Abstract
Agonistic anti-Fas antibodies and multimeric recombinant Fas ligand (FasL) preparations show high tumoricidal activity against leukemic cells, but are unsuitable for clinical application due to unacceptable systemic toxicity. Consequently, new antileukemia strategies based on Fas activation have to meet the criterion of strictly localized action at the tumor-cell surface. Recent insight into the FasL/Fas system has revealed that soluble homotrimeric FasL (sFasL) is in fact nontoxic to normal cells, but also lacks tumoricidal activity. We report on a novel fusion protein, designated scFvCD7:sFasL, that is designed to have leukemia-restricted activity. ScFvCD7:sFasL consists of sFasL genetically linked to a high-affinity single-chain fragment of variable regions (scFv) antibody fragment specific for the T-cell leukemia-associated antigen CD7. Soluble homotrimeric scFvCD7:sFasL is inactive and acquires tumoricidal activity only after specific binding to tumor cell-surface-expressed CD7. Treatment of T-cell acute lymphoblastic leukemia (T-ALL) cell lines and patient-derived T-ALL, peripheral T-cell lymphoma (PTCL), and CD7-positive acute myeloid leukemia (AML) cells with homotrimeric scFvCD7:sFasL revealed potent CD7-restricted induction of apoptosis that was augmented by conventional drugs, farnesyl transferase inhibitor L-744832, and the proteasome inhibitor bortezomib (Velcade; Millenium, Cambridge, MA). Importantly, identical treatment did not affect normal human peripheral-blood lymphocytes (PBLs) and endothelial cells, with only moderate apoptosis in interleukin-2 (IL-2)/CD3-activated T cells. CD7-restricted activation of Fas in T-cell leukemic cells by scFvCD7:sFasL revitalizes interest in the applicability of Fas signaling in leukemia therapy.
Introduction
Despite advances in T-cell leukemia therapy, only a minority of patients achieves long term tumor-free survival with conventional chemotherapy at the cost of significant and often irreversible toxic side effects.1 Therefore, new therapeutic approaches with enhanced tumor selectivity and more favorable toxicity profiles are urgently needed. Several promising targeted approaches have been developed, including naked antibodies,2,3 immunotoxins,4,5 and various cancer-selective small inhibitory molecules.6,7 Furthermore, certain members of the tumor necrosis factor (TNF) superfamily show promising proapoptotic activity toward various human leukemias and lymphomas.
Fas ligand (FasL), a prominent member of the TNF superfamily, shows superior antileukemia activity. FasL is present on lymphocytes and monocytes/macrophages as a type II transmembrane protein, (memFasL). Fas, the cognate receptor for FasL, belongs to the family of transmembrane proteins known as death receptors. Death receptors can detect the presence of specific extracellular death signals and rapidly trigger cellular destruction by apoptosis. Fas expression at the cell surface is observed in biopsies and cell lines derived from a variety of tumors. Moreover, the antitumoral effects of various chemotherapeutic drugs have been attributed partly to p53-mediated up-regulation of Fas and FasL.8-12 Fas signaling is also known to be a key element in the effector phase of a cytotoxic T lymphocyte (CTL) response against tumor cells.
Like other members of the TNF superfamily, the extracellular domain of FasL can be proteolytically cleaved into a soluble homotrimeric form13-15 (sFasL).
Early attempts to exploit Fas agonists such as anti-Fas antibodies and multimeric recombinant FasL preparations for therapy revealed extremely potent tumoricidal effects toward isolated primary tumor cells and cell lines.13,16-19 However, in vivo application of most Fas antagonists was associated with acute lethality in mice,20-22 thereby excluding therapeutic evaluation in humans. Nevertheless, the principal feasibility of therapeutic Fas activation in cancer therapy was clearly demonstrated in mice that lack a functional FasL/Fas system (lpr/gld mice),23 and by treatment of xenografted tumors with human Fas-specific antibodies.24
Recent studies have revealed that certain recombinant FasL preparations contain oligomeric, multimeric, and even aggregated sFasL forms and that these forms are responsible for the observed systemic toxicity.25 In contrast, homotrimeric sFasL is not toxic to normal cells and may even antagonize the function of membrane-bound FasL.25-27 Importantly, homotrimeric sFasL also lacks tumoricidal activity. However, selective delivery of sFasL to a predetermined tumor-associated target antigen can restore the full apoptotic potential of sFasL.28
Recently, we demonstrated that the leukemia selectivity of homotrimeric TRAIL (TNF-related apoptosis-inducing ligand), another TNF superfamily member, can be strongly enhanced by genetically fusing it to a CD7-selective antibody fragment.29 Human CD7 is a lineage-specific antigen that is highly expressed in patients with acute T-cell leukemia and in approximately 10% of patients with acute myeloid leukemia.30-33 The function of CD7 is not yet fully understood. In normal cells CD7 expression is limited to T and myeloid cells in early hematopoietic-cell ontogeny, thymocytes, natural killer (NK) cells, and to a distinct subset of peripheral-blood T cells.34-38 Human CD7 has been used for the targeted delivery of several monoclonal antibody (mAb) toxin conjugates in both preclinical studies and clinical trials.4,5,39,40
Here we report on a novel homotrimeric sFasL fusion protein, designated scFvCD7:sFasL, with enhanced and leukemia-restricted activity toward T-cell acute lymphoblastic leukemia (T-ALL) cell lines and patient-derived T-ALL, peripheral T-cell lymphoma (PTCL), and CD7-positive acute myeloid lymphoma (AML) cancer cells. We provide evidence that homotrimeric scFvCD7:sFasL is bioactive only after specific binding to cell-surface-expressed CD7 with no toxicity toward CD7-negative cells and only moderate activity toward interleukin-2 (IL-2)/CD3-activated CD7-positive T cells.
Materials and methods
mAbs and scFv antibody fragment
mAb TH69 is a murine immunoglobulin G1 (IgG1) with specificity for human CD73 (kindly provided by Dr Martin Gramatzki, University Clinic Schleswig-Holstein, Kiel, Germany). Phagemid pCANTAB5E/scFv3A1F encoding anti-CD7 antibody fragment 3A1F41 was kindly provided by Dr Chris Pennell (Department of Laboratory Medicine and Pathology, University of Minnesota). mAb TH69 and scFv3A1F compete for binding to the extracellular domain of human CD7. FasL-neutralizing mAb Alf2.1 was purchased from Sigma-Aldrich Chemie (Zwijndrecht, the Netherlands). Flag-tagged sFasL was purchased from Alexis (10P's BVBA; Breda, the Netherlands). Flag-tagged sFasL was secondarily cross-linked by preincubation with 5-fold excess of anti-Flag mAb M2 (Alexis).
Chemotherapeutics
The cytostatic drugs used are vincristine (stock; 1 mg/mL in phosphate-buffered saline [PBS]), amsacrine (stock; 1 mM in PBS), and actinomycin D (stock; 2 mg/mL in ethanol). Farnesyl transferase inhibitor L-744832 (Merck, Darmstadt, Germany) was dissolved at 10 mM in DMSO. The proteasome inhibitor bortezomib was dissolved at 10 mM in dH2O. All final concentrations were prepared by serial dilutions in serum-free medium.
Cell lines
Human CD7-positive T-ALL cell lines Jurkat, CEM, and the CD7-negative human B-cell lymphoma cell lines Ramos and Raji were purchased from the American Type Culture Collection (ATCC; Manassas, VA). T-cell lines MOLT16 and HuT78 were a kind gift from Dr Martin Gramatzki. A CD7-positive transfectant of the Ramos cell line (Ramos.CD7) was generated previously.29 All cell lines were cultured in RPMI (Cambrex, East Rutherford, NJ) supplemented with 10% fetal calf serum (FCS) at 37°C in a humidified 5% CO2 atmosphere.
Leukocytes, PBLs, activated T cells, and HUVECs
Leukocytes were isolated from whole blood of healthy donors by standard Ammonium Chloride method.42 Peripheral-blood lymphocytes (PBLs) were isolated from whole blood of healthy donors by standard density gradient centrifugation procedures (Lymphoprep; Axis-Shield PoC As, Oslo, Norway). Freshly isolated PBLs were resuspended at 2.0 × 106 cells/mL in RPMI, supplemented with 10% human pooled serum. Activated T cells were generated by incubation of freshly isolated PBLs with anti-CD3 mAb WT32 (0.5 μg/mL) for 72 hours, followed by IL-2 stimulation (100 ng/mL) for 48 hours. Human umbilical vein endothelial cells (HUVECs) were isolated as previously described.43 HUVECs were used before culture passage no. 4 and, for experiments, were precultured in 6-well plates at 60% confluency.
Construction of scFvCD7:sFasL
Previously, we constructed the eukaryotic expression plasmid pEE14scFv: sTRAIL for rapid construction, evaluation, and stable expression of scFv:sTRAIL fusion proteins in CHO-K1 cells.44 Important features of this vector are the murine kappa light-chain leader peptide encoded upstream of 2 multiple cloning sites (MCSs) that are separated by a 26-residue in-frame linker sequence, and the glutamine synthetase selectable marker gene, which allows for amplified expression of the recombinant protein in the established industrial production cell line CHO-K1. The vector exploits the strong cytomegalovirus (CMV) promoter to drive recombinant protein expression, while the leader peptide directs secretion of the recombinant protein into the culture supernatant. In the first MCS, a 745-bp DNA fragment encoding anti-CD7 scFv3A1F derived from Phagemid pCANTAB5E/scFv3A1F was directionally inserted using the unique SfiI and NotI restriction enzyme sites. In the second MCS, soluble TRAIL (sTRAIL)-encoding cDNA was swapped for a polymerase chain reaction (PCR)-truncated 539-bp DNA fragment encoding the extracellular domain of human sFasL using restriction enzymes XhoI and HindIII and standard DNA manipulation procedures. FasL cDNA truncation was performed by PCR using proofread DNA polymerase according to standard protocol using the following primers: T1, 5′-ATCCTCGAGTCTAGTGGGAGCGGATCTACCAGCCAGATGCACACA-3′ (XhoI site is underlined); and T2, 5′-CCCAAGCTTTGCTTCTCTTAGAGCTTATATAAG-3′ (HindIII site is underlined).
Production of scFvCD7:sFasL
ScFvCD7:sFasL was expressed in CHO-K1 cells using the glutamine synthetase selection/amplification system essentially as described previously.44 Briefly, CHO-K1 cells were transfected with pEE14scFvCD7: sFasL using FuGENE-6 reagent (Roche Diagnostics, Almere, the Netherlands). Stable transfectants with amplified expression were isolated and single-cell-sorted by high-speed cell sorter (Cytomation, Ft Collins, CO). Individual clones were assessed for stable and high secretion of scFvCD7: sFasL in the absence of MSX selection reagent by FasL enzyme-linked immunosorbent assay (ELISA) according to manufacturer's recommendations (Axora, San Diego, CA). This procedure identified CHO-K1 production cell line 100B2, stably secreting scFvCD7:sFasL (1.34 μg/mL) into the medium. ScFvCD7:sFasL containing supernatant was harvested (10 000g for 10 minutes) and stored at -80°C.
Solution behavior of scFvCD7:sFasL
The solution behavior of scFvCD7:sFasL was analyzed by size-exclusion (SE) fast-performance liquid chromatography (FPLC) with a calibrated HiLoad 16/60 Superdex 200 Prep-grade column (Amersham Biosciences, Uppsala, Sweden); 5 mL supernatant derived from CHO-K1 cell line 100B2 was loaded onto the column, after which individual samples were collected at 3-minute intervals. Individual samples were analyzed for their capacity to induce apoptosis in CD7-positive FasL-sensitive MOLT16 cells.
CD7-specific binding of scFvCD7:sFasL
CD7-specific binding of scFvCD7:sFasL was analyzed by incubating 1.0 × 106 CEM cells with scFvCD7:sFasL (1.34 μg/mL) in the presence or absence of CD7-blocking mAb TH69 (5 μg/mL). CD7-specific binding was analyzed by flow cytometry with PE-conjugated anti-FasL mAb (Diaclone SAS, Besancon, France). Incubations were performed for 45 minutes at 0°C and were followed by 2 washes with serum-free medium.
CD7-restricted induction of apoptosis by scFvCD7:sFasL
Tumor cells were seeded at 0.25 × 106 cells/well in a 48-well plate and treated for 16 hours with the indicated concentrations of scFvCD7:sFasL in the presence or absence of mAb TH69 (5 μg/mL) or mAb Alf2.1 (1 μg/mL). Where indicated, cells were treated with cross-linked sFasL (Flag-tagged sFasL) or with scFvCD7:sTRAIL.29 Apoptosis was assessed by one of the assays described in “Assays used to assess apoptosis.” Percentage of specific apoptosis was calculated using the following formula: specific apoptosis = (experimental apoptosis - spontaneous apoptosis)/(100 - spontaneous apoptosis) × 100%.
Assays used to assess apoptosis
PS exposure to the outer cell membrane. Flow cytometric analysis of exposure of phosphatidylserine (PS) on the outer membrane was performed with an annexin V-FITC/PI kit (VPS Diagnostics, Hoeven, the Netherlands).
Loss of mitochondrial membrane potential (ΔΨ). ΔΨ was analyzed with the cell-permeant green-fluorescent lipophilic dye DiOC6 (Molecular Probes, Eugene, OR). After treatment, cells were harvested by centrifugation (300g for 5 minutes), incubated for 20 minutes at 37°C with 0.1 μM DiOC6 in fresh medium, washed once with PBS, and analyzed by flow cytometry.
Fluorescence microscopy of activated caspase-3. After treatment, cells were spotted on microscope slides and fixed in acetone. Active caspase-3 staining was performed with mAb 5A1 (Cell Signaling Technology, Beverly, MA) and secondary FITC-conjugated antibody (DAKO, Carpinteria, CA). DAPI was used to stain all nuclei. Specific staining was evaluated using a Quantimed 600S fluorescence microscope (Leica Camera, Solms, Germany) equipped with a Leica PL Fluotar 40×/0.70 objective lens. Imaging was performed with Lytofluor imaging medium; images were captured with a Leica DC350 FX camera, and were acquired through Leica QWin 3 software.
Detection of apoptotic DNA fragmentation. DNA fragmentation was analyzed using mAb F7-26 (Alexis) according to manufacturer's recommendations. mAb F7-26 specifically detects DNA fragmented by apoptosis without reactivity for otherwise fragmented double-stranded DNA.45
Differential quantification of apoptosis in target and bystander cells in mixed-culture experiments
For mixed-culture experiments, CD7-positive target cells were labeled with the red fluorescent dye DiI (Molecular Probes). Briefly, cells (1.0 × 106 mL-1) were incubated for 5 minutes at 37°C in serum-free medium containing 5 μM DiI, followed by 3 washes with standard medium. DiI-labeled target and nonlabeled bystander cells were mixed at indicated ratios with a final cell concentration of 0.5 × 106 cells/well in a 48-well plate. After treatment, differential fluorescent characteristics of target cells and bystander cells were used to separately evaluate apoptosis by PS exposure to the outer cell membrane or by ΔΨ.
Additive induction of apoptosis by scFvCD7:sFasL, chemotherapeutics, and small inhibitory molecules
Additive apoptotic effects of treatment of cells with scFvCD7:sFasL and various chemotherapeutics or small inhibitory molecules was determined using the cooperativity index (CI), in which the sum of apoptosis induced by single-agent treatment is divided by apoptosis induced by combination treatment. When CI was less than 1, treatment was considered synergistic; when CI equaled 1, treatment was considered additive; and when CI was greater than 1, treatment was considered antagonistic.
CD7-restricted induction of apoptosis in patient-derived leukemic cells
Blood cells derived from 4 individual patients with T-ALL (patient nos. 1 to 4), 1 patient with PTCL (patient no. 5), and 1 patient with CD7-positive AML (patient no. 6) were treated for 16 hours with scFvCD7:sFasL (150 ng/mL) in the presence or absence of mAb TH69 where indicated. In addition, blood cells derived from patient no. 6 (AML) were cotreated with either vincristine or amsacrine. Apoptosis was assessed by PS exposure to the outer cell membrane and staining for active caspase-3 as described.
Results
Fractionation and stability of homotrimeric scFvCD7:sFasL
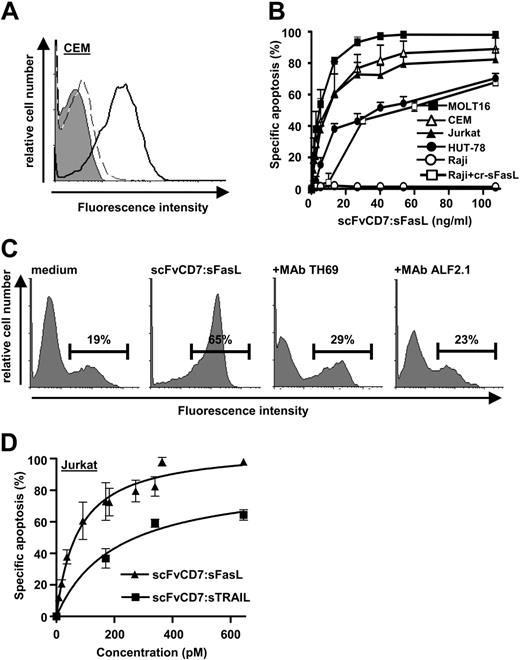
Fractionation of crude supernatant containing scFvCD7:sFasL by SE-FPLC and subsequent assessment of the apoptotic activity of each separate fraction revealed 2 peaks of apoptotic activity of 160 kDa and 700 kDa, respectively (Figure 1A). The peak corresponding to a molecular weight (MW) of approximately 160 kDa closely resembles that of the calculated MW of 147 kDa for homotrimeric scFvCD7:sFasL. Samples taken from this peak showed strong and CD7-restricted induction of apoptosis toward MOLT16 cells, which was completely abrogated by pretreatment with CD7-blocking mAb TH69. Samples taken from the 700-kDa peak showed strong apoptosis induction toward both CD7-positive MOLT16 cells and CD7-negative Ramos cells, which in contrast to the 160-kDa peak could not be inhibited by pretreatment with mAb TH69. Fractions from the 700-kDa peak were discarded. Fractions from the 160-kDa peak containing homotrimeric scFvCD7:sFasL were pooled and used for further experimental procedures and analyses. Subsequently, we analyzed for secondary formation of scFvCD7:sFasL multimers or aggregates. Prolonged storage of up to 7 days at 37°C in medium containing 15% serum did not lead to any detectable secondary formation of multimers or aggregates (data not shown). Furthermore, samples of scFvCD7:sFasL stored for up to 9 days at 37°C in the presence of serum retained potent and CD7-restricted apoptotic activity toward FasL-sensitive MOLT16 cells (Figure 1B).
CD7-restricted induction of apoptosis by homotrimeric scFvCD7:sFasL
Incubation of CEM cells with homotrimeric scFvCD7:sFasL resulted in strong and specific binding to the cell surface (Figure 2A, solid line), which was inhibited by preincubation with mAb TH69 (Figure 2A, dotted line). Treatment of a series of CD7-positive leukemic T-cell lines with serially increasing concentrations of homotrimeric scFvCD7:sFasL (1-110 ng/mL) dose-dependently increased apoptosis in all CD7-positive T-cell lines tested (Figure 2B; Jurkat, 82%; CEM, 90%; Hut78, 70%; and MOLT16, 98%). CD7-negative Raji cells were fully resistant to scFvCD7:sFasL (Figure 2B; 2.0%), but were sensitive to secondarily cross-linked sFasL (Figure 2B; 78%). Apoptosis by homotrimeric scFvCD7:sFasL was associated with apoptotic DNA-fragmentation and was inhibited by pretreatment with mAb TH69 or coincubation with FasL-neutralizing MAb Alf2.1 (Figure 2C). Interestingly, when Jurkat cells were treated with equimolar concentrations of scFvCD7:sFasL and the previously described scFvCD7:sTRAIL fusion protein,29 scFvCD7:sFasL displayed approximately 4-fold more potent apoptotic activity (Figure 2D; EC50 ∼70 pM, EC50 ∼275 pM, respectively). Similar results were obtained using CEM cells (data not shown).
Solution behavior and stability of scFvCD7:sFasL. (A) Culture medium derived from CHO-K1 production cells containing scFvCD7:sFasL was subjected to SE-FPLC using a calibrated HiLoad 16/60 FPLC column. The apoptotic activity of each individual fraction was assessed using the FasL-sensitive CD7-positive MOLT16 cells in the presence or absence of CD7-blocking mAb TH69. The horizontal bar in the graph indicates the fractions pooled for further testing. (B) scFvCD7:sFasL was stored for various lengths of time, up to 9 days, at 37°C in the presence of 15% serum. At serial time points the remaining apoptotic activity of the stored scFvCD7:sFasL was assessed by treatment of FasL-sensitive CD7-positive CEM cells for 16 hours. Apoptotic activity was assessed by ΔΨ.
Solution behavior and stability of scFvCD7:sFasL. (A) Culture medium derived from CHO-K1 production cells containing scFvCD7:sFasL was subjected to SE-FPLC using a calibrated HiLoad 16/60 FPLC column. The apoptotic activity of each individual fraction was assessed using the FasL-sensitive CD7-positive MOLT16 cells in the presence or absence of CD7-blocking mAb TH69. The horizontal bar in the graph indicates the fractions pooled for further testing. (B) scFvCD7:sFasL was stored for various lengths of time, up to 9 days, at 37°C in the presence of 15% serum. At serial time points the remaining apoptotic activity of the stored scFvCD7:sFasL was assessed by treatment of FasL-sensitive CD7-positive CEM cells for 16 hours. Apoptotic activity was assessed by ΔΨ.
CD7-restricted binding and apoptosis induction by scFvCD7:sFasL. (A) CD7-restricted binding of scFvCD7:sFasL was assessed using CD7-positive CEM cells. Cells were incubated with scFvCD7:sFasL (solid line) or with unconditioned medium (filled area). Specific binding was demonstrated by preincubating CEM cells with mAb TH69 followed by incubation with scFvCD7:sFasL (dashed line). Binding of scFvCD7:sFasL was determined by flow cytometry using PE-conjugated anti-FasL mAb. (B) CD7-restricted induction of apoptosis by scFvCD7:sFasL was assessed using CD7-positive cells (MOLT-16, CEM, Jurkat, and HUT-78) and CD7-negative Raji cells. All cell types were treated for 16 hours with increasing concentrations of scFvCD7:sFasL, after which apoptosis was assessed by ΔΨ. Additionally, Raji cells were treated with increasing concentrations of cross-linked sFasL (cr-sFasL). Indicated values are mean + SEM of 3 independent experiments. (C) Jurkat cells were treated for 16 hours with scFvCD7:sFasL (20 ng/mL) in the presence or absence of MAb TH69 or FasL-neutralizing MAb Alf2.1. Apoptosis was assessed by staining for apoptotic DNA-fragmentation using mAb F7-26. Horizontal bars indicate the percentage of apoptosis. (D) To compare the specific apoptotic activity of scFvCD7:sFasL with that of the related TRAIL fusion protein scFvCD7: sTRAIL, Jurkat cells were treated with equimolar concentrations of scFvCD7:sFasL and scFvCD7:sTRAIL for 16h. After treatment, apoptosis was assessed by ΔΨ.
CD7-restricted binding and apoptosis induction by scFvCD7:sFasL. (A) CD7-restricted binding of scFvCD7:sFasL was assessed using CD7-positive CEM cells. Cells were incubated with scFvCD7:sFasL (solid line) or with unconditioned medium (filled area). Specific binding was demonstrated by preincubating CEM cells with mAb TH69 followed by incubation with scFvCD7:sFasL (dashed line). Binding of scFvCD7:sFasL was determined by flow cytometry using PE-conjugated anti-FasL mAb. (B) CD7-restricted induction of apoptosis by scFvCD7:sFasL was assessed using CD7-positive cells (MOLT-16, CEM, Jurkat, and HUT-78) and CD7-negative Raji cells. All cell types were treated for 16 hours with increasing concentrations of scFvCD7:sFasL, after which apoptosis was assessed by ΔΨ. Additionally, Raji cells were treated with increasing concentrations of cross-linked sFasL (cr-sFasL). Indicated values are mean + SEM of 3 independent experiments. (C) Jurkat cells were treated for 16 hours with scFvCD7:sFasL (20 ng/mL) in the presence or absence of MAb TH69 or FasL-neutralizing MAb Alf2.1. Apoptosis was assessed by staining for apoptotic DNA-fragmentation using mAb F7-26. Horizontal bars indicate the percentage of apoptosis. (D) To compare the specific apoptotic activity of scFvCD7:sFasL with that of the related TRAIL fusion protein scFvCD7: sTRAIL, Jurkat cells were treated with equimolar concentrations of scFvCD7:sFasL and scFvCD7:sTRAIL for 16h. After treatment, apoptosis was assessed by ΔΨ.
Potent antitumor bystander apoptosis induction by homotrimeric scFvCD7:sFasL
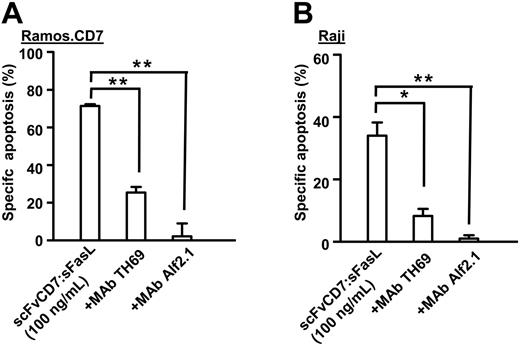
Cell-surface accretion of homotrimeric scFvCD7:sFasL on CD7-positive target cells can be exploited to cross-link Fas on neighboring tumor cells that are devoid of CD7 expression, a principle known as bystander effect.46 The proapoptotic bystander effect of homotrimeric scFvCD7:sFasL was assessed using mixed-culture experiments in which Ramos.CD7+ target cells were mixed with CD7-negative Raji bystander cells (ratio 1:1). In Ramos.CD7 target cells, scFvCD7:sFasL potently induced apoptosis (Figure 3A). In addition, in Raji bystander cells, a bystander apoptotic effect of up to 34% was detected (Figure 3B). In both target and bystander cells, apoptosis was specifically inhibited in the presence of mAb TH69 or mAb Alf2.1 (Figure 3A-B). No bystander apoptosis was observed when parental CD7-negative Ramos cells were used in this experiment (data not shown).
Absence of apoptotic activity of homotrimeric scFvCD7:sFasL toward PBLs and leukocytes
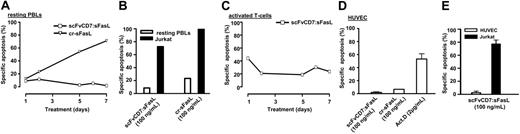
A subpopulation of normal PBLs is CD7-positive and might sustain collateral damage during treatment. Therefore, we investigated the apoptotic activity of homotrimeric scFvCD7:sFasL toward normal PBLs. To this end, PBLs were treated with excess amounts of scFvCD7:sFasL (325 ng/mL) for up to 7 days, which revealed no specific induction of apoptosis. In contrast, treatment with cross-linked sFasL (100 ng/mL) did result in increasing amounts of apoptosis in time (Figure 4A). Next, we assessed a possible “innocent” bystander apoptotic effect toward normal human leukocytes when homotrimeric scFvCD7:sFasL is present in a membrane-bound state at the cell surface of CD7-positive tumor cells (Jurkat). Treatment of mixed cultures of Jurkat cells and freshly isolated leukocytes (ratio 4:1) with homotrimeric scFvCD7:sFasL revealed no increase in apoptosis in leukocytes (Figure 4B; PBLs, 8%; Jurkat, 72%). Again, cross-linked sFasL did induce apoptosis in both Jurkat and PBLs (Figure 4B; PBLs, 23%; Jurkat, 70%).
Moderate apoptotic activity of homotrimeric scFvCD7:sFasL toward activated T cells
Treatment of anti-CD3/IL-2 activated T cells with excess amounts of homotrimeric scFvCD7:sFasL(325 ng/mL) induced apoptosis in approximately 45% of activated T cells at day 1 (Figure 4C). Up to day 7, scFvCD7:sFasL consistently induced apoptosis of approximately 15%.
No apoptotic activity toward resting HUVECs
To simulate the effect of scFvCD7:sFasL on human endothelial cells, HUVECs were treated with excess amounts of homotrimeric scFvCD7:sFasL. No apoptosis was detected after 24 hours of treatment with homotrimeric scFvCD7:sFasL (Figure 4D; 2%), whereas treatment with cross-linked sFasL or actinomycin D significantly induced apoptosis (Figure 4D; 7% and 53%, respectively).
Potent induction of apoptosis in CD7-negative bystander tumor cells. Mixed cultures of Ramos.CD7 target cells and Raji bystander cells (ratio 1:1) were treated for 16 hours with scFvCD7:sFasL (100 ng/mL) in the presence or absence of mAb TH69 or mAb Alf2.1. The differential fluorescent labeling of target and bystander cell populations was used to separately evaluate apoptosis induction by ΔΨ in Ramos.CD7 target cells (A) and Raji bystander cells (B). Indicated values are mean + SEM of 3 independent experiments. Statistical analysis was performed using 2-tailed Student t test. *P < .05; **P < .01.
Potent induction of apoptosis in CD7-negative bystander tumor cells. Mixed cultures of Ramos.CD7 target cells and Raji bystander cells (ratio 1:1) were treated for 16 hours with scFvCD7:sFasL (100 ng/mL) in the presence or absence of mAb TH69 or mAb Alf2.1. The differential fluorescent labeling of target and bystander cell populations was used to separately evaluate apoptosis induction by ΔΨ in Ramos.CD7 target cells (A) and Raji bystander cells (B). Indicated values are mean + SEM of 3 independent experiments. Statistical analysis was performed using 2-tailed Student t test. *P < .05; **P < .01.
Treatment of normal human leukocytes, activated T cells, and HUVECs with scFvCD7:sFasL. (A) Resting PBLs were subjected to treatment with scFvCD7:sFasL (325 ng/mL), or cross-linked sFasL (100 ng/mL) for up to 7 days, after which experimental apoptosis was assessed by annexin V/PI staining. Indicated values are representatives of 3 independent experiments. (B) Isolated PBLs were mixed at a ratio of 1:10 with DiI-labeled Jurkat cells. Mixed cultures were treated for 24 hours with scFvCD7:sFasL (100 ng/mL) or secondarily cross-linked sFasL (100 ng/mL). Differential fluorescent labeling of Jurkat target cells and PBLs was used to separately evaluate apoptosis induction by annexinV staining. Indicated values are representatives of 3 independent experiments. (C) Activated T cells were subjected to treatment with scFvCD7:sFasL (325 ng/mL) for up to 7 days, after which apoptosis was assessed by annexin V/PI staining. Indicated values are representatives of 3 independent experiments. (D) Resting HUVECs were treated for 24 hours with scFvCD7:sFasL (100 ng/mL), secondarily cross-linked sFasL (100 ng/mL), or actinomycin D (2 μg/mL). Apoptosis was assessed by ΔΨ. (E) Resting HUVECs were mixed with fluorescently labeled Jurkat cells (ratio 1:1) and treated with scFvCD7:sFasL (100 ng/mL) or actinomycin D (2 μg/ml) for 24 hours. Differential fluorescent labeling of Jurkat target cells and HUVEC bystander cells was used to separately evaluate apoptosis by ΔΨ.
Treatment of normal human leukocytes, activated T cells, and HUVECs with scFvCD7:sFasL. (A) Resting PBLs were subjected to treatment with scFvCD7:sFasL (325 ng/mL), or cross-linked sFasL (100 ng/mL) for up to 7 days, after which experimental apoptosis was assessed by annexin V/PI staining. Indicated values are representatives of 3 independent experiments. (B) Isolated PBLs were mixed at a ratio of 1:10 with DiI-labeled Jurkat cells. Mixed cultures were treated for 24 hours with scFvCD7:sFasL (100 ng/mL) or secondarily cross-linked sFasL (100 ng/mL). Differential fluorescent labeling of Jurkat target cells and PBLs was used to separately evaluate apoptosis induction by annexinV staining. Indicated values are representatives of 3 independent experiments. (C) Activated T cells were subjected to treatment with scFvCD7:sFasL (325 ng/mL) for up to 7 days, after which apoptosis was assessed by annexin V/PI staining. Indicated values are representatives of 3 independent experiments. (D) Resting HUVECs were treated for 24 hours with scFvCD7:sFasL (100 ng/mL), secondarily cross-linked sFasL (100 ng/mL), or actinomycin D (2 μg/mL). Apoptosis was assessed by ΔΨ. (E) Resting HUVECs were mixed with fluorescently labeled Jurkat cells (ratio 1:1) and treated with scFvCD7:sFasL (100 ng/mL) or actinomycin D (2 μg/ml) for 24 hours. Differential fluorescent labeling of Jurkat target cells and HUVEC bystander cells was used to separately evaluate apoptosis by ΔΨ.
When homotrimeric scFvCD7:sFasL is bound to the cell surface of circulating leukemic T cells, a possible innocent bystander effect toward endothelial cells might occur. We simulated this situation by treatment of mixed cultures of HUVECs and Jurkat cells (ratio 1:1) with homotrimeric scFvCD7:sFasL. In these experiments, resting HUVECs proved to be fully resistant to the possible innocent bystander effect of homotrimeric scFvCD7:sFasL (Figure 4E; resting HUVECs, 2%; Jurkat, 77%).
Additive tumoricidal effects of scFvCD7:sFasL with chemotherapeutics and small inhibitory molecules
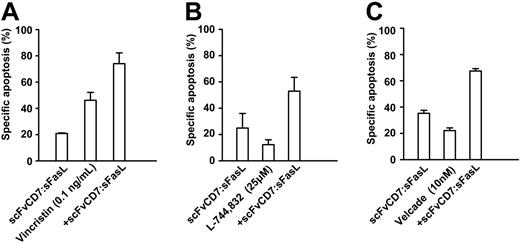
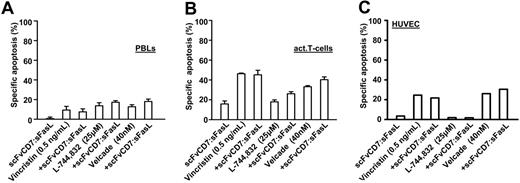
Sensitivity to FasL-induced apoptosis has previously been shown to be augmented by pre- or cotreatment with various chemotherapeutics and small inhibitory molecules. To establish whether scFvCD7:sFasL had similar properties, Jurkat cells were simultaneously treated with homotrimeric scFvCD7:sFasL and the micro-tubule inhibitor vincristine, the farnesyl transferase inhibitor L-744832, or the proteasome inhibitor bortezomib. Cotreatment with homotrimeric scFvCD7:sFasL and vincristine resulted in an increase in apoptosis compared with single-agent treatment (Figure 5A; CI = 0.9). Cotreatment with L-744832 or with bortezomib similarly increased apoptosis (Figure 5B-C, CI = 0.7 and 0.85, respectively). Identical treatment of PBLs, activated T cells, or HUVECs did not result in a significant increase in apoptosis compared with the respective chemotherapeutic agents alone (Figure 6).
Additive tumoricidal effect of scFvCD7:sFasL with several classes of antileukemia agents. Jurkat cells were treated for 16 hours with scFvCD7:sFasL (5 ng/mL) alone or in combination with vincristin (0.1 ng/mL) (A), farnesyl transferase inhibitor L-744832 (25 μM) (B), and the proteasome inhibitor bortezomib (10 nM) (C). Apoptosis induction was assessed by ΔΨ. The CI was calculated as described in “Materials and methods” and used to determine the cooperative effect of combination treatment.
Additive tumoricidal effect of scFvCD7:sFasL with several classes of antileukemia agents. Jurkat cells were treated for 16 hours with scFvCD7:sFasL (5 ng/mL) alone or in combination with vincristin (0.1 ng/mL) (A), farnesyl transferase inhibitor L-744832 (25 μM) (B), and the proteasome inhibitor bortezomib (10 nM) (C). Apoptosis induction was assessed by ΔΨ. The CI was calculated as described in “Materials and methods” and used to determine the cooperative effect of combination treatment.
Treatment in vitro of T-ALL, PTCL, and CD7-positive AML patient-derived leukemic blood samples
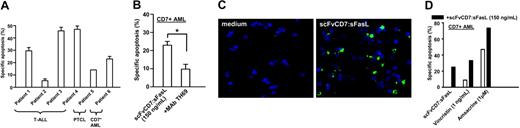
Blood samples derived from 6 patients suffering from various forms of CD7-positive leukemia were treated in vitro with homotrimeric scFvCD7:sFasL. The samples included T-ALL (patient nos. 1-4), PTCL (patient no. 5), and CD7-positive AML (patient no. 6), all containing more than 90% leukemic cells (Figure 7A). In 3 of 4 T-ALL patient samples, treatment with homotrimeric scFvCD7: sFasL resulted in a marked increase in apoptosis induction (30%, 45%, and 46% for patient nos. 1, 3, and 4, respectively). Tumor cells from T-ALL patient no. 2 were refractory to treatment (5% apoptosis). Tumor cells derived from patient no. 5 (PTCL) showed a moderate response of 14%, while treatment of tumor cells derived from patient no. 6 (CD7-positive AML) resulted in a 23% increase in apoptosis. Coincubation with mAb TH69 inhibited induction of apoptosis by homotrimeric scFvCD7:sFasL, as exemplified by the result from patient no. 6 (Figure 7B). Similar data were obtained for the other primary patient samples tested (data not shown).
After treatment, cells from patient no. 4 were analyzed for the formation of active caspase 3. Specific staining indicated the activation of caspase 3 in more than 20% of the tumor cells, whereas untreated cells showed no formation of active caspase 3 (Figure 7C).
Additive apoptotic effect of treatment of primary leukemic cells with scFvCD7:sFasL and chemotherapeutic agents
Discussion
Agonistic anti-Fas antibodies and multimeric recombinant FasL preparations show highly potent antileukemia activity. However, attempts to exploit these conventional Fas agonists for cancer therapy have met with unacceptable systemic toxicity, largely excluding Fas signaling as a therapeutic strategy for treatment of human malignancies.
Cotreatment of PBLs, activated T cells, or HUVECs with scFvCD7:sFasL and several classes of antileukemia agents. PBLs (A), activated T cells (B), and HUVECs (C) were treated with scFvCD7:sFasL (40 ng/mL) combined with vincristine (0.5 ng/mL), L-744832 (25 μM), or bortezomib (40 nM). Apoptosis was assessed by annexin V/PI staining.
Cotreatment of PBLs, activated T cells, or HUVECs with scFvCD7:sFasL and several classes of antileukemia agents. PBLs (A), activated T cells (B), and HUVECs (C) were treated with scFvCD7:sFasL (40 ng/mL) combined with vincristine (0.5 ng/mL), L-744832 (25 μM), or bortezomib (40 nM). Apoptosis was assessed by annexin V/PI staining.
The toxicity observed for sFasL preparations appears to be directly related to the presence of multimeric and aggregated sFasL species that are frequently formed during or after overexpression in primitive host cells such as Escherichia coli. Moreover, it was shown that as few as 2 adjacent trimeric (hexameric) sFasL molecules are already sufficient for Fas signaling in Raji, Jurkat, and activated T cells, as was evidenced by the formation of a death-inducing signaling complex (DISC) and subsequent apoptosis induction.47 We produced scFvCD7:sFasL in Chinese hamster ovary (CHO) cells, a currently favored industrial host-cell type for production of therapeutic recombinant proteins. We used a murine kappa light-chain leader peptide to direct produced scFvCD7: sFasL through the endoplasmic reticulum (ER) and Golgi complex, thus taking advantage of the associated stringent quality control mechanisms that facilitates secretion of correctly folded and nonaggregated scFvCD7:sFasL into the culture medium. Using SE chromatography we removed most, if not all, unwanted multimeric forms of scFvCD7:sFasL. Importantly, prolonged storage of homotrimeric scFvCD7:sFasL at 37°C in the presence of serum did not result in any detectable secondary aggregate formation, whereas apoptotic activity was retained. From this we conclude that scFvCD7:sFasL can be produced as soluble homogeneous homotrimers with no or only minimal secondary aggregate formation.
These features enable the specific targeting of biologically inactive homotrimeric scFvCD7sFasL to CD7-positive cells after which sFasL activation is induced by immobilization and multimerization on the target-cell surface. Treatment of a number of CD7-positive leukemic T-cell lines with scFvCD7:sFasL showed potent dose-dependent induction of apoptosis, which was specifically inhibited by pretreatment with mAb TH69 or coincubation with Fas-neutralizing mAb Alf2.1. Flow cytometric analysis identified that binding of scFvCD7:sFasL to Fas was barely detectable (data not shown). This observation can be explained by the fact that antibody-based proteins typically have fast-on and slow off-binding rates (for review see Hudson48 ), whereas binding of FasL to Fas (both trimers) is characterized by fast on/fast off rates49 typical for cytokine/cytokine receptor interaction. Moreover, binding of homotrimeric scFvCD7:sFasL via the antibody domains substantially benefits from the associated avidity effect of the presence of 3 scFv reading heads.50,51 Although not investigated here, this enhanced avidity can be inferred from the fact that even molar excess of mAb TH69 cannot fully abrogate the apoptotic activity of scFvCD7:sFasL. From this we concluded that biologically inactive homotrimeric scFvCD7:sFasL acquires full bioactivity only upon binding to cell-surface-expressed CD7, and that apoptosis is commenced by reciprocal cross-linking of Fas in an autocrine or paracrine manner.
Treatment of T-ALL, PTCL, and CD7-positive AML patient-derived blood samples in vitro. (A) Blood cells derived from 4 patients with T-ALL, 1 patient with PTCL, and 1 patient with CD7-positive AML were subjected to treatment with scFvCD7:sFasL (150 ng/mL) and analyzed for apoptosis induction using annexin V/PI staining. (B) Primary CD7-positive AML cells were subjected to treatment with scFvCD7:sFasL alone or in the presence of mAb TH69 for 16 hours, after which apoptosis was assessed by annexin V/PI staining. Statistical analysis was performed using 2-tailed Student t test. *P < .05. (C) Primary T-ALL cells were subjected to scFvCD7:sFasL (150 ng/mL) and then assessed for the presence of active caspase-3 using fluorescent microscopy as described in “Fluorescence microscopy of activated caspase-3.” (D) Primary CD7-positive AML cells were subjected to single-agent treatment with scFvCD7:sFasL (100 ng/mL), vincristine (10 ng/mL), amsacrine (1 μM), or to combination treatment. Apoptosis was assessed by annexin V/PI staining. The CI was used to determine the cooperative effect of combination treatment.
Treatment of T-ALL, PTCL, and CD7-positive AML patient-derived blood samples in vitro. (A) Blood cells derived from 4 patients with T-ALL, 1 patient with PTCL, and 1 patient with CD7-positive AML were subjected to treatment with scFvCD7:sFasL (150 ng/mL) and analyzed for apoptosis induction using annexin V/PI staining. (B) Primary CD7-positive AML cells were subjected to treatment with scFvCD7:sFasL alone or in the presence of mAb TH69 for 16 hours, after which apoptosis was assessed by annexin V/PI staining. Statistical analysis was performed using 2-tailed Student t test. *P < .05. (C) Primary T-ALL cells were subjected to scFvCD7:sFasL (150 ng/mL) and then assessed for the presence of active caspase-3 using fluorescent microscopy as described in “Fluorescence microscopy of activated caspase-3.” (D) Primary CD7-positive AML cells were subjected to single-agent treatment with scFvCD7:sFasL (100 ng/mL), vincristine (10 ng/mL), amsacrine (1 μM), or to combination treatment. Apoptosis was assessed by annexin V/PI staining. The CI was used to determine the cooperative effect of combination treatment.
The paracrine activation of Fas by CD7-immobilized scFvCD7: sFasL opens up the possibility of apoptosis induction in neighboring FasL-sensitive leukemia cells that have lost CD7 expression. Recently, we reported on an exceptionally strong antitumor bystander effect for an analogous scFv:sTRAIL fusion protein.46 Similarly, the paracrine activation of Fas by CD7-immobilized scFvCD7:sFasL opens up the possibility of apoptosis induction in neighboring FasL-sensitive leukemia cells that have lost CD7 expression.
In the present study, we found that scFvCD7:sFasL can induce a strong apoptotic effect toward bystander CD7-negative leukemic cells in mixed culture experiments with CD7-positive leukemic cells (Figure 3). This bystander effect was specifically inhibited by mAb TH69 or mAb Alf2.1, indicating that the bystander effect predominantly depends on activation of scFvCD7:sFasL on the cell surface of CD7-positive tumor cells. The bystander activity of scFvCD7:sFasL might be an important feature in the treatment of CD7-positive T-cell leukemia, since it has been described that treatment with anti-CD7 mAb results in CD7 down-modulation on tumor cells.41
Shedding, down-regulation, and target antigen modulation are likely to be responsible for a number of therapeutic failures observed in current antibody-based therapies. Recently, it has been reported that treatment with rituximab can result in loss of CD20 expression on lymphoma cells,52 leading to often fatal CD20-negative relapses. We speculate that treatment of non-Hodgkin lymphoma cells with a death-ligand fusion protein with specificity for CD20 may strongly benefit from the associated bystander effect. In contrast, only moderate bystander effects have been reported for immunotoxin-based strategies, most likely because these strategies usually depend on a number of necessary consecutive features, including internalization, intercellular gap junction communication, and enzymatic conversions.53,54
Previously, Samel et al28 provided proof of principle of targeted Fas signaling by fusing sFasL to a single-chain fragment of variable regions (scFv) specific for the fibroblast activation protein FAP, a tumor-associated stroma marker. The intravenous application of this novel Fas reagent in mice revealed no signs of systemic toxicity and prevented growth of xenotransplanted FAP-positive, but not FAP-negative, tumor cells. Nevertheless, from these elegant experiments it cannot be concluded that in humans a similar favorable toxicity profile will be observed.
In the current study, we explored the feasibility and safety of scFv-targeted Fas signaling in vitro by treating various CD7-positive human leukemia types in the absence or presence of normal human blood cells and endothelial cells (HUVECs). Treatment of PBLs with scFvCD7:sFasL did not induce apoptosis in any of the normal cell types present, including resting CD7-positive T cells and CD7-positive NK cells. This is a remarkable finding since specific binding of scFvCD7:sFasL to CD7 on these cells results in the local activation of scFvCD7:sFasL that is subsequently able to perform autocrine or paracrine Fas signaling. Apparently, normal cell types are relatively resistant to this form of Fas signaling. In contrast, treatment of anti-CD3/IL-2-activated T cells with homotrimeric scFvCD7:sFasL induced apoptosis in approximately 45% of activated T cells at day 1 (Figure 4C). It is well established that fratricidal Fas/FasL interactions between activated T cells are paramount to the effective resolution of an immune response.55 Although not studied here, we speculate that fusion proteins such as scFvCD7:sFasL, or those that target activation markers such as CD69, can be applied to treat T-cell-mediated autoimmunity.
Upon intravenous application in patients with leukemia, many different cell types will simultaneously encounter either free or cell-bound scFvCD7:sFasL. Binding of homotrimeric scFvCD7: sFasL to the cell surface of abundantly circulating leukemic T cells might lead to a potentially harmful innocent bystander effect toward normal cells (eg, endothelial cells). We simulated this situation using mixed-culture experiments in which HUVECs were cocultured with CD7-positive Jurkat cells (ratio 1:1) in the presence of homotrimeric scFvCD7:sFasL. In this experiment HUVECs proved to be fully resistant to a possible innocent bystander effect scFvCD7:sFasL treatment (Figure 4E).
Subsequently, we treated blood samples derived from 6 patients suffering from various forms of CD7-positive leukemias with homotrimeric scFvCD7:sFasL. Samples, all containing more than 90% leukemic cells, were derived from 4 patients with T-ALL, 1 patient with PTCL, and 1 patient with CD7-positive AML, respectively. Three of 4 T-ALL patient samples showed a marked increase in apoptosis induction (3%, 45%, and 46% for patient nos. 1, 3, and 4, respectively). Tumor cells from T-ALL patient no. 2 (5% apoptosis) were refractory to treatment. Tumor cells derived from patient no. 5 (PTCL) showed a moderate response of 14%, while treatment of tumor cells derived from patient no. 6 (CD7-positive AML) resulted in a 23% increase in apoptosis. At first glance, the therapeutic effect of scFvCD7:sFasL toward these primary tumor cells might seem rather moderate. However, ex vivo primary tumor cells typically grow in a nonsynchronized way at relatively low cell division rates. Previously, it was shown that leukemic cells are only sensitive to FasL-induced apoptosis in the G1 phase of the cell cycle.53,54 Subsequently, the effect observed ex vivo may be an underestimation of the therapeutic effect of scFvCD7:sFasL when applied in vivo. In addition, a marked variability in response was observed between patient samples, which might be related to differences in CD7 or Fas expression or, alternatively, differential expression of intracellular modulators of apoptosis such as the caspase-8 homolog cFLIP.
Various chemotherapeutic agents are known to sensitize tumor cells to Fas-mediated apoptosis at distinct levels, including receptor-proximal, mitochondrial, and/or effector-caspase level. We subjected blood samples derived from patient no. 6 (CD7-positive AML) to cotreatment with scFvCD7:sFasL and suboptimal concentrations of the chemotherapeutic agents vincristine and amsacrine that are already part of clinical practice. Cotreatment resulted in promising additive effects on apoptosis induction (Figure 7D). Importantly, identical treatment of normal PBLs, activated T cells, and resting/activated HUVECs did not result in significant increases in apoptosis compared with chemotherapy alone.
In conclusion, we describe a novel and promising anti-T-cell leukemia agent that shows strong CD7-restricted tumoricidal activity toward various CD7-positive leukemia cell types that can be augmented with various chemotherapeutic agents and small inhibitory molecules such as bortezomib. Toxicity toward normal cells appears to be restricted to a subset of activated T cells. Obviously, more research is needed to evaluate toxicity toward other cells and tissues with an emphasis on liver toxicity. New in vitro technologies, including those involving the use of human liver organ slices, appear to be appropriate for this purpose. Alternatively, scFvCD7:sFasL might be an excellent candidate for the purging of bone marrow from malignant CD7-positive cells. In each case further preclinical evaluation for scFvCD7:sFasL is warranted.
Prepublished online as Blood First Edition Paper, December 6, 2005; DOI 10.1182/blood-2005-07-2929.
Supported by grants from the Dutch Cancer Society (RUG 2002-2668 and 2005-3358).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Martin Gramatzki, Judith van der Leij, and Theo Bijma. We also thank Geert Mesander and Jelleke Dokter-Fokkens for their excellent assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal