Abstract
A major determinant in platelet production is the megakaryocyte (MK) size that is regulated both by ploidization and the increase in cytoplasmic volume at the end of maturation. Here we investigated the involvement of the mammalian target of rapamycin (mTOR) pathway in the regulation of megakaryopoiesis. We show that phosphorylation of mTOR, p70S6K1, and 4E-BP1 was diminished in thrombopoietin-cultured human MKs after rapamycin treatment. Rapamycin induced an inhibition in the G1/S transition and a decrease in the mean MK ploidy via a diminution of p21 and cyclin D3 occurring at a transcriptional level. Both cycling (2N/4N) and polyploid (8N/16N) MKs were reduced in size, with a size reduction slightly more pronounced in mature polyploid MKs than in immature ones. Rapamycin also induced a delay in the expression of MK markers and prevented the generation of proplatelet MKs. Additional experiments performed in vitro with MKs from mutant mice showed that the decrease in mean ploidy level and the delay in MK differentiation in the presence of rapamycin were less pronounced in CdknIa (p21)–/– MKs than in CdknIa (p21)+/+ MKs. These findings indicate that the mTOR pathway plays an important role during megakaryopoiesis by regulating ploidy, cell size, and maturation, in part by regulating p21 and cyclin D3.
Introduction
During megakaryocyte (MK) differentiation, the MK progenitor switches from a classic mitosis to an endomitosis corresponding to DNA replication without karyokinesis and cytokinesis. This process leads to a giant cell containing a single polylobulated nucleus with a 2N ploidy. The principal role of ploidization is to increase the MK size, particularly the volume of the cytoplasm, which may increase more strikingly than the ploidy level. As platelets arise from MK cytoplasm fragmentation, ploidization is an efficient manner to amplify the MK mass and thus platelet production.
Thrombopoietin (TPO), the ligand for the Mpl receptor, stimulates the proliferation and differentiation of MK progenitor cells in vitro and in vivo and promotes their ploidization and their cytoplasmic maturation. Although TPO is not directly involved in proplatelet formation, this growth factor increases platelet production by augmenting MK number, polyploidization, and by inducing cytoplasm maturation. The binding of TPO to Mpl activates various types of intracellular signaling pathways that play important roles in the regulation of megakaryocytopoiesis, such as the Janus kinase (JAK)/signal transducer and activator (STAT), Ras/Raf/mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI-3K), and protein kinase C (PKC) pathways. Indeed, activated STAT3 promotes the expansion of megakaryocytic progenitor cells,1 the STAT5 and Ras pathways are involved in TPO-induced proliferation, and prolonged activation of the MAPK/extracellular signal–related kinase (ERK) pathway is required for TPO-induced megakaryocytic differentiation.2 Activation of PKC-α is required for the process of proplatelet formation,3 and TPO-induced PI-3K activity is essential for optimal cell survival and cell cycling (G1/S transition) of MK progenitors.4 Protein kinase B (AKT) has been shown to be phosphorylated in normal human megakaryoblasts after stimulation by TPO in a PI-3K–dependent manner and to protect the cells from apoptosis.5
One downstream target of PI3-K/AKT/Rheb is mTOR (mammalian target of rapamycin; also known as RAFT [rapamycin and FKBP-12 target], FRAP [FKBP12-rapamycin–associated protein], or RAPT).6-8 mTOR is a Ser/Thr kinase that regulates cell growth (cell mass and cell size) and cell-cycle progression through the G1/S transition,9-11 2 processes shown to be coordinated in yeast,12 Drosophila,13,14 and mammals.15
The binding of rapamycin (Rapa), an immunosuppressive drug, to its cellular receptor FKBP12 directly inhibits mTOR-dependent downstream signaling. mTOR regulates cell growth via 2 effector proteins critical for ribosomal biogenesis and translational initiation, p70S6K1 and 4E-BP1. Activated p70S6 kinase initiates the translation of a class of mRNAs containing a tract of polypyrimidine (TOP) in their 5′ untranslated region (UTR).16 Upon phosphorylation, 4E-PB1 is inactivated and releases eIF4E, a protein that will be recruited to the translation initiation complex regulating cap-dependent translation.17 Recently, it has been demonstrated in human osteosarcoma cells that both S6K1 and 4E-PB1/eIF4E pathways are required for mTOR-dependent G1-phase progression, and that the activities of these 2 protein effectors are independent.18 However, in skeletal muscle cells, the deletion of S6K1 affects only cell size but not proliferation.19
Cell-cycle progression from G1 to S phase is controlled by D-type cyclins (D1, D2, and D3 with lineage-dependent expression) and cyclin E, which bind and activate their corresponding kinases (cdk4, cdk6 and cdk2). These complexes are involved in phosphorylation of the retinoblastoma protein (Rb) leading to activation of E2F transcription factor and progression to S phase of the cell cycle. Once phosphorylated, pRb regulates positively the transcription of cyclin D1, suggesting that progression through the G1 phase is regulated by a loop involving both Rb and cyclin D1.20 Cell cycle is regulated by the intracellular level of inhibitors of the Cip/Kip family (p27, p21 and p57), which act on the kinase activity of the G1 cyclin/cdks complexes.
Here, we investigated the involvement of mTOR pathway in megakaryopoiesis upon TPO stimulation in vitro. We show that blocking this pathway by rapamycin leads to a diminution in cell size and mean ploidy level of mature MKs, an inhibition of MK proliferation by blocking the G1/S transition, and a delay in MK maturation followed by a decrease in platelet formation. The data demonstrate that inhibition of G1/S transition and diminution in ploidy level is caused by a decrease of p21 and cyclin D3 at a transcriptional level. Using CdknIa (p21)–knockout mice, we confirmed an important role of mTOR/p21 pathway in megakaryopoiesis.
Materials and methods
Mice
Homozygous CdknIa (p21)–/– mice were a gift from Phil Leder (Harvard Medical School, Boston, MA). Homozygous CdknIa (p27)–/– mice were obtained from Andrew Koff (New York, NY) and Martin Goettlicher (Leopoldshafen-Eggenstein, Germany). CF1 mice and C57Bl/6 mice were used as controls, respectively.
Murine progenitor cells isolation
Lineage-negative (lin–) cells were purified from the femoral marrow after incubation with a mixture of rat monoclonal antibodies (mAbs) against lineage antigens (Gr1, TER119, CD11b [or Mac1], B220, and anti-CD3, all purchased from Pharmingen, San Diego, CA) and depletion with Dynabeads coupled to a mouse antibody against rat immunoglobulin (Dynal, Oslo, Norway).
Megakaryocyte culture from human CD34+ cells and murine lineage-negative cells and reagents
Cytapheresis samples from healthy individuals were obtained with their informed consent. Approval for the study was obtained from the Assistance Publique des Hôpitaux de Paris (AP-HP).
Human CD34+ cells were isolated from cytapheresis samples and incubated as previously described by Choi et al21 in serum-free medium supplemented with polyethylene glycol–recombinant human megakaryocyte growth and differentiation factor (PEG-rhuMGDF or TPO; 10 ng/mL). Murine lin– cells were grown in similar conditions. Rapamycin was purchased from Sigma (Saint Quentin Fallavier, France) and used at a concentration of 100 nM.
Western blot analysis
Cultured MKs were collected and washed twice with phosphate-buffered saline (PBS). Western blot analysis was usually performed on total protein extracts. Cells were sonicated for 10 seconds at 4°C in Laemli buffer containing 50 mM Tris HCl (pH 6.8), 4% sodium dodecyl sulfate (SDS), 20% glycerol, 100 mM dithiothreitol (DTT), and Coomassie blue. For analysis of transcription factors, Western blots were performed on separated nuclear and cytoplasmic extracts. Cells were incubated on ice for 10 minutes in buffer A (10 mM HEPES [pH 7.8]), 10 mM KCl, 2 mM MgCl2, 0.1 mM EDTA) with protease inhibitor cocktail and 10% NP40. After centrifugation for 3 minutes at 1800 g, supernatants were used as cytoplasmic extracts. Pellets were resuspended in buffer B (50 mM HEPES [pH 7.8]), 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, and protease inhibitor cocktail) and incubated on ice for 30 minutes. Nuclear debris was pelleted and the supernatants were used as nuclear extracts.
Proteins were separated on 8% to 15% SDS-PAGE, blotted onto a nitrocellulose membrane (Amersham, Buckinghamshire, United Kingdom). The following antibodies were used for immunoblotting: phospho–4E-BP1 (Thr37/46), 4E-BP1, phospho-mTOR (Ser2448), mTOR, phospho-p70 S6 kinase (Thr389), and p70 S6 kinase from Cell Signaling Technology (Ozyme, Saint Quentin Yvelines, France); anti–GATA-1 (C-20; sc-1233), anti–FLI-1 (sc-356), anti–cyclin D3 (D-7; sc-6283) and anti–HDAC-1 (H-51) from Santa Cruz Biotechnology (Santa Cruz, CA); anti-p21 from Pharmingen (Pont de Claix, France); and anti–TAL-1 (kindly provided by D. Pulford, LRF Immunodiagnostics Unit, University of Oxford, United Kingdom). Immunoblotting was performed according to the manufacturers' instruction. Immunoreactive bands were visualized by enhanced chemiluminiscence (Perbio Science, Brebières, France) after incubation with peroxidase-conjugated goat antimouse or goat antirabbit antibodies (Jackson ImmunoResearch laboratories, West Grove, PA). Coloration with Ponceau S solution (Sigma) after transfer and immunolabeling with the p70S6K antibody were used to control the quantity of proteins from TPO- and Rapa-treated cells in each experiment.
Immunolabeling for flow cytometry analysis
Human cultured CD34+ cells were rinsed in PBS, stained for 30 minutes at 4°C with an anti-CD41–APC or an anti-CD42–FITC mAb (Pharmingen, Pont de Claix, France), fixed in 0.5% paraformaldehyde for 10 minutes at room temperature, and permeabilized in 0.1% Triton X-100. Control cells were incubated with an irrelevant mouse immunoglobulin G1 (IgG1) antibody, washed twice in PBS and incubated for 30 minutes at 4°C with a FITC-conjugated goat anti–mouse IgG. Finally, the cell pellet was suspended in propidium iodide (PI) solution containing 1 × PBS, 50 μg/mL PI (Sigma), and 100 μg/mL RNAse A. The same protocol was used to stain von Willebrand factor (VWF) using a mouse anti–human VWF mAb (a gift from D. Meyer, INSERM U143, Hôpital de Bicêtre, Paris, France) as first antibody and an anti–mouse IgG–FITC as a second antibody, except that the cells were fixed and permeabilized before antibody staining. After an overnight incubation in PI solution, cell samples were analyzed on a FACSort (Becton Dickinson) equipped with an argon laser (15 mW, 480 nm excitation). About 50 000 cells were listed and analyzed with the Cellquest software package (Becton Dickinson, Mountain View, CA). The mean forward scatter height (FSC-H) of 2N, 4N, 8N, 16N, and 32N MK populations was determined as a measure of relative size.
To measure the ploidy of cultured murine MKs, cells were labeled with FITC-conjugated anti-CD41 mAb (Pharmingen, Becton Dickinson, Pont de Claix, France) after preincubation with an anti-CD16/CD32 Fc(III/II) (Pharmingen, Pont de Claix, France) to avoid nonspecific binding, washed, and incubated in a hypotonic citrate solution containing 50 μg/mL PI (Sigma) for at least 2 hours (usually overnight) at 4°C. RNAse A (50 μg/mL) was added to the cells before flow cytometry analysis.
Real-time quantitative RT-PCR
Total RNA was extracted from MKs using RNA-plus solution (Quantum Biotechnologies, Montreuil, France). Aliquots (1 μg) of total RNA were treated with RNAse free DNAse (Ambion, Huntingdon, Cambridgeshire, United Kingdom), denatured, and reverse transcribed with 200 U SuperScript II RNAse H+ reverse transcriptase (RT) (Invitrogen, Cergy Pontoise, France), as recommended by the manufacturers. Primers and internal probes for amplification of mTOR, p21, cyclin D3, TAL-1, GATA-1, and Fli-1 sequences were designed using Primer Express Software (Perkin-Elmer Applied Biosystems, Foster City, CA): mTOR: Forward 5′-CCCAGCTGCTGGAACAAAAA-3′, reverse 5′-GTCATGCCCACGTTCCTTAAC-3′, probe 5′-TGGCCCCATCACAGACTCGAATGA-3′; cyclin D3: Forward 5′-GACCATCGAAAAACTGTGCATCTA-3′, reverse 5′-CCCACTTGAGCTTCCCTAGGA-3′, probe 5′-CACGCTGTCTCTCCCCGCCAGTT-3′; p21: Forward CCTTGTGGAGCCGGAGCT-3′, reverse 5′-TTGCTGCCGCATGGG-3′, probe 5′-TCTGACATGGCGCCTCCTCTGAGT-3′; HPRT: Forward 5′-GGCAGTATAATCCAAAGATGGTCAA-3′, reverse 5′-TCAAATCCAACAAAGTCTGGCTTATAT-3′, probe 5′-CTTGCTGGTGAAAAGGACCCCACGA-3′; p27: Forward 5′-GCAATGCGCAGGAATAAGGA-3′, reverse 5′-TTTTCTTCTGTTCTGTTGGCTCTTT-3′, probe 5′-TGCAACCGATTCTTCTACTCA-3′; TAL1: Forward 5′-CTTCCCCCTATGAGATGGAGATTA-3′, reverse 5′-CCCGGCTGTTGGTGAAGAT-3′, probe 5′-CCCACACCAAAGTTGTGTGCGGC-3′; GATA-1: Forward 5′CTGGGATCACACTGAGCTTGC-3′, reverse 5′-GATTAACCTGGGCTGGTGGTT-3′, probe 5′-ACATCCCCAAGGCGGCCGA-3′; and Fli-1: Forward 5′-CCACCAACGAGAGGAGAGTCA-3′, reverse 5′-CATTGCCTCACATGCTCCTG-3′, probe 5′-CGTCCCCGCAGACCCCACAC-3′. Polymerase chain reactions (PCRs) were carried out in the ABI Prism GeneAmp 5700 Sequence Detection System (Perkin-Elmer Applied Biosystems, Foster City, CA) using the TaqMan Universal PCR Master Mix containing the specific primers (1.2 μM) and the specific probe (0.1 μM). The expression levels of p21 and cyclin D3 were expressed relative to mTOR, HPRT, and p27 and the expression levels of TAL-1, GATA-1, and FLI-1 were expressed relative to mTOR.
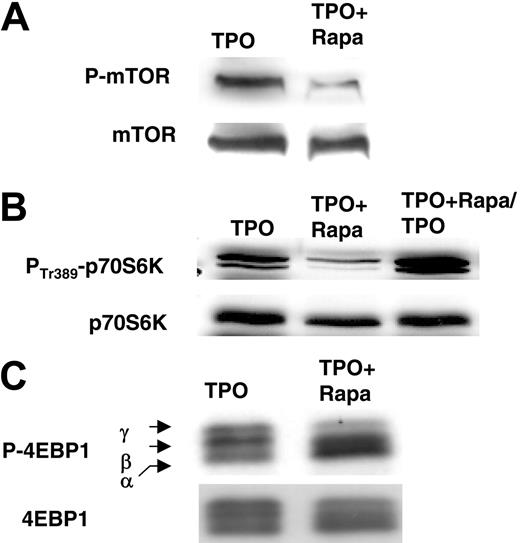
Effect of rapamycin on the phosphorylation of mTOR, p70S6K, and 4E-BP1. CD34+ cells were cultured in the presence of TPO without Rapa (TPO) or with Rapa (100 nM) added to the culture at day 0 and day 3 (TPO + Rapa). Cells were taken at day 6 to perform Western blot analyses. (A) Effect of Rapa on Ser2448 phosphorylation of mTOR. (B) Effect of Rapa on Thr389 of p70S6K. Inhibition of Thr389-phosphorylation by Rapa was reversible as shown after removing the medium containing TPO and Rapa at day 6, washing the cells twice in PBS, and restimulation for 1 day with fresh medium supplemented with TPO alone (TPO + Rapa/TPO). (C) Effect of Rapa on Thr37/46 of 4E-BP1. Accumulation of unphosphorylated (α), phosphorylated (β), and diminution of hyperphosphorylated (γ) forms is shown.
Effect of rapamycin on the phosphorylation of mTOR, p70S6K, and 4E-BP1. CD34+ cells were cultured in the presence of TPO without Rapa (TPO) or with Rapa (100 nM) added to the culture at day 0 and day 3 (TPO + Rapa). Cells were taken at day 6 to perform Western blot analyses. (A) Effect of Rapa on Ser2448 phosphorylation of mTOR. (B) Effect of Rapa on Thr389 of p70S6K. Inhibition of Thr389-phosphorylation by Rapa was reversible as shown after removing the medium containing TPO and Rapa at day 6, washing the cells twice in PBS, and restimulation for 1 day with fresh medium supplemented with TPO alone (TPO + Rapa/TPO). (C) Effect of Rapa on Thr37/46 of 4E-BP1. Accumulation of unphosphorylated (α), phosphorylated (β), and diminution of hyperphosphorylated (γ) forms is shown.
Enumeration of proplatelet MKs
MKs displaying proplatelets were defined as cells exhibiting 1 or more cytoplasmic processes with areas of constriction. The percentage of MKs with such processes was quantified by enumerating 500 cells/well (in 96-well plates) using an inverted microscope at a magnification of × 200.
Results
mTOR pathway is activated in MKs upon TPO stimulation in vitro
CD34+ cells from cytapheresis were grown in the presence of TPO and with or without the addition of Rapa at a concentration of 100 nM. To study the long-term effect of Rapa on the mTOR pathway, the compound was added at initiation of the culture, simultaneously with TPO, and again at 72 hours. Phosphorylation of mTOR and its 2 effector proteins p70S6 kinase and 4E-BP1 was analyzed on day 6 by Western blotting. In contrast to CD34+ cells grown with TPO alone, phosphorylation of mTOR was hardly detectable in cells growing in the presence of both TPO and Rapa (Figure 1A). Similarly, phosphorylation of the 2 mTOR effector proteins was partially inactivated by Rapa treatment (Figure 1B-C). This inactivation was a reversible process as shown by restoration of the phosphorylation of p70S6 kinase after Rapa deprivation and TPO restimulation (Figure 1B).
mTOR pathway is involved in the mitotic and endomitotic processes of MKs
When added at initiation of the culture and then every 72 hours, Rapa reduced 2 to 3 times the absolute number of MKs in the culture (data not shown) as well as their ploidy. This decrease in cell number was not the result of an increase in apoptosis since the percentage of Annexin V– and 7AAD-positive cells was similar at day 6 in cultures grown in the absence (11.13%) or in the presence (12.87%) of Rapa (n = 3, P < .05) (Figure 2A). In contrast, by means of BrDU incorporation, a marked decrease in cells in S and G2/M phases of the cell cycle was detected in culture treated with Rapa: from 14.66% to 10.44% for S phase and from 8.99% to 4.08% for G2/M phase at day 3 (n = 3, P < .05) and from 22.36% to 16.63% for S phase and from 5.65% to 3.63% for G2/M phase at day 6 (n = 3, P < .02) (Figure 2B), leading to an accumulation of cells in G1 in Rapa-treated culture. The prolonged mTOR inactivation also markedly decreased the mean ploidy level of CD42 expressing MKs (Figure 2C; n = 5, P < .002). However, when addition of Rapa was delayed (after day 2), no significant difference in the mean ploidy level between treated and a control culture was detected (Figure 2D; n = 3, P < .016).
Flow cytometry analysis of the effect of rapamycin on the cell cycle of megakaryocytes. CD34+ cells were cultured in the presence of TPO and Rapa (100 nM; TPO + Rapa) or without Rapa (TPO). (A) Apoptosis in cycling or resting MKs was evaluated by staining with Annexin V and 7AAD at day 6 of culture. (B) Analysis by BrDU incorporation and propidium iodide (PI) staining of G1, S, and G2/M phases of cell cycle at day 3 (D3) or day 6 (D6) in culture. (C) Rapa was added at days 0, 3, and 6 to the culture. Only CD42+ MKs were analyzed for ploidy level by PI staining at day 9 of culture. The mean ploidy was calculated from the number of cells of each ploidy level. (D) Rapa was added at day 3 and day 6 to the culture. The analysis of ploidy level was performed as in panel C. Each figure shows 1 representative analysis. Two additional experiments were performed for panels A and B and 3 for panels C and D with similar results.
Flow cytometry analysis of the effect of rapamycin on the cell cycle of megakaryocytes. CD34+ cells were cultured in the presence of TPO and Rapa (100 nM; TPO + Rapa) or without Rapa (TPO). (A) Apoptosis in cycling or resting MKs was evaluated by staining with Annexin V and 7AAD at day 6 of culture. (B) Analysis by BrDU incorporation and propidium iodide (PI) staining of G1, S, and G2/M phases of cell cycle at day 3 (D3) or day 6 (D6) in culture. (C) Rapa was added at days 0, 3, and 6 to the culture. Only CD42+ MKs were analyzed for ploidy level by PI staining at day 9 of culture. The mean ploidy was calculated from the number of cells of each ploidy level. (D) Rapa was added at day 3 and day 6 to the culture. The analysis of ploidy level was performed as in panel C. Each figure shows 1 representative analysis. Two additional experiments were performed for panels A and B and 3 for panels C and D with similar results.
These data indicate that the mTOR pathway might be involved not only during the stages when megakaryocytes divide by classic mitosis, but also during endoreduplication.
Rapamycin-induced inhibition of G1/S transition is associated with p21 and cyclin D3 decrease
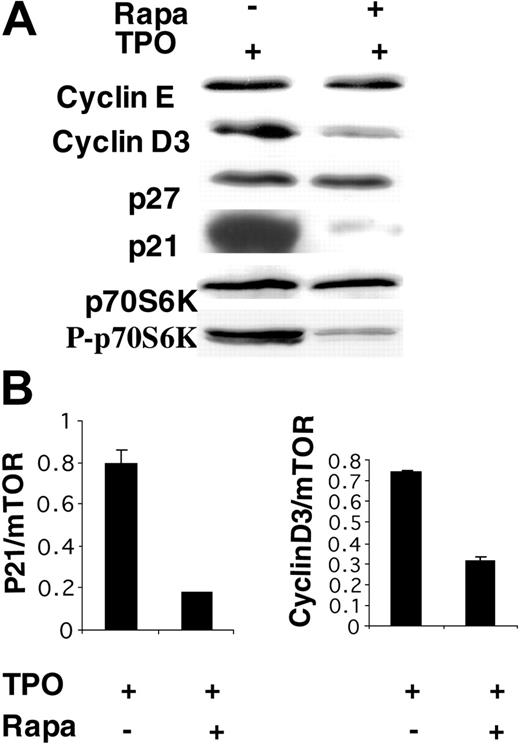
As described in Figure 2, treatment of TPO-stimulated CD34+ cells with Rapa induced a G1/S inhibition and a decrease in ploidization. To investigate precisely which cell-cycle proteins were deregulated, MKs were grown in the presence of 100 nM Rapa added to the culture at initiation and on day 3 or without Rapa. Western blot analyses were performed at day 6. In cells treated with Rapa, a decrease of cyclin D3 level and a quasi-disappearance of p21 protein were seen. Surprisingly, levels of cyclin E and p27 were unchanged. In these experiments, inhibition of the mTOR pathway was controlled by monitoring the level of phosphorylated p70S6 kinase (Figure 3A). As the mTOR pathway may regulate protein expression at different levels, we studied the level of p21 and cyclin D3 mRNAs by real-time RT-PCR. p21 and cyclin D3 mRNAs were decreased about 6- and 2-fold, respectively, when mTOR mRNA was taken as a reporter gene (Figure 3B). Similar results were obtained when HPRTI and CDKN1B were used as reporter genes (data not shown).
Rapamycin induces a diminution of cell size, a delay in MK differentiation, and a diminishing of proplatelet formation
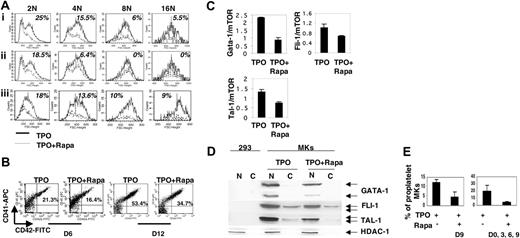
mTOR is implicated in the regulation of cell size in many cell types (reviewed in Inoki et al22 ). To study whether mTOR regulates MK cell size, MKs were analyzed by flow cytometry and the mean FSC-H was used as a surrogate parameter that correlates with cell size. This parameter was measured for each ploidy class. MKs were identified either by expression of the CD41 marker, which identifies both immature and mature MKs, and VWF, which increases during terminal differentiation. When Rapa was added at day 0, a decrease in the size of MK cells expressing CD41 was found. This decrease was more marked in the 2N/4N populations (extending to about 25% for 2N and 15% for 4N) than in 8N/16N cells (about 6% and 5%, respectively) with a very low number of 16N cells (Figure 4Ai). Comparable results were obtained in 2 additional experiments. When addition of Rapa was delayed until day 6 when the mTOR pathway was fully active and at a time when a majority of MK cells had switched to terminal differentiation, a diminution in MK size was seen only for the 2N/4N population (about 18% for 2N and 6% for 4N, n = 3; Figure 4Aii).
Effect of rapamycin on the expression of different proteins regulating cell cycle. (A) CD34+ cells were cultured in the presence of TPO without Rapa or with Rapa (100 nM) added at day 0 and day 3. Cells were analyzed by Western blot at day 6. To control the effect of Rapa, the diminution of p70S6K phosphorylation was ascertained in each experiment. Two cyclins (E and D3) and 2 inhibitors of the cycle (p21 and p27) were studied. The diminution of only cyclin D3 and p21 was detected. (B) Real-time RT-PCR was used to quantify p21 and cyclin D3 mRNA in MKs at day 6 of culture. The relative expression of p21 and cyclin D3 was calculated in comparison to mTOR mRNA, which was stable during Rapa treatment. Results are mean ± SD of triplicate determinations from a representative experiment (n = 4).
Effect of rapamycin on the expression of different proteins regulating cell cycle. (A) CD34+ cells were cultured in the presence of TPO without Rapa or with Rapa (100 nM) added at day 0 and day 3. Cells were analyzed by Western blot at day 6. To control the effect of Rapa, the diminution of p70S6K phosphorylation was ascertained in each experiment. Two cyclins (E and D3) and 2 inhibitors of the cycle (p21 and p27) were studied. The diminution of only cyclin D3 and p21 was detected. (B) Real-time RT-PCR was used to quantify p21 and cyclin D3 mRNA in MKs at day 6 of culture. The relative expression of p21 and cyclin D3 was calculated in comparison to mTOR mRNA, which was stable during Rapa treatment. Results are mean ± SD of triplicate determinations from a representative experiment (n = 4).
The major effect of Rapa on cell size was thus detected mainly on proliferating cells, which must increase their protein synthesis and cell size before dividing into 2 daughter cells. In order to perform the analysis only on postmitotic cells, Rapa was added at day 0 and then every 72 hours and FSC-H in MKs expressing high levels of VWF was measured. In these more mature MKs, Rapa treatment induced a cell-size reduction in all ploidy classes that appeared to be more marked in the 8N/16N populations labeled by the VWF than by the CD41 marker (Figure 4Ai,iii; n = 3). Taken together, these results show that the mTOR pathway not only regulates the MK size in immature proliferating cells, but also increases the size of the polyploid cells during maturation.
Proliferation and ploidization of MKs are followed by differentiation accompanied first by CD41 expression and later on by CD42 expression. As inhibition of the mTOR pathway induced a G1/S arrest and decreased ploidy levels, we studied the effects of Rapa on megakaryocytopoiesis using different markers of differentiation. When added at the initiation of culture, Rapa slightly decreased the percentage of mature MKs expressing simultaneously high level of CD41 and CD42 from 21% to 16% at day 6 of culture (Figure 4B; n = 4, P < .002). Analysis of mature MKs at day 12 of culture revealed that this diminution was not the consequence of a real blockage in differentiation because the percentage of CD41+CD42+ cells increased in a comparable manner in control (from 21% to 53%) and Rapa-treated (from 16% to 34%) cultures (Figure 4B; n = 4, P < .001). This result could be explained by an inhibition of proliferation or could be more specifically linked to a decrease in a MK transcription factor regulated at the translational level by mTOR. We thus studied the expression of 3 transcription factors involved in megakaryopoiesis, GATA-1, FLI-1, and TAL-1 at day 6 of culture. Their expression was decreased both at the mRNA (Figure 4C) and protein (Figure 4D) levels. However, as this study was performed on total cell culture and not on a purified CD41+CD42+ fraction, the decreased mRNA level of these 3 transcription factors could be explained by a reduced number of mature MKs expressing GATA-1, FLI-1, and TAL-1 in Rapa-treated culture. This suggests that the delay in MK differentiation induced by Rapa is more related to the G1/S blockage, resulting in a lower number of mature MKs than to a specific inhibition of MK differentiation. Finally, we studied the effect of Rapa treatment on in vitro formation of proplatelets. As shown in Figure 4E (right histogram), a marked decrease (about 60%) in MKs forming proplatelets was observed (n = 3, P < .01) when Rapa was sequentially added every 72 hours. Of note, in contrast to the effects on cell size and polyploidization, which require a permanent inhibition of the mTOR pathway by Rapa, delaying the addition of Rapa until day 9 of culture was still efficient to inhibit proplatelet formation (Figure 4E, left histogram; n = 3, P < .012). These data suggest that mTOR regulates proplatelet formation independently of polyploidization.
Effect of rapamycin on cell size, differentiation, and proplatelet formation of megakaryocytes. CD34+ cells were cultured in the presence of TPO and Rapa (100 nM) added at different days of culture (TPO + Rapa) or without Rapa (TPO). (A) Effect of Rapa on cell size. MKs were analyzed by flow cytometry, the mean forward scatter height was used as a parameter of cell size and was measured for each ploidy level (from 2N to 16N) determined by propidium iodide staining of MKs. (i) Rapa was added to the culture at day 0, day 3 and day 6. Only MKs expressing CD41 were analyzed on day 9. (ii) Rapa was added to the culture at day 6. Only MKs expressing CD41 were analyzed on day 9. (iii) Rapa was added to the culture at days 0, 3, and 6. Only MKs expressing von Willebrand factor (VWF) were analyzed on day 9. (B) Effect of Rapa on MK differentiation. Rapa was added to the culture at days 0, 3, 6, and 9. On day 6 (D6) and 12 (D12), the percentage of mature MKs expressing CD41 and CD42 antigens was analyzed by flow cytometry. (C) Effect of Rapa on the protein level of 3 transcription factors (GATA-1, FLI-1, and TAL-1) regulating megakaryocytopoiesis. Rapa was added to the MK culture at day 0 and day 3. Western blot analyses were performed at day 6 on nuclear (N) and cytoplasmic (C) protein extracts from total cultures treated or not with Rapa. 293 cells were used as a negative control for GATA-1, FLI-1, and TAL-1 expression and HDAC-1 was used as an internal control of nuclear protein integrity. (D) Effect of Rapa on mRNA level of GATA-1, FLI-1, and TAL-1 was evaluated by real-time RT-PCR at day 6 of culture in the presence or absence of Rapa. The relative expression of these transcription factors was calculated in comparison to mTOR mRNA, which was stable during Rapa treatment. (E) Rapa was added to the culture only at day 9 (D9; left histogram) or at days 0, 3, 6, and 9 (D0, 3, 6, 9; right histogram). At day 9, the cells were seeded at 2 × 103 cell/well in 96-well plate. At day 12, the percentage of MKs forming proplatelets was estimated by counting MKs exhibiting one or more cytoplasmic processes with areas of constriction among 500 cells. Each figure shows one representative analysis of 3 repeated experiments with similar results. Results in panels C and E are the mean ± SD of triplicate determinations from a representative experiment (n = 2).
Effect of rapamycin on cell size, differentiation, and proplatelet formation of megakaryocytes. CD34+ cells were cultured in the presence of TPO and Rapa (100 nM) added at different days of culture (TPO + Rapa) or without Rapa (TPO). (A) Effect of Rapa on cell size. MKs were analyzed by flow cytometry, the mean forward scatter height was used as a parameter of cell size and was measured for each ploidy level (from 2N to 16N) determined by propidium iodide staining of MKs. (i) Rapa was added to the culture at day 0, day 3 and day 6. Only MKs expressing CD41 were analyzed on day 9. (ii) Rapa was added to the culture at day 6. Only MKs expressing CD41 were analyzed on day 9. (iii) Rapa was added to the culture at days 0, 3, and 6. Only MKs expressing von Willebrand factor (VWF) were analyzed on day 9. (B) Effect of Rapa on MK differentiation. Rapa was added to the culture at days 0, 3, 6, and 9. On day 6 (D6) and 12 (D12), the percentage of mature MKs expressing CD41 and CD42 antigens was analyzed by flow cytometry. (C) Effect of Rapa on the protein level of 3 transcription factors (GATA-1, FLI-1, and TAL-1) regulating megakaryocytopoiesis. Rapa was added to the MK culture at day 0 and day 3. Western blot analyses were performed at day 6 on nuclear (N) and cytoplasmic (C) protein extracts from total cultures treated or not with Rapa. 293 cells were used as a negative control for GATA-1, FLI-1, and TAL-1 expression and HDAC-1 was used as an internal control of nuclear protein integrity. (D) Effect of Rapa on mRNA level of GATA-1, FLI-1, and TAL-1 was evaluated by real-time RT-PCR at day 6 of culture in the presence or absence of Rapa. The relative expression of these transcription factors was calculated in comparison to mTOR mRNA, which was stable during Rapa treatment. (E) Rapa was added to the culture only at day 9 (D9; left histogram) or at days 0, 3, 6, and 9 (D0, 3, 6, 9; right histogram). At day 9, the cells were seeded at 2 × 103 cell/well in 96-well plate. At day 12, the percentage of MKs forming proplatelets was estimated by counting MKs exhibiting one or more cytoplasmic processes with areas of constriction among 500 cells. Each figure shows one representative analysis of 3 repeated experiments with similar results. Results in panels C and E are the mean ± SD of triplicate determinations from a representative experiment (n = 2).
mTOR/p21 pathway is involved in murine MK differentiation and ploidization upon TPO stimulation
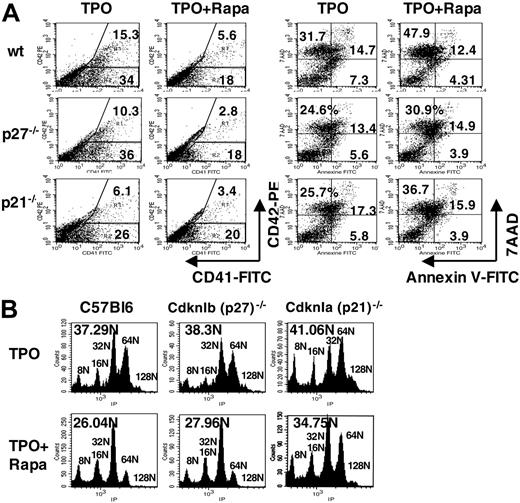
To better understand the mechanisms by which the mTOR/p21 pathway regulates MK differentiation and ploidization, murine MK progenitors obtained from wild-type (wt), CdknIb (p27)–/–, and CdknIa (p21)–/– mice were grown in the absence or in the continuous presence of 100 nM Rapa. As shown in Figure 5A, the number of CD41+CD42+ cells was about 3-fold decreased by Rapa in wt (n = 3, P < .008) and CdknIb (p27)–/– mice (n = 3, P < .008), but a little less (about 2-fold) in CdknIa (p21)–/– mice (n = 3, P < .002). As shown by annexin V and 7AAD staining, this decrease in mature MKs was not caused by an increase of apoptosis in the culture (Figure 5A) (P < .004 for wt; P < .05 for CdknIb (p27)–/–; and P < .01 for CdknIa (p21)–/– mice). We then evaluated whether p21 was involved in the decrease in ploidy induced by Rapa by comparing the ploidy level of cultured MKs from wt, CdknIb (p27)–/–, or CdknIa (p21)–/– mice. After Rapa treatment, the decrease in mean ploidy was over 30% both in wt (n = 4, P < .001) and CdknIb (p27)–/– (n = 3, P < .003) MKs, whereas it was less pronounced in CdknIa (p21)–/– MKs, only 14% (n = 3, P < .017) (Figure 5B, Table 1). These results provide strong evidences that p21 is involved in the regulation of megakaryopoiesis via the mTOR pathway.
Effect of rapamycin on murine MK ploidization
. | Difference in ploidy, % . | n . | P . |
|---|---|---|---|
| WT | 29.8 ± 1.6 | 4 | < .001 |
| CdknIb (p27)-/- | 36 ± 7 | 3 | < .003 |
| CdknIa (p21)-/- | 14 ± 3 | 3 | < .017 |
. | Difference in ploidy, % . | n . | P . |
|---|---|---|---|
| WT | 29.8 ± 1.6 | 4 | < .001 |
| CdknIb (p27)-/- | 36 ± 7 | 3 | < .003 |
| CdknIa (p21)-/- | 14 ± 3 | 3 | < .017 |
Data represents the difference in mean ploidy of murine MKs grown with TPO alone or TPO + Rapa. Results are expressed as the mean ± SEM.
Discussion
The mTOR pathway is involved in the regulation of cell growth and proliferation by activating the G1/S transition in different cell systems (reviewed in Inoki et al22 ). Herein, we investigated whether this pathway plays an important role in the regulation of megakaryocytopoiesis. Megakaryocytopoiesis is a very particular model in which, after a stage of proliferation, MK precursors switch toward an endomitotic process characterized by a series of discrete G1-S-G2 phases. Each G2 phase is followed by an incomplete mitosis leading to the absence of karyokinesis and cytokinesis. Thus, in this cellular model, at each cycle of polyploidization there is a transition from G1 to S phase, which may be regulated in way similar to a normal mitosis. In addition, polyploidization is a manner to increase cell size at even a higher level than the rise in DNA content. As platelets result from the fragmentation of MK cytoplasm, an increase in the cell volume leads to a marked increase in platelet production. Therefore, in this peculiar model, it was important to understand the role of the mTOR pathway both on the cell proliferation and cell growth.
Effect of rapamycin on murine MK differentiation, apoptosis, and ploidization. Murine Lin– cells isolated from wild-type (wt), CdknIa (p21)–/– and CdknIb (p27)–/– mice were cultured in the presence of TPO without Rapa (TPO) or with Rapa (100 nM) added at day 0 and day 3 (TPO + Rapa). Analyses were performed at day 4. (A) Flow cytometry analysis showing the percentage of mature MKs expressing CD41 and CD42 (left panel) and apoptotic cells positive for Annexin V and 7AAD (right panel). (B) DNA content in mature MKs stained with propidium iodide (PI) and analyzed by flow cytometry. The mean ploidy was calculated from the number of cells in each ploidy level. 2N and 4N cells were not considered since they could represent cells other than MK. One representative experiment is shown from 4 performed with wt and 3 performed with CdknIa (p21)–/– and CdknIb (p27)–/– mice.
Effect of rapamycin on murine MK differentiation, apoptosis, and ploidization. Murine Lin– cells isolated from wild-type (wt), CdknIa (p21)–/– and CdknIb (p27)–/– mice were cultured in the presence of TPO without Rapa (TPO) or with Rapa (100 nM) added at day 0 and day 3 (TPO + Rapa). Analyses were performed at day 4. (A) Flow cytometry analysis showing the percentage of mature MKs expressing CD41 and CD42 (left panel) and apoptotic cells positive for Annexin V and 7AAD (right panel). (B) DNA content in mature MKs stained with propidium iodide (PI) and analyzed by flow cytometry. The mean ploidy was calculated from the number of cells in each ploidy level. 2N and 4N cells were not considered since they could represent cells other than MK. One representative experiment is shown from 4 performed with wt and 3 performed with CdknIa (p21)–/– and CdknIb (p27)–/– mice.
We established that the mTOR pathway was indeed stimulated by TPO. It was previously demonstrated that TPO induced PI-3K activity in MK progenitors,4 but it was not known if the mTOR pathway, a downstream mediator in the PI-3K/AKT signaling pathway, was also activated. The only evidence for a decrease in TPO-dependent AKT and p70S6 kinase phosphorylation induced by rapamycin (Rapa), a mTOR inhibitor, was reported in the hematopoietic UT7-mpl cell line expressing exogenous TPO receptors.23 By studying the effect of Rapa on the phosphorylation of mTOR and 2 of its effector proteins, p70S6 kinase and 4E-BP1, we could demonstrate that TPO regulates this pathway in primary MKs. Inhibition of the mTOR signaling resulted in a reduction in both cell proliferation and polyploidization without inducing apoptosis. The effect of Rapa was due to an inhibition of S-phase entry, as demonstrated by BrDU treatment of the cultures. However to affect polyploidization, the mTOR pathway must be blocked by Rapa before being activated by TPO as suggested by our data, showing an absence of effects when addition of Rapa to the culture was delayed. Thus, the mTOR pathway may not be directly implicated during polyploidization, but indirectly through the inhibition of the G1/S transition in proliferative progenitors.
In different cell systems, inhibition of G1/S transition by Rapa is caused by a deregulation of cell-cycle inhibitors of the Cip/Kip family and/or cyclin E or D-type cyclins that control the G1/S transition in the cell cycle. Several reports demonstrate an accumulation of p27 in B and T cells treated by rapamycin.24-27 Surprisingly, we did not detect any modifications in the level of p27 Cdki in megakaryocytes treated with Rapa. In accordance to the results showing that T cells obtained from CdknIb (p27)–/– mice remain sensitive to Rapa,28 we show that the Rapa-induced decrease in mean ploidy level was similar whether cultured MKs were derived from CdknIb (p27)–/– or CdknIb (p27)+/+ mice. However, the decrease in mean ploidy induced by Rapa was more pronounced in CdknIa (p21)+/+ mice than in CdknIa (p21)–/– mice, suggesting an involvement of p21Cdki and its regulation by the mTOR pathway. Another molecule should be involved in this process, since CdknIa (p21)–/– MKs remained slightly sensitive to Rapa. Of note, when human MKs were treated with Rapa, we detected a diminution in p21Cdki and cyclin D3. Thus, both p21Cdki and cyclin D3 must be involved in the regulation of the cell cycle by the mTOR pathway. Cyclin D3 and p21 have already been described as targets for the mTOR pathway. A down-regulation of cyclin D3, but not cyclin D2, by Rapa has also been described in T and B cells29 and malignant B cells activated by IL-2.30 In T cells, this down-regulation was related to a translational repression of cyclin D3.29 In contrast, down-regulation of cyclin D3 in MKs was most likely due to a decreased gene transcription, though we cannot completely eliminate some inhibition of translation. Similarly, the effect of Rapa on p21 was also regulated at the transcriptional level. The inhibition was extremely important because p21 transcripts were 6-fold reduced in the presence of Rapa, suggesting that transcriptional inhibition was the predominant mechanism involved in the decreased level of p21 protein. An effect on the G1/S transition by Rapa through p21 down-regulation has already been described for T cells stimulated by IL-2,27 fibroblasts stimulated by serum and insulin,31 and, more recently, for p53-induced p21 expression by cisplatin in solid tumor cells.32 In the last 2 reports, it has been demonstrated that G1/S arrest was mediated by inhibition of p21 translation. Thus, it appears that mTOR signaling may affect the expression of the same target gene in different cell systems, but by different mechanisms (transcription, translation, or protein degradation). As Rapa does not accelerate cyclin D3 and p21 degradation in MKs, it seems that the effects in this cell type appear to be mediated through the inhibition of transcription, whereas in T cells, an inhibition of translation seems to be the predominant mechanism.
Both cyclin D3 and p21 play an important role in megakaryocytopoiesis.33,34 Cyclin D3 is expressed at a very high level in endomitotic MKs and an ectopic overexpression of cyclin D3 leads to an increase in polyploid MKs.35 In contrast, cyclin D1 is expressed at a very low level, but its ectopic overexpression also increases endomitosis.36 Thus, it is expected that an inhibition of cyclin D3 will induce a decrease in the proliferation of MK precursors as well as an inhibition of polyploidization. It was suggested that p21 plays a direct role in MK polyploidization by inducing a mitosis skipping. However, MK polyploidization is not due to a true endoreplication, but to an incomplete mitosis.37 Conversely, p21 overexpression induces an arrest in endomitosis, suggesting that p21 may be involved in the arrest of endomitosis, although CdknIa (p21)–/– mice do not display abnormalities in megakaryopoiesis.34 However, since MKs also expressed p27, it was hypothesized that p21 and p27 could have redundant functions by coupling the end of DNA replication and terminal megakaryocyte differentiation. Nevertheless, we have recently investigated megakaryopoiesis of homozygote CdknIa/CdknIb double-knockout mice and have found no defect in MK polyploidization and platelet formation (V.B., manuscript in preparation). Therefore, the precise role of p21 during megakaryocytopoiesis remains to be clarified. In different cell systems, p21 is induced by growth factors31,38 and facilitates G1 progression by increasing the binding of cyclin D to CDK4 and stabilizing the cyclinD/CDK4 complexes.31,39,40 Up-regulation of p21 was described during induction of MK differentiation of a CMK cell line by TPO and overexpression of p21 in the same cell line was sufficient to induce MK differentiation.41 Together with these observations, our results suggest that in primary MKs, the complex p21/cyclinD3/cdk4 should be crucial in G1/S transition (ie, proliferation) and in differentiation, starting when proliferation is stopped. However, the role of p21 alone during G1/S progression may be moderate in normal megakaryocytopoiesis, as cyclin D3 is present at a very high level, but could be more crucial in conditions where the level of cyclin D3 is decreased. In favor of this hypothesis, we have shown that the effect of Rapa on cell cycle was less pronounced in CdknIa (p21)–/– than in CdknIa (p21)+/+ MKs, while it has no effect on CdknIb (p27)–/– MKs. A defect in MK differentiation (lower percentage of mature MKs as attested by the CD41 and CD42 markers) was also observed when cultures were treated by Rapa. Nevertheless, this defect seems more related to a delay in differentiation than a proper blockage because MK differentiation proceeded normally but at a slower rate. Slow differentiation rate in the presence of Rapa is better explained by the inhibition in proliferation than a direct inhibition in differentiation. In favor of this hypothesis, we have studied if Rapa could specifically inhibit the translation of specific isoforms of GATA-1, TAL-1, and FLI-1. We observed a decrease in these 3 transcription factors both at the protein and mRNA levels, but which was correlated with the diminished number of MKs in the cultures. In particular, we were unable to detect the short form of TAL-1, which arises by a mTOR-regulated alternative translation initiation, and which is less effective in inducing MK differentiation.42 However this does not exclude that mTOR may regulate other transcription factors such as FOG-1, AML1, or GFI-1b, that are also involved in MK differentiation.
In addition, Rapa was able to decrease the MK cell size. This effect predominates in 2N and 4N cells, and thus in cells that may continue to proliferate. mTOR may coordinate cell growth and cell-cycle progression in MK precursors as previously shown in other cell types. However, an effect on cell size was also found in polyploid and postmitotic MKs, which express high levels of VWF. In maturing MKs, the increase in size is related to an elevated protein synthesis, which induces cytoplasm enlargement and increases platelet formation. Therefore, the effects of mTOR in this cell growth may be related to the translational control of protein synthesis via p70S6K and 4E-BP1 effectors. Finally, when mature MKs were treated by Rapa, an inhibition of proplatelet formation was noted. This inhibition was induced in the absence of changes in MK polyploidization and cell size that require mTOR inactivation by Rapa before being activated by TPO. This suggests that the mTOR pathway directly regulates this process. It is noteworthy that inhibition of the Erk/MAP kinase pathway has exactly the inverse effects, suggesting that equilibrium between these 2 signaling pathways regulates proplatelet formation (F. Auradé and Y. Chang, article in preparation). It has been already described that the mTOR pathway is involved in the organization of actin cytoskeleton and microtubule organization, although not all effects are inhibited by Rapa.43,44 It has also been shown that Rapa can also modify platelet functions such as platelet aggregation and secretion.45
Altogether, our data demonstrate that Rapa has a great effect on megakaryocytopoiesis, acting on MK proliferation, polyploidization, cell size, and proplatelet formation. It remains to determine what are the precise molecular targets of these effects. In vivo, Rapa induces a moderate thrombocytopenia in the mouse (a decrease by half in platelet count).46 In humans the thrombocytopenia is frequent, dose-dependent, but usually moderate (around 100 000 μL/mL). However, in some cases thrombocytopenia can be more severe and limiting in the prevention of graft rejection47 or in the treatment of hematologic malignancies such as myelodysplastic syndromes.48 Further understanding on how Rapa precisely inhibits the final stages of MK development may help to its use in the clinic. Our findings indicate that the mTOR pathway plays an important role in MK terminal differentiation, including platelet functions.
Prepublished online as Blood First Edition Paper, November 10, 2005; DOI 10.1182/blood-2005-07-3005.
Supported by grants from INSERM and la Ligue Nationale Contre le Cancer (équipe labellisée 2004).
H.R. and V.B. contributed equally to this work.
H.R. performed research, analyzed data, and wrote the paper; V.B. performed research and analyzed data; L.L. and B.C. performed technical help; J.L. provided purified CD34+ cells; N.D. performed the experiment and analyzed the data; and W.V. designed the research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to F. Wendling for critical reading of the manuscript and helpful suggestions, to Kirin Brewery (Tokyo, Japan) for the gift of PEG-rhuMGDF, to D. Meyer (INSERM U143, Hôpital de Bicêtre, Paris, France) for a generous gift of anti-VWF mAb, and to D. Pulford (LRF Immunodiagnostics Unit, Nuffield Department of Clinical Laboratory Sciences, University of Oxford, United Kingdom) for a generous gift of anti–TAL-1 mAb.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal