Early T-lineage progenitors (ETPs) arise after colonization of the thymus by multipotent bone marrow progenitors. ETPs likely serve as physiologic progenitors of T-cell development in adult mice, although alternative T-cell differentiation pathways may exist. While we were investigating mechanisms of T-cell reconstitution after bone marrow transplantation (BMT), we found that efficient donor-derived thymopoiesis occurred before the pool of ETPs had been replenished. Simultaneously, T lineage–restricted progenitors were generated at extrathymic sites, both in the spleen and in peripheral lymph nodes, but not in the bone marrow or liver. The generation of these T lineage–committed cells occurred through a Notch-dependent differentiation process. Multipotent bone marrow progenitors efficiently gave rise to extrathymic T lineage–committed cells, whereas common lymphoid progenitors did not. Our data show plasticity of T-lineage commitment sites in the post-BMT environment and indicate that Notch-driven extrathymic Tlineage commitment from multipotent progenitors may contribute to early T-lineage reconstitution after BMT.
Introduction
The majority of T cells are produced in the thymus after colonization by bone marrow (BM) progenitors. Because the thymus lacks self-renewing stem cells, T-lineage development is maintained through input of blood-derived progenitors.1,2 Thymic reconstitution and function can be limiting after chemotherapy or BM transplantation (BMT), during HIV infection, and in normal aging.3-7 In particular, lymphoid recovery is slow in patients undergoing BMT.3,4 Although peripheral expansion of mature T cells leads to partial immune reconstitution, recovery of a diverse immune repertoire is linked to thymic function.4,8 Understanding the molecular and cellular mechanisms of T-lineage reconstitution is essential for improving immune function after BMT and in other lymphopenic settings.
Notch signaling is essential during T-lineage development from multipotent hematopoietic progenitors.9,10 In the absence of Notch function, T-lineage development is arrested at an early stage.11 Conversely, constitutively active forms of Notch or expression of Notch ligands of the Delta-like family drive T-cell development at extrathymic sites in vivo12-14 and in coculture with BM stromal cell lines.15-17 Among the 4 mammalian Notch receptors (Notch1-4), Notch1 is required for T-lineage development.9-11 Although the key role of Notch is well established, the identity of the progenitors that first receive a Notch signal during T-lineage development is unknown (for a review, see Maillard et al10 ). In particular, it remains controversial whether Notch signals are first delivered in the thymus or whether they are delivered extrathymically, thereby driving the first steps of T-lineage specification outside of the thymus.18-22
During fetal development, T/NK-restricted progenitors arise in fetal liver and blood, suggesting that T-lineage specification can occur before thymic colonization.19,23-25 In adult mice, we found that early T-lineage progenitors (ETPs), the most primitive thymic progenitors, are likely derived from multipotent circulating Lin–Sca-1hic-Kithi (LSK) progenitors, 26,27 a population that contains previously characterized early lymphoid progenitors.28,29 Recent data indicate that the generation of ETPs depends on Notch signals that are probably delivered early after thymic entry.21,22 However, the existence of extrathymic T-lineage commitment pathways has been reported or suggested in adult mice.18,30-34 In particular, Ezine and collaborators32,33 have studied hematopoietic spleen colonies at day 12 after BMT and identified a population of donor-derived cells with phenotypic and functional characteristics of committed T-lineage progenitors, even in athymic recipients. Importantly, these progenitors could complete T-cell differentiation in the thymus when transferred intravenously, 32,33 suggesting a possible role in post-BMT thymic reconstitution. Whether this process required Notch signaling at extrathymic sites remained unknown.
In this study, we investigated T-cell reconstitution at early time points after murine BMT. We found that a wave of T lineage–committed RAG+ progenitors arose in the spleen and other peripheral lymphoid organs, but not in BM or liver, during the first weeks after BMT. These cells differentiated from purified BM progenitors, most efficiently from Flt3+ non–self-renewing multipotent LSK cells, and also from Flt3– LSK cells (enriched in long-term hematopoietic stem cells [HSCs]), but not efficiently from common lymphoid progenitors.35-37 To study the role of Notch in this process, we assessed Notch target gene expression and used a genetic model of Notch inactivation by expressing a dominant-negative inhibitor of Mastermind-like proteins (DNMAML1) that blocked Notch-mediated transcriptional activation. Our data showed that Notch signaling was active in extrathymic T lineage–committed progenitors and required for the generation of these cells. Donor-derived T-lineage progenitors appeared in peripheral lymphoid organs during a window of time after BMT when ETPs were absent or dramatically reduced in the thymus. Thus, Notch-dependent T-committed progenitors are present at multiple extrathymic sites after BMT and may provide a pool of T-cell precursors during recovery from lymphopenia.
Materials and methods
Mice
C57BL/6 (B6) and C57BL/6.Ly5.2 (Ly5SJL) mice were from Taconic (Germantown, NY). NG-BAC mice, which have GFP expression driven by Rag2 locus control elements, were kindly provided by M. Nussenzweig (Rockefeller University, New York, NY).38 All mice were females at 6 to 8 weeks of age. Experiments were performed according to protocols approved by the University of Pennsylvania Office of Regulatory Affairs.
BMT
Ly5SJL BM cells (105) were injected intravenously into B6 mice 2 to 6 hours after irradiation (900 rads). Ly5SJL mice were used as recipients of NG-BAC BM.
Retroviral transduction
The following retroviral constructs were used: MigR1 (GFP control), DNMAML1 (encoding a truncated Mastermind-like1 fused to GFP acting as a dominant-negative inhibitor of Notch signaling), 39,40 and Mig-Dtx1 (encoding the Notch modulator Deltex1 that inhibits Notch1 but not Notch2 in vivo). 40,41 Retroviral supernatant was produced as described.12 BM from 5-fluorouracil–treated B6.Ly5SJL mice was retrovirally transduced12 and transplanted into irradiated mice.
Antibodies
The following antibodies were used: FITC-conjugated anti-Ly5B6 (104), anti-Ly5SJL (A20); PE-conjugated anti-CD8α (53-6.7), anti-CD8β (H35-17.2), anti-TCRβ (H57-597), anti-TCRγ (GL3), anti-NK1.1 (PK136), anti-CD4 (RM4-5), anti-CD3 (145-2C11), anti-B220 (RA3-6B2), anti-CD19 (1D3), anti–Ter-119 (Ter-119), anti-CD11b (M1/70), anti-Gr1 (RB6-8C5), anti-CD11c (HL3), anti-Flt3 (A2F10), anti-IL7Rα (A7R34); allophycocyanin-conjugated anti-Thy1.2 (53-2.1), anti-CD4, anti-CD19, anti-CD44 (IM7), anti–c-Kit (2B8), anti-AA4.1; allophycocyanin-Cy7–conjugated anti–c-Kit, anti-CD25 (PC61); PE-Cy5.5–conjugated anti-CD44; PE-Cy7–conjugated anti-Ly5SJL and biotinylated anti-Ly5B6, anti-Ly5SJL, anti-CD25 (7D4), and anti-B220. Antibodies were from Pharmingen (San Diego, CA) or eBioscience (San Diego, CA). Biotinylated antibodies were revealed with streptavidin-Pacific blue (Molecular Probes, Eugene, OR) or streptavidin-PE-Texas red (Caltag, Burlingame, CA). The following cocktail was used to exclude lineage-positive (Lin+) cells in extrathymic tissues: anti-CD8α, CD8β, TCRβ, TCRγ, NK1.1, CD4, CD3, B220, CD19, Ter119, CD11b, Gr1, and CD11c. For the thymus, the cocktail did not include anti-CD4, as described.26
Flow cytometric sorting and analysis
Cells were stained in PBS/2% FCS after blocking with 2.4G2 supernatant and rat/mouse IgG (Sigma, St Louis, MO). Cells were sorted on a FACS DiVa (Becton Dickinson, San Jose, CA) or MoFlo (Cytomation, Fort Collins, CO) cell sorter. Analytical flow cytometry was performed on LSR II (Becton Dickinson). DAPI was used to exclude dead cells. The resulting files were uploaded into FlowJo (Tree Star, San Carlos, CA).
OP9 stromal cultures
OP9-MigR1 and OP9-DL1 cells (expressing the Notch ligand Delta-like1) were kindly provided by J. C. Zuniga-Pflucker (University of Toronto, Toronto, ON, Canada) and used as described.16 Sorted progenitor subsets were seeded into 24-well plates containing a stromal monolayer (1000-5000 progenitors/well) in the presence of mIL-7 (1 ng/mL) and huFlt3L (5 ng/mL; PeproTech, Rocky Hill, NJ). Cells were transferred on fresh stromal monolayers every 4 days. Flow cytometric analysis was gated on live GFP–CD45+ cells.
Quantitative RT-PCR
RNA was isolated using the RNEasy kit (Qiagen, Valencia, CA). cDNA was prepared with the Superscript II kit (Invitrogen, Carlsbad, CA). Real-time reverse transcription–polymerase chain reaction (RT-PCR) was performed with SYBRGreen PCR Master Mix (Applied Biosystems, Foster City, CA) and analyzed on ABI Prism 7900 (Applied Biosystems). A standard curve was generated from 10-fold dilutions of a positive control. Primer sequences are available on request.
Results
Thymic reconstitution without canonical ETPs early after BMT
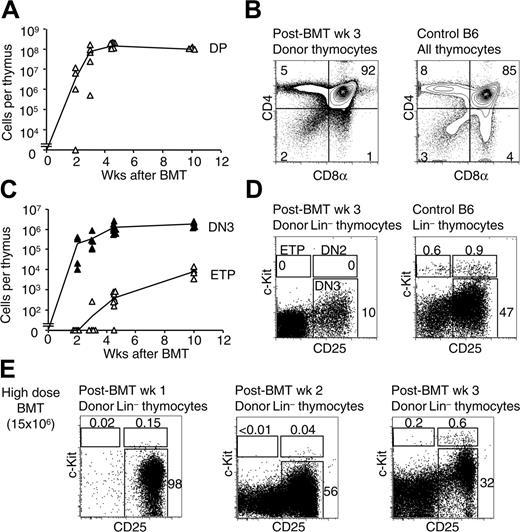
ETPs are Lin–CD25–CD44+c-Kithi thymocytes that were characterized as the earliest T-lineage progenitors in the thymus of normal adult mice.26 To evaluate whether T-lineage reconstitution after BMT involved ETPs, we assessed the kinetics of thymic reconstitution and the appearance of donor-derived ETPs after BMT (Figure 1). Adult B6 thymi were analyzed at various time points after lethal irradiation (900 rads) and BMT from B6.Ly5SJL donors (Figure 1). Thymocyte cellularity decreased markedly in the first week after BMT due to death of radiosensitive thymocytes. At 2 to 3 weeks, a wave of host-derived CD4+CD8+ double-positive (DP) thymocytes was observed (data not shown). This transient host-derived reconstitution has been attributed previously to differentiation from radio-resistant thymocytes.42 Donor-derived DP thymocytes appeared 2 to 3 weeks after BMT (Figure 1A). Their numbers rose rapidly, approximating pre-BMT numbers at 4 to 5 weeks. A representative flow cytometric analysis at 3 weeks is depicted in Figure 1B, showing substantial numbers of donor-derived CD4+CD8+ DP thymocytes and CD4+/CD8+ single-positive thymocytes. We next examined Lin– thymocyte progenitors (Figure 1C). Donor-derived CD4–CD8– double-negative 3 (DN3) thymocytes (Lin–c-Kit–CD25+) arose as early as 2 to 3 weeks after BMT, with similar kinetics as DP thymocytes (Figure 1A,C). The emergence of donor-derived ETPs was substantially delayed in comparison with DN3 and DP thymocytes. No ETPs were detected in the majority of the mice at 2 or 3 weeks after BMT (Figure 1) or at earlier time points (I.M. and W.S.P., unpublished data, July 28, 2005). At 4 weeks after BMT, the number of ETPs remained more than 10-fold lower than found later at 10 weeks, whereas the number of DN3 and DP thymocytes was essentially stable beyond 4 weeks. A representative analysis of Lin– thymocytes at 3 weeks is depicted in Figure 1D, showing substantial numbers of donor-derived DN3 thymocytes, but no ETPs. To assess whether the lack of reconstitution of the ETP pool could be overcome by a higher dose of BM, we performed BMT with 15 × 106 total BM cells (150 × higher dose; Figure 1E). Efficient reconstitution of DN2/DN3 thymocytes was observed at 1 and 2 weeks after BMT, but this did not correlate with a substantial reconstitution of the ETP pool (Figure 1E). However, ETPs started to be detected at 3 weeks in these conditions, indicating that a higher dose of infused BM slightly accelerated ETP recovery, but not during the initial 2 weeks after BMT. These observations suggested that early donor-derived thymic reconstitution in the first weeks after BMT occurred in the absence of substantial numbers of ETPs, and thus differed from steady-state thymopoiesis.
Thymic reconstitution occurs in the absence of canonical early T-lineage progenitors at early time points after BMT. (A) Reconstitution of donor-derived CD4+CD8+ DP thymocytes after BMT. BM cells (105 Ly5SJL) were transplanted into lethally irradiated (900 rads) B6 recipients. The absolute number of Ly5SJL-derived CD4+CD8+ DP thymocytes was assessed between 2 and 10 weeks after BMT. ▵ represent data from individual mice (3-8 mice/time point). The thin line is drawn between the mean value for each time point. (B) CD4/CD8 profile of donor-derived thymocytes at 3 weeks after BMT. The left contour plot is gated on donor-derived cells. The right contour plot shows the profile of a control B6 thymus. Numbers indicate the percentage of cells in each quadrant. (C) Emergence of Lin–CD25–c-Kit+ ETPs and Lin–c-Kit–CD25+ DN3 thymocytes after BMT. ▵ and ▴ represent individual data from the same mice as in panel A (▵, ETP; ▴, DN3). (D) Flow cytometric analysis of donor-derived Lin– thymocytes at 3 weeks after BMT, using 105 BM cells, in comparison with control B6 thymus. (E) Donor-derived Lin– thymocytes at 1, 2, or 3 weeks after BMT, using a high dose of BM cells (15 × 106). Numbers indicate the percentage of cells in the boxed regions.
Thymic reconstitution occurs in the absence of canonical early T-lineage progenitors at early time points after BMT. (A) Reconstitution of donor-derived CD4+CD8+ DP thymocytes after BMT. BM cells (105 Ly5SJL) were transplanted into lethally irradiated (900 rads) B6 recipients. The absolute number of Ly5SJL-derived CD4+CD8+ DP thymocytes was assessed between 2 and 10 weeks after BMT. ▵ represent data from individual mice (3-8 mice/time point). The thin line is drawn between the mean value for each time point. (B) CD4/CD8 profile of donor-derived thymocytes at 3 weeks after BMT. The left contour plot is gated on donor-derived cells. The right contour plot shows the profile of a control B6 thymus. Numbers indicate the percentage of cells in each quadrant. (C) Emergence of Lin–CD25–c-Kit+ ETPs and Lin–c-Kit–CD25+ DN3 thymocytes after BMT. ▵ and ▴ represent individual data from the same mice as in panel A (▵, ETP; ▴, DN3). (D) Flow cytometric analysis of donor-derived Lin– thymocytes at 3 weeks after BMT, using 105 BM cells, in comparison with control B6 thymus. (E) Donor-derived Lin– thymocytes at 1, 2, or 3 weeks after BMT, using a high dose of BM cells (15 × 106). Numbers indicate the percentage of cells in the boxed regions.
A wave of T-lineage progenitors in the spleen after BMT
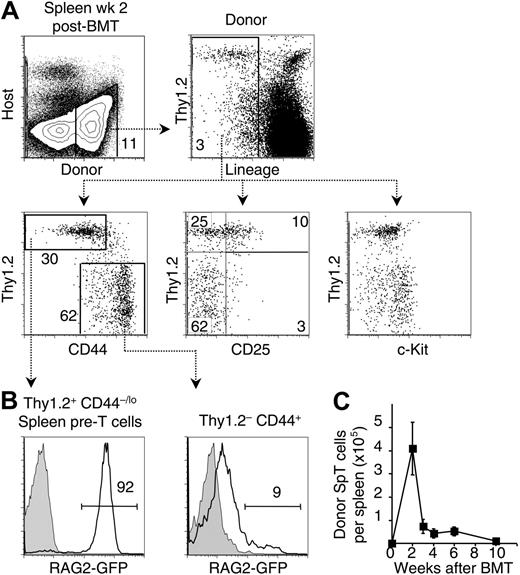
Ezine's group identified a population of donor-derived cells in day-12 spleen colonies after BMT that had features of T lineage–restricted progenitors and could complete T-lineage maturation in the thymus after intravenous delivery.32,33 To evaluate this pathway, we transferred B6.Ly5SJL BM cells into lethally irradiated B6 recipients. We first studied donor-derived cells in the spleen 12 to 14 days after BMT. Most Ly5-expressing donor-derived cells expressed lineage (Lin) markers including erythroid-, myeloid-, and lymphoid-specific antigens (Figure 2A). This included a population of Thy1.2+Lin+ cells that likely derived from mature T cells in donor BM. However, a distinct population of Thy1.2+ cells was apparent among Lin– donor-derived cells, as reported.32 The Lin– population was characterized by staining for CD44, c-Kit, and CD25. Expression of Thy1.2 and CD44 identified 2 populations of Lin– cells: Thy1.2–CD44+ cells previously described as multipotent and Thy1.2+CD44–/lo cells reported to be T-lineage restricted, and referred to as spleen pre-T cells (SpT) by Ezine and coworkers.32,33 c-Kit was expressed at low levels in the SpT population, whereas its distribution appeared bimodal in the Thy1.2– fraction and included c-Kithi cells, indicating heterogeneity of this population. Expression of CD25, a defining marker for the DN2/DN3 stages of thymocyte development, was detected mainly on the SpT cells, although at lower levels than on DN2/DN3 thymocytes. Overall, SpT cells shared many phenotypic characteristics with DN thymocytes, as described.33
To determine whether donor-derived splenic progenitor populations expressed the lymphoid-specific gene rag2, we performed BMT using BM from NG-BAC mice. NG-BAC mice express GFP under the control of rag2 locus control elements,38 a reliable early marker of lymphoid specification.28 At 2 weeks, GFP expression was present in about 90% of donor-derived SpT cells (Figure 2B). A small percentage of Thy1.2–CD44+ cells expressed GFP (∼10%), consistent with a less advanced stage of lymphoid differentiation and indicating heterogeneity within this population.
To evaluate the importance of extrathymic T-lineage differentiation over time, we tracked the absolute number of SpT cells at various time points (Figure 2C). This number peaked at 2 weeks after BMT, reaching up to 5 × 105 cells/spleen. Significant numbers of SpT cells were detectable at 6 weeks and decreased to very low levels by 10 weeks. Together, these data corroborate the findings from Ezine's group, but show that T-lineage progenitors are present for an extended period of time and are not restricted to day-12 spleen colonies.
A transient wave of T-lineage progenitors is found in the spleen after BMT. (A) Characterization of donor-derived cells in the spleen at 2 weeks after BMT. A significant fraction of donor-derived Lin– cells expressed the T-lineage marker Thy1.2. Concomitant staining of donor-derived Lin– cells with Thy1.2/CD44, Thy1.2/CD25, and Thy1.2/c-Kit is shown. Lin–Thy1.2+CD44–/lo cells are referred to as spleen pre-T cells (SpT), as described.32 Data are representative of 5 experiments. (B) Expression of RAG as an indicator of lymphoid specification. B6.Ly5SJL recipient mice were given transplants of BM from rag2-GFP reporter mice.38 At 2 weeks, a high percentage of GFP+ cells was observed among SpT cells, whereas GFP expression in the Thy1.2–CD44+ population was much lower. Dashed lines are drawn between panels A and B to graphically depict the identity of the SpT and Thy1.2–CD44+ populations, although the data were generated in similar but separate experiments. Data are representative of 2 experiments. Open histograms indicate cells derived from a rag2-gfp donor; shaded histograms, cells derived from a nontransgenic littermate. (C) Absolute number of donor-derived SpT cells in the spleen at various time points after BMT. Data are shown as mean ± 2 SEM for 3 to 8 mice per time point.
A transient wave of T-lineage progenitors is found in the spleen after BMT. (A) Characterization of donor-derived cells in the spleen at 2 weeks after BMT. A significant fraction of donor-derived Lin– cells expressed the T-lineage marker Thy1.2. Concomitant staining of donor-derived Lin– cells with Thy1.2/CD44, Thy1.2/CD25, and Thy1.2/c-Kit is shown. Lin–Thy1.2+CD44–/lo cells are referred to as spleen pre-T cells (SpT), as described.32 Data are representative of 5 experiments. (B) Expression of RAG as an indicator of lymphoid specification. B6.Ly5SJL recipient mice were given transplants of BM from rag2-GFP reporter mice.38 At 2 weeks, a high percentage of GFP+ cells was observed among SpT cells, whereas GFP expression in the Thy1.2–CD44+ population was much lower. Dashed lines are drawn between panels A and B to graphically depict the identity of the SpT and Thy1.2–CD44+ populations, although the data were generated in similar but separate experiments. Data are representative of 2 experiments. Open histograms indicate cells derived from a rag2-gfp donor; shaded histograms, cells derived from a nontransgenic littermate. (C) Absolute number of donor-derived SpT cells in the spleen at various time points after BMT. Data are shown as mean ± 2 SEM for 3 to 8 mice per time point.
Lymphoid potential of post-BMT splenic progenitors
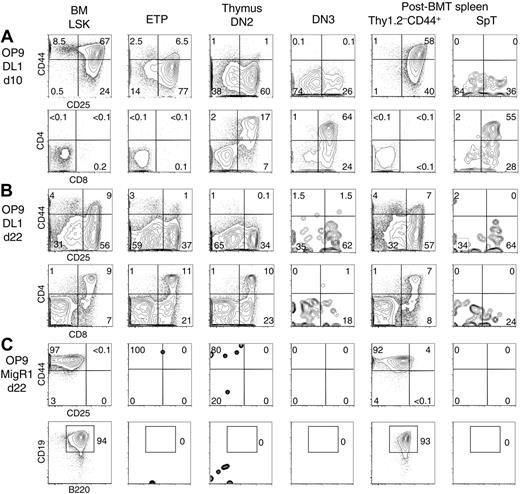
We next compared the lymphoid potential of splenic donor-derived Lin– populations to BM LSK progenitors and thymic ETP, DN2, and DN3 cells (Figure 3). Differentiation was assessed after plating on OP9-MigR1 (supporting B-lineage development) or OP9-DL1 cells (supporting T-lineage differentiation). Kinetics of lymphoid development in this system correlate with the functional maturity of progenitors.16,18,22 BM LSK cells and spleen Thy1.2–CD44+ cells underwent T-lineage development in OP9-DL1 cultures with similar kinetics (Figure 3A-B). At day 10, both subsets had progressed to CD44+CD25+ DN2 and CD44–CD25+ DN3 stages of differentiation. CD4+CD8+ DP cells arose later after 22 days. Both subsets retained the ability to produce B cells in OP9 cultures, as reported (Figure 3C).33 Based on kinetics and outcome of differentiation, Lin–Thy1.2–CD44+ progenitors were similar to BM LSK cells. In contrast, SpT cells gave rise to an early production of CD4+CD8+ DP cells that peaked at day 10 and paralleled the differentiation of thymic DN3 cells. No B potential was detected, consistent with the T-lineage restriction of this subset.32 ETPs and DN2 progenitors differentiated more slowly but produced T-lineage progeny for a longer period of time. These results showed that SpT cells were functionally similar to DN3 thymocytes.
Tissue distribution and extent of differentiation
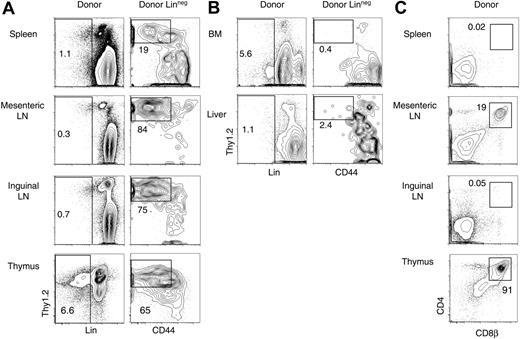
The spleen is a major site of hematopoiesis early after BMT in mice and, probably, in humans.43 To evaluate whether donor-derived T-lineage progenitors could be found in other extrathymic locations, we studied post-BMT spleen, inguinal lymph nodes, mesenteric lymph nodes (MLNs), liver, and BM by flow cytometry (Figure 4). Donor-derived Lin–Thy1.2+CD44–/lo cells were present in the spleen, as described in Figure 1, and also in inguinal and MLNs (Figure 4A). In contrast, few if any of these cells were found in the BM or liver (Figure 4B), indicating that these organs did not provide a supportive environment for the generation of T-lineage progenitors after BMT. Importantly, the immunophenotype of Lin–Thy1.2+CD44–/lo cells in spleen and lymph nodes overlapped with a large fraction of the cells in the developing cohort of Lin– thymocytes.
Lymphoid developmental potential of post-BMT T-lineage progenitor populations in OP9 and OP9-DL1 cultures. BM LSK cells, as well as thymic ETP, DN2, and DN3 cells, were compared witih post-BMT donor-derived Lin–Thy1.2–CD44+ and Lin–Thy1.2+CD44–/lo (SpT) cells, as defined in Figure 2. Sorted progenitor populations were cultured with OP9-MigR1 or OP9-DL1 BM stromal cell lines, as described.16 Flow cytometric analysis was performed at various time points after seeding. The data are shown after gating on CD45+GFP– live cells. Numbers indicate the percentage of cells in the boxed region. (A) CD44/CD25 and CD4/CD8α profile of the cells after 10 days of coculture on OP9-DL1 cells. (B) Similar analysis at day 22 of coculture. Differentiation of SpT cells paralleled DN3 thymocyte differentiation, peaking early at day 10 and then decreasing. The differentiation of Thy1.2–CD44+ cells was similar to BM LSK cells. (C) CD44/CD25 and CD19/B220 staining at day 22 of culture on OP9-MigR1 stromal cells. Both BM LSK and post-BMT Thy1.2–CD44+ cells gave rise to CD19+B220+ B cells, whereas other progenitors had no in vitro B-lineage potential.
Lymphoid developmental potential of post-BMT T-lineage progenitor populations in OP9 and OP9-DL1 cultures. BM LSK cells, as well as thymic ETP, DN2, and DN3 cells, were compared witih post-BMT donor-derived Lin–Thy1.2–CD44+ and Lin–Thy1.2+CD44–/lo (SpT) cells, as defined in Figure 2. Sorted progenitor populations were cultured with OP9-MigR1 or OP9-DL1 BM stromal cell lines, as described.16 Flow cytometric analysis was performed at various time points after seeding. The data are shown after gating on CD45+GFP– live cells. Numbers indicate the percentage of cells in the boxed region. (A) CD44/CD25 and CD4/CD8α profile of the cells after 10 days of coculture on OP9-DL1 cells. (B) Similar analysis at day 22 of coculture. Differentiation of SpT cells paralleled DN3 thymocyte differentiation, peaking early at day 10 and then decreasing. The differentiation of Thy1.2–CD44+ cells was similar to BM LSK cells. (C) CD44/CD25 and CD19/B220 staining at day 22 of culture on OP9-MigR1 stromal cells. Both BM LSK and post-BMT Thy1.2–CD44+ cells gave rise to CD19+B220+ B cells, whereas other progenitors had no in vitro B-lineage potential.
We then assessed whether T-lineage development could proceed to the CD4+CD8+ DP stage at extrathymic sites (Figure 4C). No or very few donor-derived DP cells were detected in the spleen or inguinal lymph nodes. In contrast, significant numbers of donor-derived CD4+CD8αβ+ DP cells were present in MLNs (up to 20% of donor-derived cells). MLNs preferentially host extrathymic T-lineage development in nude mice and other mouse models, suggesting that they contain supportive elements that can better replace thymic function than other extrathymic sites.44-46 Together, these data showed the presence of a substantial pool of T progenitors in peripheral lymphoid organs after BMT.
Splenic T-lineage progenitors derive most efficiently from Flt3+ LSK cells
T-lineage progenitors in the spleen after BMT could derive from the expansion of rare T lineage–committed cells in the infused BM or from differentiation of multipotent progenitors. Therefore, we isolated BM progenitor subsets and transplanted them into lethally irradiated recipients, together with syngeneic BM to ensure survival (Figure 5). We evaluated the following subsets: Flt3– LSK cells (containing self-renewing hematopoietic stem cells), Flt3+ LSK cells (containing non–self-renewing multipotent progenitors), and common lymphoid progenitors (CLPs; Lin–IL-7Rα+AA4.1+Sca-1loc-Kitlo).35-37,47,48 All 3 types of progenitors generated donor-derived cells in the spleen after 2 weeks, although at a low frequency for CLPs (Figure 5A). When B-lineage and myeloid potential were assessed (Figure 5B), Flt3– LSK cells were found to generate myeloid progeny but very few B-lineage cells at this early time point. Flt3+ LSK progenitors efficiently gave rise to both myeloid and B-lineage cells, whereas CLPs nearly exclusively generated B cells. Extrathymic T-lineage potential was assessed by evaluating SpT cells (Figure 5C,D). Flt3+ LSK progenitors were the most efficient progenitor subset at generating SpT cells. Flt3– LSK cells had lower although still significant activity at these early time points. Their contribution to extrathymic T-lineage development could also be detected later, especially in lymph nodes (data not shown). In contrast, CLPs had minimal or no activity, even though the number of CLPs transferred in this experiment was higher than the number of CLPs infused with total BM in other experiments reported in this paper. Altogether, these data indicate that LSK cells but not CLPs are the relevant progenitors for extrathymic T-lineage development after BMT.
Tissue distribution and extent of differentiation of post-BMT T-lineage progenitors. B6 mice were lethally irradiated and reconstituted with Ly5SJL BM cells. Three weeks later, cell suspensions from the spleen, MLNs, inguinal lymph nodes, BM, liver, and thymus were assessed by flow cytometry for the presence of donor-derived cells with phenotypic characteristics of T-lineage progenitors (SpT cells, as defined in Figure 2). The 3-week time point was chosen to evaluate the overall extent of prethymic differentiation. Data are representative of 2 experiments. Numbers indicate the percentage of cells in the boxed region. (A) Presence of donor-derived Lin–Thy1.2+ pre-T cells in the spleen, lymph nodes, and thymus. (B) Absence of donor-derived SpT cells in the BM and liver. (C) Generation of donor-derived CD4+CD8+ DP cells in the MLNs, but not in other lymph nodes or in the spleen after BMT.
Tissue distribution and extent of differentiation of post-BMT T-lineage progenitors. B6 mice were lethally irradiated and reconstituted with Ly5SJL BM cells. Three weeks later, cell suspensions from the spleen, MLNs, inguinal lymph nodes, BM, liver, and thymus were assessed by flow cytometry for the presence of donor-derived cells with phenotypic characteristics of T-lineage progenitors (SpT cells, as defined in Figure 2). The 3-week time point was chosen to evaluate the overall extent of prethymic differentiation. Data are representative of 2 experiments. Numbers indicate the percentage of cells in the boxed region. (A) Presence of donor-derived Lin–Thy1.2+ pre-T cells in the spleen, lymph nodes, and thymus. (B) Absence of donor-derived SpT cells in the BM and liver. (C) Generation of donor-derived CD4+CD8+ DP cells in the MLNs, but not in other lymph nodes or in the spleen after BMT.
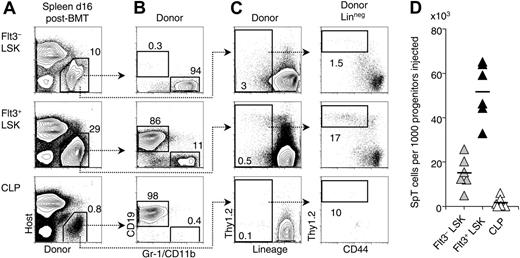
Post-BMT splenic T-lineage progenitors are most efficiently derived from Flt3+ LSK BM cells. Progenitor populations were sorted from Ly5SJL BM and transplanted into lethally irradiated B6 recipient mice together with 105 B6 BM cells to ensure survival. The following populations were assessed: Flt3– LSK cells, Flt3+ LSK cells, and CLPs (Lin–IL-7Rα+AA4.1+Sca-1loc-Kitlo).47,48 The number of cells injected was as follows: Flt3+ LSK, 500; Flt3– LSK, 250; CLPs, 750. These numbers were chosen to be close to or higher than the respective number of each progenitor subset in 105 BM cells. (A) Donor versus host Ly5 staining of spleen cell suspensions at day 16 after BMT. The high percentage of Ly5– cells is due to the presence of erythroid progenitors. (B) Myeloid (Gr-1/CD11b+) and B-lineage (CD19+) progeny of the sorted B6.Ly5SJL progenitor subsets. (C) Generation of T-lineage progenitors as defined in Figure 2. (D) Relative potency Flt3– LSK, Flt3+ LSK cells, and CLPs in the generation of SpT cells. Each triangle represents an individual recipient (n = 6). The mean is shown as a horizontal bar. The difference between each progenitor subset was statistically significant (P < .01, Student t test).
Post-BMT splenic T-lineage progenitors are most efficiently derived from Flt3+ LSK BM cells. Progenitor populations were sorted from Ly5SJL BM and transplanted into lethally irradiated B6 recipient mice together with 105 B6 BM cells to ensure survival. The following populations were assessed: Flt3– LSK cells, Flt3+ LSK cells, and CLPs (Lin–IL-7Rα+AA4.1+Sca-1loc-Kitlo).47,48 The number of cells injected was as follows: Flt3+ LSK, 500; Flt3– LSK, 250; CLPs, 750. These numbers were chosen to be close to or higher than the respective number of each progenitor subset in 105 BM cells. (A) Donor versus host Ly5 staining of spleen cell suspensions at day 16 after BMT. The high percentage of Ly5– cells is due to the presence of erythroid progenitors. (B) Myeloid (Gr-1/CD11b+) and B-lineage (CD19+) progeny of the sorted B6.Ly5SJL progenitor subsets. (C) Generation of T-lineage progenitors as defined in Figure 2. (D) Relative potency Flt3– LSK, Flt3+ LSK cells, and CLPs in the generation of SpT cells. Each triangle represents an individual recipient (n = 6). The mean is shown as a horizontal bar. The difference between each progenitor subset was statistically significant (P < .01, Student t test).
Active Notch signaling in extrathymic T-lineage progenitors
Our findings showed that post-BMT T-lineage progenitors differentiated from BM LSK progenitors at extrathymic sites. Because we have previously shown that Notch signaling is active in early T-lineage progenitors in the thymus and is critical for their generation,22 we assessed whether active Notch signaling was present in extrathymic T-lineage progenitors. Donor-derived Thy1.2–CD44+ and SpT cells were sorted 2 weeks after BMT and compared with BM LSK cells and thymic ETP, DN2, DN3, and CD4+CD8+ DP cells. RNA was subjected to real-time RT-PCR for the Notch targets Hes1 and Dtx1 and the Notch receptors Notch1, Notch2, and Notch3 (Figure 6). Hes1 and Dtx1 transcripts were rare or undetectable, respectively, in BM LSK cells. Their expression was up-regulated in ETPs and in subsequent DN thymocyte fractions, reaching maximum levels at the DN3 stage. As reported, Notch target mRNA levels decreased markedly in DP thymocytes.49,50 Expression of Notch target genes in post-BMT SpT progenitors was in the range found in early thymocyte progenitors. The same pattern applied to Notch1 and Notch3 mRNAs, which are positively regulated by Notch1 signaling51 (I.M. and W.S.P., unpublished data, June 3, 2005), whereas similar amounts of Notch2 mRNA were detected in all fractions examined. Expression of Notch targets in splenic Thy1.2–CD44+ cells did not differ from the low expression found in BM LSK cells. This correlated with the phenotypic and functional properties of Thy1.2–CD44+ cells, which were comparable to those of BM LSKs (Figures 2, 3). However, this Thy1.2–CD44+ population is heterogeneous and may contain a fraction of cells undergoing active Notch signaling. Additional gene expression analysis in post-BMT SpT cells revealed amounts of Gata3, CD3ϵ, and Tcf1 transcripts similar to those in early thymocyte fractions, consistent with a comparable stage of differentiation. However, PTcra mRNA was lower in SpT cells than in DN3 thymocytes, as reported.32 These findings suggest that the post-BMT SpT cells were exposed to Notch signaling at a similar intensity as DN thymocytes were exposed to in the thymic environment.
Notch signaling is required to generate extrathymic T-lineage progenitors
Because SpT cells had hallmarks of Notch signaling, we assessed whether the Notch pathway was essential for T-lineage differentiation at extrathymic locations. We transduced BM hematopoietic stem cells with the following GFP-tagged retroviruses: a control retrovirus (MigR1), a retrovirus encoding the pan-Notch inhibitor DNMAML1, or a retrovirus encoding the Notch modulator Deltex1 (MigDtx1). DNMAML1 is a dominant-negative inhibitor of Mastermind-like proteins (MAMLs). MAMLs are critical transcriptional coactivators for all Notch receptors (Notch1-4).52 We have previously shown that DNMAML1 inhibited Notch1- and Notch2-mediated cell fate decisions in vivo, while Dtx1 blocked Notch1-mediated T-lineage development but not Notch2-mediated marginal zone B-cell development.40 Two weeks after transplantation of MigR1-, DNMAML1-, or MigDtx1-transduced BM, the spleen was analyzed by flow cytometry for GFP expression in subsets of donor-derived Lin– progenitors (Figure 7). GFP expression in donor-derived Lin+Thy1.2– cells was measured to control for retroviral transduction efficiency. In MigR1 recipients, GFP expression was comparable in Lin+Thy1.2– cells (internal control), SpT cells, and Thy1.2–CD44+ cells (Figure 7A). In contrast, no GFP expression was detected in the Lin–Thy1.2+ SpT fraction when the donor BM had been transduced with the Notch inhibitor DNMAML1, indicating that the generation of these cells was completely Notch dependent (Figure 7B,D). GFP expression was preserved in DNMAML1-transduced Thy1.2–CD44+ cells, although decreased significantly in comparison with the internal control. When inguinal lymph nodes and MLNs of DNMAML1 recipients were analyzed, a similar Notch dependence was observed for pre-T cells arising in these organs (I.M. and W.S.P., unpublished data, July 21, 2005). Finally, GFP expression was significantly reduced but not abolished in SpT cells when the mice were reconstituted with MigDtx1-transduced BM (Figure 7C,D). Because Deltex1 inhibited Notch1- but not Notch2-mediated lymphoid cell fate decisions in vivo,40 these data suggested that cooperative signaling through Notch1 and Notch2 occurred during extrathymic T-lineage differentiation after BMT. This hypothesis was explored by assessing the gene expression profile of control MigR1- or MigDtx1-transduced SpT cells (Figure 7E). Lower amounts of Notch1, Notch3, Hes1, and Ptcra mRNA were observed in MigDtx1-transduced SpT cells, consistent with a reduced intensity of Notch signaling. In contrast to Notch1, Notch2 mRNA levels were not decreased and even slightly increased in MigDtx1-transduced cells. These results support the hypothesis that Notch1 and Notch2 can cooperate during extrathymic T-lineage development, and that expression of Dtx1 interferes specifically only with Notch1-mediated signals.
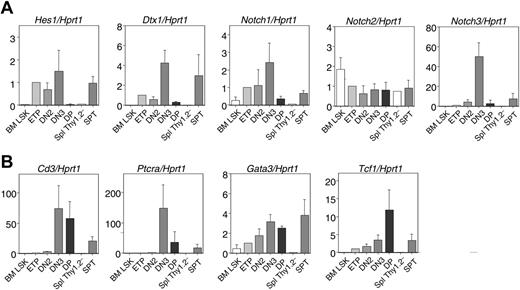
Expression of Notch target genes and T-lineage markers in extrathymic T-lineage progenitors. Real-time quantitative RT-PCR was performed on RNA from sorted spleen T-lineage progenitors at 2 weeks after BMT (spleen Thy1.2–CD44+ and SpT cells as defined in Figure 2). BM LSK, thymic ETP, and DN2 and DN3 and CD4+CD8+ DP cells from B6 mice were analyzed for comparison. Numbers on the y-axis are arbitrary units with the amount of mRNA found in ETPs set to 1. Data are shown as mean ± SD of 3 to 4 independent samples assessed in duplicate (except for Thy1.2–CD44+ cells, 1 sample). (A) Expression of the Notch target genes Dtx1 and Hes1, as well as the Notch receptors Notch1, Notch2, and Notch3, after normalization for Hprt1. (B) Expression of the T-lineage differentiation markers CD3ϵ and Ptcra, of the transcription factor Gata3, and of the Wnt target gene Tcf1.
Expression of Notch target genes and T-lineage markers in extrathymic T-lineage progenitors. Real-time quantitative RT-PCR was performed on RNA from sorted spleen T-lineage progenitors at 2 weeks after BMT (spleen Thy1.2–CD44+ and SpT cells as defined in Figure 2). BM LSK, thymic ETP, and DN2 and DN3 and CD4+CD8+ DP cells from B6 mice were analyzed for comparison. Numbers on the y-axis are arbitrary units with the amount of mRNA found in ETPs set to 1. Data are shown as mean ± SD of 3 to 4 independent samples assessed in duplicate (except for Thy1.2–CD44+ cells, 1 sample). (A) Expression of the Notch target genes Dtx1 and Hes1, as well as the Notch receptors Notch1, Notch2, and Notch3, after normalization for Hprt1. (B) Expression of the T-lineage differentiation markers CD3ϵ and Ptcra, of the transcription factor Gata3, and of the Wnt target gene Tcf1.
Altogether, our findings indicate that the generation of all extrathymic T-lineage progenitors after BMT requires Notch signaling. This requirement is absolute for the more differentiated DN3-like SpT cells. The smaller decrease in GFP expression among DNMAML1-derived Thy1.2–CD44+ cells (Figure 7B,D) suggests that this heterogeneous population may contain a subset of Notch-dependent T-lineage progenitors at an earlier differentiation stage.
Discussion
In adult mice, early T-lineage development proceeds from a subset of thymic ETPs that likely derive from circulating multipotent LSK cells.26,27 However, alternative pathways of T-lineage differentiation may exist during ontogeny and in lymphopenic environments.24,25,46 Thymic reconstitution after BMT necessitates colonization of a depleted thymus by a fresh wave of progenitors. Our data show that early after BMT, thymic reconstitution proceeded efficiently to produce substantial numbers of donor-derived CD4+CD8+ DP thymocytes before the ETP compartment was replenished. This suggested that other pathways of T-lineage differentiation may be used after BMT. For this reason, we examined the characteristics and extent of extrathymic T-lineage development, a phenomenon that was reported in day-12 spleen colonies by Ezine and collaborators.32 We showed that T-lineage progenitors were present not only in the spleen, but also in other peripheral lymphoid organs at early time points after BMT. The generation of extrathymic T-lineage progenitors occurred through Notch-dependent differentiation from BM LSK progenitors. The magnitude of this phenomenon peaked in the spleen 2 weeks after BMT and extended over a longer time period, during which thymic reconstitution was observed.
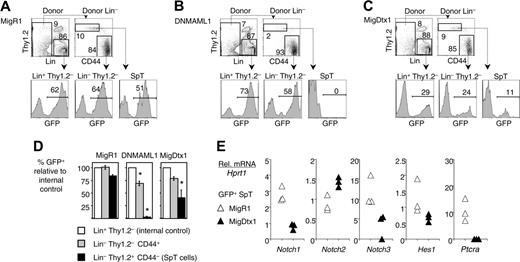
Extrathymic T-lineage progenitors are Notch dependent. B6 mice were reconstituted with Ly5SJL BM cells after transduction with a retrovirus expressing green fluorescent protein (GFP) (MigR1), the pan-Notch inhibitor DNMAML1, or the Notch modulator Deltex1 (MigDtx1). All retroviruses expressed a GFP tag. Spleen cells were analyzed 2 weeks after BMT. Data are representative of 2 experiments with at least 4 mice/group. (A) GFP expression after infusion of MigR1-transduced progenitors. Donor-derived Lin+ Thy1.2– cells were used as an internal control for the efficiency of retroviral transduction. The level of GFP expression was comparable in this population and in SpT and Thy1.2–CD44+ cells as defined in Figure 2. (B) GFP expression after infusion of DNMAML1-transduced progenitors was absent from the SpT population, indicating that its generation was Notch dependent. GFP expression was modestly reduced among Thy1.2–CD44+ cells. (C) GFP expression after infusion of Dtx1-transduced progenitors was reduced but not abolished in SpT and Thy1.2–CD44+ cells. (D) Graphical representation of the results shown in Figure 5B-C. The percentage of transduction in the Lin+Thy1.2– population (internal control) is normalized to 100%. The percentage of GFP+ cells in the SpT and Thy1.2–CD44+ populations is shown as a fraction of the internal control. Data are shown as mean ± SEM. * indicates a significant difference as compared with the internal control (P < .01, Student t test). (E) Relative expression of Notch1, Notch2, Notch3, Hes1, and Ptcra mRNA at day 14 after BMT in sorted MigR1 or MigDtx1-transduced SpT cells, showing that Dtx1 reduces the intensity of Notch signaling and decreases Notch1 but not Notch2 mRNA levels. Data are shown in arbitrary units after normalization for Hprt1 mRNA. Each triangle shows the mRNA level measured in triplicate in SpT cells from a pool of 2 recipient spleens (6 recipients in each group).
Extrathymic T-lineage progenitors are Notch dependent. B6 mice were reconstituted with Ly5SJL BM cells after transduction with a retrovirus expressing green fluorescent protein (GFP) (MigR1), the pan-Notch inhibitor DNMAML1, or the Notch modulator Deltex1 (MigDtx1). All retroviruses expressed a GFP tag. Spleen cells were analyzed 2 weeks after BMT. Data are representative of 2 experiments with at least 4 mice/group. (A) GFP expression after infusion of MigR1-transduced progenitors. Donor-derived Lin+ Thy1.2– cells were used as an internal control for the efficiency of retroviral transduction. The level of GFP expression was comparable in this population and in SpT and Thy1.2–CD44+ cells as defined in Figure 2. (B) GFP expression after infusion of DNMAML1-transduced progenitors was absent from the SpT population, indicating that its generation was Notch dependent. GFP expression was modestly reduced among Thy1.2–CD44+ cells. (C) GFP expression after infusion of Dtx1-transduced progenitors was reduced but not abolished in SpT and Thy1.2–CD44+ cells. (D) Graphical representation of the results shown in Figure 5B-C. The percentage of transduction in the Lin+Thy1.2– population (internal control) is normalized to 100%. The percentage of GFP+ cells in the SpT and Thy1.2–CD44+ populations is shown as a fraction of the internal control. Data are shown as mean ± SEM. * indicates a significant difference as compared with the internal control (P < .01, Student t test). (E) Relative expression of Notch1, Notch2, Notch3, Hes1, and Ptcra mRNA at day 14 after BMT in sorted MigR1 or MigDtx1-transduced SpT cells, showing that Dtx1 reduces the intensity of Notch signaling and decreases Notch1 but not Notch2 mRNA levels. Data are shown in arbitrary units after normalization for Hprt1 mRNA. Each triangle shows the mRNA level measured in triplicate in SpT cells from a pool of 2 recipient spleens (6 recipients in each group).
Our surprising finding that early thymic reconstitution after BMT appeared independent of ETPs may have several possible explanations. First, seeding of the thymus by extremely rare ETP-like cells could be sufficient to establish a cohort of developing thymocytes in the post-BMT setting. Second, even if significant numbers of progenitors are recruited, the irradiated thymus may constitute an environment in which the most primitive T-lineage progenitors never accumulate as ETPs, but instead have a shortened half-life or rapidly differentiate into downstream progeny. Third, relevant T-lineage progenitors may be different in the post-BMT setting and in the steady-state situation. Such atypical progenitors may phenotypically and functionally differ from physiologic progenitors. An interesting possibility is that these progenitors could be more advanced in their differentiation, thus entering the thymus downstream of ETPs, or differentiating rapidly to subsequent stages of development.
Although we cannot formally distinguish the relative contribution of these different scenarios, we have been unable to detect substantial reconstitution of the ETP pool after irradiation and BMT at any time point between 1 day and 2 weeks after BMT, even after intravenous injection of high numbers of BM cells. This led us to examine the possibility that an extrathymic pool of atypical T-lineage progenitors may contribute to T-lineage reconstitution after BMT. We found that Notch-dependent T-lineage development proceeded at extrathymic sites precisely during the window of time in which donor-derived T-lineage reconstitution was observed in the thymus in the absence of ETPs. This process gave rise to a range of extrathymic T-lineage progenitors that were phenotypically and functionally very similar to the cohort of developing DN thymocytes, as shown in spleen colonies by Ezine and coworkers.32,33 Furthermore, Ezine's group demonstrated that Thy1.2–CD44+ and SpT cells from the post-BMT spleen were efficient at generating CD4+CD8+ DP cells in the thymus after intravenous injection.33 Thus extrathymic T-lineage progenitors have the capacity to enter the thymus as DN2/DN3-like progenitors. These cells represent a large pool of T-lineage progenitors that need to be considered as important potential players in the post-BMT setting. An important future area of research will be to determine to what extent such noncanonical pathways of T-lineage differentiation and the dramatically reduced numbers of ETPs, respectively, contribute to early T-lineage reconstitution after BMT. Of note, it is possible that alternative pathways could be preferentially used under conditions of low stem cell numbers, because these cells may preferentially home to extrathymic niches when numbers are limiting.
Notch is a key molecule regulating early T-lineage development, in addition to GATA-3, Runx family members, E2A, and Wnt (for a review, see Rothenberg and Taghon53 ). Our data show that extrathymic T-lineage development after BMT, similar to intrathymic T-cell development, requires Notch signaling. Thus, the requirement for Notch in developing T cells is not limited to the thymus. These data imply that appropriate Notch receptors must be expressed by progenitors colonizing the spleen and other lymphoid organs, that Notch ligands must be expressed in the lymphoid microenvironment, and that direct contact occurs between Notch ligand and receptor expressing cells.
Notch1 is the critical receptor for early T-lineage development in the thymus. Lack of Notch1 results in an early block in T-lineage development that is not compensated by the remaining receptors (Notch2-4),11 whereas Notch2-4 single deficient mice have no reported defects in T-cell development.50,54,55 In contrast, our data in the post-BMT setting suggest that additional Notch receptors may cooperate with Notch1 for the development of extrathymic T-lineage progenitors, because the process was completely inhibited by the pan-Notch inhibitor DNMAML1, but only partially by Deltex1, a Notch modulator that inhibits Notch1 but not Notch2 in vivo.40 This hypothesis was supported by the gene expression profile of Deltex1-transduced SpT cells, which showed a decrease in Notch1 but not Notch2 transcripts, consistent with a reduced Notch signaling intensity. Thus, we favor the possibility that extrathymic T-cell development requires both Notch1 and Notch2. This will have to be further investigated in mice lacking single Notch receptors. Regarding the requirement for Notch ligands, Delta-like (Dll) family members have been described as essential for T-lineage development.15,16,56 Because post-BMT donor-derived T-cell development proceeds to the CD4+CD8+ DP stage, at least in MLNs, it is likely that Dll ligands are efficiently presented extrathymically, probably by radioresistant host cells. Recent findings indicate that the lymph node stroma expresses Dll-1 at levels comparable to those found in the thymic stroma.57 Although these Notch ligands may be expressed constitutively, whether their level and availability are limiting remains to be investigated.
Our kinetic data showed that the number of donor-derived DN3-like T-lineage progenitors peaked in the spleen 2 weeks after BMT and progressively decreased to very low levels by 10 weeks. This raised the question of whether extrathymic T-lineage commitment also occurs in normal mice. Strober's group30,31 reported the existence of rare Thy1.2+ T-committed progenitors in normal adult BM. Terra et al57 have shown limited abortive T-lineage development in normal MLNs. It is possible that the post-BMT setting may increase the efficiency of a phenomenon that otherwise occurs at a low level, which could be related to structural or molecular changes in peripheral lymphoid organs after BMT. First, BM progenitors may gain better access to spleen and lymph nodes after BMT, and their direct contact with the stroma may be enhanced by lymphopenia. Extrathymic T-cell development is repressed in the absence of lymphopenia, a phenomenon that may limit extrathymic T-lineage development in normal mice and late after BMT.46 Second, irradiation itself or its consequences may change the lymphoid microenvironment, inducing the expression of Notch ligands, Wnt molecules, or other supportive factors.
Identifying the BM progenitors that are capable of early lymphoid differentiation after BMT is critical to understand the regulation of this process and to improve immune recovery. Primitive BM LSK progenitors were more efficient than CLPs at generating T-lineage progenitors at extrathymic sites early after BMT. In contrast to LSK cells, CLPs47 nearly exclusively gave rise to B-lineage cells in the spleen after BMT. Although CLPs have T-lineage potential, they are inefficient T progenitors that may contribute predominantly to B lymphopoiesis in vivo.26 A potential role for the administration of CLPs has been suggested previously in a mouse model of BMT.58 However, substantial numbers of CLP-derived T cells were observed only in a model of allogeneic transplantation, and not in a syngeneic setting similar to our experimental system. The Flt3+ subset of LSK cells was most efficient at generating extrathymic T-lineage progenitors at early time points. This subset corresponds to non–self-renewing multipotent progenitors with lymphoid and myeloid but reduced erythroid and megakaryocytic potential.35-37 It also contains the majority of early lymphoid progenitors defined by Rag reporter activity.28 In addition, the Flt3+ LSK compartment overlaps in part with a subset of LSK cells expressing CD62L, which has been shown to possess potent lymphoid potential.29 It is possible that Flt3-mediated signals play an important role in early T-lineage differentiation from LSK progenitors after BMT. Fry et al59 reported that exogenous Flt3L after BMT in mice enhanced both thymus-independent and thymus-dependent T-lineage reconstitution. Although the thymus-independent activity involved an effect of Flt3L on dendritic cells, driving proliferation of T cells, the thymus-dependent activity resulted in increased generation of naive T cells and was characterized by increased thymic cellularity 2 weeks after BMT. It will be interesting to study whether Flt3L exerts such effects through its activity on extrathymic steps of T-lineage differentiation. Alternatively, we have shown recently that a small fraction of ETPs is Flt3L responsive.22 It is thus possible that Flt3L could enhance reconstitution of the ETP pool that is otherwise markedly delayed after BMT.
Altogether, our findings open new perspectives into the molecular and cellular mechanisms of T-lineage reconstitution after BMT. They suggest that pathways of T-lineage development exhibit plasticity in adult mice undergoing such a profound lymphopenic stress. Better understanding of early T-lineage reconstitution may provide new therapeutic interventions in patients undergoing BMT.
Prepublished online as Blood First Edition Paper, January 5, 2006; DOI 10.1182/blood-2005-08-3454.
Supported by grants from the National Institutes of Health (NIH; RO1 AI059621 to A.B. and RO1AI047833 to W.S.P.) and by grants from the Swiss Society for Grants in Medicine and Biology and the Damon Runyon Cancer Research Foundation (DRG-102-05; I.M.). Additional support was provided by NIH training grant T32-AI-055428 (B.A.S.), a predoctoral training grant from the Cancer Research Institute (T.F.), a SCOR grant from the Leukemia and Lymphoma Society (W.S.P.), and a Health Research Faculty Development Block Grant from the Commonwealth of Pennsylvania (A.B. and W.S.P.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Stephen G. Emerson and members of the Pear laboratory for critical reading of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal