Abstract
Bone marrow-derived antigen-presenting cells (APCs) play a central role in the induction of tolerance to tumor antigens expressed by B-cell lymphomas. Here we show that in vivo disruption of this APC-mediated tolerogenic mechanism unveils an intrinsic ability of malignant B cells to efficiently present tumor antigens to antigen-specific CD4+ T cells, resulting in a strong antitumor effect. This intrinsic antigen-presenting ability of malignant B cells is, however, overridden by tolerogenic bone marrow-derived APCs, leading instead to T-cell unresponsiveness and lack of antitumor effect. These results highlight the concept that therapeutic strategies aimed at enhancing the antigen-presenting function of B-cell lymphomas might not succeed unless the tolerogenic mechanisms mediated by bone marrow-derived APCs are disrupted in the first place.
Introduction
B-cell malignancies, including indolent and aggressive non-Hodgkin lymphomas, are the transformed counterparts of cells that are capable of activating antigen-specific T cells. Similar to normal B cells, malignant B cells express major histocompatibility complex (MHC) class I and II molecules and low but inducible levels of adhesion and costimulatory molecules.1-3 Indeed, much of what we have learned about the basic cell biology of MHC class I and II antigen processing and presentation, normal antigen-presenting cell (APC) homing, adhesion, and T-cell costimulation has been gleaned from analysis of B-cell lines.4-7 B-cell malignancies seem, therefore, well equipped to provide the necessary signals for activation of tumor-antigen-specific T cells.
A number of studies have demonstrated that malignant B cells can process and present antigen to T cells in vitro, including the presentation of epitopes derived from their own unique immunoglobulin idiotype to CD4+ and CD8+ T cells.4,8,9 Furthermore, cross-linking of CD40 on lymphoma cells induces the upregulation of adhesion and costimulatory molecules, resulting in a markedly enhanced T-cell response to B-cell tumors in vitro.10 It is paradoxical that in spite of their intrinsic antigen-presenting capabilities, B-cell tumors fail to be eliminated in the very same compartment—lymph nodes—in which tumor-antigen-specific T-cell responses are initiated.
Reminiscent of the previously published in vitro studies, we have found that a murine B-cell lymphoma cell line, A20, transfected to express the model antigen influenza hemagglutinin (HA), efficiently activates HA-specific CD4+ T cells from T-cell receptor (TCR) transgenic mice in vitro.11 However, when these same transgenic T cells are adoptively transferred to mice bearing A20HA lymphoma, the observed outcome is the induction of antigen-specific CD4+ T-cell anergy rather than T-cell activation.12 One potential explanation for these findings is that perhaps B-cell tumors are not efficient APCs in vivo and their direct encounter with T cells is responsible for the induction of T-cell anergy. More recent studies using parent-into-F1 bone marrow (BM) chimeras ruled out this possibility, pointing instead to cross-presentation by host APCs, not lymphoma cells themselves, as the dominant mechanism responsible for the induction of tumor-antigen-specific CD4+ T-cell tolerance.3 This involvement of a “third party” in vivo, represented by host APCs, provided an explanation for the divergent outcomes in T-cell responses to malignant B cells in the in vitro (T-cell activation) and in vivo (T-cell tolerance) settings.
Although our previous studies have indicated that the intrinsic APC capabilities of B-cell lymphomas have no influence on the induction of T-cell tolerance in vivo,3 questions that remain are whether malignant B cells are capable of directly presenting tumor antigens in vivo and whether this putative T-cell/B-cell interaction could lead to T-cell activation rather than to tolerance. To answer these questions, we evaluated lymphoma growth and antigen-specific T-cell function in experimental models in which cross-presentation by BM-derived APCs is not operative and malignant B cells are the only cells capable of presenting tumor antigen to CD4+ T cells. The results of this analysis demonstrate that direct presentation of tumor antigens by B-cell lymphoma in vivo leads to T-cell activation and to the development of effective antilymphoma immunity.
Materials and methods
Mice
Male BALB/c severe combined immunodeficiency (SCID) or C57BL/6 SCID mice, aged 6 to 8 weeks, were purchased from Jackson Laboratories (Bar Harbor, ME). BALB/c, C57BL/6, and BALB/c × C57BL/6 F1 mice were obtained from the National Institutes of Health (Frederick, MD). TCR transgenic mice expressing an αβ T-cell receptor specific for amino acids 110-120 from influenza hemagglutinin presented by I-Ed were a generous gift from H. von Boehmer.13 Transgenic mice used in these experiments were heterozygous for the transgene. All experiments involving the use of mice were performed in accordance with protocols approved by the Animal Care and Use Committees of the University of South Florida College of Medicine and the Johns Hopkins University School of Medicine.
Construction of bone marrow chimeric mice
Femurs and tibiae were obtained from BALB/c SCID mice and C57BL/6 SCID mice, and bone marrow was harvested by flushing the bones with RPMI at 4°C. Single-cell suspensions were obtained by passing bone marrow cells through a cell strainer (BD Biosciences, Franklin Lakes, NJ). Cells were washed 3 times in sterile Hanks balanced salt solution (HBSS), and 4 × 106 BM cells from either BALB/c SCID mice (H-2d) or C57BL/6 SCID mice (H-2b) were injected into the tail veins of irradiated (10 Gy [1000 rad]) BALB/cxC57BL/6 F1 (H-2dxb) recipients. A 3-month period was allowed for immune reconstitution before adoptive transfer of T cells. To assess for donor chimerism, one mouse from each group was killed, and the splenocytes were stained for I-Ed and I-Ab using the mAb 14.4.4 and the mAb Y3P, respectively. This was followed by FITC goat-anti-mouse IgG2a secondary antibody (BD Biosciences) and analysis on a FACScalibur (BD Biosciences) using FlowJo software (Tree Star, Ashland, OR).
Tumor cells
A20 cells were obtained from ATCC (Rockville, MD). A20HA was generated by electroporation-mediated plasmid transfection, and transfected cells were selected and grown as previously reported.11 For tumor challenge, cells were washed 3 times in sterile HBSS and injected into the tail veins of either immune-reconstituted mice or SCID mice in a total volume of 0.2 mL, 1 × 106 tumor cells per mouse.
Adoptive transfer of antigen-specific T cells
For the adoptive transfer of anti-HA-specific CD4+ T cells, we mated BALB/c TCR transgenic mice to C57BL/6 mice to generate H-2dxb F1 TCR transgenic offspring. It was necessary to use F1 TCR transgenic donors to ensure that any nontransgenic T cells transferred to the chimeras or SCID mice were not alloreactive to the recipient, thus triggering graft-versus-host disease (GVHD). The transgenic donor population was obtained from the thymi of H-2dxb F1 TCR transgenic animals to avoid any contaminating MHC class II-bearing APCs. CD8+ T cells and double-negative thymocytes were depleted using the antibodies 3.155 and J.11.d.2, respectively. The percentage of T cells doubly positive for CD4 and the clonotypic TCR was determined as described in “Flow cytometric analysis.” Cells were washed 3 times in sterile HBSS, and 1 × 106 naive CD4+ anti-HA TCR+ T cells were injected into the tail veins of immune reconstituted chimeric mice or SCID mice. For the generation of activated anti-HA-specific CD4+ T cells, 5 × 107 transgenic CD4+ T cells obtained from the thymi of H-2dxb F1 TCR mice were cultured in vitro for 72 hours with 1 × 108 lethally irradiated H-2dxb F1 splenocytes in the presence of 12.5 μg HA peptide (amino acids 110-120; SFERFEIFPKE). Cells were then washed and counted, and 1 × 106 activated CD4+ anti-HA TCR+ T cells were injected into the tail veins of the experimental mice. Tumor challenge was delayed for 4 weeks after the transfer of activated T cells to allow for the establishment of memory anti-HA CD4+ T cells.
Reisolation of clonotypic T cells after in vivo transfer
Clonotypic CD4+ T cells injected into tumor-free mice or mice challenged with A20HA tumors were reisolated from the spleens and lymph nodes 3 weeks after tumor challenge. In all experiments, unless otherwise noted, 3 mice per group were analyzed individually. On the day of analysis, T cells were purified by passage through nylon mesh and centrifugation on a Ficoll gradient (Amersham Pharmacia, Picataway, NJ), followed by passage through nylon wool.
Flow cytometric analysis
T cells were stained with FITC-conjugated goat anti-mouse CD4 (Caltag, Burlingame, CA) and biotinylated rat anti-clonotypic TCR antibody mAb 6.5, followed by PE-conjugated streptavidin (Caltag). Fifty thousand gated events were collected on a FACScalibur (BD Biosciences) and were analyzed using FlowJo software (Tree Star). Values represent the mean ± SE of the percentage of cells expressing the clonotypic TCR. Background staining of splenocytes from naive F1 mice is usually less than 0.1%.
Cytokine release
Purified T cells (4 × 104/well) from the different experimental groups were cultured with fresh F1 splenocytes (8 × 104/well) to which 12.5 μg/mL HA peptide was or was not added. Forty-eight hours later, supernatants were collected and stored at -70°C until assayed for IL-2 and IFN-γ by ELISA (R&D Systems, Minneapolis, MN). Values represented the mean ± SE of triplicate cultures from 3 mice in each group.
BrdU incorporation
BrdU (BD Biosciences) was diluted to 0.8 mg/mL in drinking water and was given to the experimental animals starting 2 weeks after tumor challenge until the end of the experiment. Every other day, the drinking water was changed with freshly made BrdU solution. BrdU incorporation on clonotypic T cells was determined by staining purified T cells with PE-conjugated anti-CD4 (BD Biosciences) and biotinylated rat anti-clonotypic TCR antibody mAb 6.5, followed by APC-conjugated streptavidin (BD Biosciences). Cells were then fixed, permeabilized, and stained for BrdU with the BrdU Flow Kit (BD Biosciences), according to the manufacturer's instructions. Fifty thousand gated events were collected on a FACScalibur (BD Biosciences) and were analyzed using FlowJo software (Tree Star) by first gating on the clonotypic T-cell population and then assessing the intensity of staining for BrdU.
Immunohistochemical studies
Tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin. Deparaffinized tissue sections were immersed in 1% H2O2 for 10 minutes to block endogenous peroxidase activity and were subjected to epitope retrieval treatment by heating of the slides in a commercial steamer with 0.1 M sodium citrate. Sections were stained with goat anti-mouse CD3-ϵ (Santa Cruz Biotechnology, Santa Cruz, CA) or rat anti-mouse B220 (Caltag) with the use of Vectastain ABC Kits (Vector Laboratories, Burlingame, CA). Double immunohistochemistry was performed first for B220 and then for CD3-ϵ; the epitope retrieval step was repeated after the first staining to quench any biotin or peroxidase activity. Stained sections were mounted in Vectamount (Vector Laboratories) and analyzed under a Leica DMLB 100S microscope (Leica Microsystems, Wetzlar, Germany) using either a 20 ×/0.50 HC PL Fluotar or a 40 ×/0.75 HCX PL Fluotar objective lens (Leica Microsystems). Photographs were taken with an RT Color Spot camera (Diagnostic Instruments, Sterling Heights, MI) and analyzed with Image-Pro Plus 5.0 software (Media Cybernetics, Silver Spring, MD).
In vivo T-cell depletion
Animals were depleted of CD4+ and CD8+ T cells by intraperitoneal injection of anti-CD4 (GK1.5 hybridoma) and anti-CD8 (3.155 hybridoma) ascitic fluid. Antibodies (200 μL ascitic fluid/dose) were injected on days -7, -4, and -1 before tumor challenge, followed by weekly injections until the end of the experiment.
Functional assessment of dendritic cells
CD11c+ DCs were purified from collagenase-digested spleens using CD11c microbeads and LS columns (both from Miltenyi Biotech, Auburn, CA) according to the manufacturer's instructions. DCs (2 × 105) were then plated with HA peptide (12.5 μg/mL) and purified T cells (1 × 106) from the spleens of TCR-transgenic animals. Four hours later, GolgiStop (BD Biosciences) was added to the wells. Ten hours after plating, cells were stained with PerCP-conjugated anti-mouse CD4 (BD Biosciences) and biotinylated rat anti-clonotypic TCR antibody mAb 6.5, followed by PE-conjugated streptavidin (Caltag). After fixation and permeabilization with Cytofix/Cytoperm Plus Kit (BD Biosciences), the cells were stained with FITC-conjugated anti-mouse IL-2 (BD Biosciences) and APC-conjugated anti-mouse IFN-γ (BD Biosciences). Flow cytometric analysis was performed as described.
Statistical analysis
Two-way analysis of variance (ANOVA) was used to evaluate the magnitudes of tumor-induced effects on cytokine production by clonotypic T cells. Differences in survival were assessed with the log-rank test.
Results
Rejection of B-cell lymphoma by antigen-specific CD4+ T cells in the absence of tolerogenic mechanisms mediated by APCs
In previous studies, the use of parent SCID-into-F1 bone marrow chimeras allowed us to unambiguously demonstrate the dominant role of APCs in the induction of tumor-antigen-specific T-cell tolerance during the progression of a B-cell lymphoma expressing a model tumor antigen (A20HA). In this system, naive anti-HA CD4+ transgenic T cells adoptively transferred into H-2dSCID→H-2dxb tumor-bearing chimeras, in which host APCs expressed the restricted element I-Ed required for the presentation of the HA antigen, were rendered fully unresponsive. In sharp contrast, in H-2bSCID→H-2dxb tumor-bearing chimeras, in which only malignant B cells displayed the restricted element required for antigen presentation, CD4+ HA-specific T cells remained functional. However, no significant differences in tumor growth rates were observed in either set of tumor-bearing chimeras.3 This failure of naive antigen-specific CD4+ T cells to reject A20HA lymphoma led us to explore whether memory antigen-specific CD4+ T cells would be better in controlling lymphoma growth. Therefore, we adoptively transferred 1 × 106 in vitro activated anti-HA transgenic T cells into H-2bSCID→H-2dxb chimeras or H-2dSCID→H-2dxb chimeras. Before adoptive transfer, the activated phenotype of these transgenic T cells was confirmed by their increased expression of CD69 compared with nonstimulated anti-HA CD4+ T cells (data not shown). Four weeks after T-cell transfer, all the animals were challenged with A20HA B-cell lymphoma. The rationale to wait 4 weeks was to allow the in vitro-activated anti-HA CD4+ T cells to become memory T cells in vivo.
In the first set of tumor-bearing chimeras (H-2dSCID→H-2dxb), cross-presentation of the model tumor antigen (HA) by BM-derived APCs and direct presentation by B-cell lymphoma cells were operative. In the second set of chimeras (H-2bSCID→H-2dxb), cross-presentation was disrupted, and only malignant B cells were capable of presenting tumor antigen to memory anti-HA CD4+ T cells. As seen in Figure 1, the adoptive transfer of in vitro activated anti-HA transgenic T cells into H-2dSCID→H-2dxb chimeric mice did not result in any tumor protection (Figure 1, open triangles). In sharp contrast, when only malignant B cells could present the tumor antigen (H-2bSCID→H-2dxb chimeras), the adoptive transfer of activated anti-HA CD4+ T cells resulted in lymphoma rejection in 60% of the animals (Figure 1, black squares). No such difference in tumor growth was observed in the absence of adoptively transferred antigen-specific CD4+ T cells (data not shown).
Rejection of A20HA B-cell lymphoma in the absence of cross-presentation mediated by BM-derived APCs. H-2dxb F1 mice were lethally irradiated (10 Gy [1000 rad]), and each received a graft consisting of 4 × 106 bone marrow cells from either BALB/c SCID (H-2d) or C57BL/6 SCID (H-2b) donors. Three months after bone marrow reconstitution, either group of chimeras intravenously received 1 × 106 in vitro activated CD4+ TCR-transgenic T cells (H2dxb) specific for HA/IEd. Four weeks later, all the mice were challenged intravenously with 1 × 106 A20HA cells, and they were inspected twice weekly for the development of tumors. Six mice were included in each group (P < .01). Data are representative of 3 experiments with similar results.
Rejection of A20HA B-cell lymphoma in the absence of cross-presentation mediated by BM-derived APCs. H-2dxb F1 mice were lethally irradiated (10 Gy [1000 rad]), and each received a graft consisting of 4 × 106 bone marrow cells from either BALB/c SCID (H-2d) or C57BL/6 SCID (H-2b) donors. Three months after bone marrow reconstitution, either group of chimeras intravenously received 1 × 106 in vitro activated CD4+ TCR-transgenic T cells (H2dxb) specific for HA/IEd. Four weeks later, all the mice were challenged intravenously with 1 × 106 A20HA cells, and they were inspected twice weekly for the development of tumors. Six mice were included in each group (P < .01). Data are representative of 3 experiments with similar results.
Phenotypic and functional characteristics of antigen-specific CD4+ T cells in the presence or absence of cross-presentation of tumor antigens
To better understand the survival advantage observed in Figure 1, we determined next the fate and functional characteristics of naive and in vitro activated anti-HA transgenic CD4+ T cells transferred to H-2dSCID→H-2dxb or H-2bSCID→H-2dxb chimeras. We followed an experimental design similar to that in Figure 1. The only difference was that tumor-bearing chimeras and tumor-free controls were killed 3 weeks after challenge with A20HA. In addition, we assessed in vivo T-cell proliferation by feeding a cohort of mice BrdU from day 14 after tumor challenge until the end of the experiment (day 21).
Previously, we have shown that adoptive transfer of clonotype-positive T cells into A20HA-bearing mice resulted in initial clonal expansion, followed by contraction, of this T-cell population. Although the peak of this expansion occurred 6 days after T-cell transfer, by day 20 there was still a slightly higher number of clonotype-positive T cells in A20HA-bearing mice than in tumor-free animals. However, as early as 1 week after adoptive transfer, antigen-specific CD4+ T cells displayed impaired proliferative response (as determined by 3H-thymidine incorporation) and cytokine production in response to cognate antigen in vitro.12 Reminiscent of these findings in H-2d recipients, we found that by day 21 after T-cell transfer, the percentage of antigen-specific T cells was also above baseline in H-2dSCID→H-2dxb tumor-bearing chimeras compared with tumor-free animals (Figure 2A, left). As determined by in vivo BrdU incorporation, only a small percentage of clonotype-positive T cells were still dividing in H-2dSCID→H-2dxb tumor-bearing chimeras (14.3% vs 10.3% in tumor-free chimeras) (Figure 2B, left). Furthermore, T cells from this group displayed significant impairment in IL-2 production in response to in vitro restimulation with cognate antigen compared with clonotype-positive T cells from tumor-free chimeras (Figure 2C, left). A divergent functional outcome was observed in antigen-specific CD4+ T cells reisolated from H-2bSCID→H-2dxb chimeras. First, in the absence of cross-presentation by APCs, a slight clonal expansion of antigen-specific CD4+ T cells was observed in tumor-bearing chimeras compared with tumor-free mice (Figure 2A, right). This finding suggested that T cells from tumor-bearing chimeras might have encountered antigen in vivo, most likely by interacting with malignant B cells. This putative B-cell/T-cell interaction is further supported by the strong in vivo proliferation of clonotype-positive T cells in tumor-bearing mice compared with tumor-free H-2bSCID→H-2dxb chimeras (63.4% vs 17.3% BrdU incorporation, respectively) (Figure 2B, right). Finally, anti-HA T cells from tumor-free or tumor-bearing H2bSCID→H-2dxb chimeras produced similar levels of IL-2 (Figure 2C, right), in sharp contrast to the impaired IL-2 production displayed by T cells from tumor-bearing H-2dSCID→H-2dxb chimeras (Figure 2C, left).
Phenotypic and functional changes associated with antigen recognition by naive CD4+ T cells in the presence or absence of cross-presentation of tumor antigens. Three months after bone marrow reconstitution, H-2d SCID→ H-2dxb (left panel) or H-2b SCID→ H-2dxb (right panel) chimeras received 1 × 106 naive anti-HA CD4+ TCR transgenic T cells intravenously. Four weeks later, half the animals in each set of chimeras were challenged with 1 × 106 A20HA tumor cells given intravenously. In a cohort of tumor-bearing and tumor-free chimeras, BrdU was added to the drinking water on day 14 after tumor injection. Three weeks after tumor challenge, all the animals were killed, and T cells were purified from their spleens, as described in “Materials and methods.” (A) Purified splenic T cells were analyzed by 2-color flow cytometry staining for CD4 compared with anti-HA TCR clonotype (mAb 6.5). Three mice were included per group. Values represent mean ± SE of percentage of T cells expressing the clonotypic TCR for 3 mice/group. Shown is a representative experiment of 3 independent experiments with similar results. (B) Representative FACS profile of BrdU labeling on anti-HA CD4+ T cells isolated from H-2d SCID→ H-2dxb or H-2b SCID→ H-2dxb tumor-bearing chimeras. Purified splenic T cells were stained with PE-conjugated anti-CD4 and biotinylated rat anti-clonotypic TCR mAb 6.5 followed by APC-conjugated streptavidin. Cells were then fixed, permeabilized, and stained for BrdU using the BrdU Flow Kit, and 50 000 gated events were collected on FACScalibur and were analyzed using FlowJo software. (C) Purified T cells (4 × 104/well) from the mice in panel A were mixed with fresh splenocytes (8 × 104/well) from F1 mice, to which 12.5 μg HA peptide was added. Forty-eight hours later, supernatants were collected and assayed for IL-2 by ELISA. Data represent mean ± SE of IL-2 production (pg/mL) per well from 3 mice in each group. *Statistically significant difference between tumor-bearing and tumor-free mice (P < .05). Shown is a representative experiment of 3 independent experiments with similar results.
Phenotypic and functional changes associated with antigen recognition by naive CD4+ T cells in the presence or absence of cross-presentation of tumor antigens. Three months after bone marrow reconstitution, H-2d SCID→ H-2dxb (left panel) or H-2b SCID→ H-2dxb (right panel) chimeras received 1 × 106 naive anti-HA CD4+ TCR transgenic T cells intravenously. Four weeks later, half the animals in each set of chimeras were challenged with 1 × 106 A20HA tumor cells given intravenously. In a cohort of tumor-bearing and tumor-free chimeras, BrdU was added to the drinking water on day 14 after tumor injection. Three weeks after tumor challenge, all the animals were killed, and T cells were purified from their spleens, as described in “Materials and methods.” (A) Purified splenic T cells were analyzed by 2-color flow cytometry staining for CD4 compared with anti-HA TCR clonotype (mAb 6.5). Three mice were included per group. Values represent mean ± SE of percentage of T cells expressing the clonotypic TCR for 3 mice/group. Shown is a representative experiment of 3 independent experiments with similar results. (B) Representative FACS profile of BrdU labeling on anti-HA CD4+ T cells isolated from H-2d SCID→ H-2dxb or H-2b SCID→ H-2dxb tumor-bearing chimeras. Purified splenic T cells were stained with PE-conjugated anti-CD4 and biotinylated rat anti-clonotypic TCR mAb 6.5 followed by APC-conjugated streptavidin. Cells were then fixed, permeabilized, and stained for BrdU using the BrdU Flow Kit, and 50 000 gated events were collected on FACScalibur and were analyzed using FlowJo software. (C) Purified T cells (4 × 104/well) from the mice in panel A were mixed with fresh splenocytes (8 × 104/well) from F1 mice, to which 12.5 μg HA peptide was added. Forty-eight hours later, supernatants were collected and assayed for IL-2 by ELISA. Data represent mean ± SE of IL-2 production (pg/mL) per well from 3 mice in each group. *Statistically significant difference between tumor-bearing and tumor-free mice (P < .05). Shown is a representative experiment of 3 independent experiments with similar results.
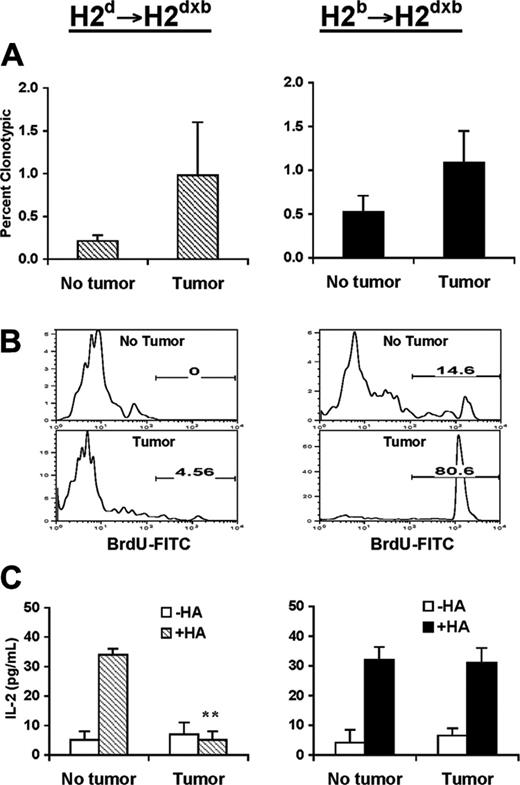
The dominant effect of BM-derived APCs in the induction of T-cell tolerance was not limited to naive antigen-specific CD4+ T cells (Figure 2, left panel); a similar functional outcome (T-cell unresponsiveness) was observed in memory antigen-specific T cells reisolated from H-2dSCID→H-2dxb tumor-bearing chimeras (Figure 3, left panel). In spite of the increased percentage of antigen-specific CD4+ T cells in the spleens of these animals (Figure 3A, left), by 3 weeks after tumor challenge few (4.56%) antigen-specific T cells were still proliferating in vivo (Figure 3B, left). Furthermore, T cells displayed blunted IL-2 production in response to in vitro restimulation with cognate antigen (Figure 3C, left), indicative that memory T cells were also rendered tolerant in H-2dSCID→H-2dxb tumor-bearing chimeras. In contrast, in the absence of cross-presentation, memory CD4+ T cells displayed strong antigen-specific responses as determined by their clonal expansion (Figure 3A, right), sustained in vivo proliferation (80.6% vs 14.6 in tumor-free chimeras) (Figure 3B, right), and preserved the ability to produce IL-2 in response to cognate antigen (Figure 3C, right).
Naive and memory antigen-specific CD4+ T cells were rendered fully unresponsive when cross-presentation mechanisms mediated by BM-derived APCs were operative in vivo. In the absence of such a mechanism, naive and memory antigen-specific T cells seemed to encounter tumor antigen directly on malignant B cells, resulting in antigen-specific T-cell proliferation and in preservation, rather than impairment, of their ability to produce IL-2 in response to cognate antigen.
Phenotypic and functional changes associated with antigen recognition by memory CD4+ T cells in the presence or absence of cross-presentation of tumor antigens. Three months after bone marrow reconstitution, H-2d SCID→ H-2dxb (left panel) or H-2b SCID→ H-2dxb (right panel) chimeras received 1 × 106 in vitro activated anti-HA CD4+ TCR transgenic T cells intravenously. One month later, half the animals in each group were challenged with 1 × 106 A20HA tumor cells given intravenously. Three weeks after tumor challenge, all the animals were killed, and T cells were purified from their spleens, as described in “Materials and methods.” (A) Purified splenic T cells were analyzed by 2-color flow cytometry staining for CD4 compared with anti-HA TCR clonotype (mAb 6.5). Values represent mean ± SE of percentage of T cells expressing the clonotypic TCR for 3 mice/group. Shown is a representative experiment of 3 independent experiments with similar results. (B) Representative FACS profile of BrdU labeling on anti-HA CD4+ T cells isolated from H-2d SCID→ H-2dxb or H-2b SCID→ H-2dxb tumor-bearing chimeras. (C) Purified T cells (4 × 104/well) from the mice in panel A were mixed with fresh splenocytes (8 × 104/well) from F1 mice to which 12.5 μg HA peptide was or was not added. Forty-eight hours later, supernatants were collected and assayed for IL-2 by ELISA. Data represent mean ± SE of IL-2 production (pg/mL) per well from 3 mice in each group. *Statistically significant difference between tumor-bearing and tumor-free mice (P < .01). Shown is a representative experiment of 3 independent experiments with similar results.
Phenotypic and functional changes associated with antigen recognition by memory CD4+ T cells in the presence or absence of cross-presentation of tumor antigens. Three months after bone marrow reconstitution, H-2d SCID→ H-2dxb (left panel) or H-2b SCID→ H-2dxb (right panel) chimeras received 1 × 106 in vitro activated anti-HA CD4+ TCR transgenic T cells intravenously. One month later, half the animals in each group were challenged with 1 × 106 A20HA tumor cells given intravenously. Three weeks after tumor challenge, all the animals were killed, and T cells were purified from their spleens, as described in “Materials and methods.” (A) Purified splenic T cells were analyzed by 2-color flow cytometry staining for CD4 compared with anti-HA TCR clonotype (mAb 6.5). Values represent mean ± SE of percentage of T cells expressing the clonotypic TCR for 3 mice/group. Shown is a representative experiment of 3 independent experiments with similar results. (B) Representative FACS profile of BrdU labeling on anti-HA CD4+ T cells isolated from H-2d SCID→ H-2dxb or H-2b SCID→ H-2dxb tumor-bearing chimeras. (C) Purified T cells (4 × 104/well) from the mice in panel A were mixed with fresh splenocytes (8 × 104/well) from F1 mice to which 12.5 μg HA peptide was or was not added. Forty-eight hours later, supernatants were collected and assayed for IL-2 by ELISA. Data represent mean ± SE of IL-2 production (pg/mL) per well from 3 mice in each group. *Statistically significant difference between tumor-bearing and tumor-free mice (P < .01). Shown is a representative experiment of 3 independent experiments with similar results.
Disruption of tolerogenic cross-presentation unveils an intrinsic ability of B-cell lymphomas to directly prime antigen-specific CD4+ T cells in vivo
Our findings suggest that in the absence of cross-presentation mechanisms, B-cell lymphomas themselves could present tumor antigens to CD4+ T cells in vivo. However, it could be argued that in the process of generating the chimeras, some H-2dxb F1 APCs might have survived lethal irradiation and been present after immune reconstitution. This is especially relevant for the reconstituted H-2bSCID→H-2dxb F1 chimeras, in which antigen presentation by residual host APCs rather than by malignant B cells could be a confounding factor. To completely rule out this possibility and to prove that direct T-cell priming by malignant B cells occurs in vivo, we evaluated the fate and function of naive anti-HA transgenic T cells transferred directly to C57BL/6 SCID (H-2b) or BALB/c SCID (H-2d) mice. In this experimental system, BM-derived APCs from C57BL/6 SCID did not have the restricted element, I-Ed, required for presentation of the model antigen to transgenic T cells; therefore, only the malignant B cells expressing HA (A20HA) were capable of presenting the antigen to anti-HA CD4+ T cells.
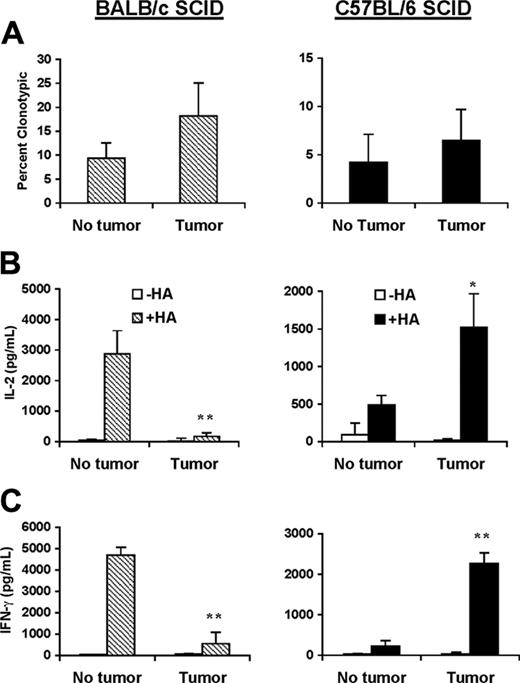
Similar to our results in the H-2dSCID→H-2dxb chimeras, adoptive transfer of antigen-specific T cells into BALB/c SCID tumor-bearing mice (tolerogenic mechanisms mediated by BM-derived APCs are operative) invariably led to a dramatic loss of the ability of antigen-specific CD4+ T cells to produce IL-2 and IFN-γ in response to in vitro restimulation with HA peptide (Figure 4, left panel). In sharp contrast, antigen-specific CD4+ T cells reisolated from C57BL/6 SCID lymphoma-bearing mice (cross-presentation is not operative) displayed significantly higher production of IL-2 and IFN-γ in response to cognate peptide than clonotype-positive T cells from tumor-free mice (Figure 4, right panel). Given that in these mice the only cells capable of presenting the model tumor antigen to CD4+ T cells are themselves malignant B cells, our results clearly confirmed that B-cell lymphomas have an intrinsic ability to effectively activate naive antigen-specific T cells in vivo.
Effective priming of naive CD4+ T cells by B-cell lymphomas occurs in the absence of cross-presentation. BALB/c SCID (left panel) or C57BL/6 SCID (right panel) mice received 1 × 106 naive anti-HA CD4+ TCR transgenic T cells from F1 donors given intravenously. One day later, half the animals in each group were challenged with 1 × 106 A20HA tumor cells given intravenously. Three weeks after tumor challenge, all the animals were killed and T cells were purified from their spleens. (A) T cells were analyzed by 2-color flow cytometry staining for CD4 compared with anti-HA TCR clonotype (mAb 6.5). Three mice were included per group. Values represent mean ± SE of percentage of T cells expressing the clonotypic TCR. Shown is a representative experiment of 3 independent experiments with similar results. Purified T cells (4 × 104/well) from the mice in panel A were mixed with fresh splenocytes (8 × 104/well) from F1 mice to which 12.5 μg HA peptide was or was not added. Forty-eight hours later, supernatants were collected and assayed for IL-2 (B) or IFN-γ (C) by ELISA. Data represent mean ± SE of cytokine production (pg/mL) per well from 3 mice in each group. Asterisks indicate statistically significant differences between tumor-bearing and tumor-free mice (*P < .05; **P < .01). Shown is a representative experiment of 3 independent experiments with similar results.
Effective priming of naive CD4+ T cells by B-cell lymphomas occurs in the absence of cross-presentation. BALB/c SCID (left panel) or C57BL/6 SCID (right panel) mice received 1 × 106 naive anti-HA CD4+ TCR transgenic T cells from F1 donors given intravenously. One day later, half the animals in each group were challenged with 1 × 106 A20HA tumor cells given intravenously. Three weeks after tumor challenge, all the animals were killed and T cells were purified from their spleens. (A) T cells were analyzed by 2-color flow cytometry staining for CD4 compared with anti-HA TCR clonotype (mAb 6.5). Three mice were included per group. Values represent mean ± SE of percentage of T cells expressing the clonotypic TCR. Shown is a representative experiment of 3 independent experiments with similar results. Purified T cells (4 × 104/well) from the mice in panel A were mixed with fresh splenocytes (8 × 104/well) from F1 mice to which 12.5 μg HA peptide was or was not added. Forty-eight hours later, supernatants were collected and assayed for IL-2 (B) or IFN-γ (C) by ELISA. Data represent mean ± SE of cytokine production (pg/mL) per well from 3 mice in each group. Asterisks indicate statistically significant differences between tumor-bearing and tumor-free mice (*P < .05; **P < .01). Shown is a representative experiment of 3 independent experiments with similar results.
To further prove that a direct T cell/B-cell interaction actually occurs in vivo, we analyzed by immunohistochemistry (IHC) the spleen sections from C57BL/6 SCID tumor-bearing mice adoptively transferred with transgenic T cells. As shown in Figure 5A, T cells were in close contact with malignant B cells in the spleens of these animals (inset). Next, we assessed the fate of these T cells by evaluating whether they were capable of infiltrating lymphoma nodules that developed in the livers of C57BL/6 SCID tumor-bearing mice or BALB/c SCID tumor-bearing mice used as controls. Although an almost complete absence of T cells was found in the lymphoma nodules of BALB/c SCID (Figure 5B, left panel), adoptively transferred T cells were clearly infiltrating lymphoma nodules in C57BL/6 SCID mice (Figure 5B, right panel).
Rejection of A20 wild-type lymphoma in the absence of cross-presentation of tumor antigens
To extend our observations beyond the TCR transgenic experimental system, we determined next whether animals with normal T-cell repertoires but disrupted cross-presentation mechanisms were capable of rejecting wild-type B-cell tumors in vivo. With the use of bone marrow cells from BALB/c or C57BL/6 immunocompetent donors, we generated 2 sets of BM chimeras in which APCs expressed H-2d or H-2b. In this model, the bone marrow graft also provided the necessary precursors for T-cell generation in the thymus of the recipient animal. Given that H-2d and H-2b elements are present in the thymi of H-2dxb mice, the reconstituted T-cell repertoire would contain H-2d-restricted and H2b-restricted mature T cells, irrespective of the BM-graft haplotype. Because A20 B cells express H-2d but not H-2b, only the H-2d-restricted T cells would be able to recognize tumor antigens directly on the lymphoma cells and, therefore, could potentially elicit an antitumor immune response. As seen in Figure 6A,in the presence of APCs able to tolerize H-2d-restricted tumor-reactive T cells (H-2d→H-2dxb chimeras), lymphoma developed in all the animals. In sharp contrast, a significant delay in the growth of A20 tumor was observed in H-2b→H-2dxb chimeras. Long-term follow up (up to 150 days) revealed that in the absence of cross-presentation to H2d-restricted T cells, 40% of these mice rejected A20 lymphoma. This antitumor effect was not NK-mediated because depletion of this cell population in H-2b→H-2dxb chimeras did not change the observed survival outcome (data not shown). Furthermore, we ruled out the possibility that H-2d alloreactive T cells in reconstituted H-2b→H-2dxb chimeras could be responsible for A20WT rejection because T cells from these chimeras did not proliferate in mixed-leukocyte reaction (MLR) in the presence of irradiated H-2d, H-2b, or H-2dxb cells whereas they proliferated vigorously in the presence of third-party H-2k cells (data not shown). Instead, in vivo depletion studies using anti-CD4 or anti-CD8 antibodies confirmed that CD4+ T cells were mainly responsible for the antitumor effect observed (Figure 6B). Therefore, when cross-presentation was disrupted, direct interaction between malignant B cells and CD4+ T cells led to significant antitumor immunity.
Tumor infiltration with T cells in the absence of tolerogenic APCs. (A) C57BL/6 SCID mice were adoptively transferred intravenously with 1 × 106 naive anti-HA CD4+ T cells. One day later, they were challenged with 1 × 106 A20HA given intravenously. Twenty-one days after tumor challenge, mice were humanely killed, and histologic sections of their spleens were stained with anti-B220 antibody (violet) to identify tumor cells and with anti-CD3-ϵ (brown) to identify T cells by immunohistochemistry. Slides were then counterstained with hematoxylin (blue). Close contact between tumor cells and T cells is revealed (inset). (B) BALB/c SCID (left) or C57BL/6 SCID (right) mice were challenged with 1 × 106 A20HA intravenously on day 0. Fourteen days later, tumor-bearing mice were adoptively transferred with 1 × 106 anti-HA-specific CD4+ T cells. One week later, mice were humanely killed, and their livers were removed. Serial histologic sections were performed and stained as detailed.
Tumor infiltration with T cells in the absence of tolerogenic APCs. (A) C57BL/6 SCID mice were adoptively transferred intravenously with 1 × 106 naive anti-HA CD4+ T cells. One day later, they were challenged with 1 × 106 A20HA given intravenously. Twenty-one days after tumor challenge, mice were humanely killed, and histologic sections of their spleens were stained with anti-B220 antibody (violet) to identify tumor cells and with anti-CD3-ϵ (brown) to identify T cells by immunohistochemistry. Slides were then counterstained with hematoxylin (blue). Close contact between tumor cells and T cells is revealed (inset). (B) BALB/c SCID (left) or C57BL/6 SCID (right) mice were challenged with 1 × 106 A20HA intravenously on day 0. Fourteen days later, tumor-bearing mice were adoptively transferred with 1 × 106 anti-HA-specific CD4+ T cells. One week later, mice were humanely killed, and their livers were removed. Serial histologic sections were performed and stained as detailed.
Impaired antigen-presenting capabilities of dendritic cells from lymphoma-bearing mice
Our in vivo results have unambiguously shown that BM-derived APCs induced unresponsiveness in naive and memory antigen-specific T cells and that this mechanism of tumor-antigen presentation prevails over direct presentation by malignant B cells. Although several BM-derived cells express MHC class II, only DCs have been shown to be strategically located in secondary lymphoid organs and to present antigens to naive CD4+ T cells in vivo.14 Such an antigen presentation could lead, however, to T-cell priming or T-cell tolerance.15 Given these findings, we evaluated next whether alterations in the antigen-presenting capabilities of DCs from A20 lymphoma-bearing mice could explain, at least in part, the state of T-cell unresponsiveness observed in these mice. CD11c+ DCs were isolated from the spleens of A20-bearing mice or tumor-free mice, and their phenotypes and ability to present cognate antigen to CD4+ T cells were evaluated in vitro. No differences in the expression of MHC class II, B7.1, B7.2, or CD40 was found among CD11c+ DCs from lymphoma-bearing and tumor-free mice (data not shown). Functional analysis of these APCs revealed that CD4+ T cells encountering cognate antigen in DCs from lymphoma-bearing mice produced significantly less IL-2 (3.54% vs 9.94%) and IFN-γ (0.20% vs 0.66%) than clonotype-positive T cells from tumor-free controls, as assessed by intracellular cytokine staining (Figure 7). It is plausible that these functionally impaired CD11c+ DCs could have represented the APC subpopulation involved in the induction of CD4+ T-cell tolerance in B-cell lymphoma-bearing mice.
Discussion
These findings demonstrate that tumor-antigen-specific CD4+ T cells can be efficiently primed in vivo by malignant B cells, leading to antilymphoma immunity only when unopposed by the dominant-negative effect of cross-presentation mediated by BM-derived APCs.
In the immune response against tumors, it is likely that malignant cells themselves and BM-derived APCs present tumor antigens to antigen-specific T cells. This explanation is more plausible for tumors that were derived from cells with intrinsic antigen-presenting capabilities and that have developed in the same organs in which antigen-specific T-cell responses are initiated, as is the case for B-cell lymphomas. As shown here, the fate of tumor-antigen-specific CD4+ T cells is divergent when tumor antigens are presented by lymphoma cells or by BM-derived APCs. Although T-cell priming and generation of effective immunity are elicited by direct tumor-antigen presentation by B-cell lymphomas, cross-presentation by BM-derived APCs leads instead to antigen-specific T-cell tolerance. The latter mechanism is clearly dominant over direct presentation because induction of T-cell unresponsiveness and tumor growth represents the ultimate outcome for B-cell lymphomas in vivo.
Rejection of A20 wild-type tumors occurs in the absence of tolerogenic APCs and is CD4+ dependent. Bone marrow cells (4 × 106) from BALB/c mice (H2d)or C57BL/6 mice (H2b) were injected intravenously into lethally irradiated F1 recipients (H2dxb). (A) Three months after bone marrow reconstitution, mice from H-2d → H-2dxb or H-2b→ H-2dxb chimeras were injected intravenously with 1 × 106 A20WT tumor cells. Ten mice were included in each group, and they were inspected twice weekly for the development of tumor (P < .01 for the comparison between both chimeras). (B) H-2b→ H-2dxb chimeras were treated with anti-CD4- or anti-CD8-depleting antibodies (3 times per week or received no treatment [no depletion]) before challenge with A20WT tumors. Mice received depleting antibodies once a week thereafter until the end of the experiment. Ten mice were included in each group (P < .01 for CD4-depleted vs -nondepleted groups).
Rejection of A20 wild-type tumors occurs in the absence of tolerogenic APCs and is CD4+ dependent. Bone marrow cells (4 × 106) from BALB/c mice (H2d)or C57BL/6 mice (H2b) were injected intravenously into lethally irradiated F1 recipients (H2dxb). (A) Three months after bone marrow reconstitution, mice from H-2d → H-2dxb or H-2b→ H-2dxb chimeras were injected intravenously with 1 × 106 A20WT tumor cells. Ten mice were included in each group, and they were inspected twice weekly for the development of tumor (P < .01 for the comparison between both chimeras). (B) H-2b→ H-2dxb chimeras were treated with anti-CD4- or anti-CD8-depleting antibodies (3 times per week or received no treatment [no depletion]) before challenge with A20WT tumors. Mice received depleting antibodies once a week thereafter until the end of the experiment. Ten mice were included in each group (P < .01 for CD4-depleted vs -nondepleted groups).
Dendritic cells from A20 lymphoma-bearing mice have impaired antigen-presenting capabilities. BALB/c mice were injected intravenously with 1 × 106 A20 WT lymphoma cells. Three weeks later, animals were killed and CD11c+ splenic DCs were isolated by magnetic sorting. Purified anti-HA T cells (2 × 106) were then plated for 10 hours with 2 × 105 DCs from lymphoma-bearing (right) or control tumor-free (left) mice in the presence or absence of cognate peptide. Each dot plot is gated on the CD4+/6.5+ clonotypic population and shows the percentage of anti-HA T cells positive for IL-2 or IFN-γ, as determined by intracellular cytokine staining. DCs were obtained from 4 mice, and the experiment was repeated twice with similar results.
Dendritic cells from A20 lymphoma-bearing mice have impaired antigen-presenting capabilities. BALB/c mice were injected intravenously with 1 × 106 A20 WT lymphoma cells. Three weeks later, animals were killed and CD11c+ splenic DCs were isolated by magnetic sorting. Purified anti-HA T cells (2 × 106) were then plated for 10 hours with 2 × 105 DCs from lymphoma-bearing (right) or control tumor-free (left) mice in the presence or absence of cognate peptide. Each dot plot is gated on the CD4+/6.5+ clonotypic population and shows the percentage of anti-HA T cells positive for IL-2 or IFN-γ, as determined by intracellular cytokine staining. DCs were obtained from 4 mice, and the experiment was repeated twice with similar results.
Several factors might account for the predominant effect of cross-presentation over direct tumor-antigen presentation. First, although B-cell lymphomas arise in the same organs in which T-cell responses are elicited, it is plausible that, at least early during lymphoma development, either cell population would be confined to its respective B-cell or T-cell zone within the secondary lymphoid organs, making direct interaction unlikely. Second, the known migratory capabilities of BM-derived APCs might favor the exposure of antigen-specific T cells to antigen cross-presented by APCs migrating from the tumor site (B-cell zone). Such an early encounter, which usually occurs in the absence of inflammation, has been shown to result in the induction of T-cell tolerance rather than T-cell activation.15 Noninflammatory BM-derived APCs, with their low levels of MHC, costimulatory molecules, and other adhesion molecules that participate in T-cell priming, can induce a “partial” T-cell activation state that, in the absence of additional signals capable of sustaining this initial response or in the presence of dominant-suppressive mechanisms, is rapidly followed by T-cell unresponsiveness.3,16 The potential role of such a suppressive mechanism(s) has been recently highlighted by our demonstration that antigen presentation by host APCs led to the induction of regulatory T cells (TRegs) that dominantly suppress activated Th1 effector cells primed by A20HA cells directly.17
Our findings that memory CD4+ T cells are also rendered unresponsive in tumor-bearing mice in which both mechanisms of tumor-antigen presentation are operative (H-2d into H-2dxb chimeras or H-2dSCID) further confirmed the dominant-negative effect of cross-presentation over direct presentation. Memory T cells, because of their migratory capabilities,18,19 are likely to encounter tumor antigens in malignant B cells residing in B-cell zones and in BM-derived APCs cross-presenting tumor antigens. Although it is plausible that some memory T cells could be directly activated by malignant B cells, the fact that the overall outcome is tolerance induction suggests that most T cells might have encountered tumor antigen on APCs or that they are actively suppressed by APC-induced TRegs.17 Needless to say, the demonstration that memory CD4+ T cells are also rendered unresponsive during the in vivo growth of B-cell tumors has sobering implications for immunotherapy of this disease and provides one important explanation for the observed limited efficacy of T-cell adoptive therapy against B-cell malignancies.
B cells, macrophages, and DCs are all BM-derived cells that express MHC class II molecules and, as such, are capable of presenting tumor antigen to antigen-specific CD4+ T cells. However, several lines of evidence have pointed to DCs as playing a central role in initiating T-cell responses in vivo given their superior efficiency in antigen uptake, processing, MHC-peptide complex display, and migration to the T-cell areas of secondary lymphoid organs.14 Such a DC-T-cell encounter in vivo has not always led to T-cell priming and could result instead in the induction of T-cell unresponsiveness.15,20 Presentation of tumor antigens by DCs seems, therefore, to be the most likely mechanism involved in lymphoma-induced CD4+ T-cell tolerance. Interestingly, we have found that CD11c+ DCs from lymphoma-bearing mice are impaired in their antigen-presenting capabilities (Figure 7), suggesting that lymphoma-induced T-cell tolerance could be attributed, at least in part, to suboptimal tumor-antigen presentation by CD11c+ DCs. We are assessing whether these cells are tolerogenic in vivo and whether the impairment in antigen presentation is restricted to a specific DC subpopulation.
The finding that malignant B cells can efficiently prime antigen-specific CD4+ T cells is not surprising, considering the resemblance to their normal counterparts. Normal B cells have long been known to interact with CD4+ T cells during physiologic immune responses in a process that involves the presentation of peptide-MHC class II complexes, along with costimulatory signals to antigen-specific T cells.21,22 Similarly, some studies have shown that malignant B cells display well-preserved antigen-presenting capabilities.3,11,23,24 In contrast to these findings, others have found that B cells not only fail to activate naive T cells but may also induce unresponsiveness of antigen-specific T cells.10,25-27 Therefore, significant controversy remains about the ability of B cells to stimulate T-cell responses in vivo. The results presented here provide support for the intrinsic priming ability of B cells and indicate that such a T-cell/B-cell interaction in vivo is associated with the elicitation of antilymphoma immunity (Figures 1, 6). Although we realize that not all B-cell lymphomas have preserved antigen-presenting capabilities, our results indicate that even when they do, as is the case for A20, host APC-dependent tolerance is dominant over B-cell-dependent T-cell activation.
For many years, a number of different approaches have been used to enhance the antigen-presenting capabilities of malignant B cells, mainly by genetically modifying B cells to enforce the expression of immunologically relevant molecules such as adhesion/costimulatory molecules or cytokines.11,28-30 Although these strategies can induce systemic immunity in vivo, the effect observed has been transient and insufficient to induce effective lymphoma eradication. One potential explanation for the limited success of these strategies is that they have focused on enhancing the APC function of the malignant B cells while ignoring the other important component represented by the dominant effect of cross-presentation of tumor antigens.
Therefore, from a therapeutic perspective, it is likely that elicitation of effective antilymphoma immunity will require a rational combination of therapeutic approaches aimed at enhancing the antigen-presenting function of B cells with strategies targeting the remarkable barrier imposed by tolerogenic cross-presentation mediated by BM-derived APCs.
Prepublished online as Blood First Edition Paper, December 8, 2005; DOI 10.1182/blood-2005-07-3014.
Supported by Public Health Service grants CA87583 and CA100850 (E.M.S.) and P50CA96888 and PO1CA15396 (H.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
E.M.S. is a Junior Faculty of the Lymphoma Research Foundation of America. H.L. is a Stohlman Scholar of the Leukemia and Lymphoma Society of America.

![Figure 1. Rejection of A20HA B-cell lymphoma in the absence of cross-presentation mediated by BM-derived APCs. H-2dxb F1 mice were lethally irradiated (10 Gy [1000 rad]), and each received a graft consisting of 4 × 106 bone marrow cells from either BALB/c SCID (H-2d) or C57BL/6 SCID (H-2b) donors. Three months after bone marrow reconstitution, either group of chimeras intravenously received 1 × 106 in vitro activated CD4+ TCR-transgenic T cells (H2dxb) specific for HA/IEd. Four weeks later, all the mice were challenged intravenously with 1 × 106 A20HA cells, and they were inspected twice weekly for the development of tumors. Six mice were included in each group (P < .01). Data are representative of 3 experiments with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/7/10.1182_blood-2005-07-3014/5/m_zh80070693340001.jpeg?Expires=1763460691&Signature=AIJEtQXDVUeuO7oUJWSwdJZciP1wmX9lomkHqVYB~VwAmH0ivNuPaQj8aIHH88UrHK7RZ8-FgA0ZD02PcweKBV3F-RXsiLQymZqcD2D2HW9sK1~tM6eOAzg6TovsG2RPsbh9MGy8BkKO5vYCRrYR~QE4Zb0gB579MQlNN6jsTb0xs~hbGJw3dlsMiqcjhV0Hz8Z6ifvjsF1sBX0RDE8mcdwGg7ll~MJ-RxkGrUV9M-U2zLQvvF4rPHweFu8wcIlDolEA2EQqVg2EOWBpYhMqWcddQdZrwhZ9hTVlnuiS2kiL5AXL1dEuLfDt7TrnIQt-zAwUgaeVTxP-adyjC7kF-w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 6. Rejection of A20 wild-type tumors occurs in the absence of tolerogenic APCs and is CD4+ dependent. Bone marrow cells (4 × 106) from BALB/c mice (H2d)or C57BL/6 mice (H2b) were injected intravenously into lethally irradiated F1 recipients (H2dxb). (A) Three months after bone marrow reconstitution, mice from H-2d → H-2dxb or H-2b→ H-2dxb chimeras were injected intravenously with 1 × 106 A20WT tumor cells. Ten mice were included in each group, and they were inspected twice weekly for the development of tumor (P < .01 for the comparison between both chimeras). (B) H-2b→ H-2dxb chimeras were treated with anti-CD4- or anti-CD8-depleting antibodies (3 times per week or received no treatment [no depletion]) before challenge with A20WT tumors. Mice received depleting antibodies once a week thereafter until the end of the experiment. Ten mice were included in each group (P < .01 for CD4-depleted vs -nondepleted groups).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/7/10.1182_blood-2005-07-3014/5/m_zh80070693340006.jpeg?Expires=1763460691&Signature=Y2S3yQxJ2eepGD6~Hn2zjOhnOxjDWt~pYoh9eDJn6X3G8jTcfSwqVUCMgJ~EOQXpbZ5wDrHIulZn-GHzA6aJwGxsSQzW5qh9BHrKzKdH-4nUFEMi~gJkly39-BVyqxJSXxPW2mXelF5T9X21WvpHiEZe92DXKBTYPV7ewvqOYoTMlp1OH22BmpFkdYII7NqfGLG7fvxrDAh5kKVC2fIxVTuoL54UrJK7eNTiYpGb0YwEIgNGrwIzk-9pWmb2vIDy-qV-obHgmmwEtTa7hX1OXPEY8CBj2uESlL-DcvUZPyL-XEwd4tNUBv~njgsVAxjjM1GRZALKfvHf4PIiTtlkHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal