Abstract
By retroviral overexpression of the Notch-1 intracellular domain (ICN) in human CD34+ hematopoietic stem cells (HSCs), we have shown previously that Notch-1 signaling promotes the T-cell fate and inhibits the monocyte and B-cell fate in several in vitro and in vivo differentiation assays. Here, we investigated whether the effects of constitutively active Notch-1 can be mimicked by overexpression of its downstream target gene HES1. Upon HES-1 retroviral transduction, human CD34+ stem cells had a different outcome in the differentiation assays as compared to ICN-transduced cells. Although HES-1 induced a partial block in B-cell development, it did not inhibit monocyte development and did not promote T/NK-cell-lineage differentiation. On the contrary, a higher percentage of HES-1-transduced stem cells remained CD34+. These experiments indicate that HES-1 alone is not able to substitute for Notch-1 signaling to induce T-cell differentiation of human CD34+ hematopoietic stem cells.
Introduction
Studies with Notch-1-deficient and transgenic mice have shown that signaling through the Notch-1 transmembrane receptor is necessary and sufficient for T-cell commitment.1-3 In humans, we showed that retroviral overexpression of the intracellular domain of Notch-1 (ICN) in cord-blood CD34+ cells results in inhibition of B-cell development both in vitro and in vivo. Instead, cells are forced to differentiate along the T/NK pathway, resulting in ectopic development of T cells in the bone marrow (BM) of mice injected with ICN-transduced human hematopoietic stem cells (HSCs).4
A technique to direct HSCs toward the T-cell lineage could be of great therapeutic value to develop strategies to enhance T-cell development from transplanted donor HSCs after myeloablative therapy. Manipulation of HSCs with ICN is not an option however, since constitutive Notch-1 expression eventually leads to the development of T-cell neoplasms,5 and mutations in the NOTCH1 gene have been involved in most cases of human T-cell acute lymphoblastic leukemia (T-ALL).6 Understanding the cellular events leading to T-cell commitment after ICN overexpression might lead to the identification of an effector molecule that does not cause tumor development when overexpressed in HSCs.
An important target gene of Notch-1 signaling is the basic helix-loop-helix transcription factor HES1,7 which is expressed in thymocytes and thymic stroma and is essential for normal T-cell development.8,9 HES-1 is up-regulated in hematopoietic precursors after ICN overexpression10 and after activation of endogenous Notch-1 signaling by binding with the Delta-like-1 ligand.11,12 Here, we investigated whether HES-1 is the mediator of the effects on human hematopoietic differentiation seen previously with ICN overexpression.
Study design
Production of HES-1 retrovirus
Human HES-1 cDNA was cloned in the retroviral vector pLZRS-IRES-EGFP (LIE)13 upstream of the internal ribosomal entry site (IRES). ICN-LZRS was prepared by subcloning ICN from the ICN-MSCV construct used before.4 Infectious retrovirus was produced by transfection of Phoenix-A cells.14 Expression of HES-1 protein was verified by Western blotting on nuclear extracts of HES-1-transduced HEK-T cells using a polyclonal rabbit anti-HES-1 antibody.
Sorting of CD34+Lin- cells, retroviral transduction, and differentiation assays
CD34+ cells were isolated from cord-blood mononuclear cells by positive selection with EasySep magnetic beads (StemCell Technologies, Vancouver, BC, Canada), and CD34+ Lin- cells were sorted after staining with anti-human CD34-APC, CD3-FITC, CD14-FITC, CD19-FITC, or CD19-PE monoclonal antibodies (Becton and Dickinson Immunocytometry Systems, San Jose, CA) and CD56-FITC (Serotec, Oxford, United Kingdom). The purity was always more than 98%. Retroviral transduction and coculture on MS-5 stromal cells were performed as described.4 Labeling of cells with the indicated antibodies and flow cytometric analysis were performed as described.4
Calculation of absolute cell numbers
Absolute cell numbers were calculated from the total viable cell number as counted with the hemocytometer, the frequency of enhanced green fluorescence protein+ (EGFP+) cells and the frequency of the indicated subpopulations as measured by flow cytometry. Because transduction efficiencies were different for LIE, HES-1, and ICN, these numbers were multiplied by a factor to normalize to 1 × 103 EGFP+ cells at the start of all cultures.
Statistical analyses
The paired Student t test was used with a significance level of .05.
Results and discussion
Overexpression of HES-1 reduces B-cell differentiation but does not promote T/NK-cell differentiation
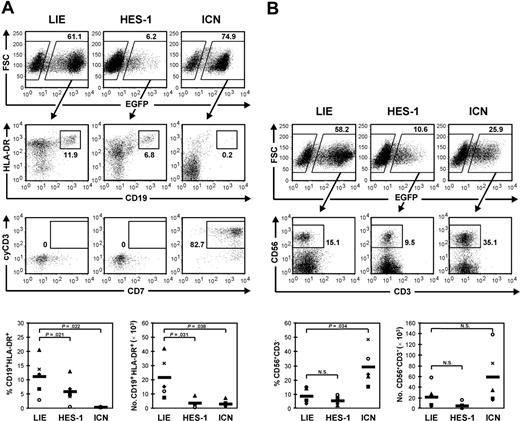
Cord-blood CD34+ cells were transduced with comparable efficiencies by the HES-1 and ICN retrovirus (17.0% ± 5.9% and 19.6% ± 7.5% after 1 day, respectively). The transduction efficiency for the control virus LIE was higher, that is, 47.2 % ± 12.4%. As anticipated, B-cell development in MS-5 cocultures was dramatically blocked by ICN overexpression (Figure 1A). In the cultures initiated with HES-1-transduced cells, B-cell differentiation was not completely suppressed but yet significantly reduced (Figure 1A). This reduction was correlated with the level of HES-1 expression and was not caused by a block at an immature B-cell stage (data not shown). These results indicate that the up-regulation of HES-1 in response to Notch-1 signaling might aid to the inhibition of B-cell development but is not the main mediator of this effect.
Whereas overexpression of ICN drives T-cell development, as shown by the massive generation of CD7+cyCD3+ T/NK progenitors under B-cell conditions in MS-5 cocultures, HES-1 overexpression did not mimic this effect (Figure 1A). Furthermore, HES-1, unlike ICN, did not promote NK-cell development in MS-5 cocultures with specific cytokines for NK-cell development (Figure 1B). Therefore, the Notch-1 signal instructing the CD34+ cell to commit to the T/NK lineage is probably not or not solely mediated by HES-1.
Our results are in line with those of Kawamata et al,15 who also noticed a partial block in B-cell development but no extrathymic T-cell development in the BM of mice transplanted with HES-1- or HES-5-transduced mouse BM Lin- precursors. Also, Maillard et al16 communicated that overexpression of HES-1 in Notch-1-deficient murine precursors cannot rescue T-cell development in fetal thymus organ culture (FTOC). Combined with our data, it is clear that overexpression of HES-1 is not sufficient to impose T-cell differentiation on either murine or human HSCs.
Overexpression of HES-1 reduces B-cell differentiation but does not promote T/NK-cell differentiation. (A) Flow cytometric analysis of MS-5 cocultures with cytokine Mix 2 (SCF and IL-7) for B-cell development. Percentages of B cells (CD19+HLA-DR+) and T/NK progenitor cells (CD7+cyCD3+) in the EGFP+-gated fraction of cultures initiated with LIE-, HES-1-, or ICN-transduced CD34+ cord-blood cells are indicated. Dot plots shown are representative of 5 independent experiments with cells from 5 different donors. (B) Flow cytometric analysis of MS-5 cocultures with cytokine Mix 3 (SCF, IL-2, IL-15) for NK-cell development. Percentage of NK cells (CD56+CD3-) in the EGFP+-gated fraction is indicated. Dot plots shown are representative of 5 independent experiments with cells from 5 different donors. Scatterplots show relative and absolute numbers of B cells and NK cells generated in 5 independent experiments. Each symbol represents an individual experiment. The mean of 5 experiments is represented by thick horizontal lines in each bottom panel. P values obtained with the paired Student t test are shown when significant; N.S. indicates a nonsignificant P value.
Overexpression of HES-1 reduces B-cell differentiation but does not promote T/NK-cell differentiation. (A) Flow cytometric analysis of MS-5 cocultures with cytokine Mix 2 (SCF and IL-7) for B-cell development. Percentages of B cells (CD19+HLA-DR+) and T/NK progenitor cells (CD7+cyCD3+) in the EGFP+-gated fraction of cultures initiated with LIE-, HES-1-, or ICN-transduced CD34+ cord-blood cells are indicated. Dot plots shown are representative of 5 independent experiments with cells from 5 different donors. (B) Flow cytometric analysis of MS-5 cocultures with cytokine Mix 3 (SCF, IL-2, IL-15) for NK-cell development. Percentage of NK cells (CD56+CD3-) in the EGFP+-gated fraction is indicated. Dot plots shown are representative of 5 independent experiments with cells from 5 different donors. Scatterplots show relative and absolute numbers of B cells and NK cells generated in 5 independent experiments. Each symbol represents an individual experiment. The mean of 5 experiments is represented by thick horizontal lines in each bottom panel. P values obtained with the paired Student t test are shown when significant; N.S. indicates a nonsignificant P value.
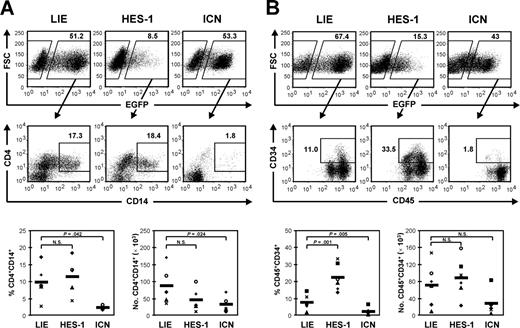
HES-1 overexpression has no influence on myeloid differentiation but maintains CD34+ cells
Culturing CD34+ cells on MS-5 in the presence of SCF, FL3, TPO, IL-2, IL-7, and IL-15 (Mix 6) allows their expansion and differentiation toward monocytes. We showed earlier that ICN expression blocks both HSC expansion and differentiation toward monocytes.4 This was confirmed in the current experiments (Figure 2). HES-1-transduced CD34+ cells, on the other hand, generated comparable frequencies of monocytes as the control (Figure 2A). However, a significantly higher percentage of HES-1-transduced CD34+ cells remained CD34+, and absolute numbers of CD34+ cells were not reduced compared to the control (Figure 2B). The frequency of CD34+ cells also correlated with the level of HES-1 expression (data not shown). Although recent studies indicate that HES-1 maintains stem cells,17,18 phenotypic analysis of the CD34+ population from our MS-5 cultures did not point to a specific increase of cells with a stem-cell-like phenotype (ie, CD34brightCD133+) (data not shown).
Conclusion
Our experiments indicate that HES-1 is not the sole mediator of T-cell commitment induced by ICN overexpression. Based on the data from Kawamata et al, HES-5 also is not the downstream effector molecule of ICN.15 Deltex-1, which is also up-regulated after ICN overexpression (Deftos et al10 and own observation) cannot be the downstream mediator either, since forced expression of Deltex-1 in hematopoietic progenitors results in B-cell development at the expense of T-cell development.19 More target genes of Notch signaling are being discovered, however. HERP1 and HERP2, which are structurally very similar to HES, are both up-regulated by Notch signaling. They can form heterodimers with HES proteins that bind with greater affinity to the promoter of downstream genes and have a stronger repression activity than the respective homodimers.20 This might explain why overexpression of HES-1 alone has little effect. Possibly one or more other transcription factors that are up-regulated by ICN overexpression must be overexpressed together with HES-1. HERP1 (Hey1) and HERP2 (Hey2) knock-out mice have been established recently,21 so it will be interesting to find out whether T-cell development is influenced in these mice. Another possible scenario is that ICN drives cells to the T/NK pathway by up-regulating T/NK-committed genes. For example, the pre-TCR- α chain (PTCRA) gene has been shown to be a direct target gene of Notch-1 in T cells.10,22 Furthermore, several studies point to the existence of CBF1, suppressor of hairless Lag-1 (CSL)-independent Notch signaling pathways.23 Although the CSL pathway is active in ICN-transduced cord blood CD34+ cells, as shown by the up-regulation of HES-1, it is possible that the effects of ICN on differentiation are mediated by CSL-independent mechanisms.
HES-1 overexpression has no influence on myeloid differentiation but maintains CD34+ cells. Flow cytometric analysis of MS-5 cocultures with cytokine Mix 6 (SCF, FL3, TPO, IL-2, IL-7, and IL-15). Percentages of CD4+CD14+ monocytes (A) and CD45+CD34+ cells (B) in the EGFP+-gated fraction of cultures initiated with LIE-, HES-1-, or ICN-transduced CD34+ cord-blood cells are indicated. Dot plots shown are representative of 5 to 6 independent experiments with cells from 5 to 6 different donors. Scatterplots show relative and absolute numbers of monocytes and CD34+ cells generated in 5 to 6 independent experiments. Each symbol represents an individual experiment. The thick horizontal lines in each bottom panel represent the mean. P values obtained with the paired Student t test are shown when significant; N.S. indicates a nonsignificant P value.
HES-1 overexpression has no influence on myeloid differentiation but maintains CD34+ cells. Flow cytometric analysis of MS-5 cocultures with cytokine Mix 6 (SCF, FL3, TPO, IL-2, IL-7, and IL-15). Percentages of CD4+CD14+ monocytes (A) and CD45+CD34+ cells (B) in the EGFP+-gated fraction of cultures initiated with LIE-, HES-1-, or ICN-transduced CD34+ cord-blood cells are indicated. Dot plots shown are representative of 5 to 6 independent experiments with cells from 5 to 6 different donors. Scatterplots show relative and absolute numbers of monocytes and CD34+ cells generated in 5 to 6 independent experiments. Each symbol represents an individual experiment. The thick horizontal lines in each bottom panel represent the mean. P values obtained with the paired Student t test are shown when significant; N.S. indicates a nonsignificant P value.
Prepublished online as Blood First Edition Paper, December 1, 2005; DOI 10.1182/blood-2005-05-1815.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the staff of the Bloedtransfusiecentrum Oost-Vlaanderen for the supply of umbilical cord blood samples and Marie-José De Bosscher for lymphoprepping and freezing them. We are grateful to Dr T. Sudo (Toray Industries Inc, Kamakura, Japan) for his kind gift of the anti-HES-1 antibody, to Nancy De Cabooter and Els Demecheleer for DNA sequencing, to Christian De Boever for help with artwork, and to Caroline Collier for animal care.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal