Abstract
ADAM-9, a member of the adisintegrin and metalloproteinase family, contains both metalloproteinase and disintegrin domains. Myeloma cell lines express ADAM-9; however, its function and role in the pathophysiology of multiple myeloma is unknown. The aim of this study was to establish whether primary myeloma cells express ADAM-9, whether ADAM-9 regulates IL-6 production in human osteoblasts (hOBs), whether ADAM-9 interacts with specific integrin heterodimers, and the identity of downstream signaling pathways. Primary myeloma cells demonstrated increased expression of ADAM-9 (P < .01). ADAM-9 promoted a 5-fold increase in IL-6, but not IL-1β mRNA, and a dose- and time-dependent increase in IL-6 production by hOBs (P < .01). IL-6 induction was inhibited by an antibody to the αvβ5 integrin (P < .01) but not by antibodies to other integrin heterodimers. ADAM-9 was shown to bind directly to the αvβ5 integrin on hOBs. Antibodies to ADAM-9 and αvβ5 integrin inhibited myeloma cell–induced IL-6 production by hOBs (P < .01). Furthermore, inhibitors of p38 MAPK and cPLA2, but not NF-κB and JAK2, signaling pathways inhibited ADAM-9–induced IL-6 production by hOBs (P < .01). These data demonstrate that ADAM-9, expressed by myeloma cells, stimulates IL-6 production in hOBs by binding the αvβ5 integrin. This may have important consequences for the growth and survival of myeloma cells in bone.
Introduction
Multiple myeloma is a disease characterized by the growth of malignant plasma cells within the bone marrow microenvironment. The growth and survival of myeloma cells is regulated by complex interactions between the tumor cells and the cells of the local environment, particularly bone marrow stromal cells and osteoblasts. These interactions play an important role in regulating production of critical regulators of myeloma-cell growth and survival such as interleukin-6 (IL-6)1-4 and factors implicated in the development of myeloma bone disease such as the ligand for receptor activator of NF-κB and osteoprotegerin.5-8 Although studies have demonstrated that these interactions are dependent upon cell-to-cell contact, the identities of the molecules involved are poorly understood. A number of molecules have been implicated in regulating IL-6 production, such as CD44 and CD56, whereas other molecules may regulate RANKL and OPG production, such as the α4β1 integrin.7 However, these molecules have often been shown to be only partially responsible, suggesting that other factors may also play a critical role. Equally, the downstream signaling pathways are often poorly defined.
We have shown that myeloma cells express ADAM-9 (MDC-9/meltrin-γ),9 a member of the ADAM (adisintegrin and metalloproteinase) or MDC (metalloproteinase/disintegrin/cysteine-rich) family. The ADAM family is a family of membrane-anchored proteins that contain a number of characteristic domains.10,11 These include the presence of a signal sequence followed by a prodomain, a metalloproteinase domain, a disintegrin-like domain, a cysteinerich region, a transmembrane domain, and a short cytoplasmic tail. Members of the ADAM family have been implicated in a number of important cellular processes including proteolysis, cell-to-cell and cell-to-matrix interactions, cell fusion, and cell signaling.11-14 Although studies have often focused upon the proteolytic activity of members of this family, there is increasing evidence to suggest that they may play a role in cell adhesion. The disintegrin-like domains of members of this family contain the same conserved cysteine residues found in the soluble snake venom disintegrins. Thus, members of the ADAM family may have the capacity to interact directly with integrins. ADAM-15 is the only ADAM family member to contain an RGD (arginine-glycine-aspartate) motif in the predicted binding site and mediates adhesion of a number of cell types via interactions with αvβ3 integrin or α5β1 integrin.15,16 All other members of the family contain alternative sequences in the predicted binding site. ADAM-2 (fertilin-β) plays a role in sperm-egg fusion17-19 by binding the α6β1 integrin on the egg membrane.20-22 Moreover, ADAM-12 and ADAM-23 have been reported to bind the integrins α9β1 and αvβ3, respectively.23,24 ADAM-9 can mediate adhesion of fibroblasts via interactions with the α6β1 integrin25 and myeloma cells can bind to ADAM-9 via αvβ5 integrin9 . These data demonstrate that non–RGD-containing members can associate with specific integrins. This raises the possibility that ADAM-9 could mediate cell-to-cell communication between myeloma cells and cells found in the bone marrow microenvironment such as osteoblasts to regulate production of critical survival signals such as IL-6.
Therefore, the aim of the present study was to determine whether primary myeloma cells express ADAM-9 and whether this was involved in regulating cell-to-cell communication between myeloma cells and cells found in the bone marrow microenvironment, particularly osteoblasts. In addition, we sought to identify the integrins with which ADAM-9 may interact and downstream signaling pathways.
Patients, materials, and methods
Materials and reagents
Rabbit anti–human ADAM-9 disintegrin domain polyclonal antibody (AB19025), mouse anti–human αvβ5-integrin monoclonal antibody immobilized on immunoaffinity gel matrix (GEM1961), mouse anti–human αvβ3-integrin monoclonal antibody (LM609, MAB1976Z), mouse anti–human αvβ5-integrin monoclonal antibody (P1F6, MAB1961Z), mouse anti–human αvβ6-integrin monoclonal antibody (MAB2077Z), and recombinant αvβ5 integrin in Triton X-100 (CC1025) were obtained from Chemicon International (Temecula, CA). RPMI 1640 medium, l-glutamine, 2-mercaptoethanol, sodium pyruvate, modified Eagle medium (MEM) nonessential amino acids, phosphate-buffered saline (PBS), and Versene (0.53 mM EDTA in PBS) were from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Equitech-Bio (Kerrville, TX). DuoSet IL-6 enzyme-linked immunosorbent assay (ELISA) development kit (DY206), recombinant soluble human ADAM-9 (939-AD-020), and mouse anti–human ADAM-9 ectodomain monoclonal antibody (MAB939) were from R&D Systems (Minneapolis, MN). Histopaque 1077, trypan blue, dimethylsulfoxide (DMSO), paraformaldehyde, and phorbol 12-myristate 13-acetate (PMA) were from Sigma-Aldrich (St Louis, MO). Cy2-conjugated affinipure goat anti–mouse IgG and Cy5-conjugated affinipure goat anti–rabbit IgG were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Vectashield mounting medium for fluorescence microscopy with 4,6-diamidino-2-phenylindole (DAPI; H-1200) was obtained from Vector Laboratories (Burlingame, CA). Fluorescein isothiocyanate (FITC)–conjugated secondary antibody, anti–immunoglobulin κ or λ light chain conjugated with R-phycoerythrin (PE), and f(ab)2 fragment conjugated with R-phycoerythrin were from DAKO (Glostrup, Denmark).
Isolation and culture of myeloma cells
The human multiple myeloma cell lines RPMI-8226 and U266 and the lymphoblastoid cell lines HS Sultan and ARH-77 were obtained from European Collection of Animal Cell Cultures (Porton Down, United Kingdom). The myeloma cell line JJN-3 was a gift from Prof I. Franklin (University of Glasgow, Glasgow, United Kingdom). Cells were grown in RPMI 1640 medium supplemented with 10% FBS, 2 mM l-glutamine, 5 mM 2-mercaptoethanol, 2 mM sodium pyruvate, and 0.1 mM MEM nonessential amino acids (called supplemented media) in a humidified atmosphere of 5% CO2/95% air at 37°C as described previously.26 Bone marrow aspirates were obtained from 9 patients with multiple myeloma, at diagnosis, under local ethics approval (Sheffield Research Ethics Committee protocol number 94/173, University of Sheffield, United Kingdom). Informed consent was provided in accordance with the Declaration of Helsinki. The bone marrow mononuclear cells were isolated by density gradient centrifugation using histopaque 1077 and used for subsequent flow cytometric and immunocytochemical analysis.
Isolation and culture of human osteoblast-like cells
Trabecular bone explants were obtained from patients undergoing joint replacement surgery and primary bone cells were obtained using the method described previously.27 Human osteoblasts (hOBs) were incubated in the presence or absence of either recombinant soluble ADAM-9 protein (200 nM), recombinant disintegrin-like ADAM-9 protein (D9; 1-200 μg/mL), recombinant disintegrin-like ADAM-9 control protein (D9C; 1-200 μg/mL), or RPMI-8226 myeloma cells (1 × 106 cells/well). In some studies hOBs were incubated with purified rabbit anti–human ADAM-9 polyclonal antibody (5 μg/mL); anti–human αvβ3 integrin, αvβ5 integrin, or αvβ6 integrin antibodies (20 μg/mL); or isotype control IgG (20 μg/mL) prior to addition of recombinant proteins or cells. In further studies, hOBs were incubated with cell signaling inhibitors SB-203580 (10 μM) for p38 mitogen-activated protein kinase (MAPK), PD-98059 (10 μM) for a mitogen-activated and extracellular regulated kinase (MEK or ERK1/2) MAPK, arachidonyltrifluoromethyl ketone (AACOCF3;20 μM) for cytosolic phospholipase A2 (cPLA2), Rp-diastereomer of adenosine 3′,5′-phosphorothioate (RP-cAMP; 10 μM) and SN50 (10 μg/mL) for NF-κB or AG490 (5 μM) for Janus kinase 2 (JAK2) pathways prior to addition of proteins or cells. The cells were incubated at 37°C for 24 hours. The supernatant was collected and IL-6 concentration measured by ELISA. In some experiments, hOBs were cultured in the presence or absence of D9 (10 μg/mL) or D9C (10 μg/mL) for 6 hours prior to extraction of RNA for Northern hybridization studies.
Western blot analysis of ADAM-9 expression
ADAM-9 expression in myeloma cell lines was examined by Western blot analysis. Briefly, cells were lysed in TBS buffer containing 1% Nonidet P-40, 1 mM EDTA, and 1:200 diluted proteinase inhibitor set III (AEBSF [4-(2-aminoethyl)-benzene sulfonyl fluoride hydrochloride, 250 μM], aprotinin [400 nM], (-)-N-[(2S, 3R)-3-amino-2-hydroxy-4-phenylbutyryl]-l-leucine methyl ester [bestatin, 25 μM], acetyl-l-leucyl-l-leucyl-l-argininal [leupeptin, 20 μM], n-(3-carboxyoxirane-2-carbonyl)-leucyl-amino(4-guanido) butane [E-64, 7.5 μM], and pepstatin A [5 μM]). Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose membrane. The membrane was then incubated with rabbit anti–human ADAM-9 polyclonal antibody (5 μg/mL), which was detected with goat antirabbit secondary antibody (Kirkegaard & Perry Laboratories, Gaithersburg, MD) conjugated with horseradish peroxidase (HRP) and the supersignal West Pico Chemiluminescent substrate system (Pierce Biotechnology, Rockford, IL).
In some studies release of ADAM-9 from RPMI-8226 cells following stimulation with PMA, an activator of ectodomain shedding,28,29 was examined. FBS-free conditioned media was collected following treatment of RPMI-8226 cells with or without PMA (100 nM) or in the presence or absence of 1:200 diluted proteinase inhibitor set III at 37°C for 2 hours. The media were concentrated, proteins were precipitated with ammonium sulfate, and samples were subjected to 12.5% SDS-PAGE electrophoresis and Western blot analysis as described.
In further studies, the ability of ADAM-9 to bind the αvβ5 integrin was determined. Immunaffinity gel matrix beads with an anti–αvβ5-integrin monoclonal antibody, covalently attached, were incubated with 1% bovine serum albumin in PBS for nonspecific blocking. After washing, the beads were then incubated with or without recombinant αvβ5 integrin. The beads were washed and incubated with 500 nM recombinant ADAM-9 for 20 minutes. The washed samples were then separated by electrophoresis on a 10% Bis-Tris precast gel (Invitrogen), transferred to a nitrocellulose membrane, and examined by Western blotting using the anti–ADAM-9 antibody.
Flow cytometric analysis of ADAM-9 expression
ADAM-9 protein expression by myeloma cell lines and primary myeloma cells was also examined by flow cytometry. Cells (1 × 106) were incubated with rabbit anti–human ADAM-9 antibody (5 μg/mL) or isotype control IgG (5 μg/mL), followed by FITC-conjugated goat anti–rabbit IgG secondary antibody. Dual-color analysis was performed to identify myeloma cells in bone marrow samples by also incubating cells with PE-conjugated anti–immunoglobulin κ or λ light chain or control f(ab)2 fragment. Cells were analyzed for FITC and PE fluorescence on a FACSCalibur flow cytometer (BD Pharmingen, Bedford, MA).
In some studies the effect of PMA was also examined. Cells (1 × 106 cells/mL) were incubated with PMA (100 nM) or vehicle (0.001% DMSO) at 37°C for 2 hours. Cells were collected and stained with purified rabbit anti–ADAM-9 antibody as described.
The ability of ADAM-9 and αvβ5 integrin to interact directly was also examined by flow cytometry. hOBs were incubated in FBS-free media in the presence or absence of recombinant ADAM-9 (500 nM) for 10 minutes. In some studies cells were first incubated with the anti–human αvβ5-integrin–blocking antibody (20 μg/mL), or isotype control IgG (20 μg/mL), at room temperature for 10 minutes. Cells were collected and then incubated with purified rabbit anti–human ADAM-9 polyclonal antibody (5 μg/mL). The samples were then incubated with Cy2-coupled affinipure goat anti–mouse IgG secondary antibody and analyzed with a FACSCalibur flow cytometer.
Fluorescence immunocytochemical analysis of ADAM-9 and αvβ5-integrin expression
Dual-color fluorescence immunocytochemistry (FIC) was performed to examine ADAM-9 expression by light chain–positive (cIgLC+) and –negative (cIgLC-) bone marrow mononuclear cells from patients with multiple myeloma. Bone marrow mononuclear cells were fixed in 4% para-formaldehyde cytocentrifuged onto glass slides and incubated with rabbit anti–human ADAM-9 polyclonal antibody (5 μg/mL) or isotype control IgG (5 μg/mL). Cells were washed, incubated with secondary antibody conjugated with FITC for 20 minutes, and then incubated with a 1:20 dilution of anti–immunoglobulin κ or λ light chain or control f(ab)2 fragment conjugated with PE. Slides were mounted in Vectashield mounting medium for fluorescence, with DAPI for nuclear staining, and examined using a DMRB fluorescence microscope and QWin image analysis software (Leica, Milton Keynes, United Kingdom). Images were captured using a Nu200 cooled CCD camera (Photometrics, Tucson, AZ) system.
Colocalization of ADAM-9 and αvβ5 integrin on hOBs was also examined by FIC. Cells were incubated with or without recombinant ADAM-9 (500 nM). After washing, the cells were fixed in ethanol and incubated with anti–human ADAM-9 polyclonal antibody (5 μg/mL) and anti–human αvβ5-integrin antibody (5 μg/mL) or mouse and rabbit isotype control IgG (5 μg/mL). The cells were incubated with Cy5-coupled affinipure goat anti–rabbit IgG and Cy2-coupled affinipure goat anti–mouse IgG secondary antibodies, counterstained with DAPI, and examined by fluorescence microscopy.
Northern hybridization
Northern blot analysis was performed by separating 15 μg of total RNA on a 1% agarose–formaldehyde gel. RNA was transferred to positively charged nylon membrane (Boehringer Mannheim, Mannheim, Germany) by capillary blotting and fixed by UV cross-linking (Stratalinker; Stratagene, Cambridge, United Kingdom). cDNA probes for IL-6 and IL-1β were labeled with 32P-dCTP (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) using a random priming method (random primed DNA labeling kit; Boehringer Mannheim). Hybridization was performed in Quickhyb (Stratagene, Amsterdam, The Netherlands) using 50 to 100 ng cDNA probe. Membranes were exposed to X-ray film at -80°C and reprobed with a cDNA for GAPDH to standardize RNA loading.
Measurement of IL-6 and IL-1β by ELISA
IL-6 concentration in the supernatant was measured using a DuoSet ELISA Development kit (DY206) from R&D Systems. IL-1β was measured using a commercially available ELISA (R&D Systems).
Preparation of recombinant disintegrin domain of ADAM-9 (D9)
A cDNA encoding the disintegrin domain of ADAM-9 was amplified by reverse-transcriptase–polymerase chain reaction (PCR) and cloned into a pGEX glutathione S-transferase (GST) fusion vector. Recombinant ADAM-9 disintegrin protein was expressed and fused to GST in Escherichia coli as described previously.9 The fusion protein was purified by affinity chromatography and the GST removed by cleavage with thrombin. Recombinant disintegrin protein (D9) was separated from contaminating GST by additional affinity chromatography steps. A negative control (D9C) was prepared by subjecting an empty vector to identical expression and purification steps.
Statistical analysis
Data are the mean of quadruplicate determinations and 95% confidence interval (CI). Each experiment has been repeated on a minimum of 2 separate occasions. In each case, data from a single representative experiment are shown. Multiple comparisons were performed with a one-way analysis of variance followed by the Dunnett test for treatment versus control comparisons. Pair-wise comparisons were carried out by performing nonparametric Mann-Whitney U test or by Wilcoxon matched pairs test. In each analysis, differences were considered statistically significant for P values less than .05. All statistical tests were 2-sided.
Results
Human myeloma cells express ADAM-9
Western blot analysis demonstrated expression of a single product of approximately 115 kDa corresponding to the ADAM-9 precursor protein in each of the myeloma and lymphoblastoid cell lines examined (Figure 1A). Flow cytometric analysis of ADAM-9 expression in RPMI-8226 cells confirmed that ADAM-9 is highly expressed in human myeloma cells (Figure 1B). Analysis of ADAM-9 expression on live cells, which represents the cell-surface expression, demonstrated the expression of membrane-associated ADAM-9 (Figure 1B). Similar staining was observed in each of the other cell lines (data not shown).
To determine whether primary myeloma cells, defined by expression of restricted immunoglobulin light chain, express the ADAM-9 protein, FIC and flow cytometry analysis were performed. FIC demonstrated the presence of light chain–producing cells in the bone marrow of patients with multiple myeloma (Figure 1C-D). All light chain–producing cells stained positively for expression of ADAM-9 protein (Figure 1C,E). Flow cytometric analysis confirmed expression of ADAM-9 in the light chain–positive population of cells (cIgLC+; Figure 1E). However, expression of ADAM-9 was significantly stronger in cIgLC+ cells when compared with light chain–negative cells (cIgLC-; P < .01; Figure 1F). The average mean fluorescence value ± 95% CI in 9 patient samples was 80.6 ± 44.6 in the cIgLC- population of cells whereas it was 170.7 ± 104.5 in the cIgLC+ population.
Human myeloma cells shed disintegrin domain containing ADAM-9 protein
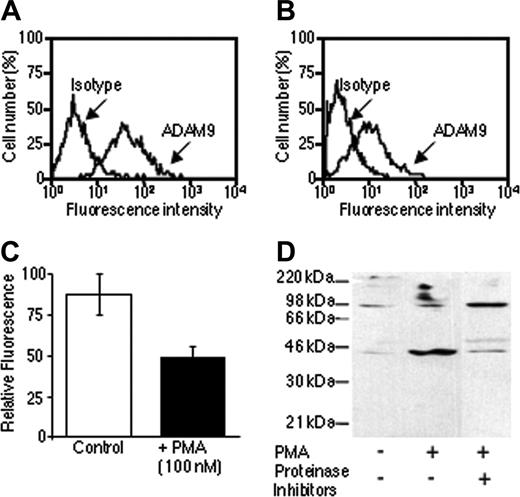
Flow cytometric and Western blot analysis were also performed to determine whether soluble forms of ADAM-9 exist. PMA treatment caused a decrease of approximately 50% in ADAM-9 surface expression in RPMI-8226 myeloma cells (P < .05; Figure 2A-C). Media conditioned by RPMI-8226 cells contained both 44-kDa and 90-kDa products (Figure 2D) and PMA was shown to increase the production of the soluble 44-kDa ADAM-9 product. Since the antibody to ADAM-9 was raised against the disintegrin domain, this is consistent with the protein containing the disintegrin domain and the cysteine-rich region. This protein would be predicted to have a molecular weight of 30.7 kDa without glycosylation although there are 2 predicted glycosylation sites in this region, which would increase this size. It is unclear whether the 90-kDa product was ADAM-9 shed from cell surface and containing the prodomain, metalloproteinase, disintegrin, and cysteine-rich domains or is a product generated by alternative mRNA splicing, as this was not increased by PMA treatment. Proteinase inhibitors appeared to inhibit PMA-induced soluble 44-kDa ADAM-9 production, which is consistent with proteinase-dependent release, but increase the amount of 90-kDa product (Figure 2D).
Human myeloma cells express ADAM-9. (A) Western blot analysis of ADAM-9 expression in human myeloma cells demonstrated a single product. (B) Flow cytometric analysis of ADAM-9 expression (red histograms) in RPMI-8226 cells demonstrated expression in both permeabilized (Perm) and nonpermeabilized (Nonperm) cells when compared with an isotype control (blue histograms). (C) Two-color fluorescence immunocytochemical analysis of bone marrow mononuclear cells demonstrated expression of the restricted light chain (red) staining in a proportion of mononuclear cells. Light chain–positive cells were shown to stain positively for ADAM-9 (green). Nuclei were counterstained with DAPI and examined at an objective magnification of × 40 (0.7 numeric aperture [NA]). (D) Flow cytometric analysis of bone marrow mononuclear cells showing cIgLC- and cIgLC+ staining. (E) Flow cytometric analysis demonstrated ADAM-9 expression in both cIgCL- (green histogram) and cIgLC+ (red histogram) cells. (F) Analysis of mean fluorescence values (±95% CI) for cIgCL- and cIgCL+ cells isolated from patients with multiple myeloma demonstrated significantly greater expression in cIgLC+ cells. *P < .01 by Wilcoxon matched pairs test.
Human myeloma cells express ADAM-9. (A) Western blot analysis of ADAM-9 expression in human myeloma cells demonstrated a single product. (B) Flow cytometric analysis of ADAM-9 expression (red histograms) in RPMI-8226 cells demonstrated expression in both permeabilized (Perm) and nonpermeabilized (Nonperm) cells when compared with an isotype control (blue histograms). (C) Two-color fluorescence immunocytochemical analysis of bone marrow mononuclear cells demonstrated expression of the restricted light chain (red) staining in a proportion of mononuclear cells. Light chain–positive cells were shown to stain positively for ADAM-9 (green). Nuclei were counterstained with DAPI and examined at an objective magnification of × 40 (0.7 numeric aperture [NA]). (D) Flow cytometric analysis of bone marrow mononuclear cells showing cIgLC- and cIgLC+ staining. (E) Flow cytometric analysis demonstrated ADAM-9 expression in both cIgCL- (green histogram) and cIgLC+ (red histogram) cells. (F) Analysis of mean fluorescence values (±95% CI) for cIgCL- and cIgCL+ cells isolated from patients with multiple myeloma demonstrated significantly greater expression in cIgLC+ cells. *P < .01 by Wilcoxon matched pairs test.
Human myeloma cells shed ADAM-9. (A) Flow cytometric analysis of ADAM-9 expression on the surface of RPMI-8226 myeloma cells. (B) Flow cytometric analysis of ADAM-9 expression on the surface of RPMI-8226 myeloma cells incubated in the presence of PMA. (C) Comparison of mean fluorescence values (±95% CI) from cells incubated in the presence or absence of PMA demonstrated a reduction in ADAM-9 expression in PMA-treated cells. (D) Western blot analysis of ADAM-9 in media conditioned by RPMI-8226 cells incubated in the presence or absence of PMA and/or proteinase inhibitors. PMA stimulated an increase in the 44-kDa product that was blocked with proteinase inhibitors.
Human myeloma cells shed ADAM-9. (A) Flow cytometric analysis of ADAM-9 expression on the surface of RPMI-8226 myeloma cells. (B) Flow cytometric analysis of ADAM-9 expression on the surface of RPMI-8226 myeloma cells incubated in the presence of PMA. (C) Comparison of mean fluorescence values (±95% CI) from cells incubated in the presence or absence of PMA demonstrated a reduction in ADAM-9 expression in PMA-treated cells. (D) Western blot analysis of ADAM-9 in media conditioned by RPMI-8226 cells incubated in the presence or absence of PMA and/or proteinase inhibitors. PMA stimulated an increase in the 44-kDa product that was blocked with proteinase inhibitors.
ADAM-9 mediates myeloma-cell induction of IL-6 expression by hOBs in an αvβ5-integrin–dependent manner
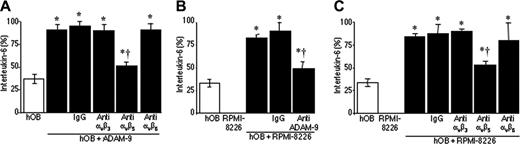
Human myeloma cells have been shown to stimulate bone marrow stromal cells and osteoblasts to produce IL-6 in a cell-to-cell–contact manner.27,30 To establish whether ADAM-9 could promote IL-6 production by osteoblasts, hOBs were treated with recombinant soluble ADAM-9. hOBs treated with ADAM-9 demonstrated a 2.5-fold increase in the amount of IL-6 produced (Figure 3A; P < .01). Since ADAMs contain disintegrin domains and can interact with integrins, we investigated whether blocking antibodies raised to specific integrins could block ADAM-9–induced IL-6 production. While antibodies against αvβ3 integrin and αvβ6 integrin had no effect on ADAM-9–mediated IL-6 production, an antibody against αvβ5 integrin reduced ADAM-9–enhanced IL-6 production by 40% (Figure 3A; P < .01).
To establish whether ADAM-9 expression by myeloma cells could mediate myeloma cell–induced IL-6 production by osteoblasts, myeloma cells were incubated with osteoblasts in the presence or absence of an anti–ADAM-9 antibody. When cultured with hOBs, RPMI-8226 cells promoted a significant increase in IL-6 production in cultures of hOBs when compared with hOBs cultured alone (Figure 3B; P < .01). However, when hOBs were incubated with an antibody to ADAM-9 prior to addition of RPMI-8226 cells, concentrations of IL-6 were reduced by approximately 45% (Figure 3B; P < .01), suggesting that ADAM-9 may at least in part contribute to myeloma cell–induced IL-6 production. To confirm that this was mediated by an interaction with the αvβ5 integrin, myeloma cells were cultured with hOBs in the presence or absence of blocking integrin antibodies. Isotype control or antibodies against αvβ3 integrin or αvβ6 integrin had no effect on RPMI-8226–induced IL-6 production in hOBs with values being 2.6-fold, 2.7-fold, and 2.4-fold higher than hOBs cultured alone, respectively (P < .01 in each case; Figure 3C). However, when cells were first incubated with an αvβ5-integrin antibody, IL-6 production was reduced significantly (P < .01; Figure 3C). Values remained significantly higher than control, suggesting that the interaction with the αvβ5 integrin was partly involved in the myeloma cell–induced IL-6 production.
ADAM-9 mediates IL-6 production by human osteoblasts in an αvβ5-integrin–dependent manner. (A) Human osteoblasts were cultured in the presence or absence of recombinant ADAM-9 and either a control IgG or the presence of blocking antibodies to different integrins. (B) Human osteoblasts were cultured in the presence or absence of RPMI-8226 myeloma cells and either a control IgG or the presence of ADAM-9–blocking antibody. (C) Human osteoblasts were cultured in the presence or absence of RPMI-8226 cells and either an IgG control antibody or antibodies to various integrins. In each case, after 24 hours, IL-6 was measured by ELISA. Data are the means of samples from a representative experiment (n = 4-8), and error bars are 95% CI. IL-6 production is shown as percentage of the mean + 95% CI of the maximum bar in the graph. A Mann-Whitney U test was used for pair-wise comparisons. *P < .01 compared with hOBs alone; and †P < .01 compared with IgG.
ADAM-9 mediates IL-6 production by human osteoblasts in an αvβ5-integrin–dependent manner. (A) Human osteoblasts were cultured in the presence or absence of recombinant ADAM-9 and either a control IgG or the presence of blocking antibodies to different integrins. (B) Human osteoblasts were cultured in the presence or absence of RPMI-8226 myeloma cells and either a control IgG or the presence of ADAM-9–blocking antibody. (C) Human osteoblasts were cultured in the presence or absence of RPMI-8226 cells and either an IgG control antibody or antibodies to various integrins. In each case, after 24 hours, IL-6 was measured by ELISA. Data are the means of samples from a representative experiment (n = 4-8), and error bars are 95% CI. IL-6 production is shown as percentage of the mean + 95% CI of the maximum bar in the graph. A Mann-Whitney U test was used for pair-wise comparisons. *P < .01 compared with hOBs alone; and †P < .01 compared with IgG.
ADAM-9 and αvβ5 integrin interact directly in vitro
Since ADAM-9 and RPMI-8226 myeloma cells promote production of IL-6 by hOBs and this can be inhibited by interfering with the activity of either ADAM-9 or αvβ5 integrin, we hypothesized that these 2 molecules interact directly. To test this, ADAM-9 was incubated with immunoaffinity beads bound with αvβ5 integrin, immunoprecipitated, and subjected to Western blot analysis of ADAM-9 activity. Beads without αvβ5 integrin served as the control. Only limited ADAM-9 activity was detected in the absence of the αvβ5 integrin (Figure 4A lane 3). However, addition of ADAM-9 to αvβ5-integrin–coated beads increased ADAM-9 activity, indicating that ADAM-9 may be associating directly with the αvβ5 integrin (Figure 4A lane 4). To determine whether ADAM-9 could form a complex with the αvβ5 integrin on hOBs, flow cytometric analyses were performed. hOBs were incubated with ADAM-9 in the presence or absence of anti–αvβ5-integrin antibody. Addition of ADAM-9 to hOBs demonstrated a 39% increase in fluorescence intensity compared with that of untreated cells (Figure 4B). Addition of anti–αvβ5-integrin monoclonal antibody completely inhibited the binding of the ADAM-9 to hOBs (Figure 4B). Furthermore, FIC analysis showed that ADAM-9 and αvβ5 integrin are colocalized in individual cells (Figure 4C). ADAM-9 was not detected on untreated osteoblasts but was detected when added directly to cells.
ADAM-9 mediates induction of IL-6 in hOBs via the disintegrin domain
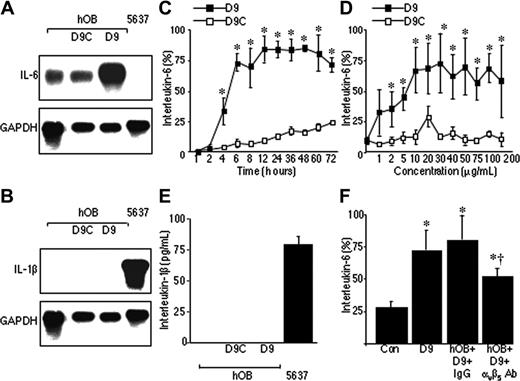
These data suggest that ADAM-9 mediates induction of IL-6 in osteoblasts by specifically interacting with the αvβ5 integrin; however, it was unclear whether this was mediated by the ADAM-9 disintegrin domain. Short-term treatment of osteoblasts (6 hours) with the disintegrin domain protein (D9) demonstrated a significant increase in IL-6 mRNA compared with unstimulated cells or cells incubated with a control protein (Figure 5A). Densitometric analysis demonstrated that this treatment resulted in an approximately 5-fold increase in the level of IL-6 mRNA. The effect of D9 on IL-6 was not mediated indirectly via interleukin-1β, since D9 did not affect IL-1β mRNA or protein expression by hOBs (Figure 5B,E). The D9 protein was shown to promote a significant increase in the release of IL-6 from hOBs (Figure 5C-D; P < .001). D9 stimulated a detectable increase in IL-6 release from hOBs after only 4 hours (P < .01) and this reached a maximum at 12 hours (P < .01; Figure 5C), which was maintained through to 72 hours. The D9 protein also caused a dose-dependent increase in production of IL-6 by these cells (Figure 5D; P < .001). Increased production of IL-6 by hOBs was observed at a concentration of 1 μg/mL of D9 (P < .01). Maximum induction of IL-6 was observed with 10 μg/mL of D9 protein (P < .01). Finally, we confirmed that D9 acts through the αvβ5 integrin, since D9-induced production of IL-6 by hOBs could be inhibited by an antibody against the αvβ5 integrin by 36% compared with isotype IgG control (Figure 5F; P < .01). Blocking antibodies against α2 integrin, α5 integrin, β1 integrin, β3 integrin, or RGD-containing peptides had no effect on D9-induced production of IL-6 by hOBs (data not shown).
ADAM-9 interacts with αvβ5 integrin directly. (A) Western blot analysis of ADAM-9 immunoprecipitated with αvβ5 integrin. Lane 1, protein marker; lane 2, recombinant ADAM-9 alone; lane 3, αvβ5-integrin antibody–conjugated beads, in the absence of recombinant αvβ5 integrin, showing only trace amounts of ADAM-9 reactivity; lane 4, ADAM-9 immunoprecipitated with the αvβ5-integrin antibody–conjugated beads, in the presence of αvβ5 integrin, showing strong staining for ADAM-9. (B) Flow cytometric analysis of hOBs incubated with or without control IgG or anti–αvβ5-integrin antibody prior to addition of recombinant ADAM-9. Isotype control antibody is shown in white histograms and ADAM-9 staining in gray histograms. The αvβ5-integrin antibody prevented ADAM-9 staining. (C) FIC of hOBs stained for ADAM-9 (green staining) and αvβ5 integrin (red staining). Nuclei were counterstained with DAPI. Colocalization of ADAM-9 and αvβ5 integrin is shown in the right panel as orange staining. Cells were examined with an objective magnification of × 100 (1.3 NA).
ADAM-9 interacts with αvβ5 integrin directly. (A) Western blot analysis of ADAM-9 immunoprecipitated with αvβ5 integrin. Lane 1, protein marker; lane 2, recombinant ADAM-9 alone; lane 3, αvβ5-integrin antibody–conjugated beads, in the absence of recombinant αvβ5 integrin, showing only trace amounts of ADAM-9 reactivity; lane 4, ADAM-9 immunoprecipitated with the αvβ5-integrin antibody–conjugated beads, in the presence of αvβ5 integrin, showing strong staining for ADAM-9. (B) Flow cytometric analysis of hOBs incubated with or without control IgG or anti–αvβ5-integrin antibody prior to addition of recombinant ADAM-9. Isotype control antibody is shown in white histograms and ADAM-9 staining in gray histograms. The αvβ5-integrin antibody prevented ADAM-9 staining. (C) FIC of hOBs stained for ADAM-9 (green staining) and αvβ5 integrin (red staining). Nuclei were counterstained with DAPI. Colocalization of ADAM-9 and αvβ5 integrin is shown in the right panel as orange staining. Cells were examined with an objective magnification of × 100 (1.3 NA).
p38 MAP kinase and/or phospholipase A2 signaling pathways are involved in ADAM-9/αvβ5-integrin–induced production of IL-6 by hOBs
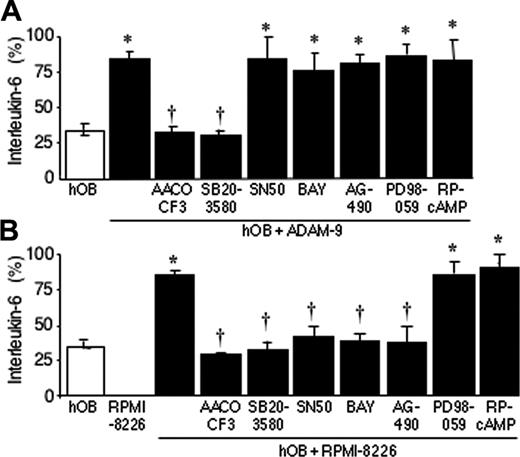
To identify downstream signaling pathways involved in ADAM-9/αvβ5-integrin– or RPMI-8226 cell–stimulated production of IL-6 by hOBs, we used inhibitors of specific signaling. Pretreatment of hOBs with the p38 MAPK inhibitor SB203580 or cPLA2 inhibitor AACOCF3 prior to addition of recombinant ADAM-9 reduced the level of IL-6 produced by hOBs to untreated control levels (Figure 6A; P < .01). However, the induction of IL-6 production by ADAM-9 was not inhibited by PD98059, an MEK1 inhibitor that inhibits phosphorylation of ERK 1/2; Figure 6A). Similarly, NF-κB inhibitors BAY 11-7082 ((E)-3-[(4-methylphenylsulfonyl]-2-propenenitrile)) and SN50, JAK2 inhibitor AG490, and cyclic AMP antagonist Rp-cAMP had no effect on ADAM-9–induced IL-6 production by hOBs (Figure 6A). This would be consistent with the p38 MAPK and/or cPLA2 pathways being involved in ADAM-9/αvβ5-integrin signaling. We also investigated whether the same signaling pathways were involved in RPMI-8226–induced production of IL-6 by hOBs. PD98059 and Rp-cAMP did not affect production of IL-6, whereas SB203580 and AACOCF3 completely blocked RPMI-8226–induced production of IL-6 by hOBs (Figure 6B), suggesting the same signaling pathways (p38 MAPK and cPLA2) might be involved in ADAM-9/αvβ5-integrin–and RPMI-8226–induced IL-6 production by hOBs. However, NF-κB inhibitors BAY 11-7082 and SN50 and JAK2 inhibitor AG490 also significantly reduced IL-6 production, suggesting that these 2 signaling pathways are also involved in RPMI-8226–induced IL-6 production by hOBs (Figure 6B).
The disintegrin domain of ADAM-9 mediates interactions with the αvβ5 integrin and induces interleukin-6 production (hOB). (A) Northern blot analysis of IL-6 mRNA expression in hOBs incubated alone or in the presence of recombinantADAM-9 disintegrin domain (D9) or control protein (D9C). The 5637 cell line served as a negative control. Blots were reprobed with GAPDH to demonstrate equal loading between D9 and control protein. (B) Northern blot analysis of IL-1β mRNA expression in hOBs incubated alone or in the presence of recombinant D9 or D9C control protein. The 5637 cell line served as a positive control. (C) hOBs were incubated in the presence of recombinant D9 (10 μg/mL) or D9C, and IL-6 concentrations were measured by ELISA at the stated time points. (D) hOBs were incubated in the presence of increasing concentrations of recombinant D9, or control protein D9C, for 24 hours, and IL-6 concentrations were measured by ELISA. Data are the means of samples from a representative experiment (n = 4-8), and error bars are 95% CI. IL-6 production is shown as percentage of the mean + 95% CI of the maximum bar in the graph. *P < .001 compared with untreated cells by the Dunnett test. (E) hOBs were incubated alone or in the presence of recombinant D9 (10 μg/mL) or D9C for 24 hours, and IL-1β concentrations were determined by ELISA. The 5637 cells were cultured in the same way and served as a control. (F) hOBs were incubated alone (con) or in the presence of recombinant D9 with or without IgG or αvβ5-integrin antibody. IL-6 concentrations were determined by ELISA. *P < .01 compared with control; and †P < .01 compared with IgG (Mann-Whitney U test).
The disintegrin domain of ADAM-9 mediates interactions with the αvβ5 integrin and induces interleukin-6 production (hOB). (A) Northern blot analysis of IL-6 mRNA expression in hOBs incubated alone or in the presence of recombinantADAM-9 disintegrin domain (D9) or control protein (D9C). The 5637 cell line served as a negative control. Blots were reprobed with GAPDH to demonstrate equal loading between D9 and control protein. (B) Northern blot analysis of IL-1β mRNA expression in hOBs incubated alone or in the presence of recombinant D9 or D9C control protein. The 5637 cell line served as a positive control. (C) hOBs were incubated in the presence of recombinant D9 (10 μg/mL) or D9C, and IL-6 concentrations were measured by ELISA at the stated time points. (D) hOBs were incubated in the presence of increasing concentrations of recombinant D9, or control protein D9C, for 24 hours, and IL-6 concentrations were measured by ELISA. Data are the means of samples from a representative experiment (n = 4-8), and error bars are 95% CI. IL-6 production is shown as percentage of the mean + 95% CI of the maximum bar in the graph. *P < .001 compared with untreated cells by the Dunnett test. (E) hOBs were incubated alone or in the presence of recombinant D9 (10 μg/mL) or D9C for 24 hours, and IL-1β concentrations were determined by ELISA. The 5637 cells were cultured in the same way and served as a control. (F) hOBs were incubated alone (con) or in the presence of recombinant D9 with or without IgG or αvβ5-integrin antibody. IL-6 concentrations were determined by ELISA. *P < .01 compared with control; and †P < .01 compared with IgG (Mann-Whitney U test).
p38 MAP kinase and phospholipase A2 pathways are involved in ADAM-9–induced production of IL-6 by hOBs. hOBs were incubated in the presence or absence of inhibitors of p38 MAPK (SB-203580, 10 μM), MEK (PD-98059, 10 μM), cPLA2 (AACOCF3, 20 μM), cAMP (RP-cAMP, 10 μM), NF-κB (BAY 11-7082, 10 μM; and SN50, 10 μg/mL), or JAK2 (AG490 5 μM) for 30 minutes before addition of ADAM-9 (200 nM; A) or RPMI-8226 cells (5 × 105; B). The cells were incubated for a further 24 hours, and IL-6 concentrations were determined by ELISA. Data are the means of samples from a representative experiment (n = 4-8), and error bars are 95% CI. IL-6 production is shown as percentage of the mean + 95% CI of the maximum bar in the graph. A Mann-Whitney U test was used for the pair-wise comparisons. *P < .01 samples compared with respective untreated hOBs (□); †P < .01 samples compared with ADAM-9–treated hOBs (A) or hOBs cultured with RPMI-8226 cells (B).
p38 MAP kinase and phospholipase A2 pathways are involved in ADAM-9–induced production of IL-6 by hOBs. hOBs were incubated in the presence or absence of inhibitors of p38 MAPK (SB-203580, 10 μM), MEK (PD-98059, 10 μM), cPLA2 (AACOCF3, 20 μM), cAMP (RP-cAMP, 10 μM), NF-κB (BAY 11-7082, 10 μM; and SN50, 10 μg/mL), or JAK2 (AG490 5 μM) for 30 minutes before addition of ADAM-9 (200 nM; A) or RPMI-8226 cells (5 × 105; B). The cells were incubated for a further 24 hours, and IL-6 concentrations were determined by ELISA. Data are the means of samples from a representative experiment (n = 4-8), and error bars are 95% CI. IL-6 production is shown as percentage of the mean + 95% CI of the maximum bar in the graph. A Mann-Whitney U test was used for the pair-wise comparisons. *P < .01 samples compared with respective untreated hOBs (□); †P < .01 samples compared with ADAM-9–treated hOBs (A) or hOBs cultured with RPMI-8226 cells (B).
Discussion
In the present study, we have demonstrated that ADAM-9 is expressed by both human myeloma cell lines and primary myeloma cells. Flow cytometric analysis of patient samples clearly indicates a significantly higher level of expression in malignant plasma cells than in other cell populations present in the bone marrow, suggesting that ADAM-9 may play a critical role in the biology of these cells. ADAM-9 was found on the cell membrane, making it ideally placed to play an important role in cell-to-cell and cell-to-matrix interaction.
We also demonstrated that recombinant soluble ADAM-9 could promote the production of IL-6 by primary hOBs. Treatment of hOBs with recombinant ADAM-9 resulted in a significant increase in IL-6 production by hOBs. Furthermore, incubation of hOBs with the disintegrin domain from ADAM-9 (D9) stimulated an increase in the IL-6 mRNA expression, which was associated with a concentration- and time-dependent increase in IL-6 protein production. The effect of the disintegrin domain of ADAM-9 on production of IL-6 appears to be specific to primary hOBs, as the D9 protein was unable to promote an increase in IL-6 release from human myeloma cells, SaOS-2 osteosarcoma cells, or HT-1080 fibrosarcoma cells (data not shown). Both SaOS-2 and HT-1080 cells produce IL-6 constitutively and can be induced to upregulated production with external stimuli (data not shown). This suggests that these cells may not express the molecule(s) responsible for mediating ADAM-9–induced IL-6 production. The ADAM-9–induced up-regulation of IL-6 was not a general activation signal, as ADAM-9 had no effect on IL-1β mRNA levels or protein production. This would also argue against the increase in IL-6 being mediated indirectly via another molecule such as IL-1β.
ADAM-9 clearly had the ability to promote IL-6 production by hOBs and this appeared to be mediated through binding the αvβ5 integrin. Soluble ADAM-9– or D9 protein–induced production of IL-6 by hOBs was inhibited by an antibody to the αvβ5 integrin but not by antibodies to the αvβ3 integrin, αvβ6 integrin, or other integrin subunits including α2, α5, β1, or β3. Furthermore, soluble ADAM-9 protein was immunoprecipitated by an αvβ5-integrin antibody, and binding of soluble ADAM-9 to cell-associated αvβ5 integrin was inhibited by the same antibody. In addition, FIC demonstrated colocalization of both proteins at the cellular level. Thus, these lines of evidence support the concept that ADAM-9 mediates its effects on osteoblasts by binding the αvβ5 integrin. This is consistent with our previous studies demonstrating that human myeloma cells, but not lymphoblastoid cells, bind to immobilized D9 protein via αv β5 integrin9 . ADAM-9 has also been shown to interact with the α6β1 integrin on fibroblasts, suggesting it may have more than 1 integrin partner.25 Although in the present study we did not examine the effect of antibodies to α6 integrin on ADAM-9–induced IL-6 production by hOBs, an antibody to the β1 integrin had no effect, suggesting that in osteoblasts α6β1 integrin is not mediating the induction of IL-6 production. Thus, these studies support the suggestion that ADAM-9 and perhaps other ADAM family members are able to interact with more than one integrin heterodimer and that this may be cell type specific. Interestingly, previous studies have shown that αv-containing integrins bind to a number of extracellular matrix molecules, including vitronectin and osteopontin,31,32 and that binding can be inhibited with RGD-containing peptides. However, in the present study RGD-containing peptides were unable to inhibit ADAM-9–induced IL-6 production by hOBs. These data suggest that the binding site within the αvβ5 integrin that mediates this interaction maybe distinct from the RGD-binding site. This is consistent with recent reports that have demonstrated that other members of the ADAM family, including ADAM-12, which binds the α9β1 integrin, and ADAM-23, which binds the αvβ3 integrin, do so in an RGD-independent manner.23,24
A number of studies have demonstrated that myeloma cells promote the release of IL-6 from bone marrow stromal cells and osteoblasts in a contact-dependent manner.33,34 Barille et al30 demonstrated that the induction of IL-6 was partly mediated by CD44 and CD56, suggesting that other factors may also play a role. In the present study we have shown that myeloma cells express ADAM-9 and that ADAM-9 by interacting with the αvβ5 integrin on hOBs can promote IL-6 production. We also demonstrated that both an anti–ADAM-9 antibody and an antibody to αvβ5 integrin were able to partially prevent the induction of IL-6 in hOBs induced by contact with myeloma cells. This suggests that these novel interactions may play an important role in regulating IL-6 production in myeloma.
In addition to demonstrating that ADAM-9/αvβ5-integrin interactions are important in regulating IL-6 production in osteoblasts, this study also provides evidence to suggest that the p38 MAPK and cPLA2 pathways may mediate downstream signaling. The p38 MAPK inhibitor SB203580 but not a MEK inhibitor, PD95058, inhibited ADAM-9–induced IL-6 secretion in hOBs. The involvement of p38 MAPK on IL-6 secretion has been observed with other agonists of IL-6 in osteoblasts and other cell types, and SB203580 has also been shown to inhibit IL-1β–induced IL-6 production in osteoblast-like cells.35 Moreover, TNF-α–induced IL-6 production in MG-63 osteosarcoma cells and in bone marrow stromal cells also occurs via the p38 MAPK pathway.36,37 However, studies have shown that, depending upon the stimulant and cell type, MEK can be involved in the regulation of IL-6 expression, although this was not the case in this study.38-40 Interestingly, we also found that AACOCF3, a specific inhibitor of cPLA2, inhibited IL-6 production induced by ADAM-9, which is consistent with a role for the cPLA2 pathway in mediating the ADAM-9 response. This is supported by data from Wang et al41 who reported that blocking cPLA2 with antisense oligonucleotides lead to a reduction in IL-6 production by lipopolysaccharide (LPS)–induced HeLa cells. Furthermore, p38 MAPK has been reported to phosphorylate cPLA2 and may promote the generation of PGE2, which may contribute to IL-6 production.42,43 Thus, it is likely that ADAM-9 induces IL-6 production by activation of p38 MAPK to phosphorylate cPLA2, which contributes to the release of arachidonic acid for the generation of PGE2 (Figure 7). Studies have shown that PKA/cAMP might be downstream of cPLA2/PGE2. Rp-cAMP, an antagonist of cyclic AMP, had no effect on ADAM-9–induced IL-6 production by hOBs, suggesting that PKA pathway is not involve in ADAM-9–induced IL-6 production by hOBs. In addition to the p38 MAPK and cPLA2 pathways, which are involved in ADAM-9–induced production of IL-6 by hOBs, JAK2 and NF-κB pathways are also involved in myeloma cell–induced IL-6 production by hOBs. This is consistent with other studies that showed that JAK2 and NF-κB pathways participate in IL-6 production by other stimulants and cell types.40,42
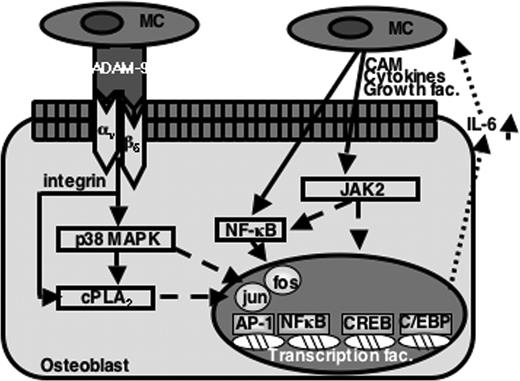
In conclusion, this study has demonstrated that human myeloma cells express ADAM-9 protein and this is able to interact directly with the αvβ5 integrin on osteoblasts to regulate IL-6 production via the p38 MAPK and cPLA2 pathways. Taken together, these studies have identified a novel role for ADAM-9 in cell-to-cell communication, particularly in regulating IL-6 production in osteoblasts. This may play a critical role in the development of multiple myeloma. These data raise the possibility of targeting ADAM-9 and/or the αvβ5 integrin to prevent these interactions and make the bone marrow microenvironment a less a favorable environment for the growth and survival of myeloma cells.
A model for the mechanism of ADAM-9–mediated IL-6 production by osteoblasts in multiple myeloma. Myeloma cells (MC) express ADAM-9, which interacts directly with αvβ5 integrin on osteoblasts to induce IL-6 production via the p38 MAPK and cPLA2 pathways. Myeloma cells also regulate IL-6 production in osteoblasts via alternative mediators through JAK2 and NF-κB pathways. CAM indicates cell adhesion molecules.
A model for the mechanism of ADAM-9–mediated IL-6 production by osteoblasts in multiple myeloma. Myeloma cells (MC) express ADAM-9, which interacts directly with αvβ5 integrin on osteoblasts to induce IL-6 production via the p38 MAPK and cPLA2 pathways. Myeloma cells also regulate IL-6 production in osteoblasts via alternative mediators through JAK2 and NF-κB pathways. CAM indicates cell adhesion molecules.
Prepublished online as Blood First Edition Paper, December 22, 2005; DOI 10.1182/blood-2005-09-3830.
Supported by the Leukaemia Research Fund and the Intramural Research Program of the National Institute of Dental and Craniofacial Research, National Institutes of Health.
A.K. and M.Z. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. Human myeloma cells express ADAM-9. (A) Western blot analysis of ADAM-9 expression in human myeloma cells demonstrated a single product. (B) Flow cytometric analysis of ADAM-9 expression (red histograms) in RPMI-8226 cells demonstrated expression in both permeabilized (Perm) and nonpermeabilized (Nonperm) cells when compared with an isotype control (blue histograms). (C) Two-color fluorescence immunocytochemical analysis of bone marrow mononuclear cells demonstrated expression of the restricted light chain (red) staining in a proportion of mononuclear cells. Light chain–positive cells were shown to stain positively for ADAM-9 (green). Nuclei were counterstained with DAPI and examined at an objective magnification of × 40 (0.7 numeric aperture [NA]). (D) Flow cytometric analysis of bone marrow mononuclear cells showing cIgLC- and cIgLC+ staining. (E) Flow cytometric analysis demonstrated ADAM-9 expression in both cIgCL- (green histogram) and cIgLC+ (red histogram) cells. (F) Analysis of mean fluorescence values (±95% CI) for cIgCL- and cIgCL+ cells isolated from patients with multiple myeloma demonstrated significantly greater expression in cIgLC+ cells. *P < .01 by Wilcoxon matched pairs test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/8/10.1182_blood-2005-09-3830/4/m_zh80080694180001.jpeg?Expires=1769169660&Signature=PGxbiK8uAgwPef1BOcvdze9BX24b0BCjymK2IAicgcJGWLgjZF7M-D09EjJPT0Vbfg5D4IRc-03isdZG2meEFtAyiBt12I5M9U-WZ~foV43eOdAawj~ck8wFX6ymYf~zRfhiEa5vE2JMTUJq63X6vBNLqFtzeR607guSSg8v59VS9WndMtS7fC9iI2lmiSkWEIKU7R0bBu4ogPXKnNWkpbRiJYtQbXFmcwJpt8fWOSx-pqa1h23xkd-5mPhaVh8RGoj820Es-aN5IH5uh6XStd07sw3kUO8b1b9yFeOoQk25OePJAqrOX-kTTpAeHbUCXpyNDQNG4ws642cmohjVZg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal