs did not induce a transient Ca2+ influx. ATP and RANTES/CCL5 induced robust Ca2+ influx.
Chemotactic effects of adenosine. (A) Semiquantitative RT-PCR revealed the expression of A1, A2a, A2b, and A3 receptors on human neutrophils, monocytes, and lymphocytes. (B) Adenosine (from left, 0, 0.1, 1, 10, 100, 1000 μM) and CPA (from left, 0, 0.01, 0.1, 1, 10, 100 μM), but not CGS21680 (from left, 0, 0.1, 1, 10, 100, 1000 μM) induced chemotaxis by human monocytes. (*P < .05). (C) Adenosine and its analogs did not induce a transient Ca2+ influx. ATP and RANTES/CCL5 induced robust Ca2+ influx.
We further investigated the capacity of adenosine to induce chemotaxis. Adenosine induced a modest chemotactic response by human monocytes (Figure 1B). The dose-response curve followed a typical bell-shaped curve and the chemotaxis index reached a maximum of 2.5 at 0.1 mM adenosine (P < .05). In contrast, RANTES/CCL5, a potent proinflammatory chemokine, induced a robust chemotactic response, consistent with previous reports.8 Specific agonists were used to determine which adenosine receptor was involved. CPA, a specific agonist of the A1 receptors, elicited monocyte chemotaxis at a concentration of 1 μM, whereas monocytes failed to respond to CGS21680, a specific agonist of A2a receptor (Figure 1B). A weak but statistically significant CPA-induced chemotaxis was also detected for neutrophil and lymphocytes (data not shown). Therefore, adenosine is capable of inducing the migration of some leukocyte populations in vitro, probably by interacting with the A1 receptors.
We further assessed the potency of adenosine as a chemoattractant byaCa2+ influx assay because this response is associated with robust chemoattractant receptor activation. However, weak chemoattractants are typically incapable of inducing Ca2+ influx.20 RANTES/CCL5 at 100 ng/mL induced a transient Ca2+ influx, consistent with previous reports (Figure 1C).24 The adenosine receptor agonists did not induce a detectable Ca2+ influx in human monocytes and neutrophils, suggesting that Gi-coupled A1 and A3 adenosine receptor activation was incapable of generating sufficient IP3 to release calcium storage in endoplasmic reticulum (ER; Figure 1C and data not shown). In contrast, ATP elicited a marked and prolonged calcium influx, likely due to the activation of P2X receptors.25 These data, in agreement with chemotaxis analysis, suggest that adenosine is a weak chemoattractant. Furthermore, the level of A1 and A3 adenosine receptor-induced signal transduction in leukocytes was modest.
Treatment by adenosine induced heterologous desensitization of RANTES/CCL5 receptors
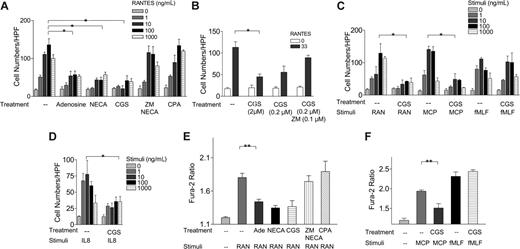
RANTES/CCL5 is a major proinflammatory chemokine that regulates leukocyte trafficking.7,8,24 In a Boyden chamber assay, RANTES/CCL5, at a concentration from 0 to 1000 ng/mL, induced a typical bell-shaped chemotactic response (Figure 2A).8,24 Pretreatment with 0.1 mM adenosine at 37°C for 30 minutes impaired this RANTES/CCL5-induced chemotaxis (Figure 2A). NECA at 10 μM, a potent activator of both A1 and A2a receptors, also inhibited CCL5-induced chemotaxis, at a level similar to that induced by adenosine. We determined which subtype of adenosine receptor is involved in heterologous desensitization by using A1 and A2a receptor-specific agonists, CPA and CGS21680, respectively. Pretreatment with CGS21680 at 2 μM, an A2a receptor-specific agonist, induced a similar potent inhibitory effect on the RANTES response (Figure 2A). In contrast, the A1 receptor agonist, CPA, had no effect. Furthermore, ZM241385, an A2a receptor-specific antagonist, at 10 μM, reversed the NECA-induced inhibitory effects (Figure 2A). CGS21680 at 0.2 μM also induced a potent inhibitory effect, which was reversed by pretreatment with 0.1 μM ZM241385 (Figure 2B). In the absence of pretreatment, a mixture of CGS21680 with RANTES did not show any inhibitory effects, indicating that the inhibitory effects of adenosine and its analogs did not result from their direct binding to RANTES receptors (data not shown). It typically took at least 30 minutes of pretreatment with adenosine compounds to elicit inhibitory effects on chemotaxis. These data suggested that A2a receptors mediate adenosineinduced desensitization of RANTES receptors. We expanded our investigation to other chemoattractant receptors and found that CGS21680 effectively down-regulated CCL3/MIP-1α, CCL2/MCP-1, but not fMLP-induced chemotaxis of human monocytes (Figure 2C and our unpublished data, January 2006). Furthermore, CGS21680 pretreatment also desensitized CXCL8/IL-8-induced chemotaxis of human neutrophils (Figure 2D).
Role of A2a receptor in adenosine-induced heterologous desensitization of RANTES receptors. (A) Effects of adenosine (0.1 mM), NECA (10 μM), CGS21680 (2 μM), ZM241385 (2 μM)/NECA (10 μM), and CPA (0.3 μM) on RANTES-induced chemotaxis of human monocytes. (B) Effects of CGS21680 at 2 μM and 0.2 μM, CGS21680 (0.2 μM)/ZM241385 (0.1 μM) on RANTES-induced chemotaxis of human monocytes. (C) Effects of CGS21680 (2 μM) on RANTES (RAN), MCP-1 (MCP), and fMLF-induced chemotaxis of human monocytes. (D) Effects of CGS21680 (2 μM) on CXCL8/IL-8-induced chemotaxis of human neutrophils. (E) Effects of adenosine (Ade, 0.1 mM), NECA (10 μM), CGS21680 (2 μM), ZM241385 (2 μM)/NECA (10 μM), and CPA (0.3 μM) on RANTES-induced (50 ng/mL) Ca2+influx in human monocytes. (F) Effects of CGS21680 (2 μM) on MCP-1- and fMLF-induced Ca2+influx (*P < .001, by 2-way ANOVA analysis; **P < .02, Student t analysis).
Role of A2a receptor in adenosine-induced heterologous desensitization of RANTES receptors. (A) Effects of adenosine (0.1 mM), NECA (10 μM), CGS21680 (2 μM), ZM241385 (2 μM)/NECA (10 μM), and CPA (0.3 μM) on RANTES-induced chemotaxis of human monocytes. (B) Effects of CGS21680 at 2 μM and 0.2 μM, CGS21680 (0.2 μM)/ZM241385 (0.1 μM) on RANTES-induced chemotaxis of human monocytes. (C) Effects of CGS21680 (2 μM) on RANTES (RAN), MCP-1 (MCP), and fMLF-induced chemotaxis of human monocytes. (D) Effects of CGS21680 (2 μM) on CXCL8/IL-8-induced chemotaxis of human neutrophils. (E) Effects of adenosine (Ade, 0.1 mM), NECA (10 μM), CGS21680 (2 μM), ZM241385 (2 μM)/NECA (10 μM), and CPA (0.3 μM) on RANTES-induced (50 ng/mL) Ca2+influx in human monocytes. (F) Effects of CGS21680 (2 μM) on MCP-1- and fMLF-induced Ca2+influx (*P < .001, by 2-way ANOVA analysis; **P < .02, Student t analysis).
Ca2+ flux is mediated by chemokine receptors via a signaling pathway distinct from the chemotactic pathway. Therefore, if adenosine and its analogs desensitize chemokine-induced Ca2+ flux, it will suggest that the desensitization is likely to be at the level of chemokine receptors. RANTES/CCL5 at 100 ng/mL induced a transient but marked Ca2+-ion influx in human monocytes, as indicated by an increase in the Fura-2 ratio from 1.18 ± 0.03 to 1.79 ± 0.07 (Figure 2E). Pretreatment with 0.1 mM adenosine, 10 μM NECA, 2 μM CGS21680, but not 0.3 μMCPA, also impaired CCL5/RANTES-induced Ca2+-ion influx (Figure 2E). ZM241385 blocked NECA-induced desensitization effects. Pretreatment with CGS21680 also desensitized MCP-1-induced Ca2+ flux (Figure 2F). No inhibitory effect was detected on fMLF-induced Ca2+-ion influx follow treatment with CGS21680 (Figure 2F). Taken together, our results suggest that prior activation of A2a receptors causes heterologous desensitization of a number of chemokine receptors, including RANTES receptors.
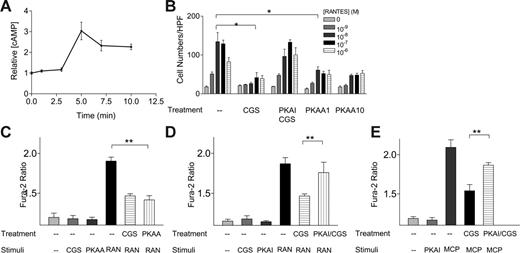
Role of PKA in A2a receptor-mediated heterologous desensitization of RANTES receptors. (A) Effect of CGS21680 on cAMP accumulation in human monocytes. (B) Effect of a potent PKA inhibitor, cAMPs, Rp-isomer, TEA salt, at 100 μM (PKAI), on the inhibitory effects of CGS21680 (2 μM) on RANTES-induced chemotaxis. Effect of the potent PKA activator, N6-benzoyl-cAMP, at 1 and 10 μM (PKAA), on RANTES-induced chemotaxis in the absence of CGS21680. (C) Effects of PKAA at 1 μM on RANTES-induced Ca2+ influx. (D) Effects of a PKA inhibitor, cAMPs, Rp-isomer (PKAI), at 100 μM on CGS21680-induced inhibitory effects on RANTES-mediated Ca2+ influx. (E) Effects of a PKA inhibitor, cAMPs, Rp-isomer (PKAI), at 1 μM on CGS21680-induced inhibitory effects on MCP-1-mediated Ca2+-influx. *P < .001, by 2-way ANOVA analysis; **P < .03, by Student t analysis.
Role of PKA in A2a receptor-mediated heterologous desensitization of RANTES receptors. (A) Effect of CGS21680 on cAMP accumulation in human monocytes. (B) Effect of a potent PKA inhibitor, cAMPs, Rp-isomer, TEA salt, at 100 μM (PKAI), on the inhibitory effects of CGS21680 (2 μM) on RANTES-induced chemotaxis. Effect of the potent PKA activator, N6-benzoyl-cAMP, at 1 and 10 μM (PKAA), on RANTES-induced chemotaxis in the absence of CGS21680. (C) Effects of PKAA at 1 μM on RANTES-induced Ca2+ influx. (D) Effects of a PKA inhibitor, cAMPs, Rp-isomer (PKAI), at 100 μM on CGS21680-induced inhibitory effects on RANTES-mediated Ca2+ influx. (E) Effects of a PKA inhibitor, cAMPs, Rp-isomer (PKAI), at 1 μM on CGS21680-induced inhibitory effects on MCP-1-mediated Ca2+-influx. *P < .001, by 2-way ANOVA analysis; **P < .03, by Student t analysis.
Involvement of PKA in heterologous desensitization induced by A2a receptors
We investigated the molecular mechanism of A2a receptormediated heterologous desensitization. On stimulation by 2 μM CGS21680, human monocytes responded with a 3-fold increase in the level of intracellular cAMP within 5 minutes, consistent with previous reports showing that A2a receptors activate adenylyl cyclase through coupling to Gs proteins (Figure 3A). Elevation of cAMP typically activates PKA, a family of Ser/Thr kinases that plays a pleiotropic role in regulating cell function. In the absence of adenosine analogs, 1 or 10 μM N6-benzoyl cAMP, a membranepermeable specific PKA activator (PKAA), is sufficient to induce a marked inhibition of RANTES-mediated chemotaxis (Figure 3B). “cAMPs, Rp-isomer” at 100 μM, a specific inhibitor of PKA (PKAI), reversed the inhibitory effects of CGS21680, suggesting that PKA is required for A2a receptor-mediated heterologous desensitization (Figure 3B). We further examined the effects of PKA inhibitors and activators in RANTES-elicited Ca2+-ion influx. As shown in Figure 3C, the PKAA at 1 μM was sufficient to inhibit RANTES-elicited Ca2+ influx in the absences of adenosine compounds (Figure 3C). In contrast, the PKAI at 100 μM partially reversed CGS21680 inhibition of RANTES-induced Ca2+-ion influx (Figure 3D). Furthermore, PKAI also blocked CGS21680 inhibition of MCP-1 induced Ca2+ flux (Figure 3E). Our data suggested that activation of A2a receptors induced an increase in intracellular cAMP, and activation of PKA was sufficient and necessary for A2a receptor-mediated desensitization of RANTES receptors.
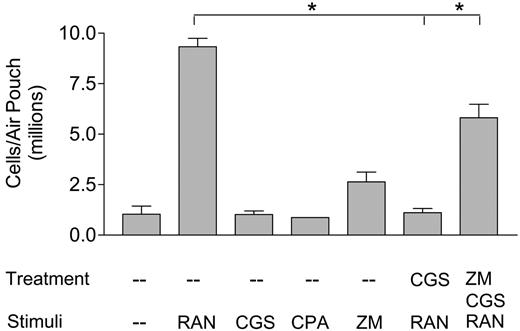
Treatment with CGS21680 blocked leukocyte recruitment in vivo
An air pouch model in mice was used to assess the inhibitory effects of adenosine receptor agonists on leukocyte trafficking in vivo. The result show that RANTES/CCL5 at 1 μg/mL induced an 8-fold increase in the numbers of leukocytes infiltrating each air pouch, indicative of its high chemotactic potency (Figure 4). In contrast, CGS21680 at 20 μMorCPAat 3 μM did not induce an influx of leukocytes, consistent with our previous observation that adenosine played a limited role in mediating chemotaxis in vitro (Figure 1). A 30-minute pretreatment with 20 μM CGS21680 almost totally blocked RANTES-induced leukocyte recruitment, which is consistent with A2a receptor-mediated heterologous desensitization of RANTES receptors in vivo. ZM241385 at 30 μM, an A2a receptor-specific antagonist, enhanced the cell numbers in air pouches by 2-fold through an unknown mechanism. When CGS21680 was administered with ZM241385, the inhibitory effects with CGS21680 were significantly reversed, further indicating that A2a receptors were involved in CGS21680-elicited inhibition of leukocyte trafficking. These in vivo air pouch experiments supported the physiologic significance of our in vitro observation that adenosine interferes with the chemotactic responses of leukocytes.
In vivo assay of RANTES-induced leukocyte recruitment into murine air pouches. The air pouches were initially treated with PBS, 20 μM CGS21680, or 30 μM ZM241385 together with 20 μM CGS21680 for 30 minutes, then stimulated with either 1 μg/mL RANTES, 20 μM CGS21680, 3 μM CPA, or the combination of CGS21680 and 30 μM ZM241385, for 4 hours. The cells were then harvested in PBS supplemented with EDTA and heparin, stained, and counted. (This graph represents data from one of 3 in vivo experiments, each bar represents 4 mice; *P < .01.)
In vivo assay of RANTES-induced leukocyte recruitment into murine air pouches. The air pouches were initially treated with PBS, 20 μM CGS21680, or 30 μM ZM241385 together with 20 μM CGS21680 for 30 minutes, then stimulated with either 1 μg/mL RANTES, 20 μM CGS21680, 3 μM CPA, or the combination of CGS21680 and 30 μM ZM241385, for 4 hours. The cells were then harvested in PBS supplemented with EDTA and heparin, stained, and counted. (This graph represents data from one of 3 in vivo experiments, each bar represents 4 mice; *P < .01.)
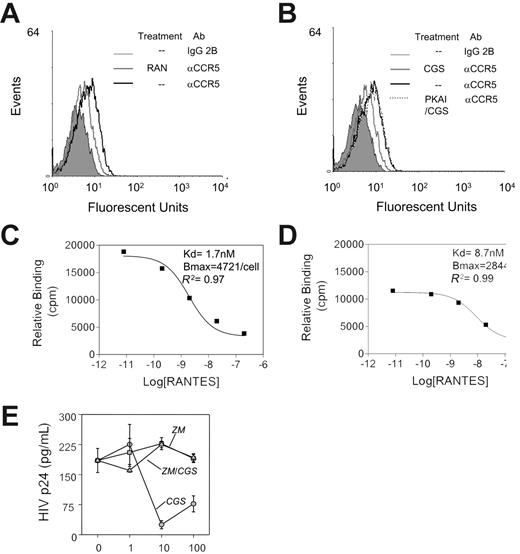
Effect of activation of A2a receptors on CCR5-mediated HIV-1 viral infection. (A) FACS analysis revealed a decrease in the CCR5 level on activated human monocytes after pretreatment with RANTES for 30 minutes at 37°C. (B) FACS analysis revealed a decrease in the CCR5 level on activated human monocytes after pretreatment with CGS21680 (2 μM) for 30 minutes at 37°C. Pretreatment with cAMPs, Rp-isomer (PKAI) at 100 μM reversed the inhibitory effects by CGS21680. (C) Homologous competition binding analysis, using 125 I-labeled RANTES, on the binding affinity (Kd) and binding sites (Bmax) on human monocytes. (D) Treatment with CGS21680 (2 μM) decreased RANTES binding affinity by 5-fold and RANTES binding sites by 39% (2-way ANOVA analysis of panel C versus panel D yield P < .001). (E) Effect of pretreatment with CGS21680 on the susceptibility of monocytes to R5 HIV-1. Monocytes were pretreated with either CGS21680 (CGS), the adenosine receptor antagonist ZM241385 (ZM), or both (ZM/CGS) at concentrations from 1 to 100 μM, and after 1 hour, the monocytes were exposed to HIV-1 strain JRFL. Cells were washed after 1 hour and returned to culture for 48 hours, and the supernatants were collected. The HIV p24 level in the supernatants was determined by ELISA. Data are representative of 6 experiments.
Effect of activation of A2a receptors on CCR5-mediated HIV-1 viral infection. (A) FACS analysis revealed a decrease in the CCR5 level on activated human monocytes after pretreatment with RANTES for 30 minutes at 37°C. (B) FACS analysis revealed a decrease in the CCR5 level on activated human monocytes after pretreatment with CGS21680 (2 μM) for 30 minutes at 37°C. Pretreatment with cAMPs, Rp-isomer (PKAI) at 100 μM reversed the inhibitory effects by CGS21680. (C) Homologous competition binding analysis, using 125 I-labeled RANTES, on the binding affinity (Kd) and binding sites (Bmax) on human monocytes. (D) Treatment with CGS21680 (2 μM) decreased RANTES binding affinity by 5-fold and RANTES binding sites by 39% (2-way ANOVA analysis of panel C versus panel D yield P < .001). (E) Effect of pretreatment with CGS21680 on the susceptibility of monocytes to R5 HIV-1. Monocytes were pretreated with either CGS21680 (CGS), the adenosine receptor antagonist ZM241385 (ZM), or both (ZM/CGS) at concentrations from 1 to 100 μM, and after 1 hour, the monocytes were exposed to HIV-1 strain JRFL. Cells were washed after 1 hour and returned to culture for 48 hours, and the supernatants were collected. The HIV p24 level in the supernatants was determined by ELISA. Data are representative of 6 experiments.
Activation of adenosine receptors blocked HIV infection
The likelihood that adenosine desensitizes CCR5 suggested that it may thus interfere with R5 HIV-1 infection of leukocytes. This would be consistent with results obtained from the study of heterologous desensitization of CCR5 induced by other G proteincoupled receptors.26-28 We assessed the inhibitory effects of CGS21680 on the affinity and the copy numbers of RANTESbinding sites on human monocytes. RANTES exhibits a very high affinity for both CCR1 and CCR5, at a dissociation constant of 10-9 nM. Results of fluorescence-activated cell sorting (FACS) analysis revealed a decrease in membrane CCR5 level of activated monocytes after pretreatment with RANTES or CGS21680, indicative of homologous desensitization of RANTES or heterologous desensitization by CGS21680, respectively (Figure 5A-B). Semiquantitative RT-PCR did not detect a change of CCR5 mRNA after CGS21680 treatment, suggesting that the desensitization effects of adenosines are not due to a decrease in CCR5 transcription (our unpublished results, January 2006). To confirm the FACS analysis results, we directly measured the RANTES binding on cell surfaces of activated monocytes. Pretreatment with 2 μM CGS21680 caused a 39% decrease in cell-surface RANTES-binding sites and a 5-fold decrease in the binding affinity, indicative of the impairment of RANTES receptor function (Figure 5C-D). Heterologous desensitization of CCR5 often results in an inhibition in HIV infection.26,27 We reasoned that the desensitization of CCR5 following preincubation with the adenosine A2 receptor agonist CGS21680 suggests that these cells may exhibit altered susceptibility to HIV infection. To test this possibility, we treated monocytes with CGS21680 for 60 minutes to allow for heterologous desensitization of CCR5 and then subjected these cells to infection with the R5 HIV-1 strain JRFL. Our data (Figure 5E) show that CGS21680 pretreatment resulted in a significant reduction in the level of R5 HIV infectivity and replication. The cross-desensitization appears to be dependent on adenosine receptor A2 based on the failure of CGS21680 to mediate an effect in the presence of the antagonist ZM241385. These results correlate well with the results described, which showed that CGS21680 induced heterologous desensitization of the function of CCR5.
Discussion
It has been reported that adenosine is chemotactic and may therefore promote leukocyte recruitment to inflammatory sites.16 We confirmed that this effect was dependent on A1 adenosine receptors. Although Gi-coupled A1 receptors were expressed by human monocytes, neutrophils, and lymphocytes, they only mediated weak chemotaxis and failed to elicit a transient Ca2+ flux. Not unexpectedly, our results show that CPA, an agonist for A1 receptor, failed to recruit leukocytes to the air pouch. Thus, both in vitro and in vivo data suggest that A1 receptors do not play a significant proinflammatory role.
On the other hand, the reported anti-inflammatory effects of adenosine were more readily substantiated. Treatment with adenosine impaired chemokine receptor-mediated chemotaxis and Ca2+ flux. Similar inhibitory effects were obtained with the A2a receptorspecific agonist, CGS21680, and the CGS2680 effect was blocked with ZM241385, indicating that adenosine-induced inhibitory effects were mediated by A2a receptors. In vivo studies using an air pouch model confirmed that CGS21680 was a potent inhibitor of leukocyte recruitment, supporting a physiologic role of adenosineinduced heterologous desensitization. We tested Adora2a-/- mice to further confirm whether CGS21680-mediated inhibitory effects were A2a receptor dependent. To our surprise, RANTES failed to induce a leukocyte recruitment to air pouches in these mice (data not shown). We speculate that the reduced capacity of the mice to vasodilate might have contributed to this reduced leukocyte infiltration into air pouches. We are currently examining this phenomenon further.
Chemokine receptor-mediated heterologous desensitization typically involves activation of Gi proteins and subsequent induction of PKC.9 Phosphorylation of the cytoplasmic tail and intracellular loops of chemokine receptors uncouples their interaction with downstream G proteins, which may result in receptor internalization.9,10 Previous studies have established the essential role of PKA in adrenergic receptor-mediated heterologous desensitization.29 Our data suggest that PKA is required for adenosine-induced heterologous desensitization of chemokine receptors through Gscoupled A2a receptors. The mechanistic consequence of activation of A2a receptors was to elevate intracellular [cAMP], an activator of PKA. The fact that N6-benzoyl cAMP desensitized RANTES receptors and “cAMPs, Rp-isomer” blocked the CGS21680-induced inhibitory effects suggested that PKA was sufficient and necessary for A2a receptor-mediated heterologous desensitization. Prolonged treatment with CGS21680 impairs both RANTES- and MCP-1-induced chemotaxis and Ca2+ flux. Loss of chemokineinduced function can be partly explained by decrease in the binding affinity and sites of chemokine receptors. However, it is still unclear whether adenosine-activated PKA can directly phosphorylate chemokine receptors. Additional studies are needed to map out the substrates of PKA phosphorylation and the precise biochemical basis for cross-talk mechanism. In addition to directly modifying activities of chemokine receptors, previous reports showed that activation of A2a receptors inhibited chemokine-induced expression of integrin, which might also contribute to an impairment of chemotaxis.30
PKC-mediated heterologous desensitization between G proteincoupled receptors follows a hierarchy. For example, certain receptors, such as the fMLF receptor, have a higher capacity to desensitize other receptors and are poorly desensitized. For example, treatment with fMLF causes a substantial phosphorylation and internalization of C5a and IL-8 receptors, resulting in over 50% inhibition of their function.31,32 In contrast, IL-8 receptors are unable to fully cross-desensitize fMLF receptors. A similar hierarchy may exist for adenosine-induced heterologous desensitization. For example, CGS21680 significantly down-regulates RANTES- and MCP-1-induced chemotaxis, but not fMLP-induced chemotaxis.
It has been well established that adenosine nonredundantly limits inflammation by activating A2a receptors.17 Two general pathways have been proposed to explain the adenosine-induced immunosuppressive activity: the activation of A2a receptors dampens T-cell function in general, or adenosine inhibits the secretion of proinflammatory cytokines and superoxide anion (O -2) from monocytes, neutrophils, and other cells.12,13,15,17-19 Our data suggest a novel anti-inflammatory activity of A2a receptors, which results in inhibition of leukocyte trafficking. The lack of leukocyte infiltration in a liver damage model in Adora2a-/- mice further supports our conclusion.17 The relative contribution of each mechanism to the suppression of inflammation is still unclear. However, our results suggest that A2a receptors impose suppressive effects at multiple levels of immune responses. During the initial stage of inflammation, inhibition of superoxide anion (O -2) secretion by neutrophils and phagocytosis by macrophages limits the extent of innate immune response. At a later stage, A2a receptors dampen adaptive immunity by down-regulating the production of 2 pivotal proinflammatory TH1 cytokines, INF-γ and TNF. Desensitization of chemokine receptors provides an additional level of regulation to limit the potential damaging aspects of inflammation. Thus, we propose that adenosine receptor cross-talk suppresses inflammation in a temporally and spatially concerted fashion in part by interfering with inflammatory cell trafficking.
Prepublished online as Blood First Edition Paper, March 7, 2006; DOI 10.1182/blood-2005-06-2599.
Supported by National Institutes of Health grants T32DA07237, DA-06650, DA14230, DA-16544, and DA-13429; in part with federal funds from the National Cancer Institute under contract no. NO1-CO-12400; and by the National Natural Science Foundation of China (no. 30400401).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal