Abstract
To study the role of the stress-induced “readthrough” acetylcholinesterase splice variant, AChE-R, in thrombopoiesis, we used transgenic mice overexpressing human AChE-R (TgR). Increased AChE hydrolytic activity in the peripheral blood of TgR mice was associated with increased thrombopoietin levels and platelet counts. Bone marrow (BM) progenitor cells from TgR mice presented an elevated capacity to produce mixed (GEMM) and megakaryocyte (Mk) colonies, which showed intensified labeling of AChE-R and its interacting proteins RACK1 and PKC. When injected with bacterial lipopolysaccharide (LPS), parent strain FVB/N mice, but not TgR mice, showed reduced platelet counts. Therefore, we primed human CD34+ cells with the synthetic ARP26 peptide, derived from the cleavable C-terminus of AChE-R prior to transplantation, into sublethally irradiated NOD/SCID mice. Engraftment of human cells (both CD45+ and CD41+ Mk) was significantly increased in mice that received ARP26-primed CD34+ human cells versus mice that received fresh nonprimed CD34+ human cells. Moreover, ARP26 induced polyploidization and proplatelet shedding in human MEG-01 promegakaryotic cells, and human platelet engraftment increased following ex vivo expansion of ARP26-treated CD34+ cells as compared to cells expanded with thrombopoietin and stem cell factor. Our findings implicate AChE-R in thrombopoietic recovery, suggesting new therapeutic modalities for supporting platelet production.
Introduction
The number of circulating blood cells is tightly regulated by cytokines and chemokines capable of immediate response to various stimuli.1 Adjustment to changing needs involves rapid mobilization of cells from the bone marrow (BM) and the vascular marginal pool in response to inflammation, stress, or injury.2 An example is inflammation-inducible hematopoiesis, which was thoroughly studied in murine models using bacterial lipopolysaccharide (LPS), the main cell-wall component of gram-negative bacteria.3 LPS is an endotoxin that stimulates an acute inflammatory response via the CD14 receptor and the Toll-like receptor-4 (TLR4) found on monocytes and tissue macrophages.4 LPS-TLR4 interaction initiates a signal transduction cascade that leads to the release of pro-inflammatory cytokines,3 including tumor necrosis factor (TNF)–α, interleukin (IL)–1β, –6, and –8, and others. These cytokines activate the mobilization of hematopoietic cells from the BM5 and set in motion the migration of leukocytes from blood vessel walls, increasing their numbers in the circulation.6 The net result of this process is an immediate and dramatic increase in the number of circulating peripheral blood (PB) cells, needed to mount the immune response, accompanied by a corresponding decrease in BM cell numbers, which in turn induces a compensatory increase in their production.7
Many factors are involved in abating the inflammatory response and allowing the recovery of hemostasis. Acetylcholine (ACh), one of these factors, acts by attenuating the secretion of pro-inflammatory cytokines at the posttranscriptional level via activation of nicotinic receptors on tissue macrophages.8 Circulating acetylcholinesterase (AChE) controls the levels of ACh, suggesting promotion of the inflammatory process under AChE excess.9 There are 3 C-terminally variant forms of AChE: synaptic (S), erythrocytic (E), and readthrough (R). All are ubiquitously expressed in hematopoietic cell lineages, especially in megakaryocytes (Mks) and erythrocytes.10-12 Importantly, AChE contributes to hematopoiesis processes. In rats, the fraction of AChE-positive BM cells increases following the induction of thrombocytopenia. In both primary cell cultures and live mice, antisense suppression of AChE gene expression modified Mk development.13,14
Platelet production is a self-regulated process. Thrombocytopenia, a reduction in platelets, stimulates the production of thrombopoietin (TPO) and promotes megakaryocytopoiesis.7 As platelet counts return to normal, TPO is effectively cleared from the circulation. This involves TPO binding to its receptor, c-mpl, and its uptake into platelets and Mk.1,15 TPO is the main physiologic growth factor for Mk proliferation, differentiation, and platelet production. Nevertheless, c-mpl-/- and TPO-/- knockout mice have a residual 10% of normally functioning Mks and platelets. This cannot be attributed to IL-6, -11, and leukemia inhibitory factor (LIF), which also are known to induce Mk differentiation,16-19 suggesting the involvement of other factor(s) in Mk differentiation.20 Chemokine-mediated interactions of Mk progenitors with sinusoidal BM endothelial cells (BMECs) recently have been shown to promote TPO-independent platelet production, supporting this notion.2
Pancytopenia and prolonged thrombocytopenia remain significant clinical problems for patients undergoing BM transplantation. Engraftment of transplanted BM is usually accomplished within 2 to 3 weeks, during which period the patient is susceptible to life-threatening infections and bleeding. Platelet recovery after autologous stem cell or cord blood (CB) transplantation is significantly delayed (up to 6 weeks after transplantation) and is attributable to insufficient Mk precursors in the grafts21,22 rather than low TPO levels.23,24
Based on our previous findings that the stress-induced AChE-R variant and its cleavable C-terminal peptide, ARP, stimulate the proliferation of CD34+ hematopoietic progenitor cells,12 we hypothesized that AChE-R and/or ARP may facilitate the proliferation and differentiation of Mk and subsequent platelet production, following stress and in BM transplantation. We therefore compared the baseline hematopoiesis and responses to low doses of LPS in TgR transgenic mice expressing human AChE-R to those of the age-matched FVB/N parent strain mice. To explore the possibility that the thrombopoietic effects of AChE-R involved ARP, we further tested the effect of synthetic ARP26 on human MEG-01 promegakaryocytic cells and on the posttransplantation recovery of blood cells and platelets in sublethally irradiated NOD/SCID mice. Our findings support the notion that AChE-R and ARP play pivotal roles in hematopoiesis and thrombopoiesis and propose a potential new strategy and possible future use of ARP26 for improving postengraftment thrombopoiesis.
Materials and methods
Animal models
The animal ethics committees of The Hebrew University (approval no. NS-02-29 molecular and cellular biology of long-term stress response) and the Weizmann Institute of Science approved the use of animals in this study.
Transgenic mice. TgR mice expressing human (h) AChE-R were generated by injecting a DNA construct including the proximal cytomegalovirus (CMV) promoter-enhancer followed by exons 2, 3, 4, pseudointron 4′, exon 5 of the human ACHE gene (accession no. M55040), and an SV40 polyadenylation signal25 into fertilized eggs of FVB/N mice. This transgene presented unimpaired Mendelian inheritance over 5 generations.26 Strain- and age-matched FVB/N mice served as controls.
To generate inflammatory reaction, 5 μg LPS of Escherichia coli origin (Sigma, St Louis, MO) was injected intraperitoneally in 400 μL of phosphate buffered saline (PBS, Biological Industries, Beth Haemek, Israel).
NOD/SCID mice. Nonobese diabetic SCID (NOD/SCID) mice were maintained under defined flora conditions in the animal facility at the Weizmann Institute (Rehovot, Israel) in sterile intraventilated cages (IVC; Techniplast, Buguggiate, Italy). Mice were sublethally irradiated with 375 cGy at 67 cGy/min from a 60Co source. Twenty-four hours later, they were injected with 100 000 human cord blood (CB) CD34+ cells by intravenous injection in 400 μL Hank balanced salt solution (HBSS, Biological Industries). Mice were killed between 2 and 6 weeks after transplantation. Samples of peripheral blood (PB, orbital bleed) and BM (femur bone) were removed and human engraftment assessed.
Human cell sources
All human material used in this study was approved by the Hospital Human Experimentation Ethics Committee of the Tel-Aviv Sourasky Medical Center in accordance with the Helsinki accords. All participants gave written informed consent to partake in the study. CB cells were retrieved from human umbilical cords of newborns of uncomplicated full-term pregnancies as described12 in anticoagulant citrate dextrose solution formula A–supplemented bags (Baxter, Deerfield, IL). Mononuclear cells (MNCs) were separated using a 2-step technique.27 CD34+ stem cells were purified using a CD34+ progenitor cell isolation kit (PE, Miltenyi Biotec GmbH, Gladbach, Germany) according to the manufacturer's instructions.
MEG-01 cells were maintained in Iscove minimal Dulbecco medium (IMDM, Biological Industries), supplemented with 10% horse serum.28 For experiments cells were plated at a density of 1 × 106 cells/mL in 6-well plates (Nalge Nunc International), incubated with the noted agents for 18 hours, fixed with fresh 4% paraformaldehyde in PBS (phosphate buffer 01 M; pH 7.4 and 0.9% NaCl) for 1 hour, and resuspended in PBS and kept at 4°C until staining.
RT-PCR analyses
Total BM RNA was purified (RNeasy kit, Qiagen, Hilden, Germany) and treated with DNase I (Qiagen) according to the manufacturer's protocols. Reverse transcription (RT) involved 400 ng RNA, 1 μM of each dNTP (Sigma), 10 μM DTT (Sigma), 2 μL RT buffer × 5 (Sigma), 2.5 μM random hexamers (1 U/μL, Sigma), 40 U in 1 μL RNase inhibitor (Roche Molecular Biochemicals, Indianapolis, IN), 2.5 U/μL Superscript reverse transcriptase (Sigma), and DDW to make a total volume of 10 μL. RT was for 45 seconds at 42°C, 5 seconds at 90°C. Polymerase chain reaction (PCR) was performed as previously described using selective primers for AChE-R.11 Briefly, 4 μL PCR buffer × 10 (Sigma), 2.5 μL of each primer (10 mM, Sigma), 0.5 μL TAQ polymerase (Sigma), and DDW to a final volume of 40 μL were added to the RT product. β-Actin was used as a standard housekeeping transcript.
Acetylthiocholine (AThCh) hydrolyzing activity
Mouse plasma samples were separated from the nucleated cell fraction by centrifugation at 4300 rpm (2000g, 20 minutes), sterilized through a 0.2 μm pore size filter, and stored in aliquots at -70°C until use. BM cells were washed with PBS (Sigma) and resuspended in low salt detergent buffer (300 mM NaCl, 0.5% Triton X-100, 50 mM Tris HCl, pH 7.6) containing a protease inhibitor cocktail (Roche Molecular Biochemicals). AThCh activity was measured as previously described.29
Progenitor colony assays
GEMM- and GM-CFUs. Mouse BM MNCs were cultured at 2 × 105 cells per 35-mm tissue culture dish (Corning, NY) in IMDM (Biological Industries) supplemented with 0.8% methylcellulose (Sigma-Aldrich, St Louis, MO), 10% fetal calf serum (FCS, Biological Industries), and 5 × 10-4 M 2-beta-mercaptoethanol (2-ME) (Sigma), 5 ng/mL recombinant murine-granulocyte macrophage–colony stimulating factor (rmu-GM-CSF, R&D Systems, Minneapolis, MN), 10 ng/mL rmu–stem cell factor (rmu-SCF, R&D), U/mL rhu-erythropoietin (rhu-EPO, R&D), and rmu-IL-3 (rmu-IL-3, R&D) in 5% CO2 at 37°C. Colonies of more than 40 cells were counted at day 10. Colonies containing red cells and heterogeneous cell sizes were counted as GEMM-CFUs, while colonies containing only white cells and more homogeneous in cell size were counted as GM-CFUs.
CFU-Mks. 105 BM MNC per 35-mm dish were cultured in McCoy Medium (Biological Industries) supplemented with 0.3% agar (Difco, Detroit, MI), 10% FCS, and 10-4 M 2-ME, 2 ng/mL rmu-thrombopoietin (rmu-TPO, R&D), and 10 ng/mL rmu-SCF in 5% CO2 at 37°C for 10 days. For AChE activity staining, plates were placed into an oven for 2 hours at 45°C with Whatmann no. 1 filter paper discs carefully placed over the agar layer. The filter paper was then gently removed and plates incubated with AChE substrate (10 mg acetylthiocholine [AThCh] iodide dissolved in 15 mL of 0.1 M dibasic sodium phosphate, 1 mL of 0.5 M sodium citrate, 2 mL of 30 mM cupric sulfate, and 2 mL of 5 mM potassium ferricyanide) for up to 24 hours at room temperature or until colonies turned brown in color.
Quantification of cytokine levels
Mouse TPO, EPO, tumor necrosis factor-alpha (TNF-α) and IL-6 levels in plasma of TgR and FVB/N mice were determined using Quantikine murine enzyme-linked immunosorbent assay (ELISA) kits (R&D), according to the manufacturer's instructions.
Immunohistochemistry
BM cell smears were fixed for 15′ with methanol, washed 3 times with PBS and then 3 times with 100 mM glycine to quench autofluorescence. Blocking buffer included 1% donkey serum (Santa Cruz Biotechnology, Santa Cruz, CA) or 1% goat serum (Santa Cruz) for 30 minutes at room temperature. Antibodies against human AChE-R (Rabbit, 0.6 μg/slide),30 PKC ϵ (mouse, 0.5 μg/slide) (BD Biosciences, Palo Alto, CA), and RACK1 (mouse, 0.25 μg/slide) (BD Biosciences) were incubated for 60′ with blocking buffer. TBST (Tris buffered saline with 0.2% Tween 20) was used to wash slides after each antibody incubation. For detection, biotin-SP–conjugated affiniPure goat anti–mouse IgM or donkey anti–rabbit IgG (1:200, Jackson ImmunoResearch Laboratories, West Grove, PA), and Cy3-conjugated streptavidin (1:200, Jackson ImmunoResearch Laboratories) were each incubated for 30 minutes at room temperature. May-Grünwald staining was performed to morphologically identify Mks.
MEG-01 cell suspension samples (25 μL each) were placed on 18-mm coverslips coated with poly-L-ornithine and allowed to dry at room temperature. Briefly, paraformaldehyde-fixed cells were incubated with 3% H2O2 in PBS for 30 minutes, followed by a PBS wash and incubation with a blocking buffer containing 5% bovine serum albumin (BSA), 0.8% Triton X-100 in PBS (1 hour at room temperature). Primary antibodies, polyclonal ant-activated caspase-3 (Cell Signaling Technology), anti-ARP,30 or SC3531 (BD Biosciences) were incubated overnight at 4°C. Detection was performed with the use of horseradish peroxidase–ABC kit (Vectastain, Vector Labs, Burlingame, CA). Cells were mounted in immunomount (Thermo-Shandon, Pittsburgh, PA) and analyzed using a Zeiss Axioplan microscope (Oberkochen, Germany) equipped with a Plan-Neofluar 40 ×/0.75 objective lens. A CRI Real-14 digital camera was used to capture images (Cambridge Research and Instrumentation, Woburn, MA). Images were acquired through ImagePro 4.5.1 software (Media Cybernetics, Silver Spring, MD), and were processed with Adobe Photoshop 5.5 (Adobe Systems, San Jose, CA).
Cell cycle analysis
MEG-01 cells (2 × 106) were fixed in 100% ethanol at 4°C overnight, washed twice in 0.5% BSA in PBS, resuspended in 1 mL of staining solution (PBS containing 0.05 mg/mL propidium iodide, and 1 mg/mL RNAse), and incubated at 37°C for 30 minutes. DNA content was analyzed using a FACS Calibur flow cytometer and CellQuest software (BD Biosciences).
TUNEL staining
In situ nick-end labeling of fragmented DNA (TUNEL) was performed using a DeadEnd kit (Promega, Madison, WI). MEG-01 cell counts were determined on a Zeiss Axiophot microscope, using a magnification of 400 ×. The results are expressed as the average ± SEM of the percentage of positive cells in 4 independent fields in the same coverslip (n = at least 100 cells/field).
Microscopy
Scanning electron. MEG-01 cells were washed in PBS, fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) for 1 hour at room temperature, postfixed for 30 minutes in 1% OsO4 in 0.1 M sodium cacodylate buffer (pH 7.2), dehydrated in ethanol, and then critical-point-dried using CO2. Samples were sputter-coated with gold and analyzed with a JEOL 5800 scanning electron microscope (JEOL, Tokyo, Japan).
Transmission electron. MEG-01 cells were fixed for 1 hour in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) at room temperature. Cells were then washed in PBS, pelleted, and postfixed for 1 hour in 1% OsO4 in 0.1 M sodium cacodylate buffer plus 5 mM CaCl2 and 0.8% potassium ferricyanide at room temperature. The cells were then dehydrated in acetone and embedded in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate and analyzed with a JEOL 1210 electron microscope.
Expansion and priming cultures
For cell-priming experiments, 100 000 fresh CB CD34+ cells were supplemented with 2 nM of peptide, ARP26 (2 nM, synthetic peptide with the AChE-R C-terminal sequence),12 ASP43 (2 nM synthetic peptide with the AChE-S C-terminal sequence),12 or no supplement for 2 hours and injected into mice without washing. For cell cultures, CB CD34+ cells were expanded in liquid cultures in the presence of one of the following growth supplements: ARP26, ASP43, rhu-TPO (1 ng/mL) (R&D) together with rhu-SCF (50 ng/mL; Genzyme Diagnostic, Cambridge, MA), or no supplement (control). Liquid cultures were initiated and maintained in 24-well tissue culture plates (1 × 105 cells/well in 1 mL). Cells were grown for 10 days at 37°C in 5% CO2 in a fully humidified atmosphere in IMDM supplemented with 5% autologous CB plasma. At 3-day intervals, cultures were supplemented with the same growth factor(s), and cells were counted by trypan blue exclusion and diluted to maintain cultures at concentrations no higher then 100 000 cells/mL.32 Cultured cells were injected into NOD/SCID mice at a concentration of 100 000 or 200 000 together with 100 000 unexpanded fresh CD34+ cells per mouse as indicated.
Detection of human cell engraftment in NOD/SCID mice
NOD/SCID mice were killed 2 to 6 weeks after transplantation, and PB and BM were analyzed following lysis of mature red blood cells (RBCs) with FACS lysis buffer (BD Bioscience). Cells (5 × 106) were incubated with human antibodies anti-CD41a–FITC (Beckman/Coulter, Fullerton, CA), anti-CD34–PE (BD Bioscience), and anti-CD45 PerCP (BD Bioscience) (30 minutes, 4°C). To follow human platelet engraftment, PB of NOD/SCID mice was stained with anti–human CD41a-FITC and anti–mouse CD41a–PE (BD Bioscience), and a specific platelet gate was placed at acquisition.
At least 500 000 events per sample were acquired with a BD FACS Calibur (BD Bioscience). Data analysis used Cell Quest and Cell Quest Pro software (BD Bioscience). Matched isotype controls for all antibodies were used to detect background fluorescence (supplied by Caltag and BD Bioscience). All human antibodies were pretested on naive mice that did not receive a transplant to test for any cross-reactivity.
To detect human-originated cells, BM DNA was extracted (QIAprep Spin Miniprep Kit, Qiagen) according to manufacturer instructions. DNA samples (100 ng, 2 mL) were incubated, according to instructions of Roche, the manufacturer, in 10 mL containing 1 mL Light Cycler DNA master hybridization probe (Roche Molecular Biochemicals), 1 mL primers (5 mM sense and 5 mM antisense), 1 mL probes (5 mM anchor and 5 mM sensor), 1.2 mL MgCl2 (3 mM), and nuclease-free water. Primer and probe sequences used to detect human and mouse TNFα are listed in Table 1.33 PCR involved 45 cycles (95°C for 10 seconds, 65°C for 7 seconds, and at 72°C for 20 seconds). Standard curves were generated by mixing mononuclear cells (MNCs) from human CB together with mouse BM, total number of cells being 5 × 106 per concentration with mixtures of 0%, 0.5%, 1%, 2%, 5%, 10%, 20%, 40%, 60%, 80%, and 100% human cells. The human probe and primer were found negative in naive mice33 and used to detect amounts of human DNA in the NOD/SCID mice that received transplants.
The DNA sequence of the primers and probes
TNFα . | 5′-3′ sequence . |
|---|---|
| Human primers | |
| Sense primer | AGGAACAGCACAGGCCTTAGTG |
| Antisense primer | AAGACCCCTTCCAGATAGATGG |
| Human probe donor | GCCCCTCCACCCATGTGCTCC–FL |
| Human probe recipient | CACCCACCACCATCAGCCGCATC–AC |
| Mouse primers | |
| Sense primer | GGCTTTCCGAATTCACTGGAC |
| Antisense primer | CCCCGGCCTTCCAAATAAA |
TNFα . | 5′-3′ sequence . |
|---|---|
| Human primers | |
| Sense primer | AGGAACAGCACAGGCCTTAGTG |
| Antisense primer | AAGACCCCTTCCAGATAGATGG |
| Human probe donor | GCCCCTCCACCCATGTGCTCC–FL |
| Human probe recipient | CACCCACCACCATCAGCCGCATC–AC |
| Mouse primers | |
| Sense primer | GGCTTTCCGAATTCACTGGAC |
| Antisense primer | CCCCGGCCTTCCAAATAAA |
PCR template, primers, and HybProbes are single stranded. One HybProbe is labeled with the fluorescent donor dye fluorescein-sensor (FL), the other one is labeled with an acceptor dye (LCRed 640, Anchor [AC]). The donor dye is excited by blue light of 470 nm and emits green light of 530 nm. Nucleotide sequences are based on sequence of the human and mouse tumor necrosis factor gene (accession numbers M26331 and Y00467).33
Results
AChE-R overexpression in bone marrow and blood cells
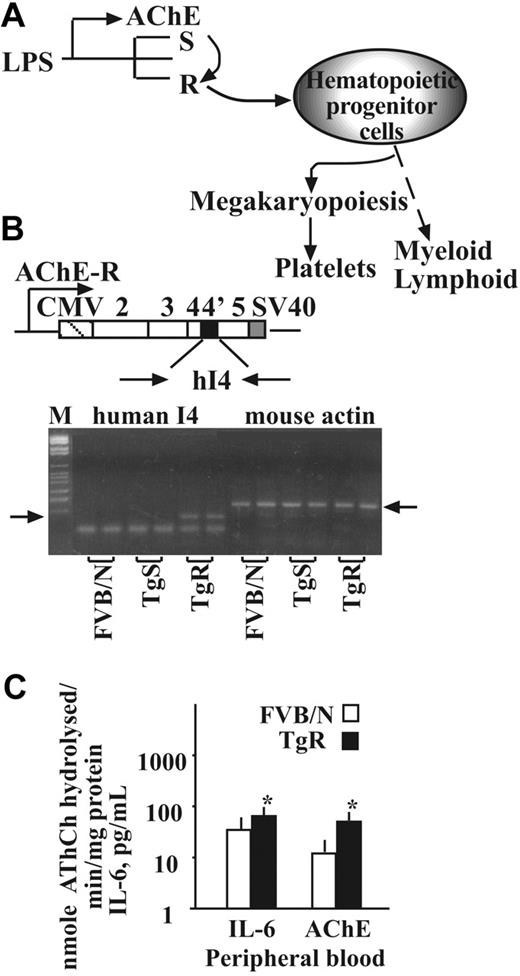
In brain neurons, a stress-induced switch from production of AChE-S to the -R variant elevates soluble AChE-R levels29,34 through the function of the splicing factor protein SC35.31 We hypothesized that this shift, which also occurs in blood cells,9 may reduce the control over pro-inflammatory cytokine production by circulating ACh and the nicotinic α7 AChR.35 This would predictably initiate progenitor cell expansion12 (Figure 1A). We used the TgR transgenic mice expressing hAChE-R as a model of a chronic splicing shift toward AChE-R. RT-PCR analysis detected human AChE-R mRNA in the BM of TgR mice but not in strain-matched FVB/N mice or in the TgS mice overexpressing the hAChE-S variant (Figure 1B).
Transgene facilitation of hematopoietic regulators. (A) The proposed concept involves stress-induced switch from production of AChE-S to the -R variant, resulting in hematopoietic progenitor cell expansion toward the megakaryocyte lineage and increased platelet counts. (B) Human (h) AChE-R DNA construct inserted into the FVB/N mouse genome. hAChE-R cDNA-derived 100-base pair product was successfully amplified in bone marrow DNA of TgR but not TgS or FVB/N mice (n = 12, left arrow). A mouse actin product (130 base pair, right arrow) appeared in all 3 tested lines, FVB/N, TgR, and TgS. (C) Transgene-induced changes in IL-6 and AChE activity (± SEM; *P < .01, Student t test).
Transgene facilitation of hematopoietic regulators. (A) The proposed concept involves stress-induced switch from production of AChE-S to the -R variant, resulting in hematopoietic progenitor cell expansion toward the megakaryocyte lineage and increased platelet counts. (B) Human (h) AChE-R DNA construct inserted into the FVB/N mouse genome. hAChE-R cDNA-derived 100-base pair product was successfully amplified in bone marrow DNA of TgR but not TgS or FVB/N mice (n = 12, left arrow). A mouse actin product (130 base pair, right arrow) appeared in all 3 tested lines, FVB/N, TgR, and TgS. (C) Transgene-induced changes in IL-6 and AChE activity (± SEM; *P < .01, Student t test).
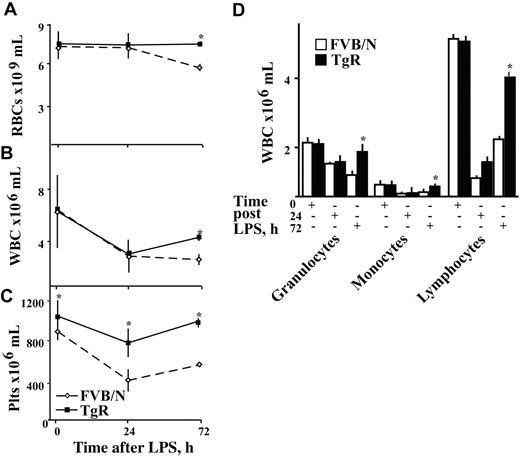
Shorter post-LPS hematopoietic recovery in TgR mice. Shown are RBC (× 109) (A), WBC (× 106) (B), and platelet (Plts × 106) (C) counts per mL of FVB/N (dashed line) and TgR (solid line) mice (n = 25) in peripheral blood. (D) Results of morphologic examination of peripheral blood smears from TgR and FVB/N mice, at different time points after LPS injection, as indicated. Asterisks denote significant differences, and results are presented as mean ± SD of WBCs × 106 per mL of blood (P < .02, n = 10). (+) indicates the time point after LPS treatment where quantitation was performed.
Shorter post-LPS hematopoietic recovery in TgR mice. Shown are RBC (× 109) (A), WBC (× 106) (B), and platelet (Plts × 106) (C) counts per mL of FVB/N (dashed line) and TgR (solid line) mice (n = 25) in peripheral blood. (D) Results of morphologic examination of peripheral blood smears from TgR and FVB/N mice, at different time points after LPS injection, as indicated. Asterisks denote significant differences, and results are presented as mean ± SD of WBCs × 106 per mL of blood (P < .02, n = 10). (+) indicates the time point after LPS treatment where quantitation was performed.
AChE-R excess is associated with elevated basal and post-stress platelet counts
Basal levels of RBC and white blood cell (WBC) counts were similar in both TgR and FVB/N mice (Figure 2A,B). In contrast, platelet counts were significantly higher in TgR mice (894 ± 87 vs 1051 ± 160 × 109/mL, Student t test, P < .001, n = 25, Figure 2C). Manual differential counts of WBC subpopulations showed similar distributions into granulocytes, monocytes, and lymphocytes in TgR and FVB/N mice (Figure 2D), reflecting selective thrombocytosis under chronic AChE-R overexpression.
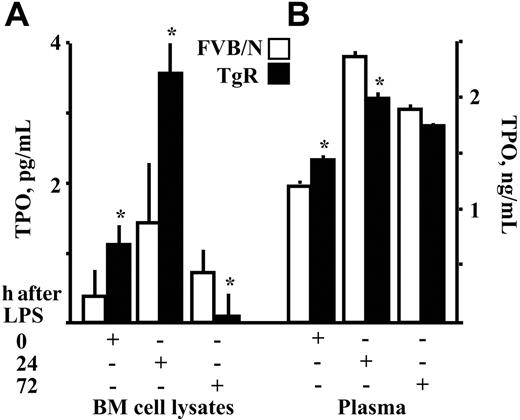
Changes in TPO levels in response to LPS injection. Thrombopoietin (TPO) levels were measured in (A) bone marrow cell lysates and (B) plasma from TgR and FVB/N mice. Asterisks denote significantly different values. Results are presented as mean ± SD (P < .04, n = 10). (+) indicates the time point where quantitation was performed.
Changes in TPO levels in response to LPS injection. Thrombopoietin (TPO) levels were measured in (A) bone marrow cell lysates and (B) plasma from TgR and FVB/N mice. Asterisks denote significantly different values. Results are presented as mean ± SD (P < .04, n = 10). (+) indicates the time point where quantitation was performed.
To study the relevance of AChE-R for regulating the changes in blood cell numbers in response to acute inflammation, LPS was injected intraperitoneally. RBC counts predictably36-38 dropped up to 72 hours after LPS in control FVB/N but not TgR mice (Figure 2A). WBC counts dropped in both strains, but cell counts recovered considerably faster in TgR mice, reaching significantly higher levels than those of FVB/N control mice by 72 hours after LPS injection (P < .02, n = 10, Figure 2B,D). Platelet counts in FVB/N control mice dropped significantly, as expected, to thrombocytopenic levels between 24 and 72 hours. In contrast, platelet counts in TgR mice were only slightly reduced and returned to normal values within 72 hours (P < .001, n = 10, Figure 2C).
AChE-R overexpression modulates TPO and inflammatory cytokine levels
To further study our observation of elevated platelet counts in TgR mice, TPO concentrations were measured in the plasma and BM cell extracts from TgR and FVB/N mice. Values were significantly higher in both BM and plasma from TgR mice (P = .013, .04, respectively, compared to FVB/N control mice [Figure 3A]), suggesting that these mice can serve as a model of chronic inflammation.38-40 TPO levels in the TgR bone marrow increased by 24 hours after LPS injection (P = .002), followed by a decline toward 72 hours (P = .02, n = 10, Figure 3A). Both changes were larger than those occurring in FVB/N mice. In plasma, the initially high basal TPO levels of TgR mice were maintained 24 hours after LPS (P = .01, n = 10). However, at this time point, plasma TPO levels of FVB/N mice rose to significantly higher values than those of TgR mice, possibly due to the corresponding dramatic drop in platelet numbers (Figure 2C). At 72 hours, TPO levels decreased slightly but remained higher than normal in both mouse strains (Figure 3B).
AChE-R excess associates with higher pro-inflammatory cytokine levels
To study the possible effects of AChE-R on inflammatory reactions, we measured the levels of pro-inflammatory cytokines in plasma and BM extracts from TgR and FVB/N mice. Both AChE activity and IL-6, but not TNF-α, levels were significantly higher in the plasma of TgR mice as compared with FVB/N controls (Figure 2A,B).
Two hours after LPS, TgR mice showed significantly higher levels of TNFα in plasma (834 ± 231 pg/mL, P < .04, n = 10) but significantly lower levels in BM, as compared to FVB/N mice (120 ± 66 vs 334 ± 81, P < .01, n = 10, 2), possibly because the main production of TNFα occurs in peripheral blood. hAChE-R expression was accompanied by elevated levels of catalytically active AChE and the pro-inflammatory cytokine IL-6 in the serum of TgR as compared with strain- and age-matched FVB/N parent-strain mice (Figure 1C), compatible with our initial hypothesis. In contrast, IL-6 levels were comparable in TgR and FVB/N mice after LPS injection (Tables 2,3), compatible with the notion of a pre-existing active inflammatory state in the TgR strain.
Cytokine production and AChE catalytic activity in LPS-injected TgR mice: inflammatory cytokine levels after LPS
. | TNFα (pg/mL) . | . | . | . | IL-6 (pg/mL) . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Plasma, time after LPS . | . | BM, time after LPS . | . | Plasma, time after LPS . | . | BM, time after LPS . | . | ||||||
. | 0 h . | 2 h . | 0 h . | 2 h . | 0 h . | 2 h . | 0 h . | 2 h . | ||||||
| TgR mice | 0 | 834 ± 231 | 30 ± 4.2 | 120 ± 66 | 34 ± 2.5 | 1418 ± 62 | 35 ± 4.3 | 507 ± 135 | ||||||
| FVB/N mice | 0 | 538 ± 217 | 23 ± 8.3 | 334 ± 81 | 20 ± 4.5 | 1378 ± 40 | 8 ± 2.7 | 498 ± 331 | ||||||
| P | NS | .04 | NS | .01 | .01 | NS | .001 | NS | ||||||
. | TNFα (pg/mL) . | . | . | . | IL-6 (pg/mL) . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Plasma, time after LPS . | . | BM, time after LPS . | . | Plasma, time after LPS . | . | BM, time after LPS . | . | ||||||
. | 0 h . | 2 h . | 0 h . | 2 h . | 0 h . | 2 h . | 0 h . | 2 h . | ||||||
| TgR mice | 0 | 834 ± 231 | 30 ± 4.2 | 120 ± 66 | 34 ± 2.5 | 1418 ± 62 | 35 ± 4.3 | 507 ± 135 | ||||||
| FVB/N mice | 0 | 538 ± 217 | 23 ± 8.3 | 334 ± 81 | 20 ± 4.5 | 1378 ± 40 | 8 ± 2.7 | 498 ± 331 | ||||||
| P | NS | .04 | NS | .01 | .01 | NS | .001 | NS | ||||||
TNFα and IL-6 levels were measured before and 2 hours after LPS injection. Data are expressed as means ± SD.
NS indicates not significant.
Cytokine production and AChE catalytic activity in LPS-injected TgR mice: AThCh hydrolysing activity/min/mg of protein after LPS
. | BM, time after LPS . | . | . | Plasma, time after LPS . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | 0 h . | 24 h . | 72 h . | 0 h . | 24 h . | 72 h . | ||||
| TgR mice | 20 ± 3.0 | 14.2 ± 4.3 | 13.1 ± 1.8 | 87 ± 1.5 | 31.7 ± 12.8 | 7.7 ± 0.4 | ||||
| FVB/N mice | 14 ± 2.7 | 6.3 ± 1.9 | 11.0 ± 0.7 | 15 ± 1.5 | 27.0 ± 13.1 | 9.3 ± 1.0 | ||||
| P | .02 | < .001 | NS | .001 | NS | NS | ||||
. | BM, time after LPS . | . | . | Plasma, time after LPS . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | 0 h . | 24 h . | 72 h . | 0 h . | 24 h . | 72 h . | ||||
| TgR mice | 20 ± 3.0 | 14.2 ± 4.3 | 13.1 ± 1.8 | 87 ± 1.5 | 31.7 ± 12.8 | 7.7 ± 0.4 | ||||
| FVB/N mice | 14 ± 2.7 | 6.3 ± 1.9 | 11.0 ± 0.7 | 15 ± 1.5 | 27.0 ± 13.1 | 9.3 ± 1.0 | ||||
| P | .02 | < .001 | NS | .001 | NS | NS | ||||
AChE catalytic activity assessed by its AThCh hydrolyzing activity/min/mg of protein was measured in plasma and bone marrow cell extracts (BM) of LPS-injected mice (n = 10). Shown are average concentrations ± SD in plasma or BM proteins.
NS indicates not significant.
Enhanced proliferative potential in TgR bone marrow progenitors
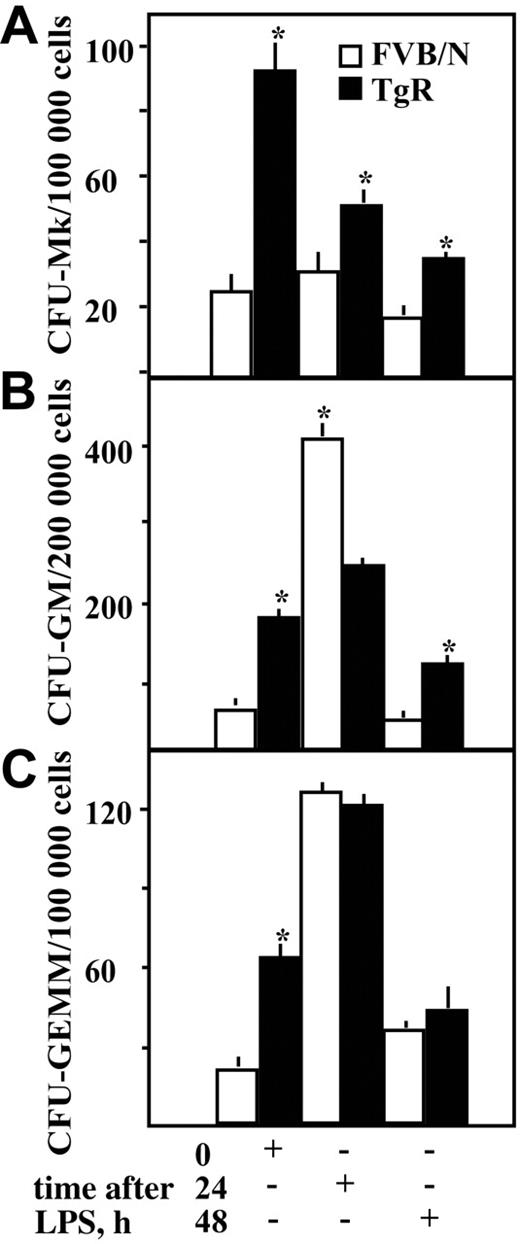
The proliferative potential of BM progenitor cells was evaluated by clonogenic assays using growth factors to support the development of the specific hematopoietic lineages. Colonies were classified as colony-forming units (CFU)–Mk, CFU-granulocyte/macrophage (GM), or CFU-granulocyte/erythrocyte/monocyte/Mk (GEMM) and were counted 10 to 14 days after plating. TgR mice showed significantly higher baseline numbers of CFU-Mk, -GM, and -GEMM hematopoietic progenitor cells as compared to FVB/N controls (P ≤ .003, n = 12, Figure 4). Following LPS injection, TgR mice maintained significantly higher numbers of Mk progenitors (P < .001, n = 12, Figure 4A). In FVB/N mice, the number of CFU-GMs was predictably elevated at 24 hours after LPS36,37 (P = .01, n = 12, Figure 4B) but decreased noticeably by 48 hours. In contrast, TgR CFU-GM numbers decreased 48 hours after LPS yet remained higher than those of FVB/N controls (P = .03, n = 12, Figure 4B). The more modest increase in TgR CFU-GM colonies could, perhaps, be due to the chronic exposure to AChE-R, continuously exploiting the proliferative potential of myeloid progenitor cells. After LPS, the numbers of multipotential CFU-GEMM colonies were similar in TgR and FVB/N mice (NS, n = 12, Figure 4C).
AChE-R overexpression associates with elevated megakaryocytic PKCϵ
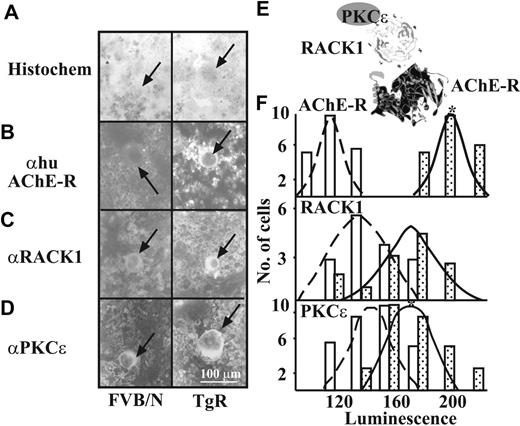
AChE-R was reported to interact with the scaffold protein RACK1 and with its target proteins, protein kinase C βII (PKC βII)41 or PKC ϵ.42 PKC ϵ has been implicated in the programming of megakaryocytic lineage commitment and potentiates the transcription factor GATA-1.43 To study a potential AChE-R/PKCϵ–RACK1 interaction in Mks, we labeled AChE-R, PKCϵ, and RACK1 in BM smears from TgR and FVB/N mice (Figure 5).
TgR Mks, detected in BM smears by the May-Grünwald staining (Figure 5A), predictably expressed higher AChE-R labeling than Mks from FVB/N mice (212.3 ± 15.0 vs 130.9 ± 18.3 luminescence units, P < .001, n = 50, Figure 5B,F and Table 4). Intriguingly, RACK1 labeling intensity was discernibly, although insignificantly, elevated in TgR Mks, as compared to FVB/N mice (162.3 ± 49.2 vs 153.4 ± 21.0, NS, n = 50, Figure 5C,F and Table 4). No differences in the number of PKC ϵ–labeled Mks were detected in TgR mice (data not shown); nevertheless, the intensity of PKC ϵ labeling was significantly higher as compared to FVB/N mice (187.7 ± 22.2 vs 160.9 ± 19.7 luminescence units, P < .001, n = 50, Figure 5D,F and Table 4). Thus, AChE-R interaction with RACK1 and with PKCϵ (Figure 5E) emerged as a putative mechanism for increased intracellular signaling in TgR Mks, compatible with our previous findings in glioblastoma cells42 and MEG-01 cells.28
Luminescence intensity of human AChE-R, RACK1, and PKCϵ in Mks
Antibody . | FVBN . | TgR . | P . |
|---|---|---|---|
| None | 116.8 ± 8.7 | 122.4 ± 11.8 | NS |
| Hu AChE-R | 130.94 ± 18.26 | 212.3 ± 15.0 | < .001 |
| RACK1 | 153.4 ± 21.0 | 162.3 ± 49.2 | NS |
| PKCϵ | 160.9 ± 19.7 | 187.8 ± 22.7 | < .001 |
Antibody . | FVBN . | TgR . | P . |
|---|---|---|---|
| None | 116.8 ± 8.7 | 122.4 ± 11.8 | NS |
| Hu AChE-R | 130.94 ± 18.26 | 212.3 ± 15.0 | < .001 |
| RACK1 | 153.4 ± 21.0 | 162.3 ± 49.2 | NS |
| PKCϵ | 160.9 ± 19.7 | 187.8 ± 22.7 | < .001 |
Luminescence levels (from 1, low luminescence, to 220, bright) plus or minus SD were determined using an upright Zeiss microscope, ImagePro image capture, and Adobe Photoshop V 5.5 analysis for each megakaryocyte stained in the bone marrow smears (n = 50 per antibody).
NS indicates not significant using Student t test. Background staining was detected by incubation with no primary antibody.
Facilitated progenitor cells' potential in TgR mice. Committed colony-forming units of (A) megakaryocyte (CFU-Mk), (B) granulocytic/monocytic (CFU-GM), and (C) multipotential (CFU-GEMM) progenitors were quantified in a semisolid colony formation assay. Asterisks denote significantly different values. Assays were set up in triplicates from bone marrow preparations (4 mice per time point). Values represent mean ± SD. (+) indicates the time point where quantitation was performed. Asterisks denote significantly different values (P < .05, Student t test).
Facilitated progenitor cells' potential in TgR mice. Committed colony-forming units of (A) megakaryocyte (CFU-Mk), (B) granulocytic/monocytic (CFU-GM), and (C) multipotential (CFU-GEMM) progenitors were quantified in a semisolid colony formation assay. Asterisks denote significantly different values. Assays were set up in triplicates from bone marrow preparations (4 mice per time point). Values represent mean ± SD. (+) indicates the time point where quantitation was performed. Asterisks denote significantly different values (P < .05, Student t test).
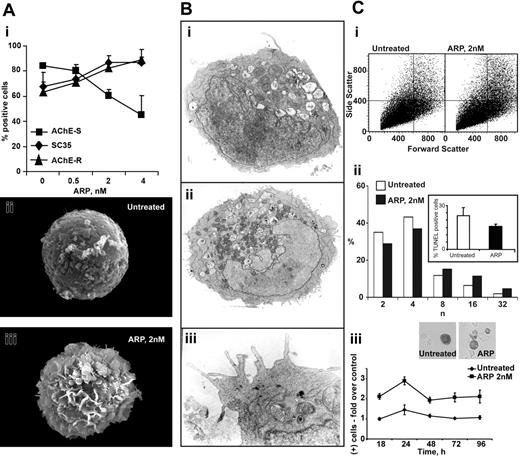
ARP26 promotes preplatelet formation and polyploidization in human MEG-01 promegakaryocytic cells
AChE-R is C-terminally cleaved to yield a free peptide, ARP. In human CD34+ progenitors, a synthetic version of this peptide, ARP26, promotes megakaryocytopoiesis12 and elevates endogenous AChE gene expression.44 To more deeply explore these effects, we incubated a promegakaryocytic cell line MEG01 with increasing doses of ARP26 and used immunolabeling to follow the consequences. ARP26 increased the splice factor SC35, which predictably led to a shift from AChE-S to AChE-R mRNA45 with a plateau at 2 nM ARP26 (Figure 6Ai). Within 24 hours, scanning electron microscopy revealed the appearance of demarcation membranes and the initiation of shedding events resembling platelet formation in ARP26-treated cells (Figure 6Aii,iii). TUNEL analysis excluded the possibility of cell death, and transmission electron microscopy demonstrated that treated cells contained apparently intact nuclei and mitochondria, and highlighted thin peripheral extensions about to demarcate (Figure 6Bi-iii). Importantly, ARP treatment further initiated nuclear polyploidization in treated cells while increasing caspase3 activation (Figure 6Ci-iii), known to associate with megakaryocytopoiesis.28 These experiments suggested relevance of AChE-R in human cells as well.
AChE-R potentiates engraftment potential in NOD/SCID mice
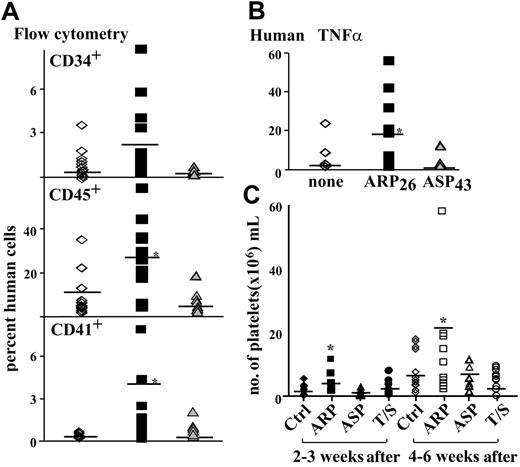
Next, we wished to determine whether AChE-R and/or ARP26 could improve engraftment of transplanted BM cells and recovery from thrombocytopenia in a NOD/SCID mouse transplantation model. Human CB CD34+ cells were primed for 2 hours prior to injection with ARP26, a synthetic peptide comprising 26 amino acids of the C-terminal sequence of AChE-R,44 or ASP43, a 43-amino acid sequence derived from the C terminus of AChE-S.46 At 2 nM, the optimal concentration for stimulating hematopoietic stem cell proliferation,47 human CB CD34+ cells (1 × 105) were injected into NOD/SCID mice irradiated 24 hours earlier. Cells were either primed and supplemented with ARP26 or primed and supplemented with ASP43. Untreated cells served as controls. Mice were killed 6 weeks after transplantation, and single BM cell suspensions extracted from the femur bones were assessed for the presence of human hematopoietic cells. Monoclonal antibodies against human CD45, CD34, and CD41 were used to assess engraftment efficacy of the transplanted human cells. Fractions of human CD34+ cells in the posttransplantation BM of NOD/SCID mice were similar in all groups (Figure 7A). However, significantly more human CD45+ cells were found in the BM of NOD/SCID mice that were injected with ARP26 together with ARP26-primed CD34+ cells (P = .02, n = 12, 16, and 8 mice, respectively, Figure 7A). Fractions of human Mks (CD41+) were higher in the BM of NOD/SCID mice that received ARP26-primed cells as compared with ASP43-primed or nonprimed human cells (P = .03, n = 12, 16, and 8 mice, respectively, Figure 7A). These results demonstrate a significantly better engraftment of transplanted ARP26-primed human CD34+ cells, when injected with ARP26, as compared with non–ARP26-treated cells.
AChE-R, RACK1, and PKC ϵ expression in megakaryocytes. (A) BM smears were stained with May-Grünwald (A) and specific antibodies to detect AChE-R (B), RACK1 (C), and PKCϵ (D). Arrows indicate labeled cells. (E) Illustration of the putative interaction between the 3 proteins. (F) Population distributions of Mk-labeling intensities for AChE-R, RACK1, and PKCϵ. □ represent FVB/N, and  , TgR mice BM-labeling intensities. Note the shift to the right, indicating increased levels of these 3 proteins in Mks from TgR as compared with FVB/N mice. n = 50 cells per test. *P < .01, Student t test.
, TgR mice BM-labeling intensities. Note the shift to the right, indicating increased levels of these 3 proteins in Mks from TgR as compared with FVB/N mice. n = 50 cells per test. *P < .01, Student t test.
AChE-R, RACK1, and PKC ϵ expression in megakaryocytes. (A) BM smears were stained with May-Grünwald (A) and specific antibodies to detect AChE-R (B), RACK1 (C), and PKCϵ (D). Arrows indicate labeled cells. (E) Illustration of the putative interaction between the 3 proteins. (F) Population distributions of Mk-labeling intensities for AChE-R, RACK1, and PKCϵ. □ represent FVB/N, and  , TgR mice BM-labeling intensities. Note the shift to the right, indicating increased levels of these 3 proteins in Mks from TgR as compared with FVB/N mice. n = 50 cells per test. *P < .01, Student t test.
, TgR mice BM-labeling intensities. Note the shift to the right, indicating increased levels of these 3 proteins in Mks from TgR as compared with FVB/N mice. n = 50 cells per test. *P < .01, Student t test.
Quantitative PCR with human specific probes was used to assess the relative presence of human DNA in the BM of NOD/SCID mice. Significant differences could be observed between mice that received transplants of ARP26-primed CD34+ cells as compared to cells primed with ASP43 or nonprimed cells (P = .015, Figure 7B). This suggested that the increased engraftment reflected sustained presence of more nucleated cells of human origin.
Increased human platelet production in NOD/SCID mice transplanted with ARP26-expanded CD34+ cells are known to differentiate in culture, producing many committed progenitors, but have limited capacity for long-term engraftment in NOD/SCID mice. Therefore, freshly isolated CD34+ cells are needed to enable long-term engraftment.48,49 On the other hand, differentiated blood cells can be a better source for platelets. To explore ARP26 effects on platelet recovery in NOD/SCID mice, we therefore attempted to ex vivo expand committed Mk progenitor cells in the stem cell graft prior to transplantation. CD34+ cells were incubated for 10 days in medium supplemented with 10% plasma and 2 nM ARP26, 2 nM ASP43, or TPO (10 ng/mL) and SCF (50 ng) (growth factors optimal for Mk commitment). Cells with no growth factor supplement served as control. The cultured, more mature CD34+ cells (between 1 and 2 × 105) were injected, aiming to facilitate early platelet production, together with 100 000 fresh CB CD34+ cells, used to maintain long-term engraftment. Early platelet engraftment (2-3 weeks after transplantation) and late platelet engraftment (4 and 6 weeks after transplantation) were analyzed. Incubating CD34+ cells with ARP26, ASP43, or TPO and SCF did not augment engraftment of human CD45+, CD34+, or CD41+ cells (data not shown). However, the expansion enabled us to test the effect on platelet production from the injected differentiated cells. Full blood cell counts were performed on the NOD/SCID mice that received transplants, and the presence of human platelets was monitored using platelet-specific human antibodies and flow cytometry. In NOD/SCID mice that received cells expanded with ARP26, as compared with control cells, we observed a trend toward higher human platelet numbers, both early (between 2 and 3 weeks) and late (4-6 weeks) after engraftment. Importantly, platelet counts were significantly higher in ARP26-expanded as compared with ASP43-expanded cells (mean, 1.27 × 106/mL control vs 2.87 × 106/mL ARP26-expanded vs 0.96 × 106/mL ASP43-expanded vs 1.57 × 106/mL TPO/SCF-expanded group, P < .05; significantly higher human platelet numbers engrafted in mice transplanted with ARP26-expanded cells, Figure 7C) and at the late-transplanted stage (mean, 5.09 × 106/mL control vs 22.03 × 106/mL ARP26-expanded vs 5.42 × 106/mL ASP43-expanded vs 3.29 × 106/mL TPO/SCF-expanded group, P < .05; significantly higher human platelet numbers present in the peripheral blood of mice that received transplants, Figure 7B). These observations were compatible with the hypothesis that the injected differentiated Mks facilitated platelet production in the engrafted mice and that the enhanced AChE-R production by these cells supported a shift toward megakaryocytopoiesis, which culminated in higher platelet counts at the later test time.
ARP26 promotes proplatelet formation and nuclear polyploidization in human MEG01 promegakaryocytic cells. (A) ARP26 dose-response and proplatelet formation. (i) Shown are the effects of the noted ARP26 doses following 24 hours' incubation with MEG01 cells. Note increase in the splice factor SC35 and corresponding substitution of AChE-S mRNA with AChE-R mRNA, which appears to plateau under 2 nM ARP26. (ii, iii) Scanning electron microscopy demonstration of proplatelet-like demarcations in ARP26-treated cells. Shown are representative untreated (ii) and an ARP26-treated cells (iii) for 24 hours after treatment. (B) Transmission electron microscopy. (i) Control cell. Inset: TUNEL analysis of control and treated cells, excluding the possibility of ARP26-induced apoptosis. (ii) ARP26-treated cell. Note intact nuclear membrane, numerous mitochondria, and peripheral membrane demarcations. (iii) Enlarged section, highlighting the numerous demarcations. (C) Enhanced nuclear polyploidization and caspase 3 activation under ARP26 treatment of MEG01 cells. (i) Flow cytometric analysis. Note increase in both side and forward scatter of ARP26-treated as compared to control (CTR) cells. (ii) Nuclear polyploidization. Note that within 24 hours, ARP26 treatment increases the fractions of cells with 16 n and 32 n nuclei by approximately 2-fold while decreasing the fractions of cells with 2 n and 4 n nuclei. (iii) Caspase 3 activation. Throughout the 4 days after treatment, ARP26 consistently enhances the levels of activated caspase-3. Error bars indicate standard evaluation of the mean (SEM).
ARP26 promotes proplatelet formation and nuclear polyploidization in human MEG01 promegakaryocytic cells. (A) ARP26 dose-response and proplatelet formation. (i) Shown are the effects of the noted ARP26 doses following 24 hours' incubation with MEG01 cells. Note increase in the splice factor SC35 and corresponding substitution of AChE-S mRNA with AChE-R mRNA, which appears to plateau under 2 nM ARP26. (ii, iii) Scanning electron microscopy demonstration of proplatelet-like demarcations in ARP26-treated cells. Shown are representative untreated (ii) and an ARP26-treated cells (iii) for 24 hours after treatment. (B) Transmission electron microscopy. (i) Control cell. Inset: TUNEL analysis of control and treated cells, excluding the possibility of ARP26-induced apoptosis. (ii) ARP26-treated cell. Note intact nuclear membrane, numerous mitochondria, and peripheral membrane demarcations. (iii) Enlarged section, highlighting the numerous demarcations. (C) Enhanced nuclear polyploidization and caspase 3 activation under ARP26 treatment of MEG01 cells. (i) Flow cytometric analysis. Note increase in both side and forward scatter of ARP26-treated as compared to control (CTR) cells. (ii) Nuclear polyploidization. Note that within 24 hours, ARP26 treatment increases the fractions of cells with 16 n and 32 n nuclei by approximately 2-fold while decreasing the fractions of cells with 2 n and 4 n nuclei. (iii) Caspase 3 activation. Throughout the 4 days after treatment, ARP26 consistently enhances the levels of activated caspase-3. Error bars indicate standard evaluation of the mean (SEM).
Enhanced human blood cell engraftment in NOD/SCID mice. 100 000 human CB CD34+ cells were injected into the tail vein of pre-irradiated NOD/SCID mice with no priming of cells (none, ⋄) or following priming of cells with ARP26 for 2 to 4 hours and injection with human ARP26 (▪) or ASP43 (▴). Bone marrow was harvested 6 weeks after transplantation. (A) CD34+, CD45+, and CD41+ human cells were detected using flow cytometry and monoclonal antibodies, n = 12, 16, and 8 mice, respectively. (B) Quantitative real-time PCR using human TNFα primers to detect human DNA in the mouse bone marrow. Sensitivity limit was 10%. n = 12, 16, and 8 mice, respectively. Asterisks denote significant differences. Lines represent mean values. (C) Precultured CD34+ cells expanded with ARP26 improve platelet counts. 100 000 human CD34+ cells were injected into mice (n = 9) together with 100 000 to 200 000 CD34+ cells cultured for 10 days with no supplement (control, ctrl), 2 nM ARP26, 2 nM ASP43, or human TPO/SCF (T/S). Human platelets per mL of mouse blood were quantified using anti CD41, specific for human platelets. The mean differences (denoted by lines) between groups were large with statistical differences observed in the experimental group of mice that received CD34+ cells stimulated with synthetic peptide ARP in vitro for 10 days together with fresh CB CD34+ cells (asterisks).
Enhanced human blood cell engraftment in NOD/SCID mice. 100 000 human CB CD34+ cells were injected into the tail vein of pre-irradiated NOD/SCID mice with no priming of cells (none, ⋄) or following priming of cells with ARP26 for 2 to 4 hours and injection with human ARP26 (▪) or ASP43 (▴). Bone marrow was harvested 6 weeks after transplantation. (A) CD34+, CD45+, and CD41+ human cells were detected using flow cytometry and monoclonal antibodies, n = 12, 16, and 8 mice, respectively. (B) Quantitative real-time PCR using human TNFα primers to detect human DNA in the mouse bone marrow. Sensitivity limit was 10%. n = 12, 16, and 8 mice, respectively. Asterisks denote significant differences. Lines represent mean values. (C) Precultured CD34+ cells expanded with ARP26 improve platelet counts. 100 000 human CD34+ cells were injected into mice (n = 9) together with 100 000 to 200 000 CD34+ cells cultured for 10 days with no supplement (control, ctrl), 2 nM ARP26, 2 nM ASP43, or human TPO/SCF (T/S). Human platelets per mL of mouse blood were quantified using anti CD41, specific for human platelets. The mean differences (denoted by lines) between groups were large with statistical differences observed in the experimental group of mice that received CD34+ cells stimulated with synthetic peptide ARP in vitro for 10 days together with fresh CB CD34+ cells (asterisks).
Discussion
To explore the putative contribution of cholinergic signaling to mammalian thrombopoiesis, we combined the TgR transgenic mouse model with inflammatory response analyses and BM engraftment studies. Our findings demonstrate increased thrombopoiesis in response to increased production of the stress-induced AChE-R protein and attribute part of the thrombopoietic process to ARP, the cleavable C-terminal peptide of AChE-R, and its interaction with the scaffold protein RACK1 and PKCϵ. This allowed us to extend the concept of what has been defined by others as “the inflammatory reflex”35 to the realm of thrombopoiesis, further supporting the notion of ex vivo augmentation for facilitating posttransplantation thrombopoiesis.
The kidneys and the liver both produce thrombopoietin (TPO), the plasma levels of which are regulated through receptor-mediated uptake, internalization, and catabolism by Mk and platelets.50 TPO levels are tightly controlled under normal conditions and increase only when Mk and platelet production is needed. TPO levels are higher than normal in patients with reactive thrombocytosis (high platelet counts),51,52 for example, in patients with acute bleeding or with acute or chronic inflammation.51,52 Thrombocytosis associated with chronic inflammatory conditions is related to increases in IL-6 levels.39 TPO mRNA and protein levels increased following IL-6 administration to mice and in cancer patients receiving IL-6.53 However, IL-6 treatment failed to increase megakaryopoiesis in vitro and in vivo.19
The involvement of AChE-R and its C-terminal peptide, ARP, in thrombopoiesis is relatively subtle as compared with that of TPO. AChE-R is expressed in Mks10-12 and promotes Mk proliferation in vitro.47 While the C-termini of mouse and human AChE-R share very little sequence homology, murine AChE-R cross-reacts with anti–human ARP antibodies, and hAChE-R affects murine neuronal progenitors.54,55 In our current study we found a significant increase in TPO levels as well as higher platelet counts in TgR mice overexpressing the stress-induced AChE-R splice variant, as compared to the strain-matched FVB/N mice. LPS administration induced a rapid fall in platelet counts in both TgR and FVB/N control mice. We found platelet recovery to be considerably faster in TgR mice than in their strain-matched controls. Moreover, TgR mice showed faster WBC recovery than controls following LPS-induced inflammation and maintained normal RBC values while control FVB/N mice became pancytopenic for at least 72 hours after LPS injection. These differences could be attributed to the augmented capacity of TgR BM progenitors to proliferate and differentiate into pluripotent CFU-GEMM, CFU-GM, and CFU-Mk. The LPS-induced fall in RBC counts can probably be attributed to enhanced degradation (due to decreased deformability and increased osmotic fragility), consumption into micro-aggregates and reduced production (under bone marrow suppression of erythropoietin levels).56,57 The rapid leukocyte decrease is likely due to tissue migration.5,58 Together, these observations support the notion that AChE-R plays an active role in the proliferation of hematopoietic progenitors, especially Mks, without compromising their ability to differentiate into the various hematopoietic lineages.
Our findings highlight the AChE-R effect as a bimodal one. Extracellularly, it predictably reduces ACh levels, that way facilitating the production of pro-inflammatory cytokines with growth factor capacities in response to inflammatory signals; intracellularly, it interacts with partner signaling proteins, promoting cell proliferation in yet another pathway. The AChE-R partner protein RACK1 binds to PKC ϵ.42 PKC ϵ was shown to induce megakaryocytic differentiation in HEL and K562 cells43 in primary human hematopoietic stem cells.59,60 Moreover, TPO increases PKC ϵ expression in mouse Mks,61 whereas blocking PKC activation inhibits platelet formation62 and suppresses megakaryocytopoiesis in MEG01 cells.28 Thus, the elevated levels of AChE-R and PKC ϵ in Mks from TgR mice as well as the higher plasma TPO levels in these mice support the notion of a cholinergic promotion of thrombopoietic signal transduction through both the hydrolytic and the nonenzymatic features of AChE-R, involving 2 signaling pathways.
Lack of proliferating Mk progenitors in BM grafts,21,22 alloimmunization, and refractoriness to platelet transfusions63,64 impede recovery of patients with severe thrombocytopenia after bone marrow transplantation. TPO, the physiologic regulator of thrombopoiesis, has not been clinically effective due to the paucity of Mk progenitors in the grafts.24 MGDF, the pegylated form of TPO, was retracted due to immunogenicity in healthy donors who developed anti-TPO antibodies and became severely thrombocytopenic.65
ARP26, a synthetic peptide derived from AChE-R C-terminus, previously was shown to facilitate the proliferation and differentiation of very early CD34+ myeloid precursors and Mk progenitor cells. Ex vivo, ARP26 treatment was shown to yield considerably higher numbers of platelet-producing Mks than in untreated cells.47 Our current findings present proplatelet formation and nuclear polyploidization effects in ARP26-treated MEG-01 cells and enhanced platelet formation following ARP priming in vivo, in NOD/SCID mice. Increasing the numbers of Mk precursors in the graft should shorten the extended nadir period of severe thrombocytopenia, promoting successful engraftment of long-term repopulating stem cells, the appropriate targets for endogenous or exogenous TPO.
Priming CB CD34+ cells with ARP26 increased significantly the number of human CD45+ cells found in the mouse BM 6 weeks after transplantation. Quantitative PCR analysis confirmed larger content of the human TNFα gene (as a nuclear marker of human-originating cells) in mice that received transplants of ARP26-primed cells. Additionally, incubating CB CD34+ cells for 10 days with 2 nM ARP26 improved the recovery from thrombocytopenia in NOD/SCID mice. CD34+ cells placed in culture lose their ability for long-term engraftment due to differentiation and commitment manifested by the acquisition of the CD38 marker.49,66 Nevertheless, these cells produce more AChE-R12 and can hence support megakaryopoiesis when mixed with immature CD34+, providing clear engraftment advantages to CD45+, CD34+, and CD41+ Mk human cells. Our current study proposes a possible novel strategy to facilitate thrombopoiesis by exposing stem cells to ARP, hopefully improving stem cell engraftment and shortening posttransplantation thrombocytopenia.
Prepublished online as Blood First Edition Paper, December 27, 2005; DOI 10.1182/blood-2005-08-3240.
H.S. and V.D. designed the study; H.S. directed the study in Jerusalem and E.N. directed the work at the Sourasky Medical Center; M.P., C.P., and C.G.-S. performed the study; and T.L. contributed the bone marrow transplantation experiments.
M.P. and C.P. contributed equally to this study.
Supported by grants from the European Community (EC) Alternative Splicing Network of Excellence (LHS 2004-.1.1.5-3 (H.S.), DIP-G 3.22 and the Israel Science fund (618-02) (H.S.), the United States–Israel Binational Science Foundation (1999/115) (H.S. and C.P.), the Deutsches Krebsforschungszentrum (DKFZ), and the Israel Ministry of Science (MOS) (V.D.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Ms Shoshana Baron, Dr Sigi Kay, and Dr Orit Kollet for expert technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal