In this study, we demonstrate the involvement of DNAM-1-triggering receptor and its ligands, poliovirus receptor (PVR) and Nectin-2, in natural killer (NK) cell-mediated lysis of dendritic cells (DCs). The surface expression of both ligands was up-regulated in DCs as compared to monocytes. It reached maximal densities after DC maturation induced by different stimuli including lipopolysaccharide (LPS), poly I:C, flagellin, and CD40L. Both immunohistochemical analysis and confocal microscopy revealed expression of DNAM-1 ligands by DCs in lymph nodes in which they were localized in the parafollicular T-cell region and surrounded the high endothelial venules. Remarkably, in cytolytic assays, DNAM-1 cooperated with NKp30 in the NK-mediated killing of both immature and mature DCs and the degree of contribution of DNAM-1 appeared to correlate with the surface densities of its specific ligands PVR and Nectin-2.
Introduction
Natural killer (NK) cells and dendritic cells (DCs) are important players of the innate immunity representing a first line of defense against pathogens or tumors.1-5 After encountering pathogens and their products (eg, lipopolysaccharide, [LPS]) immature DCs (iDCs) undergo a process of maturation leading to the ability to function as antigen-presenting cells (APCs) allowing efficient T-cell priming.1,2 In response to virus-infected or tumor-transformed cells, NK cells rapidly react through cytotoxicity and release of cytokines and chemokines, which, in turn, can activate or recruit multiple cell types.6,7
NK cells are equipped with activating receptors that once engaged by the specific ligands on target cells induce NK cytolytic activity.5 The function of activating receptors is under the control of inhibitory NK receptors (iNKRs) specific for HLA class I molecules.8,9 In humans, 2 main groups of HLA class I-specific receptors have been identified: the killer immunoglobulin-like receptors (KIRs, CD158) are clonally distributed receptors that recognize shared allelic determinants of classical HLA-A, -B, or -C molecules, whereas the CD94/NKG2A heterodimeric receptor interacts with HLA-E.10,11 The general concept is that major histocompatibility complex (MHC) class I-deficient aberrant cells are susceptible to NK-mediated lysis, whereas normal cells are protected from NK cells by the expression of HLA class I molecules.12 However, DCs represent a remarkable exception among normal cells.13,14 Indeed, whereas mature DCs (mDCs) express high levels of HLA class I molecules, iDCs are characterized by low amounts of surface HLA class I molecules. Thus, in an autologous setting, mDCs are protected from NK cytotoxicity, whereas iDCs are highly susceptible to lysis by NK cells and in particular by the CD94/NKG2A+KIR- NK cell subset.15 Importantly, the ability to kill iDCs would render NK cells capable of negatively selecting DCs that during maturation do not acquire the capability of optimal antigen presentation and T-cell priming.15-17 It is of note that in an allogeneic setting, both iDCs and mDCs might be susceptible to NK-mediated lysis, provided that some NK cells express KIRs that are mismatched with the HLA class I alleles of DCs15 (referred to as “alloreactive” NK cells). The clinical relevance of the NK cell alloreactivity was recently emphasized in haploidentical bone marrow transplantation (BMT), in which KIR/HLA class I mismatches existed in the donor-versus-recipient direction.18 Alloreactive NK cells were able not only to eliminate residual leukemic cells, preventing leukemic relapse, but also the patient's DCs, thus avoiding donor T-cell priming and development of graft-versus-host disease (GvHD).19 In the case of inefficient KIR/HLA class I inhibitory interactions, activating NKRs are allowed to transduce triggering signals, resulting in NK cell activation and killing of different targets including virus-infected cells, tumors, and DCs. Among the NK-activating receptors, NKp30, belonging to the natural cytotoxicity receptor (NCR) family, is selectively expressed by NK cells and plays a major role in the NK-mediated killing of DCs on recognition of still undefined ligands.20,21 However, so far little is known about the existence of additional receptor-ligand interactions that might contribute to the process of NK-mediated recognition of DCs.
DNAM-1 is a triggering receptor that is expressed by virtually all human NK cells and T lymphocytes and monocytes.22 DNAM-1 specifically recognizes poliovirus receptor (PVR; CD155) and Nectin-2 (CD112), two closely related molecules belonging to the Nectin family.23 As previously reported, peripheral blood mononuclear cells (PBMCs) do not express these ligands, with the exception of monocytes characterized by low levels of both molecules at the cell surface.23
In the present study, we investigated the expression of PVR and Nectin-2 during monocyte differentiation toward iDCs and mDCs. Moreover, we show that the interaction of DNAM-1 with PVR and Nectin-2 contributes to the NK-mediated lysis of both iDCs and mDCs.
Materials and methods
Monoclonal antibodies
The following monoclonal antibodies (mAbs), produced in our laboratory, were used in this study: JT3A (IgG2a, anti-CD3), c127 (IgG1, anti-CD16), c218 (IgG1, anti-CD56), BAB281 and KL247 (IgG1 and IgM, respectively, anti-NKp46), Z231 and KS38 (IgG1 and IgM, respectively, anti-NKp44), A76 and F252 (IgG1 and IgM, respectively, anti-NKp30), BAT221 (IgG1, anti-NKG2D), GN18 and F5 (IgG3 and IgM, respectively, anti-DNAM-1), M5A10 and L95 (IgG1, anti-PVR), L14 (IgG2a, anti-Nectin-2), BAM195 (IgG1, anti-MICA), and A6-136 (IgM, anti-HLA class I).5,8,20,23,24 M295 (IgG1, anti-ULBP1), M310 (IgG1, anti-ULBP2), and M551 (IgG1, anti-ULBP3) were kindly provided by Amgen (Seattle, WA). The anti-CD1a (BL6 mAb, IgG1, PE-labeled), anti-CD14 (RM052 mAb, IgG2a, either unlabeled or FITC-labeled), anti-CD83 (HB15a mAb, IgG2b), anti-CD86 (HA5.2B7 mAb, IgG2b, PE-labeled), and anti-immunoglobulin-like transcript 3 (ILT3; ZM3.8 mAb, IgG1, PC5-labeled) were purchased from Beckman Coulter (Milan, Italy); the anti-CD20 (Leu16 mAb, IgG1; Becton Dickinson, Mountain View, CA; and L26 mAb, IgG2a; DakoCytomation, Milan, Italy), the anti-CD3 (UCHT-1 mAb, IgG1; Cymbus Byotechnology, Chandlers-Ford, United Kingdom), and the APC-conjugated anti-CD11c (S-HCL-3 mAb, IgG2b; Becton Dickinson) were also used. The anti-MR (PAM-1 mAb, IgG1) was a kind gift of Alberto Mantovani (Mario Negri Institute, Milan, Italy).25
Isolation and culture of NK-cell populations
PBMCs, derived from healthy donors, were isolated on Ficoll-Hypaque gradients and peripheral blood lymphocytes (PBLs) were obtained by depleting PBMCs of plastic-adherent cells. Enriched NK cells were isolated by incubating PBLs with anti-CD3 (JT3A), anti-CD20 (Leu 16) for 30 minutes at 4°C, followed by goat antimouse-coated Dynabeads (Dynal, Oslo, Norway) for 30 minutes at 4°C and immunomagnetic depletion.24 CD3-20- cells were cultured for 7 days in the presence of 100 U/mL rIL-2 (Proleukin; Chiron, Emeryville, CA) to obtain activated polyclonal NK cell populations. To obtain NK cell clones, the purified NK cells were cultured, after limiting dilution, on irradiated feeder cells, 1.5 ng/mL PHA (Gibco, Paisley, United Kingdom) and rIL-2. The NK cell line NK92 was kindly provided by E. O. Long (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, MD).
Generation of DCs from PBMCs
PBMCs from healthy donors, either the same or different from the donors of NK cells, were resuspended in RPMI 1640 containing 10% FCS and allowed to adhere for 2 hours at 37°C in culture flasks (Corning Life Sciences, Costar, Corning, NY). The adherent fraction was cultured in the presence of rGM-CSF (50 ng/mL, Mielogen; Schering-Plough, Milan, Italy) and rIL4 (20 ng/mL; PeproTech, Rocky Hill, NJ) as described.15 After 5 days of culture, fresh medium containing GM-CSF and IL-4 was added and the incubation prolonged for another 2 days to obtain cells characterized by the CD14-CD1a+CD83- phenotype corresponding to iDCs. To generate CD14-CD1a+CD83+CD86+ mDCs, at day 5 cells were treated for 48 hours with LPS from Escherichia coli (40 ng/mL final concentration; Sigma-Aldrich, St Louis, MO) together with fresh GM-CSF and IL-4. In a set of experiments, mDCs were also obtained by treating the cells with other stimuli: poly I:C (50 μg/mL final concentration; Amersham, Biotech, Buckinghamshire, United Kingdom), flagellin (1 μg/mL final concentration; Alexis Biochemicals, Lausen, Switzerland), and coculture with 3T3/CD40L cell transfectant, kindly provided by Daniel Olive (INSERM, Marseille, France; DC/3T3 ratio of 10:1).
Cytolytic activity and flow cytofluorimetric analysis
NK cells were tested for cytolytic activity against either iDCs or mDCs (LPS induced) in a 4-hour 51Cr-release assay as previously described.15 The concentrations of the various mAbs added for masking experiments were 10 μg/mL. The effector-target (E/T) ratios are indicated in the text. NK cells were preincubated (15 minutes at room temperature [RT]) with saturating amounts of anti-NKp30 and anti-DNAM-1 mAb, either alone or in combination, and, after washing, used in the cytolytic assay. In the allogeneic setting, DCs were derived from donor no. 2 characterized by HLA class I haplotype: A2,25; B7,18 (Bw6); Cw7 (CAsn80), whereas NK cells were derived from donor no. 10 characterized by HLA class I haplotype: A10,28; B15,27 (Bw6, Bw4); Cw2,4 (CLys80). According to the KIR phenotype of donor no. 10, characterized by a high proportion of KIR2DL1+2DL2/3-3DL1+NKG2A- cells, polyclonal NK cells displayed good alloreactivity against both PHA blasts and B-EBV derived from donor no. 2 (not shown). Also the representative clone no. 19-7, characterized by NCRdull and KIR2DL1+2DS2+3DL1+ NKG2A- phenotype, was alloreactive against mDCs derived from donor no. 2, lacking any HLA class I group recognized by iNKRs.
To analyze the surface markers of DCs, cells were first preincubated for 10 minutes at 4°C with hIgG (1 mg/mL) and then stained with the appropriate mAbs followed by PE-conjugated AffiniPure F(ab′)2 goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). The reactivity of mAb with NK cells was assessed by indirect immunofluorescence using the appropriate mAb followed by PE-conjugated isotype-specific goat antimouse second reagent (Southern Biotechnology, Birmingham, AL). Samples were analyzed on a FACSCalibur flow cytometer (Becton Dickinson).
Tissues
Four human lymph nodes removed for diagnostic purposes from patients undergoing surgery for colon or renal cancer were obtained after approval from the Azienda Ospedaliero-Universitaria Careggi (Florence, Italy) Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki. Only lymph nodes without any tumor invasion were used throughout the study.
Immunohistochemistry
Immunohistochemical staining was performed as previously described26 on 4-μm cryostat sections fixed in 4% paraformaldehyde at RT for 20 minutes. Sections were subsequently exposed to 0.3% hydrogen-peroxide-methanol solution to quench the endogenous peroxidase activity. After 30 minutes of preincubation with normal horse serum (Vectastain ABC kit; Vector Laboratories, Burlingame, CA), sections were layered for 30 minutes with the purified ascitic fluid of the 2 different anti-Nectin-2 and PVR mAbs (L14 and L95 mAbs, respectively, final dilution of 10 μg/mL) followed by biotinylated horse antimouse antisera, and the avidin-biotin-peroxidase complex (Vectastain ABC kit), as described.26 As a peroxidase substrate, 3-amino-9-ethylcarbazole (AEC; Vector Laboratories) was used. Finally, sections were counterstained with Gill hematoxylin (Merck, Darmstadt, Germany) and mounted with Kaiser glycerol gelatin (Merck). All incubations were performed at RT. As negative control, primary antibody was omitted or replaced with an isotype-matched control mAb of irrelevant specificity.
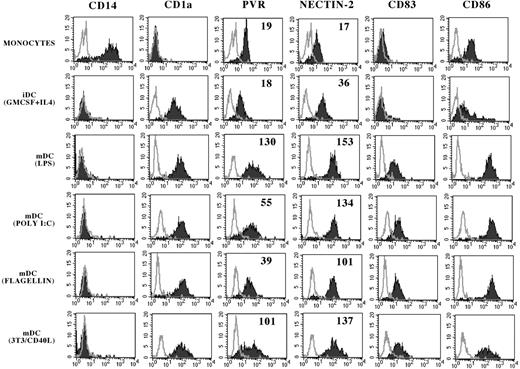
PVR and Nectin-2 expression on monocytes, iDCs, and mDCs. Monocytes (gated on PBMCs by physical parameters in FSC/SSC dot plot) or cultured iDCs or mDCs (obtained using the indicated culture conditions) were analyzed by flow cytometry with mAbs to the indicated molecules. White profiles represent isotypic negative controls. Numbers indicate the mean fluorescence intensity (MFI).
PVR and Nectin-2 expression on monocytes, iDCs, and mDCs. Monocytes (gated on PBMCs by physical parameters in FSC/SSC dot plot) or cultured iDCs or mDCs (obtained using the indicated culture conditions) were analyzed by flow cytometry with mAbs to the indicated molecules. White profiles represent isotypic negative controls. Numbers indicate the mean fluorescence intensity (MFI).
Laser confocal microscopy
Immunofluorescence staining was performed on 4-μm cryostat sections fixed in 4% paraformaldehyde at RT for 20 minutes. All subsequent steps were performed at RT. After fixation, the sections were blocked with normal horse serum for 30 minutes and then incubated for 30 minutes with primary antibody (anti-Nectin-2 or anti-PVR mAbs, 4 μg/mL) diluted in PBS with 3% BSA. After 2 washes with PBS, the sections were subsequently incubated with secondary antibody (anti-IgG-Alexa 488, 1:1500; Molecular Probes, Invitrogen, Carlsbad, CA) for 30 minutes and washed twice with PBS. In some experiments, Nectin-2, PVR, mannose receptor (MR; macrophages and DCs), von Willebrand factor (VWF; endothelial cells), CD3 (T cells), CD20 (B cells), CD11c (DCs) were investigated for colocalization on the same sections by double-label immunofluorescence. Thus, the sections were again blocked with normal horse serum for a further 30 minutes and subsequently exposed, for 30 minutes, to anti-MR (PAM-1 mAb, IgG1, 5 μg/mL), anti-CD3 (UCHT-1 mAb, IgG1, 10 μg/mL), anti-CD20 (L26 mAb, IgG2a, 50 μg/mL in PBS + 3% BSA), and anti-VWF (rabbit antihuman polyclonal antibody, 1:8000; DakoCytomation), and subsequently exposed to secondary antibody (anti-IgG1-Alexa 546, 1:1000, anti-IgG2a-Alexa 633, or goat antirabbit-Alexa 546, 1:1000 as appropriate; Molecular Probes Invitrogen). Alternatively, the sections were incubated with APC-conjugated anti-CD11c (S-HCL-3 mAb, IgG2b, 1:2; Becton Dickinson). To avoid cross-reaction between the detection system for the second mAb and the first mAb, the antimouse antibody was used at lower concentration in the second step of the double-staining protocol. Furthermore, to ascertain that the detection system for the second mAb did not cross-react with the first mAb, double-label immunofluorescence was performed by omitting the second primary antibody or replacing it with an isotype-matched control mAb with irrelevant specificity, which resulted in a single signal. All sections were mounted in antifading mounting media (Vectashield, Vector Laboratories) and examined by conventional confocal microscopy on a Zeiss LSM 510 META microscope system (Carl Zeiss, Jena, Germany).
Results
Analysis of the surface expression of PVR and Nectin-2 on immature and mature monocyte-derived DCs
Peripheral blood monocytes, detected by physical parameters in forward-side scatter (FSC/SSC) dot plot and by staining with anti-CD14 mAb, expressed low surface densities of both PVR and Nectin-2 molecules (Figure 1).23 We analyzed whether their expression was modified during differentiation toward DCs. Classical CD14-CD1a+CD83-CD86dull iDCs, obtained by culturing plastic adherent monocytes in the presence of GM-CSF and IL-4, were found to up-regulate Nectin-2 expression as compared to monocytes, whereas PVR expression was not modified (Figure 1). Next, iDCs were exposed to different stimuli promoting their maturation; these included LPS, poly I:C, flagellin, and coculture with 3T3-CD40L cell transfectant. The mDCs were characterized by the expression of CD83 as well as by up-regulation of CD86 (Figure 1) and HLA-class I molecules (not shown). Remarkably, Nectin-2 surface densities were further incremented in mDCs as compared to iDCs. Moreover, mDCs also up-regulated PVR surface expression as compared to monocytes (and iDCs). Although not shown, monocytes, iDCs, and mDCs did not display surface expression of other members of the Nectin family including Nectin-1, -3, and -4 (not shown). Thus, the surface density of both PVR and Nectin-2 is modified on culture conditions that allow the transition from peripheral blood monocytes to iDCs and mDCs.
Expression of DNAM-1 ligands was also analyzed on circulating peripheral blood DCs. The majority of DCs, detected by the ILT3+CD14neg surface phenotype,27 expressed both Nectin-2 and PVR molecules (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article).
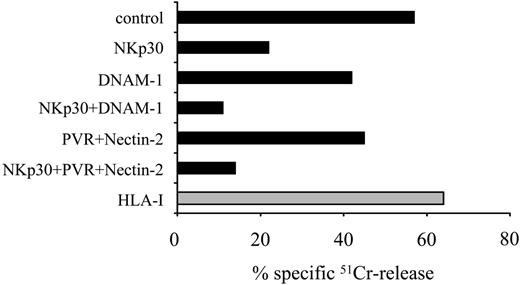
mAb-mediated disruption of DNAM-1 interaction with PVR or Nectin-2 inhibits the NK cell-mediated lysis of autologous iDCs. Activated polyclonal NK cells from the representative donor no. 8 were analyzed for cytolytic activity against autologous iDCs either in the absence or in the presence of mAbs to the indicated molecules, used alone or in combination. The E/T ratio used was 10:1. The results are representative of 3 independent experiments; the SD of the mean of the triplicates was less than 5%.
mAb-mediated disruption of DNAM-1 interaction with PVR or Nectin-2 inhibits the NK cell-mediated lysis of autologous iDCs. Activated polyclonal NK cells from the representative donor no. 8 were analyzed for cytolytic activity against autologous iDCs either in the absence or in the presence of mAbs to the indicated molecules, used alone or in combination. The E/T ratio used was 10:1. The results are representative of 3 independent experiments; the SD of the mean of the triplicates was less than 5%.
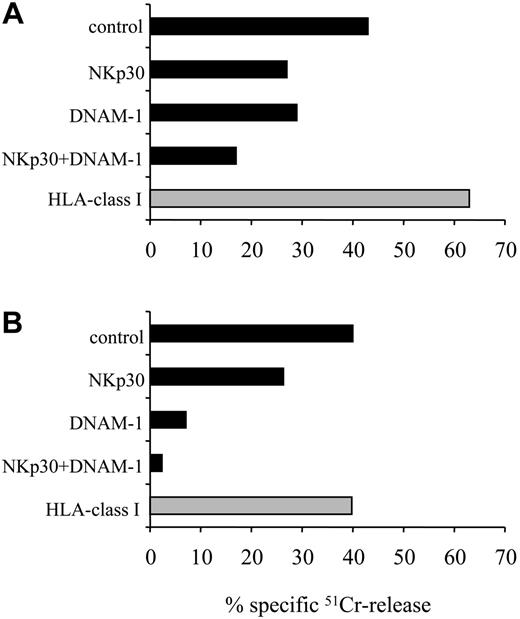
Role of DNAM-1 in the recognition of mDCs by normal NK cells in an allogeneic setting. Activated polyclonal NK cells from donor no. 10 (A) and the representative NK cell clone no. 19-7 (B), selected as “alloreactive” against donor no. 2 (see “Materials and methods”), were analyzed for their cytolytic activity against mDCs derived from this donor. Effector cells were preincubated with medium alone (control) or with mAbs to the indicated molecules, used alone or in combination. The E/T ratio used was 10:1. The results are representative of 3 independent experiments; the SD of the mean of the triplicates was less than 5%.
Role of DNAM-1 in the recognition of mDCs by normal NK cells in an allogeneic setting. Activated polyclonal NK cells from donor no. 10 (A) and the representative NK cell clone no. 19-7 (B), selected as “alloreactive” against donor no. 2 (see “Materials and methods”), were analyzed for their cytolytic activity against mDCs derived from this donor. Effector cells were preincubated with medium alone (control) or with mAbs to the indicated molecules, used alone or in combination. The E/T ratio used was 10:1. The results are representative of 3 independent experiments; the SD of the mean of the triplicates was less than 5%.
Analysis of the role of DNAM-1/PVR or Nectin-2 interaction in the recognition of DCs by normal NK cells
To analyze the role of DNAM-1/ligand interactions in the recognition of DCs, NK cells were purified from PBLs and, after 7 days of culture in the presence of IL-2, they were tested for cytolytic activity against autologous iDCs (Figure 2). In agreement with previous data, iDCs were efficiently killed and masking of NKp30 resulted in strong inhibition of lysis (Figure 2).21 Importantly, mAb-mediated masking of either DNAM-1 (on NK cells) or its ligands PVR and Nectin-2 (on iDCs) resulted in a significant down-regulation of NK cytotoxicity. Moreover, the combined masking of NKp30 and DNAM-1 or its ligands resulted in virtual abrogation of lysis.
Previous studies showed that HLA class I expression protects mDCs from lysis mediated by autologous NK cells. Thus, to analyze whether DNAM-1 plays any role in the recognition of mDCs, we used an appropriate allogeneic setting; that is, we selected KIR/HLA class I-mismatched donors as a source of NK cells and mDCs. As shown in Figure 3A, polyclonal NK cells from donor no. 10 lysed allogeneic mDCs derived from donor no. 2 (see “Materials and methods” for KIR phenotype and HLA haplotypes). Cytolytic activity was further incremented by mAb-mediated masking of HLA class I molecules, due to partial KIR matched or NKG2A-mediated inhibitory interactions. Blocking experiments using specific mAbs revealed that both NKp30 and DNAM-1 were involved in the NK-mediated lysis of mDCs. The relevance of DNAM-1 in NK-mediated killing of mDCs was particularly evident when a KIR-mismatched alloreactive NK cell clone characterized by low surface density of NKp30 was used as effector (Figure 3B).
Altogether these data show that DNAM-1 cooperates with NKp30 in recognition of iDCs and mDCs by normal NK cells.
DNAM-1-mediated recognition of PVR and Nectin-2 contributes to the NK92 cell line-mediated lysis of DCs
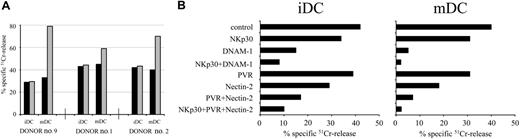
To substantiate the role of the interaction of DNAM-1 with PVR and Nectin-2 in the NK cell-mediated killing of DCs, we used as effector the NK92 cell line. Previous studies showed that NK92 cells provide a suitable tool to better analyze the role of DNAM-1/ligand interactions in killing of different target cells.23 This is consequent to the fact that NK92 cells display a simplified pattern of triggering receptors, as compared to normal NK cells. In particular, NK92 cells express normal levels of DNAM-1 but low levels of NCR including NKp30, the major triggering receptor involved in DC lysis. As shown in Figure 4A, NK92 killed both iDCs and mDCs. Because NK92 cells express CD94/NKG2A inhibitory receptors, in the presence of saturating amounts of anti-HLA class I mAb, lysis of mDCs was further incremented. Notably, unlike normal NK cells, in the absence of engagement of HLA class I-specific inhibitory receptors, NK92 cells killed more efficiently mDCs than iDCs (see, in particular, DC target cells derived from donor no. 9 in Figure 4).
To evaluate the role of the interactions between DNAM-1 and its ligands in NK92-mediated lysis of iDCs and mDCs, the cytotoxic assays were performed either in the absence or in the presence of mAbs specific for NKp30, DNAM-1, PVR, or Nectin-2 used alone or in combination (Figure 4B). Consistent with the poor expression of NKp30, the role of this receptor in NK92-mediated lysis of DCs was only marginal. On the contrary, a sharp down-regulation of NK-mediated lysis of iDCs could be detected on mAb-mediated masking of DNAM-1 (67% inhibition of lysis). Importantly, this effect was more evident when target cells were represented by mDCs (85% inhibition of lysis). Note that, according to its higher surface density, Nectin-2 appears to play a more relevant role than PVR.
NK92 cell line-mediated cytolysis of iDCs and mDCs: involvement of DNAM-1/ligand interaction. (A) The NK92 NK cell line was analyzed for its cytolytic activity against iDCs or mDCs derived from 3 representative donors either in the absence (▪) or in the presence ( ) of anti-HLA class I mAb. The E/T cell ratio used was 10:1. The results are representative of 3 independent experiments; the SD of the mean of the triplicates was less than 4%. (B) NK92 was analyzed for its capability of killing iDCs or mDCs derived from the representative donor no. 2, either in the absence or in the presence of mAbs to the indicated molecules, used alone or in combination. The E/T ratio used was 10:1. The results are representative of 3 independent experiments; the SD of the mean of the triplicates was less than 5%.
) of anti-HLA class I mAb. The E/T cell ratio used was 10:1. The results are representative of 3 independent experiments; the SD of the mean of the triplicates was less than 4%. (B) NK92 was analyzed for its capability of killing iDCs or mDCs derived from the representative donor no. 2, either in the absence or in the presence of mAbs to the indicated molecules, used alone or in combination. The E/T ratio used was 10:1. The results are representative of 3 independent experiments; the SD of the mean of the triplicates was less than 5%.
NK92 cell line-mediated cytolysis of iDCs and mDCs: involvement of DNAM-1/ligand interaction. (A) The NK92 NK cell line was analyzed for its cytolytic activity against iDCs or mDCs derived from 3 representative donors either in the absence (▪) or in the presence ( ) of anti-HLA class I mAb. The E/T cell ratio used was 10:1. The results are representative of 3 independent experiments; the SD of the mean of the triplicates was less than 4%. (B) NK92 was analyzed for its capability of killing iDCs or mDCs derived from the representative donor no. 2, either in the absence or in the presence of mAbs to the indicated molecules, used alone or in combination. The E/T ratio used was 10:1. The results are representative of 3 independent experiments; the SD of the mean of the triplicates was less than 5%.
) of anti-HLA class I mAb. The E/T cell ratio used was 10:1. The results are representative of 3 independent experiments; the SD of the mean of the triplicates was less than 4%. (B) NK92 was analyzed for its capability of killing iDCs or mDCs derived from the representative donor no. 2, either in the absence or in the presence of mAbs to the indicated molecules, used alone or in combination. The E/T ratio used was 10:1. The results are representative of 3 independent experiments; the SD of the mean of the triplicates was less than 5%.
Immunohistochemical analysis of Nectin-2 and PVR expression in human lymph nodes. Sections were immunostained with anti-Nectin-2 or anti-PVR mAbs (red), using the avidin-biotin-peroxidase method, and counterstained with the nuclear counterstain Gill hematoxylin (blue). (A) Scattered, positive cells for Nectin-2 are mainly distributed in the parafollicular T-cell areas of a lymph node (arrows; original magnification × 100; objective lens, 10 ×/0.25 numeric aperture [NA]). (B) Higher-power magnification of the area indicated in panel A reveals cells positive for Nectin-2 surrounding a vessel (arrows; original magnification × 250; objective lens, 25 ×/0.50 NA). (C) Several cells showing a stellate morphology and expressing Nectin-2 surround a vessel where endothelial cells are also stained (original magnification × 400; objective lens, 40 ×/0.70). (D) Few cells stained for PVR and lining a parafollicular T-cell area are visible (original magnification × 400; objective lens, 40 ×/0.70). Images were acquired using a Leica DMLB microscope. (Leica Microsystems, Wetzlar, Germany). A Canon Power Shot G5 digital camera (Canon, Tokyo, Japan) and Adobe Photoshop version 7.0.1 (Adobe Systems, San Jose, CA) were used to capture images.
Immunohistochemical analysis of Nectin-2 and PVR expression in human lymph nodes. Sections were immunostained with anti-Nectin-2 or anti-PVR mAbs (red), using the avidin-biotin-peroxidase method, and counterstained with the nuclear counterstain Gill hematoxylin (blue). (A) Scattered, positive cells for Nectin-2 are mainly distributed in the parafollicular T-cell areas of a lymph node (arrows; original magnification × 100; objective lens, 10 ×/0.25 numeric aperture [NA]). (B) Higher-power magnification of the area indicated in panel A reveals cells positive for Nectin-2 surrounding a vessel (arrows; original magnification × 250; objective lens, 25 ×/0.50 NA). (C) Several cells showing a stellate morphology and expressing Nectin-2 surround a vessel where endothelial cells are also stained (original magnification × 400; objective lens, 40 ×/0.70). (D) Few cells stained for PVR and lining a parafollicular T-cell area are visible (original magnification × 400; objective lens, 40 ×/0.70). Images were acquired using a Leica DMLB microscope. (Leica Microsystems, Wetzlar, Germany). A Canon Power Shot G5 digital camera (Canon, Tokyo, Japan) and Adobe Photoshop version 7.0.1 (Adobe Systems, San Jose, CA) were used to capture images.
These experimental settings further support the role of DNAM-1/ligand interactions in inducing NK-mediated lysis of both iDCs and mDCs. Moreover, the higher susceptibility to lysis of mDC appears to correlate with the up-regulation of the surface expression of Nectin-2 and PVR in these cells.
Expression of PVR and Nectin-2 by DCs in human lymph nodes
To analyze whether the DNAM-1 ligands are expressed in vivo by DCs localized in human secondary lymphoid organs, human lymph nodes were analyzed by immunohistochemistry using anti-Nectin-2- and anti-PVR-specific mAbs. Scattered cells reacting with anti-Nectin-2 mAb showed a stellate morphology and were localized in the parafollicular T-cell areas of all lymph nodes analyzed (Figure 5A-B). Nectin-2+ cells surrounded the high endothelial venules (HEVs; Figure 5C), not only in the inner cortex, but also in the interfollicular areas of the outer cortex, extending to closely beneath the marginal sinus (Figure 5A-C). In the same lymph nodes, cells expressing PVR showed a pattern of localization similar to that of Nectin-2+ cells. However, the number of cells stained by anti-PVR mAb was consistently lower and of weaker intensity than that obtained by using anti-Nectin-2 mAb (Figure 5D). Endothelial cells of HEVs as well as those belonging to other vessel types were also stained by both Nectin-2- and PVR-specific mAbs (Figure 5).28,29
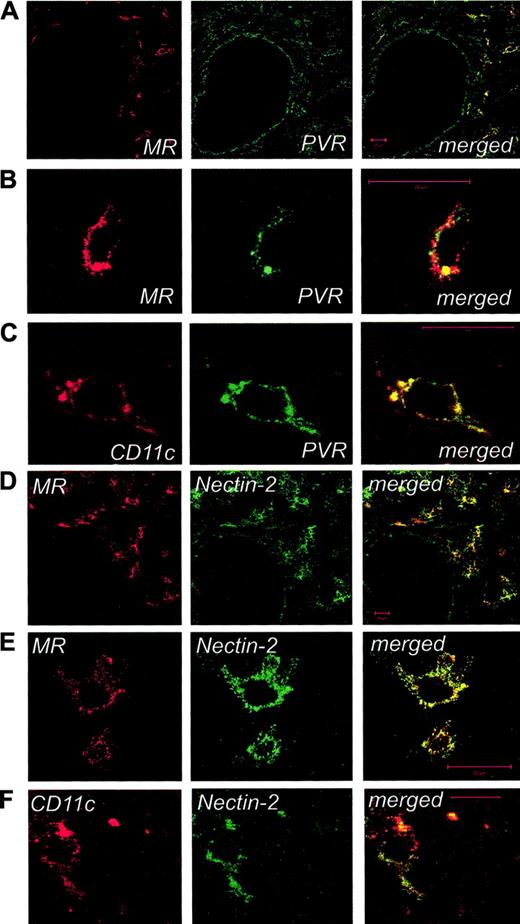
To better assess the distribution of PVR- and Nectin-2-expressing cells within the human lymph node and to identify their nature, laser confocal microscopy analysis was further applied. By this technique, high numbers of PVR- and Nectin-2-expressing cells (Figure 6), characterized by similar tissue localization, could be detected. Most cells expressing PVR and Nectin-2 (Figure 6) were identified as DCs on the basis of classical morphologic features and their costaining with mAb to MR30 and CD11c (Figure 6 at higher magnification). Staining of endothelial cells for both PVR and Nectin-2 (panels A and B, respectively) was also detected, whereas T and B lymphocytes were not stained by anti-PVR or anti-Nectin-2 mAbs (data not shown).
Laser confocal microscopy characterization of PVR- and Nectin-2-expressing cells in human lymph nodes. Human lymph nodes were stained for mannose receptor (MR; red) and PVR (A) or Nectin-2 (D; green) and were analyzed by confocal microscopy. Cells around the HEV show colocalization of DNAM-1 ligands and MR (merged images, yellow), whereas single staining for DNAM-1 ligands is visible on endothelial cells. High-power magnifications focused on single cells around the HEVs show costaining of MR and CD11c markers with PVR (B-C) and Nectin-2 (E-F). No staining was observed by using an isotype-matched control mAb with irrelevant specificity (not shown). Bar indicates 20 μm. Images in panels A-E were acquired using an LSM 510 Meta confocal microscope (Zeiss, Jena, Germany) equipped with a 40 ×/1.30 NA oil Plan-Neofluor objective lens (Zeiss). Images in panel F were acquired using an LSM 510 Meta laser scanning confocal microscope equipped with a 63 ×/1.4 NA oil Plan-Neofluor objective lens (Zeiss). LSM 510 Meta confocal microscope software version 3.0 (Zeiss) was used to capture all the images.
Laser confocal microscopy characterization of PVR- and Nectin-2-expressing cells in human lymph nodes. Human lymph nodes were stained for mannose receptor (MR; red) and PVR (A) or Nectin-2 (D; green) and were analyzed by confocal microscopy. Cells around the HEV show colocalization of DNAM-1 ligands and MR (merged images, yellow), whereas single staining for DNAM-1 ligands is visible on endothelial cells. High-power magnifications focused on single cells around the HEVs show costaining of MR and CD11c markers with PVR (B-C) and Nectin-2 (E-F). No staining was observed by using an isotype-matched control mAb with irrelevant specificity (not shown). Bar indicates 20 μm. Images in panels A-E were acquired using an LSM 510 Meta confocal microscope (Zeiss, Jena, Germany) equipped with a 40 ×/1.30 NA oil Plan-Neofluor objective lens (Zeiss). Images in panel F were acquired using an LSM 510 Meta laser scanning confocal microscope equipped with a 63 ×/1.4 NA oil Plan-Neofluor objective lens (Zeiss). LSM 510 Meta confocal microscope software version 3.0 (Zeiss) was used to capture all the images.
Discussion
DNAM-1 is a surface molecule that transduces activating signals resulting in enhancement of cytotoxicity by NK cells.22 The DNAM-1-specific ligands, PVR and Nectin-2, are highly expressed in different tumor cell lines.23 Accordingly, a role for DNAM-1/ligand interactions has been demonstrated in the NK-mediated lysis of different tumors including ex vivo derived leukemias and neuroblastoma cells.31,32
In this study, we show that monocyte-derived DCs express PVR and Nectin-2, that is, the ligands of the DNAM-1-activating receptor. In particular, Nectin-2 expression was up-regulated on iDCs, as compared with monocytes, and reached maximal surface density in mDCs, whereas up-regulation of PVR was confined to mDCs. Importantly, we also demonstrate that DNAM-1/ligand interactions are involved in NK-mediated lysis of DCs. Finally, immunohistochemistry and confocal microscopy confirmed that the DNAM-1 ligands are expressed in vivo by DCs in normal human lymph nodes.
Recent studies provided evidence that NK cells play a role in the regulation of both innate and adaptive immune responses by interacting with DCs both in inflamed peripheral tissues and in secondary lymphoid compartments. In particular, NK cells have been shown to be capable of either killing DCs or promoting their maturation.15,21,33
In an autologous setting, whereas mDCs are protected from NK-mediated lysis due to the high surface expression of HLA class I molecules, iDCs are efficiently killed by NK cells characterized by the KIR-, NKG2A+ phenotype, on engagement of the NKp30 receptor by still undefined ligands on iDCs.15,21 In the present study, we demonstrate that also DNAM-1 can contribute to killing of iDCs. This observation suggests a role for this molecule in the NK cell-mediated “quality control” of DC maturation, a process whose final outcome might be the selection of the “most fitting” DCs, that is, cells that are characterized by the high expression of HLA class I molecules and of costimulatory ligands that optimize their ability to prime T cells.34,35
Moreover, under appropriate allogeneic combinations, we could show that DNAM-1 receptor is involved also in killing of mDCs. Notably, whereas the function of NKp30 predominated when target cells were represented by iDCs, NKp30 and DNAM-1 appeared to equally contribute to recognition and lysis of mDCs. These differences correlated with differences in surface expression of the DNAM-1 ligands. Indeed, both PVR and Nectin-2 were strongly up-regulated in mDCs as compared to both iDCs and peripheral blood monocytes. We previously showed that in some healthy individuals variable fractions of NK cells expressed low surface densities of NCR (NCRdull phenotype).20,36 The NCRdull phenotype resulted in an impaired killing of tumor target cells that were primarily lysed via NCR. Importantly, however, also NK cells expressing low levels of NKp30 could maintain the ability to kill DCs thanks to DNAM-1/ligand interactions (Figure 3B). This observation might be relevant in patients with acute myeloid leukemia (AML) undergoing haploidentical BMT.19 The low incidence of GvHD in these patients has been attributed to the presence, among NK cells derived from donor's hematopoietic stem cells, of subsets characterized by the expression of KIRs that are mismatched with the HLA class I molecules of the patient. These “KIR-mismatched” NK cells were able to kill the patient's DCs. However, because it occurs in healthy individuals, a fraction of KIR-mismatched NK cells might be characterized by the NKp30dull phenotype. In this case, the NK alloreactivity against DCs of the recipient might be preserved by the presence of DNAM-1/PVR and Nectin-2 interactions.
Although the molecular mechanisms regulating NK-mediated lysis of DCs have been clarified, at least in part, those involved in NK-induced maturation and migration of DCs are still only partially defined. It is of note that engagement of NKp30 results not only in activation of cytotoxicity but also in production of cytokines such as IFN-γ and TNF-α,21 which promote DC maturation.33 This observation suggested that NKp30 could be involved not only in the process of iDC killing but also in the mechanisms that favor their progression toward mDCs. In this context, preliminary experiments showed that, unlike NKp30, the engagement of DNAM-1 did not result in significant cytokine production by NK cells (not shown). This suggests that DNAM-1/ligand interactions might be mainly involved in recognition and killing of DCs rather than in promoting their maturation.
Finally, although it is well known that mDCs express crucial chemokine receptors such as CCR7 that allow their migration to lymph nodes, little is known on additional molecular interactions regulating this process.34,35 Interestingly, it has recently been shown that stimulation of fibroblasts via PVR (by the use of specific mAb or DNAM-1 soluble receptor) inhibits cell-matrix adhesion and promotes cell migration.37 Based on this observation, it is possible that PVR could play a similar role on mDCs prior to their migration to secondary lymphoid compartments. Thus, PVR engagement by DNAM-1 (on NK and T cells) might contribute to migration of mDCs toward lymph nodes.
Prepublished online as Blood First Edition Paper, November 29, 2005; DOI 10.1182/blood-2005-07-2696.
Supported by grants awarded by Associazione Italiana per la Ricerca sul Cancro (AIRC), Istituto Superiore di Sanità (ISS), Ministero della Salute, Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR), Fondazione Compagnia di San Paolo, Turin, Italy, and European Union FP6, LSHB-CT-2004-503319-Allostem (the European Commission is not liable for any use that may be made of the information contained). S. Marcenaro is recipient of a fellowship awarded by Fondazione Italiana per la Ricerca sul Cancro (FIRC).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 5. Immunohistochemical analysis of Nectin-2 and PVR expression in human lymph nodes. Sections were immunostained with anti-Nectin-2 or anti-PVR mAbs (red), using the avidin-biotin-peroxidase method, and counterstained with the nuclear counterstain Gill hematoxylin (blue). (A) Scattered, positive cells for Nectin-2 are mainly distributed in the parafollicular T-cell areas of a lymph node (arrows; original magnification × 100; objective lens, 10 ×/0.25 numeric aperture [NA]). (B) Higher-power magnification of the area indicated in panel A reveals cells positive for Nectin-2 surrounding a vessel (arrows; original magnification × 250; objective lens, 25 ×/0.50 NA). (C) Several cells showing a stellate morphology and expressing Nectin-2 surround a vessel where endothelial cells are also stained (original magnification × 400; objective lens, 40 ×/0.70). (D) Few cells stained for PVR and lining a parafollicular T-cell area are visible (original magnification × 400; objective lens, 40 ×/0.70). Images were acquired using a Leica DMLB microscope. (Leica Microsystems, Wetzlar, Germany). A Canon Power Shot G5 digital camera (Canon, Tokyo, Japan) and Adobe Photoshop version 7.0.1 (Adobe Systems, San Jose, CA) were used to capture images.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/5/10.1182_blood-2005-07-2696/2/m_zh80050692000005.jpeg?Expires=1769095824&Signature=x0ri8-QhCQ5Mr-VoMG~UvqlzWRZr~hE0DBdD5AiimShtmCtOQFr6k-Dx2HgskLqFhmhG8EWEK60-LrI6GNznrVUrwos1Z4OCEd7lzD32q-yGRJrbs2J~cTYsk-8vleitSNEYsvlsv4xMDPOJgXd8JtqgRZ8HsBrFdlzgXC6VQa8FAY6C6pY1GaLHOpbo7g5FgHb9FBWlIapoH7YHjQbuMDkaYAO6BDiqaaUCbNQYU0F-p9GlOaQ6CcolwHy28m5RCbVX3UKYMrhkvm~L4SgT5jPYt-tbUvt-dT2VBuNDGwC4iqIkTdhKZcXbU4cEmKUz2TFiib5vuVcsJeIjuSGFOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal