Interleukin (IL)-10 and glucocorticoids (GCs) inhibit the ability of antigen-presenting dendritic cells (DCs) to stimulate T lymphocytes. We show that induction of GILZ (GC-induced leucine zipper) is involved in this phenomenon. IL-10, dexamethasone (DEX), and transforming growth factor (TGF)β stimulate GILZ production in human immature DCs derived from monocytes and from CD34+ cells. GILZ is necessary and sufficient for DEX, IL-10, and TGFβ modulation of CD80, CD83, CD86, immunoglobulin-like transcript (ILT)-3, and B7-H1 expression by DCs, and alteration of DC functions. GILZ stimulates the production of IL-10 by immature DCs and prevents the production of inflammatory chemokines by CD40L-activated DCs. In contrast, GILZ does not prevent CD40 ligand-mediated inhibition of phagocytosis, indicating that it affects some but not all aspects of DC maturation. GILZ prevents DCs from activating antigen-specific T lymphocyte responses. Administration of GCs to patients stimulates GILZ expression in their circulating antigen-presenting cells, and this contributes to the weak lymphocyte responses of GC-treated patients. Thus, regulation of GILZ expression is an important factor determining the decision of DCs whether or not to stimulate T lymphocytes, and IL-10, GCs, and TGFβ share this mechanism for influencing DC functions and the balance between immune response and tolerance.
Introduction
Dendritic cells (DCs) play a critical role in the activation of T lymphocytes through their ability to capture, process, and present antigens in association with major histocompatibility (MHC) molecules. Interaction between the costimulatory molecules CD80 and CD86, expressed by DCs, and CD28, expressed by T lymphocytes, is required for optimal T lymphocyte activation.1 Immature DCs express low levels of CD80 and CD86. They are activated by microbe-derived molecules through Toll-like receptors (TLRs), and this up-regulates CD80/CD86 expression and ensures their maturation into fully efficient antigen-presenting cells (APCs). CD40 ligation also induces DC maturation and optimal T lymphocyte stimulation. Maturation of DCs induced by TLR ligands and CD40 ligand (CD40L) involves activation of the nuclear factor (NF)-κB and p38 mitogen-activated protein (MAP) kinase pathways. DCs may also generate immune tolerance by inducing T lymphocyte unresponsiveness or apoptosis, or by inducing T lymphocytes with regulatory functions.2 The mechanisms by which antigen presentation by DCs leads to opposite patterns of T lymphocyte responses are not fully understood but they may depend on the stage of DC maturation.2-4 It was initially suggested that induction of tolerance resulted from a “default pathway,” in which antigen presentation in the presence of limited amounts of CD80/CD86 resulted in T lymphocyte anergy. This may account for tolerance induced by immature DCs in tissues devoid of infectious agents or inflammation. More recent findings, however, suggest that the induction of tolerance is a more active process, requiring the production by DCs of tolerance-inducing molecules such as interleukin (IL)-10, indoleamine 2,3-dioxygenase (IDO), B7-H1, immunoglobulin-like transcript (ILT)-3, and inducible costimulator ligand (ICOSL).2,5-7 Such molecules can be generated during the progression along an alternate pathway of DC maturation, leading to “semimature” DCs. This process may be induced by the local production of tolerance-inducing cytokines, including IL-10 and transforming growth factor (TGF)β, and by tolerance-inducing cells such as regulatory T lymphocytes. These mechanisms are not mutually exclusive, as regulatory cells produce IL-10 and TGFβ.2,8-12
Glucocorticoids (GCs) are widely used to treat disorders involving uncontrolled immune activation, and especially autoimmune diseases, allergy, and allograft rejection. Initial analyses focused on the effects of GCs on T lymphocytes to explain their therapeutic properties, but recent reports show that the effects of GCs are also due to silencing innate immune cells. In particular, GCs alter the normal maturation of DCs in response to TLR ligands and to CD40L. They down-regulate the expression of CD80, CD86, and MHC molecules and the production of IL-12, whereas they stimulate the production of IL-10.13,14 IL-10 has similar effects on DCs,15 suggesting that the 2 agents share a common mechanism of action to interfere with DC maturation.
GILZ (GC-induced leucine zipper) was identified in mice thymocytes treated with GCs, protecting them from T-cell receptor (TCR)/CD3-induced cell death.16 Both GCs and IL-10 stimulate the production of GILZ in mice and human monocytes/macrophages, and GILZ is involved in GC and IL-10 anti-inflammatory effects.17 GILZ blocks the NF-κB, MAPK, and AP-1 signal transduction pathways in several types of cells.17-20 In view of the importance of these pathways in DC maturation, we addressed whether induction of GILZ in DCs could explain why GCs and IL-10 alter the maturation of DCs and their ability to activate T lymphocytes.
Materials and methods
Antibodies and flow cytometry
The following monoclonal antibodies (mAbs) coupled with fluorescein-isothiocyanate (FITC), phycoerythrin (PE), or phycoerythrin-cyanin5 (PE-Cy5) were used: CD14-PE and CD80-PE from Becton Dickinson (Pont de Claix, France); CD3-PE, CD40-PE, CD34-PE-Cy5, CD83-PE, and ILT-3-PE-Cy5 from Beckman Coulter (Villepinte, France); CD1a-PE-Cy5, CD86-FITC or CD86-PE, HLA-DR-PE-Cy5, and CD90-FITC or CD90-PE from Pharmingen (San Diego, CA); and B7-H1-PE from Cliniscience (Montrouge, France). Negative controls were irrelevant isotype-matched mAbs. GILZ protein was labeled by incubation with a rabbit polyclonal anti-GILZ Ab21 followed by a PE-conjugated anti-rabbit F(ab′)2 (Jackson Immunoresearch, West Grove, PA). As controls, we used a rabbit polyclonal IgG (Santa Cruz Biotechnology, Santa Cruz, CA) and a PE-conjugated anti-IgG1 mAb (Immunotech, Marseilles, France). At least 104 gated events were acquired with either a FACScalibur (Becton Dickinson) or an EPICS ELITE (Beckman Coulter) and analyzed with CellQuest (Becton Dickinson) or FlowJo (TreeStar, Can Carlos, CA) softwares.
Human samples, cell purifications, and DC differentiation
Peripheral blood and leukapheresis products were collected with informed consent from the donors or patients, and the study protocol was approved by the Institutional Review Board of the South Paris Medical School. To obtain monocyte-derived DCs (MoDCs), monocytes were isolated from mononuclear cells of healthy donors by negative selection (Dynal, Compiègne, France). They were plated in culture flasks at 6 × 105 cells/mL in RPMI 1640 medium (Invitrogen, Cergy-Pontoise, France) containing 10% human AB serum (hABS), 20 ng/mL IL-4 (a kind gift from Dr K. Thielemans, Vrije University, Brussels, Belgium) and 100 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Schering Plough, Levallois-Perret, France) for 9 days, the culture medium being changed on day 3 as described,22 giving immature Mo-DCs. To induce activation and maturation of DCs, trimeric CD40L (250 ng/mL; Immunex, Seattle, WA) was added on day 7 of culture. To obtain CD34-DCs, leukapheresis samples from patients with solid tumors in remission were obtained after stem-cell mobilization with G-CSF/cyclophosphamide. CD34+ cells were purified using immunomagnetic beads as described23 and frozen in fetal calf serum (FCS) containing 10% dimethylsulfoxide until use. CD34+ cells were thawed and seeded in 12-well tissue-culture plates (Costar, Cambridge, MA) at 5 × 105 cells/mL in 2 mL RPMI 1640 medium containing 10% hABS, 1% L-glutamine, 1% antibiotics, stem-cell factor (300 ng/mL; a gift from Amgen, Thousand Oaks, CA), Flt3-ligand (50 ng/mL; a gift from Immunex), 100 ng/mL GM-CSF, and tumor necrosis factor (TNF)α (5 ng/mL; Valbiotech, Paris, France). On day 5, cells were seeded at 2.5 to 5 × 105 cells/mL and incubated for an additional 7 days in the same culture medium supplemented with 50 ng/mL IL-4, the medium being changed at day 8. In some experiments, 250 ng/mL CD40L was added on day 10. Dexamethasone (DEX; Sigma, L'isle d'Abeau, France) was used at 10-7 M, IL-10 (a gift from K. Moore, DNAX, Palo Alto, CA) at 100 ng/mL, and TGFβ (R&D Systems, Minneapolis, MN) at 1 ng/mL. The purity of freshly isolated monocytes and CD34+ cells was assessed by flow cytometry after staining of an aliquot of the preparation with CD14-PE mAb or CD34-PE-Cy5 mAb, respectively (purity > 90%). MoDCs were routinely more than 95% human leukocyte antigen (HLA)-DR+ and more than 85% CD11c+, and CD34-DCs were more than 90% HLA-DR+.
APCs were obtained from patients undergoing treatment with oral GCs (prednisone or prednisolone, 0.5 mg/kg of body weight/day) for alcoholic hepatitis (n = 7), symptomatic sinusitis (n = 2), or acute cervico-brachial neuralgia (n = 2). APCs were isolated from mononuclear cells by negative selection (Dynal) before and after 2 days of GC treatment. After selection, 91.7% ± 2.9% of cells expressed CD14 (4 experiments). The fraction of DCs (CD11c+ ILT3+ cells) was 0.26% ± 0.15% and 2.25% ± 0.20% before and after selection, respectively.
RT-PCR studies
Expression of the human GILZ gene was measured as mRNA by real-time reverse transcriptase-polymerase chain reaction (RT-PCR), and results were expressed as arbitrary units, as previously described.17
Production of GILZ lentiviral vectors
The thy1-IRES-GILZ lentiviral vector construct was obtained in 3 steps using pGILZ, a GILZ-encoding vector,17 the intermediate plasmid pMLV-thy1-IRES-TK,24 the shuttle plasmid pTRE2 (Clontech, Palo Alto, CA), and the pHIV-EF1L-thy1/GFP-W+ lentiviral vector.25 pGILZ was digested with BamHI and the ends were blunted, and then digested with NotI. The resulting GILZ cDNA was ligated into pMLV-thy1-IRES-TK, replacing the TK gene, giving pMLV-thy1-IRES-GILZ. Then, the thy1-IRES-GILZ cassette was inserted between the AvrII and NotI sites in pTRE2, giving pTRE2-thy1-IRES-GILZ. The thy1-IRES-GILZ cassette was then ligated between the MluI and SalI sites in the pHIV-EF1L-thy1/GFP-W+, giving the pHIV-EF1L-thy1/GILZ-W+ lentiviral vector (Lv GILZ). pHIV-EF1L-thy1/GILZ-W+ is a HIV1-derived vector composed of a bicistronic cassette coexpressing both the human THY1 reporter gene and GILZ by the mean of an internal ribosome entry site (IRES) sequence from the encephalomyocarditis virus under the control of the full-length elongation factor 1 alpha promoter (EF1L). pHIV-EF1L-thy1/GILZ-W+ also contains the cPPT (central polypurine tract)26 and WPRE (woodduck hepatitis virus posttranscriptional regulatory element) sequences. Lv TK was constructed by replacing the GILZ gene with the HSV1-TK gene in Lv GILZ to obtain a control construct. pCMVΔR8.91 and pMD.G, both kindly provided by D. Trono (University of Geneva, Switzerland), are HIV-derived packaging constructs which encode the HIV-1 Gag and Pol precursors and the regulatory proteins Tat and Rev and carries an envelope plasmid (env) encoding the vesicular stomatitis virus envelope G protein (VSV-G) used to pseudotype the vector particles. Lentiviral particles were produced as described elsewhere.25 Aliquots of 2 × 107 293T cells were seeded in 162-cm2 tissue-culture flasks and cotransfected using the calcium phosphate method with 9.9 μg pMD.G, 23 μg pCMVΔR8.91, and 29 μg bicistronic pHIV-EF1L-thy1/GILZ-W+ or pHIV-EF1L-thy1/TK-W+. The next day, medium was replaced with medium without serum and lentiviral supernatants were collected 24 hours later. The supernatants were spun at 800g and passed through a 0.45-μm pore-size filter (Costar, Cambridge, MA), and concentrated by ultrafiltration using Centricon Plus-80 filter devices according to the manufacturer's instructions (Centricon Plus-80, molecular weight cutoff 100 kDa; Millipore, Bedford, MA). Concentrated supernatants were stored in aliquots at -80°C until further use. Viral titers were determined using 293T indicator cells as previously described,25 and were 2 × 108 to 109 infectious particles per milliliter.
Transfer of GILZ gene
MoDCs were transduced with pGILZ or pcDNA317 by nucleofection after 7 days of culture, as described by the kit manufacturer (Amaxa System, Köln, Germany). Nucleofection efficiency, assessed using a green fluorescent protein (GFP)-reporter vector, was tested by flow cytometry 24 hours later: 59% ± 6% (3 experiments) of the cells were GFP positive. CD34-DCs were infected with lentiviral vectors on day 8 of culture. Briefly, 105 cells were suspended in 400 μL of culture medium containing lentiviral supernatants at a multiplicity of infection of 100 and 8 μg/mL protamine sulfate and centrifuged at 20°C at 1000g for 3 hours (spinoculation).25 The cells were then further cultured as described in “Human samples, cell purifications, and DC differentiation.” Transduction efficiency was assessed on day 12 of culture by flow cytometry using anti-CD90 mAbs, and was 21.9% ± 4.7% for Lv GILZ and 29.4% ± 6.6% for Lv TK (mean ± SEM of 5 separate experiments).
RNA interference experiments
The 21-nt-long interfering RNA duplexes with 2 3′-end overhanging dT nucleotides on the antisense strand were synthesized. The sequences of the antisense strands of the small interfering RNAs (siRNAs) were for GILZ siRNA (siGILZ): 5′-AAC AGC UUC ACC UGA CAA CGAdTdT; and for control siRNA (siC) with random nucleotides and no known specificity: 5′-CAU AAC GAG CGG AAG AAC GdTdT (MWG Biotech, Ebersberg, Germany; or Dharmacon, Lafayette, CO).
Nucleofection of MoDCs with siRNAs was conducted on day 6 of culture. Then, DEX, IL-10, or medium alone were added, and the samples were cultured for 24 hours. CD34-DCs were transfected with siRNAs on day 8 using the JetSi endo transfection kit (Q-Biogene, Illkirch, France) as described by the manufacturer. Twenty-four hours later, the cells were washed and cultured as described in “Human samples, cell purifications, and DC differentiation” in the presence or absence of DEX or IL-10 until day 12. For APCs, freshly isolated cells were transfected using the JetSi endo transfection kit.
Lymphocyte responses
MoDCs or APCs from Bacille Calmette-Guérin (BCG)-vaccinated individuals were loaded with purified protein derivative (PPD, 1 μg/mL; Statens Serum Institute, Copenhagen, Denmark) for 4 hours, washed, and cocultured (0.5 × 105 DCs) with 1 × 105 freshly purified autologous CD4+ T-cells in round-bottomed 96-well culture plates in RPMI + 10% hABS. CD4+ T cells were purified by negative selection using immunomagnetic beads (Dynal). In some experiments DCs were transfected with siRNAs 24 hours before the end of the MoDC culture. The response of CD4+ T lymphocytes was analyzed at the single-cell level on day 7 of the coculture using the dye Pkh26 (Sigma-Aldrich, Saint Quentin Fallavier, France), as previously described.27 For mixed leukocyte reaction (MLR), allogeneic T cells were purified from peripheral blood mononuclear cells (PBMCs) using immunomagnetic beads as described25 and frozen until use. Thirty gray-irradiated CD34-DCs were mixed in triplicate round-bottomed wells with 105 freshly thawed allogeneic T cells/well and cocultured in 200 μL RPMI + 10% hABS at 37°C for 5 days. Each CD34-DC preparation was tested against T lymphocytes from 3 unrelated donors. Cell proliferation was assayed by [3H]-TdR incorporation. Results are expressed as mean counts per minute (cpm).
ELISA assays
Production of cytokines and chemokines was assessed using enzyme-linked immunosorbent assay (ELISA) kits from Diaclone (Besançon, France) for interferon (IFN)γ and IL-10 and from R&D Systems for CCL3, CCL5, and CXCL8.
Endocytosis assay
The uptake ability of GILZ-expressing DCs was measured using FITC-conjugated dextran particles (FITC-Dx; Sigma) diluted in phosphate-buffered saline (PBS) at 10 mg/mL and used at a final concentration of 1 mg/mL in culture medium. GILZ-expressing DCs were seeded in culture medium at 1 × 106 cells/mL, kept at 37°C for 30 minutes, and then incubated with FITC-Dx at 37°C for 1 hour. Negative control of uptake was cells tested at 4°C. Then, cells were washed 4 times in cold PBS and analyzed immediately by flow cytometry. When the endocytosis of FITC-Dx was studied on lentivirally-infected CD34-DCs, cells were stained with CD90-PE mAbs prior to the uptake assay and analysis was performed on gated CD90+ CD34-DCs, which were considered transduced cells.
Results
Expression of GILZ by DCs
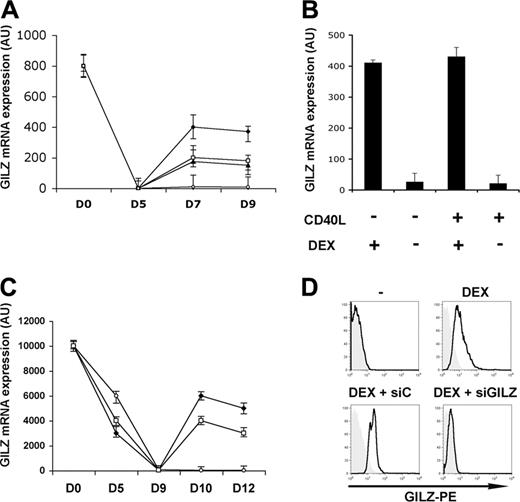
We monitored GILZ gene expression by real time RT-PCR during the differentiation of MoDCs. Freshly isolated monocytes expressed the GILZ gene. This expression disappeared after 5 days of culture in the presence of GM-CSF + IL-4, and remained undetectable both in the presence and in the absence of CD40L-induced DC maturation. Addition of DEX led to an up-regulation of GILZ gene expression. This expression was maintained even in the presence of CD40L (Figure 1A). When DEX was added on day 5 and removed on day 7, GILZ expression then disappeared by day 9, regardless of the addition of CD40L (Figure 1B). Therefore, GILZ gene induction is reversible and its persistence depends on the continuous presence of DEX. IL-10 and TGFβ also stimulated GILZ gene expression by DCs, although to a lesser extent than DEX (Figure 1A). GILZ gene expression was also studied during the differentiation of DCs from CD34+ cells (CD34-DCs). Freshly isolated CD34+ cells expressed the GILZ gene strongly and this expression was progressively lost during their dendritic differentiation. Addition of DEX or IL-10 increased GILZ gene expression, and the increase persisted after CD40L addition (Figure 1C). Therefore, DEX and IL-10 stimulated GILZ gene expression in immature DCs, and this expression persisted upon CD40L triggering. When DEX or IL-10 were added on day 7 to MoDCs or on day 10 to CD34-DCs, no induction of GILZ gene expression was observed, regardless of the addition of CD40L (data not shown), indicating that sensitivity of DCs to GCs and IL-10 was restricted to a specific step of their differentiation. Expression of GILZ by MoDCs was also studied by flow cytometry using an anti-GILZ polyclonal Ab. No GILZ was detected in immature MoDCs cultured without DEX. Addition of DEX, IL-10, or TGFβ stimulated GILZ production (Figure 1D, upper panels, and data not shown). Western blot studies gave similar findings (data not shown).
GILZ production by DCs.GILZ gene expression was determined by real-time RT-PCR. (A) DEX (♦), IL-10 (□), TGFβ (▴), or medium (○) were added to MoDC cultures on days 5 and 7, and CD40L was added on day 7. (B) DEX was added to MoDCs on day 5, cells were washed on day 7, and DEX, CD40L, or medium were added. (C) DEX (), IL-10 (), or medium (○) were added on day 9 of CD34-DC cultures, and CD40L was added on day 10. (D) GILZ expression (bold curves) in untreated MoDCs and in MoDCs treated for 24 hours with DEX, DEX + control siRNA (siC), or DEX + GILZ siRNA (siGILZ) was determined by flow cytometry. Tinted histograms correspond to the control antibody. Means (± SEM) are shown in panels A-C, and a typical experiment, in panel D. n = 5 for panels A and C, and n = 3 for panels B and D.
GILZ production by DCs.GILZ gene expression was determined by real-time RT-PCR. (A) DEX (♦), IL-10 (□), TGFβ (▴), or medium (○) were added to MoDC cultures on days 5 and 7, and CD40L was added on day 7. (B) DEX was added to MoDCs on day 5, cells were washed on day 7, and DEX, CD40L, or medium were added. (C) DEX (), IL-10 (), or medium (○) were added on day 9 of CD34-DC cultures, and CD40L was added on day 10. (D) GILZ expression (bold curves) in untreated MoDCs and in MoDCs treated for 24 hours with DEX, DEX + control siRNA (siC), or DEX + GILZ siRNA (siGILZ) was determined by flow cytometry. Tinted histograms correspond to the control antibody. Means (± SEM) are shown in panels A-C, and a typical experiment, in panel D. n = 5 for panels A and C, and n = 3 for panels B and D.
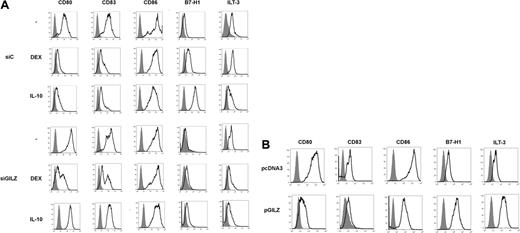
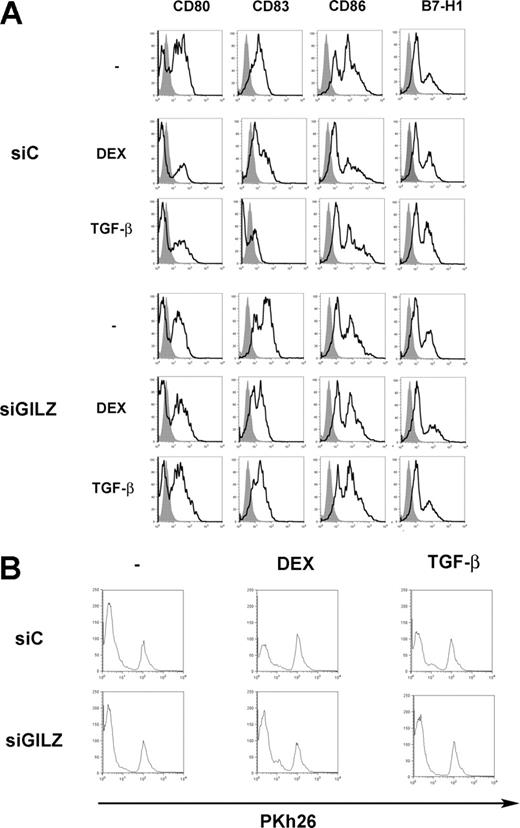
Effects of GILZ on MoDC phenotype. (A) MoDCs were treated on day 5 with DEX, IL-10, or medium, and with control (siC) or GILZ (siGILZ) siRNA. On day 7, DC phenotype was determined. One typical experiment of 3 is shown. (B) MoDCs were transduced with a control (pcDNA3) or a GILZ-encoding (pGILZ) vector on day 6 of culture, and were stimulated with CD40L on day 7. DC phenotype was determined on day 9. One representative experiment of 2 is shown. The referred marker is shown by boldface curves, and the isotype control is in gray histograms.
Effects of GILZ on MoDC phenotype. (A) MoDCs were treated on day 5 with DEX, IL-10, or medium, and with control (siC) or GILZ (siGILZ) siRNA. On day 7, DC phenotype was determined. One typical experiment of 3 is shown. (B) MoDCs were transduced with a control (pcDNA3) or a GILZ-encoding (pGILZ) vector on day 6 of culture, and were stimulated with CD40L on day 7. DC phenotype was determined on day 9. One representative experiment of 2 is shown. The referred marker is shown by boldface curves, and the isotype control is in gray histograms.
Modulation of DC phenotype by GILZ
We determined whether GILZ is involved in the phenotypic changes of MoDCs induced by DEX or IL-10. Inhibition of GILZ production was obtained by transfecting MoDCs with GILZ siRNA. The GILZ siRNA prevented the DEX- or IL-10-induced production of GILZ mRNA, as assessed by real-time RT-PCR, and of GILZ protein, as assessed by flow cytometry and by Western blotting. A control siRNA had no effect (Figure 1D, lower panels, and data not shown).
DEX and IL-10 changed the phenotype of MoDCs. When added on day 5 of culture to DCs transduced with control siRNA, they inhibited the expression of CD80, CD83, and CD86, and they stimulated the expression of B7-H1. DEX and IL-10 inconsistently inhibited the expression of MHC class II molecules and stimulated that of ILT3 (Figure 2A and data not shown). In these experiments, expression of CD83 by control cells was higher than expected for DCs cultured in the absence of differentiating agents, reflecting a partial activation of DCs by nucleofection. Transduction of DCs with GILZ siRNA prevented DEX- and IL-10-induced changes of phenotype: GILZ siRNA partially restored CD80 and CD83 expression and completely restored CD86 expression; it abolished induction of B7-H1 and ILT3 (Figure 2A). In experiments in which DEX or IL-10 inhibited MHC class II expression, this effect was reversed by GILZ siRNA (data not shown). Thus, GILZ is involved in the DEX- and IL-10-induced changes of MoDC phenotype. Next, a GILZ-encoding vector (pGILZ) was introduced into MoDCs and cell phenotype was determined 24 hours later. We verified by flow cytometry and Western blotting that nucleofection of MoDCs with pGILZ resulted in production of the GILZ protein (data not shown). The effect of GILZ on CD86 expression was inconsistent. In contrast, GILZ-producing DCs expressed lower levels of CD80 and CD83 and higher levels of B7-H1 and ILT3 than did DCs transfected with a control vector (Figure 2B). Therefore, GILZ is sufficient to cause changes of DC phenotype.
The effect of GILZ on the production of IL-10 and chemokines by DCs
Treatment of immature MoDCs with DEX stimulates their production of IL-10.14 We investigated whether GILZ was involved in this effect. DEX stimulated IL-10 production in MoDCs transfected with control siRNA. In contrast, transfection with GILZ siRNA partially prevented DEX-induced up-regulation of IL-10 production. We next tested the effect of GILZ gene transfer into MoDCs. MoDCs nucleofected with pGILZ produced approximately 6-fold more IL-10 than control cells nucleofected with pcDNA3 (Table 1). Therefore, induction of GILZ is at least partially responsible for the DEX-mediated stimulation of IL-10 production by DCs.
Role of GILZ in DEX-induced IL-10 production by MoDCs
. | DEX . | IL-10, pg/mL . |
|---|---|---|
| — | – | 176 ± 15 |
| — | + | 1068 ± 70 |
| siC | + | 1576 ± 76 |
| siGILZ | + | 487 ± 22 |
| pcDNA3 | – | 299 ± 35 |
| pGILZ | – | 1750 ± 85 |
. | DEX . | IL-10, pg/mL . |
|---|---|---|
| — | – | 176 ± 15 |
| — | + | 1068 ± 70 |
| siC | + | 1576 ± 76 |
| siGILZ | + | 487 ± 22 |
| pcDNA3 | – | 299 ± 35 |
| pGILZ | – | 1750 ± 85 |
IL-10 concentration (mean ± SEM) was measured in supernatants of immature MoDCs treated with DEX alone, with DEX and control, or GILZ siRNA, or in immature MoDCs nucleofected with a control (pcDNA3) or a GILZ-encoding (pGILZ) vector.
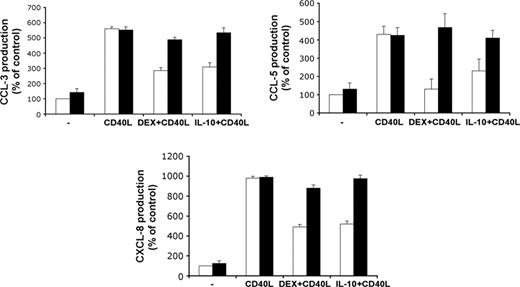
Activation of MoDCs with CD40L stimulated the production of the chemokines CCL3, CCL5, and CXCL8, but this was partially prevented by treatment with DEX or IL-10. Transfection of GILZ siRNA inhibited the effect of each DEX and IL-10 on chemokine production, whereas transfection with control siRNA had no effect (Figure 3).
Effects of GILZ on chemokine production by DCs. MoDCs were treated with control (□) or GILZ (▪) siRNA on day 5, with DEX, IL-10, or medium on days 5 and 7, and with or without CD40L on day 7. Production of CCL3, CCL5, and CXCL8 was determined on day 9. Results (mean ± SEM) are expressed as percentages of the values for controls, treated with control siRNA only (n = 3).
Effects of GILZ on chemokine production by DCs. MoDCs were treated with control (□) or GILZ (▪) siRNA on day 5, with DEX, IL-10, or medium on days 5 and 7, and with or without CD40L on day 7. Production of CCL3, CCL5, and CXCL8 was determined on day 9. Results (mean ± SEM) are expressed as percentages of the values for controls, treated with control siRNA only (n = 3).
GILZ effect on DC maturation
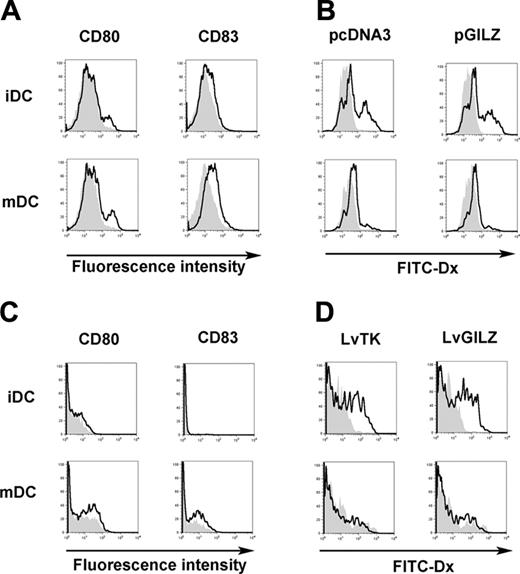
The impact of GILZ expression on DC maturation was evaluated. MoDCs were nucleofected with either pcDNA3 or pGILZ, stimulated or not with CD40L, and tested for dextran particles phagocytosis. As expected, the expression of CD80 and CD83 was lower in cells nucleofected with pGILZ than in controls (Figure 4A). In immature MoDCs, phagocytosis of dextran was similar between GILZ-expressing and control cells. CD40L-induced activation of MoDCs abolished phagocytosis to a similar extent in GILZ-expressing and control cells (Figure 4B). In these experiments, phagocytosis of dextran by immature MoDCs nucleofected with either pcDNA3 or pGILZ was lower than in cells not nucleofected, whereas expression of CD80 and CD83 was stronger (data not shown). This indicates that nucleofection induced a partial maturation of MoDCs. To avoid this, we generated GILZ-producing CD34-DCs using lentiviral-mediated transfer of the GILZ gene. A thymidine kinase-encoding lentivirus was used as control. Both lentiviruses also encoded for the CD90 reporter gene, and phenotypic changes were analyzed in CD90+ CD34-DCs. In the absence of CD40L activation, CD34-DCs expressed no CD83 and only low levels of CD80. GILZ expression partially inhibited the up-regulation of CD80 and CD83 induced by CD40L (Figure 4C). In immature CD34-DCs, phagocytosis was slightly higher in GILZ-expressing cells than in controls (Figure 4D). Activation of CD34-DCs with CD40L abolished phagocytosis in both GILZ-expressing and control cells. These findings confirm those obtained with MoDCs, showing that GILZ has dissociated effects on the maturation of DCs, preventing some but not all parameters of this process.
GILZ-expressing DCs and T lymphocyte activation
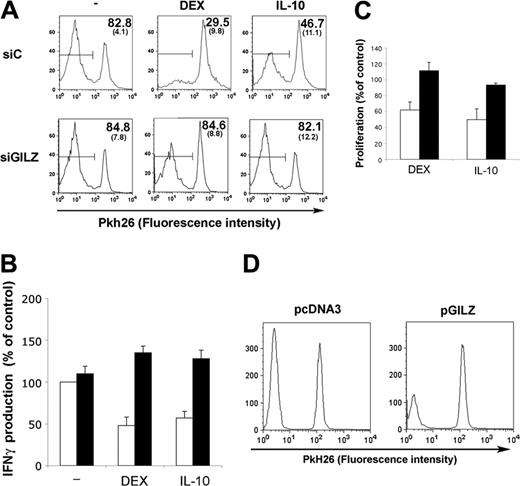
To test the ability of GILZ-expressing DCs to activate T lymphocytes, MoDCs were transfected with control or GILZ siRNA, cultured with or without DEX or IL-10, and loaded with PPD. DCs were then mixed with autologous Pkh26-labeled CD4+ T lymphocytes, and proliferation of lymphocytes was followed. Treatment of DCs with DEX or IL-10 decreased their ability to stimulate CD4+ T lymphocytes (data not shown). The effect of DEX and IL-10 was preserved when DCs were transfected with control siRNA. In contrast, GILZ siRNA abolished the effect of DEX or IL-10 on DCs, restoring a strong proliferative response to PPD (Figure 5A). The effect of GILZ expression by DCs on IFNγ production by CD4+ T lymphocytes was also tested. PPD-loaded MoDCs stimulated IFNγ production by autologous CD4+ T lymphocytes. Treating DCs with either DEX or IL-10 inhibited the production of IFNγ by CD4+ T lymphocytes. This inhibition was abolished when DCs were transfected with GILZ siRNA, whereas control siRNA had no effect (Figure 5B).
The role of GILZ on CD34-DC-induced allogeneic response was also tested. Treatment of CD34-DCs with DEX or IL-10 decreased their ability to stimulate the proliferation of allogeneic T lymphocytes. Transfection of CD34-DCs with control siRNA had no effect, regardless of the addition of DEX or IL-10. Transfection with GILZ siRNA slightly enhanced proliferation in the absence of DEX or IL-10. It totally reversed the inhibitory effect of DEX and IL-10 (Figure 5C).
We tested whether GILZ expression in DCs was sufficient to decrease CD4+ T lymphocyte stimulation. MoDCs transfected with pGILZ or a control vector were loaded with PPD, washed, and cocultured with autologous CD4+ T lymphocytes. The proliferation of CD4+ T lymphocytes was lower when GILZ-expressing DCs were used as APCs (Figure 5D). Expression of GILZ in DCs also decreased the production of IFNγ by CD4+ T lymphocytes (20.1% ± 11.3% compared with the production with control DCs; P < .05).
We tested whether TGFβ has similar effects on DCs to DEX. Treatment of DCs with TGFβ inhibited their expression of CD80, CD83, and CD86, and it up-regulated the expression of B7-H1. These effects were reversed when DCs were nucleofected with GILZ siRNA (Figure 6A). When TGFβ-treated DCs were used to present PPD, they stimulated CD4+ T lymphocytes to a lesser extent than control DCs. This defect was reversed when DCs were treated with GILZ siRNA (Figure 6B). Therefore, TGFβ-treated DCs display phenotypic and functional alterations close to those of DEX-treated DCs, and these changes also involve GILZ production by DCs.
GILZ effect on DC maturation. (A-B) MoDCs were nucleofected with either pcDNA3 or pGILZ and stimulated with CD40L or left unstimulated, creating mature DCs (mDCs) and immature DCs (iDCs), respectively. (A) CD80 and CD83 expression was analyzed in DCs transfected with pcDNA3 (bold lines) or pGILZ (gray histograms). (B) phagocytosis of FITC-Dx was analyzed at 37°C (bold lines) or at 4°C (gray histograms). (C-D) CD34-DCs were infected with LvGILZ or LvTK on day 8 of culture. CD40L was added or not on day 10. Results shown are gated on CD90+ (transduced) DCs. (C) DC phenotype was determined on day 12. Bold lines and gray histograms correspond to LvTK- and LvGILZ-infected DCs, respectively. (D) Phagocytosis of FITC-Dx was determined at 37°C (bold lines) or 4°C (gray histograms). Results are from 1 representative experiment of 3.
GILZ effect on DC maturation. (A-B) MoDCs were nucleofected with either pcDNA3 or pGILZ and stimulated with CD40L or left unstimulated, creating mature DCs (mDCs) and immature DCs (iDCs), respectively. (A) CD80 and CD83 expression was analyzed in DCs transfected with pcDNA3 (bold lines) or pGILZ (gray histograms). (B) phagocytosis of FITC-Dx was analyzed at 37°C (bold lines) or at 4°C (gray histograms). (C-D) CD34-DCs were infected with LvGILZ or LvTK on day 8 of culture. CD40L was added or not on day 10. Results shown are gated on CD90+ (transduced) DCs. (C) DC phenotype was determined on day 12. Bold lines and gray histograms correspond to LvTK- and LvGILZ-infected DCs, respectively. (D) Phagocytosis of FITC-Dx was determined at 37°C (bold lines) or 4°C (gray histograms). Results are from 1 representative experiment of 3.
GILZ-expressing DCs and antigen-specific response of CD4+ T lymphocytes. MoDCs were treated on day 5 with control (siC; □) or GILZ (siGILZ; ▪) siRNA and with DEX, IL-10, or medium. They were loaded with PPD, washed, and mixed with autologous CD4+ T lymphocytes on day 7. (A) Lymphocyte proliferation, determined by Pkh26 fluorescence intensity (1 representative experiment of 3). The mean (SEM) fraction of Pkh26low CD4+ T lymphocytes in the 3 experiments is shown in the top right corner. Horizontal bars indicate Pkh26low cells. (B) IFNγ production (mean ± SEM of 2 experiments). In the absence of PPD loading there was no proliferation and less than 250 pg/mL IFNγ. (C) CD34-DCs were treated on day 8 with control (□) or GILZ (▪) siRNA and with DEX, IL-10, or medium. On day 12, the DCs were mixed with allogeneic T lymphocytes at a 1:2 ratio. Proliferation is expressed as percentages of values for controls, to which no DEX or IL-10 was added to DCs. Results (mean ± SEM) are from 1 representative experiment of 2. Thymidine incorporation (mean ± SEM) by cells stimulated with control or GILZ siRNA-treated DCs, without DEX or IL-10 addition, was 21 984 ± 5 206 cpm and 31 842 ± 9 023 cpm, respectively. (D) MoDCs were nucleofected with pcDNA3 or pGILZ on day 6, loaded with PPD on day 7, and cocultured with Pkh26-labeled CD4+ T lymphocytes. T lymphocyte proliferation was determined 7 days later. Results are from 1 representative experiment of 6, in which the inhibition of proliferation with GILZ-expressing DCs was 50.3% ± 8.0% (mean ± SEM) compared with control DCs (P < .05).
GILZ-expressing DCs and antigen-specific response of CD4+ T lymphocytes. MoDCs were treated on day 5 with control (siC; □) or GILZ (siGILZ; ▪) siRNA and with DEX, IL-10, or medium. They were loaded with PPD, washed, and mixed with autologous CD4+ T lymphocytes on day 7. (A) Lymphocyte proliferation, determined by Pkh26 fluorescence intensity (1 representative experiment of 3). The mean (SEM) fraction of Pkh26low CD4+ T lymphocytes in the 3 experiments is shown in the top right corner. Horizontal bars indicate Pkh26low cells. (B) IFNγ production (mean ± SEM of 2 experiments). In the absence of PPD loading there was no proliferation and less than 250 pg/mL IFNγ. (C) CD34-DCs were treated on day 8 with control (□) or GILZ (▪) siRNA and with DEX, IL-10, or medium. On day 12, the DCs were mixed with allogeneic T lymphocytes at a 1:2 ratio. Proliferation is expressed as percentages of values for controls, to which no DEX or IL-10 was added to DCs. Results (mean ± SEM) are from 1 representative experiment of 2. Thymidine incorporation (mean ± SEM) by cells stimulated with control or GILZ siRNA-treated DCs, without DEX or IL-10 addition, was 21 984 ± 5 206 cpm and 31 842 ± 9 023 cpm, respectively. (D) MoDCs were nucleofected with pcDNA3 or pGILZ on day 6, loaded with PPD on day 7, and cocultured with Pkh26-labeled CD4+ T lymphocytes. T lymphocyte proliferation was determined 7 days later. Results are from 1 representative experiment of 6, in which the inhibition of proliferation with GILZ-expressing DCs was 50.3% ± 8.0% (mean ± SEM) compared with control DCs (P < .05).
In vivo induction of GILZ by GC treatment
GILZ gene expression was quantified by real-time PCR in circulating APCs from patients treated with GCs for various inflammatory disorders. Analyses were performed before and 48 hours after initiation of treatment. GC stimulated GILZ gene expression by circulating APCs (535 ± 43 AU of GILZ mRNA [mean ± SEM] vs 151 ± 35 AU before GC administration; P < .05).
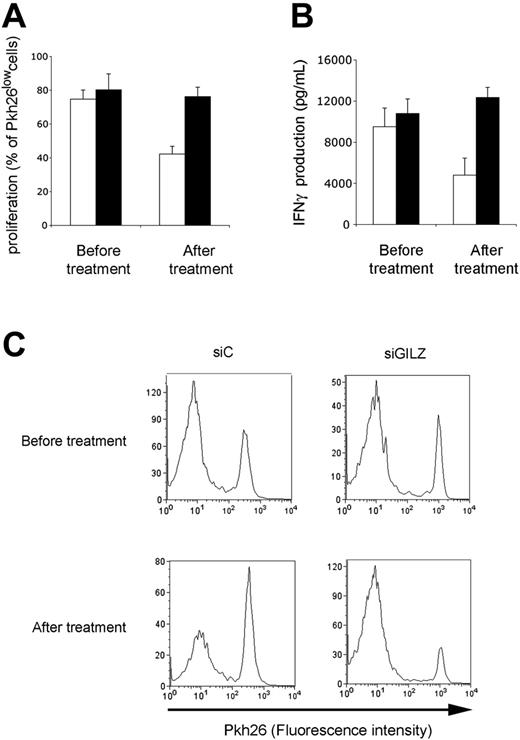
The ability of circulating APCs from GC-treated patients to stimulate an antigen-specific CD4+ T lymphocyte response was tested. APCs from GC-treated patients stimulated CD4+ T lymphocytes poorly, as assessed by proliferative and IFNγ production assays (data not shown). Findings were similar when APCs were transfected with a control siRNA. In contrast, APCs from GC-treated patients generated strong proliferation and IFNγ production when they were transfected with GILZ siRNA (Figure 7). Therefore, in GC-treated patients, GILZ expression by circulating APCs is increased, and this explains their poor effectiveness at triggering an antigen-specific CD4+ T lymphocyte response.
Discussion
GCs and IL-10 inhibit the expression of costimulatory and MHC molecules by DCs, and they prevent efficient antigen presentation to T lymphocytes. GCs and IL-10 each stimulate DC expression of molecules that directly inhibit T lymphocyte responses, including B7-H1, IL-10, and ILT3. We show that these effects of GCs and IL-10 involve a common mechanism, mediated by the induction of GILZ expression. DCs derived in vitro from monocytes or CD34+ cells do not produce GILZ spontaneously, but GILZ production is induced by either DEX or IL-10. The continuous presence of DEX is required for the modulation of DC phenotype.6,14 GILZ production also requires the continuous presence of DEX or IL-10, and stops when these agents are removed. In contrast, GILZ production is not influenced by the addition of CD40L. In MoDCs treated with DEX or IL-10, the decreased expression of costimulatory and MHC class II molecules and the increased expression of B7-H1 and ILT3 are prevented by GILZ siRNA. Moreover, GILZ gene transfer to MoDCs is sufficient to reproduce the phenotypic changes caused by DEX and IL-10.
GILZ involvement in TGFβ-induced alterations of DC functions. (A) MoDCs were treated on day 5 with DEX, TGFβ, or medium, and with control (siC) or GILZ (siGILZ) siRNA. On day 7, DC phenotype was determined. (B) MoDCs were treated on day 5 with control (siC) or GILZ (siGILZ) siRNA and with DEX, TGFβ, or medium. They were loaded with PPD, washed, and mixed with autologous CD4+ T lymphocytes on day 7. Lymphocyte proliferation was determined by Pkh26 fluorescence intensity. One typical experiment of 2 is shown.
GILZ involvement in TGFβ-induced alterations of DC functions. (A) MoDCs were treated on day 5 with DEX, TGFβ, or medium, and with control (siC) or GILZ (siGILZ) siRNA. On day 7, DC phenotype was determined. (B) MoDCs were treated on day 5 with control (siC) or GILZ (siGILZ) siRNA and with DEX, TGFβ, or medium. They were loaded with PPD, washed, and mixed with autologous CD4+ T lymphocytes on day 7. Lymphocyte proliferation was determined by Pkh26 fluorescence intensity. One typical experiment of 2 is shown.
Besides modulating the phenotype of DC, GILZ also affected their function. GILZ stimulated IL-10 production by DCs to the same extent as GCs. Both DEX and IL-10 inhibited the production of inflammatory chemokines by CD40L-stimulated DCs. This effect was not found in DCs transfected with GILZ siRNA. DEX and IL-10 inhibited DC stimulation of an antigen-specific CD4+ T lymphocyte response and an allogeneic response. RNA interference experiments showed that GILZ expression is necessary for this effect, and gene transfer experiments showed that it is sufficient. The effect of DEX, IL-10, and GILZ was stronger on DC phenotype changes than on T lymphocyte activation by DCs. The inhibition of T lymphocyte stimulation by GILZ-expressing DCs presumably results from a conjunction of effects: a decreased expression of costimulatory and MHC molecules, a decreased production of chemokines, which contribute to T lymphocyte activation,28,29 and an increased expression of IL-10, B7-H1, and ILT3. This redundancy of mechanisms may ensure a strong and consistent inhibition of T lymphocyte activation by GILZ-expressing DCs regardless of their origin and of their environmental conditions.
Effect of GILZ in GC-treated patients. Patients were tested before and after 48 hours of treatment with GCs. Their APCs were transfected with control (siC; □) or GILZ (siGILZ; ▪) siRNA, loaded with PPD, and cocultured with autologous CD4+ T lymphocytes. Proliferation (A,C) and IFNγ production (B) were determined on day 7 of culture. (A-B) Results are expressed as means ± SEM for 4 patients; (C) a typical experiment.
Effect of GILZ in GC-treated patients. Patients were tested before and after 48 hours of treatment with GCs. Their APCs were transfected with control (siC; □) or GILZ (siGILZ; ▪) siRNA, loaded with PPD, and cocultured with autologous CD4+ T lymphocytes. Proliferation (A,C) and IFNγ production (B) were determined on day 7 of culture. (A-B) Results are expressed as means ± SEM for 4 patients; (C) a typical experiment.
TGFβ also up-regulated GILZ production by DCs. TGFβ acts on T lymphocytes to prevent immune activation. However, TGFβ also affects DC functions directly,8,9 and our findings show that GILZ induction is involved in this effect. GILZ production may thus be a common mechanism of immune control shared by several immunosuppressive cytokines. Interestingly, both IL-10 and TGFβ are produced by T lymphocytes with regulatory functions,12 suggesting that these cells may control immune activation at least partly by inducing GILZ expression in DCs. Because GILZ-expressing DCs themselves produce IL-10, a self-amplifying loop may be induced locally to inhibit inflammation and immune activation.
GILZ inhibits cell activation via various molecular targets. It prevents NF-κB activation, at least partly by binding directly to its p65 subunit.17-19 It also interferes with the MAPK pathway and the induction of AP-1.19,20 These signal transduction pathways are central to the maturation of DCs, so a simple explanation of the effect of GILZ on DC function would be that it prevents DC maturation. The observation that GILZ inhibits several CD40L-induced modifications of DCs, such as chemokine production and up-regulation of costimulatory and MHC molecules, is consistent with this possibility. We also observed that GILZ prevents lipopolysaccharide (LPS)-induced maturation of DCs (data not shown). As immature DCs are poor activators of T lymphocytes and may even promote tolerance, this would account for the inhibitory effect of GILZ on T lymphocyte responses. However, GILZ does not prevent all consequences of DC maturation, as it does not affect CD40L-induced inhibition of phagocytosis. Moreover, GILZ has specific effects, such as up-regulation of IL-10 and B7-H1 expression. Expression of B7-H1 increases with DC maturation,5,7,30 and therefore induction of IL-10 and B7-H1 by GILZ is not consistent with GILZ only preventing DC maturation. These observations rather suggest that GILZ drives DCs to an alternative pathway of differentiation, leading to DCs with many features of tolerogenic DCs. The action of GILZ on DC maturation, regardless of its mechanism, presumably explains why IL-10 and TGFβ affect the function of immature DCs but are not effective on fully mature DCs.10,11,15 The inability of DEX and IL-10 to induce GILZ when added to fully differentiated DCs may also explain this phenomenon.
Since their introduction as therapy, GCs have become a cornerstone in most immunosuppressive treatments. Although their mechanisms of action remain incompletely understood, APCs are critical targets to their therapeutic effect. GCs stimulate synthesis of IκBα and thus prevent NF-κB activation,31,32 suggesting a possible mechanism of GC action in DCs. However, the intracellular effects of GCs appear much more complex than initially thought, involving several molecular targets. We show here that GC administration to humans stimulates GILZ expression by circulating APCs and that GILZ induction accounts for the poor efficacy of APCs from GC-treated patients to stimulate T lymphocytes. Indeed, neutralization of GILZ by RNA interference restores fully potent antigen presentation by APCs from GC-treated patients. By inducing GILZ in DCs, GCs may reproduce for therapeutic purposes a natural mechanism of immune regulation induced by immunosuppressive cytokines, including IL-10 and TGFβ. Evidence from genetically modified mice demonstrates that IL-10 and TGFβ are central to immune tolerance.33-37 In human and in mice, GILZ is constitutively produced by APCs (Berrebi et al17 and this work). Possibly, this constitutive expression contributes to preventing unwarranted immune activation in healthy individuals. On the other hand, GILZ expression by DCs may participate in immune defects in some disorders, and especially in cancers, which often produce IL-10, and in which IL-10-induced inhibition of CD808 and enhancement of B7-H16 expression by DCs contribute to the failure of the immune system to control the tumor. Altogether, these results show that regulation of GILZ expression plays a critical role in the decision of DC to stimulate T lymphocytes or not, and IL-10, GCs, and TGFβ share this mechanism to affect DC functions and the balance between immune response and tolerance.
Prepublished online as Blood First Edition Paper, November 17, 2005; DOI 10.1182/blood-2005-07-2760.
Supported by the Association pour la Recherche sur le Cancer, the Agence Nationale de Recherche sur le SIDA, the Université Paris-Sud, and the Action Thématique Concertée INSERM “foie et alcool.” E.M. was financially supported by the Ministère de l'Education Nationale, de la Recherche et de la Technologie and by “Ensemble Contre le SIDA”; H.H. was supported by the Agence Nationale de Recherche sur le SIDA.
N.C. and E.M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Celine Morel for her expert technical assistance. We thank Weiping Zou (Tulane University, New Orleans, LA) for critical review of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal