Abstract
We showed previously that mild real hypoxia and hypoxia-mimetic agents induced in vitro cell differentiation of acute myeloid leukemia (AML). We here investigate the in vivo effects of intermittent hypoxia on syngenic grafts of leukemic blasts in a PML-RARα transgenic mouse model of AML. For intermittent hypoxia, leukemic mice were housed in a hypoxia chamber equivalent to an altitude of 6000 m for 18 hours every consecutive day. The results show that intermittent hypoxia significantly prolongs the survival of the leukemic mice that received transplants, although it fails to cure the disease. By histologic and cytologic analyses, intermittent hypoxia is shown to inhibit the infiltration of leukemic blasts in peripheral blood, bone marrow, spleen, and liver without apoptosis induction. More intriguingly, intermittent hypoxia also induces leukemic cells to undergo differentiation with progressive increase of hypoxia-inducible factor-1α protein, as evidenced by morphologic criteria of maturating myeloid cells and increased expression of mouse myeloid cell differentiation–related antigens Gr-1 and Mac-1. Taken together, this study represents the first attempt to characterize the in vivo effects of hypoxia on an AML mouse model. Additional investigations may uncover ways to mimic the differentiative effects of hypoxia in a manner that will benefit human patients with AML.
Introduction
Angiogenesis is an essential phenotype in growth and development, wound healing, and reproduction.1,2 An inadequate amount of vessel growth contributes to ulcer formation, whereas excessive angiogenesis is relevant to a number of pathologic conditions including arthritis, psoriasis, and cancers.3-6 Leukemia, a common hematopoietic malignancy, has traditionally been regarded as a “liquid tumor” with the appearance of leukemic cells freely floating in the peripheral circulation. Accordingly, leukemia was assumed not to require angiogenesis for its growth. However, recent evidence suggests that angiogenesis is also important in the pathogenesis of numerous different hematologic malignancies, including acute myeloid leukemia (AML), chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), as well as multiple myeloma.7-12 For example, bone marrow (BM) from patients with acute promyelocytic leukemia (APL; a unique subtype of AML with a specific chromosomal translocation t(15;17) that causes the expression of a fusion protein, PML-RARα [promyelocytic leukemia retinoic acid receptor α]13,14 ) exhibited significantly increased microvessel density. Cellular levels of angiogenic factors such as vascular endothelial growth factor (VEGF) are abnormally elevated and provide an independent predictor of outcome in adults with AML.15 Also, there is a 6- to 7-fold increase in microvessel density in BM biopsies of newly diagnosed untreated ALL in children.16 Moreover, the myeloproliferative diseases (polycythemia vera, CML, and myelofibrosis) have also significantly increased neovascularity.17 In addition to VEGF, human hematopoietic cells express high levels of basic fibroblast growth factor, which is known to play a critical role in (i) tumorigenesis of solid tumors as a potent endothelial cell mitogen and in (ii) the pathophysiology of AML.1,18
On the other hand, antiangiogenic effects of chemotherapeutic and other novel drugs for the treatment of leukemia, such as all-trans retinoic acid (ATRA),19 arsenic trioxide (As2O3),20 farnesyltransferase inhibitors,21 and the tyrosine kinase inhibitor imatinib,22 might contribute to their therapeutic potential.23 Thalidomide, which exerts an antiangiogenic effect and a direct cytotoxic effect, was found to be effective in multiple myeloma and myelodysplastic syndrome in a subset of patients.24 These observations provide a conceptual basis for the future use of angiogenesis inhibitors in leukemia, perhaps first in patients in whom all conventional therapy has failed and later as an adjunct to conventional therapy.
It is rational to speculate that angiogenesis increases while antiangiogenesis decreases oxygen concentration of the BM micro-environment. Conversely, hypoxia (low oxygen tension) promotes angiogenesis as an oxygen homeostatic mechanism for adaptive response.25,26 The potential relationship between angiogenesis and prognosis of leukemia as well as the possible benefit of antiangiogenic drugs to treat leukemia promoted us to explore the effects of hypoxia and hypoxia-mimicking agents cobalt chloride (CoCl2) and desferrioxamine (DFO) on AML cells. Recently, we showed that nontoxic concentrations of CoCl2 and DFO as well as mild hypoxia induce the in vitro differentiation of AML cell lines and some fresh leukemic cells.27-29 Hypoxia was also reported to modify the proliferation and differentiation of CD34+ CML cells.30 Inspired by these interesting in vitro discoveries, the present work aims to explore in vivo effects of hypoxia on AML mice that were generated by using syngenic grafts of leukemic blasts from PML-RARα transgenic mice. The results demonstrate for the first time that in vivo intermittent hypoxia prolongs survival of the leukemic mice by tumor arrest and differentiation induction.
Materials and methods
Isolation and transplantation of leukemic cells
Leukemic cells were isolated from BM and spleen of leukemic hMRP8–PML-RARα transgenic mice (Leukemia 1111), as described,31 by flushing RPMI1640 medium (Sigma, St Louis, MO) through long bones and collecting cells from dissociated spleens. To propagate leukemia, leukemic blasts (2 × 105 or 2 × 106 viable hematopoietic cells) were injected into the tail vein of 7- to 10-week-old syngenic FVB-NICO (FVB/N) mice (Shanghai Laboratory of Animal Center, Chinese Academy of Sciences, Shanghai, China) after sublethal irradiation totaling 4.5 Gy. Animal handling was approved by the committee for humane treatment of animals at Shanghai Second Medical University.
Treatment of leukemic mice
Mice that received implants of leukemic blasts were randomly assigned to treatment. For the treatment of hypoxia-mimetic agents, CoCl2 and DFO powders with a purity of 99% (Sigma) were dissolved in normal saline as 1.5 g/L and 5 g/L stock solutions, respectively. Fifteen and 50 μg/g body weight (wt) of CoCl2 and DFO were respectively administered to leukemic mice by intraperitoneal injection every other day. Control mice were treated with intraperitoneal injection of normal saline. For hypoxia treatment, normal and leukemic mice were housed in a hypoxic chamber equivalent to an altitude of 6000 m for 18 hours every day. Mice with or without implantation of leukemic cells in normal oxygen (air) were used as controls.
Histologic and cytologic analyses
Peripheral blood was obtained from the retro-orbital venous plexus, and white blood cells (WBCs), red blood cells (RBCs), and platelets (PLs) were counted by manual methods. BM cells were obtained by flushing RPMI 1640 medium (Sigma) through mouse long bones. Blood and BM smears were prepared according to standard hematologic techniques and stained with Wright Giemsa stain. Then, cell morphologic features were examined by light microscopy (Olympus BX-51; Olympus Optical, Tokyo, Japan). Spleen and liver specimens were respectively cut into 3 parts and immediately processed for snap freezing in liquid nitrogen, fixation, and cell suspension. Spleen and liver tissues were fixed in 10% neutral buffered formalin, paraffin embedded, and stained with hematoxylin-eosin (H&E). The extent of the leukemic cell infiltration was assessed on paraffin sections. For proliferation assay, the paraffin sections were treated with mouse monoclonal antibody against proliferating cell nuclear antigen (PCNA; PC10; Santa Cruz Biotechnology, Santa Cruz, CA), followed by horseradish peroxidase (HRP)–conjugated secondary antibody. The sections were then stained by a MaxVision Kit (Maixin Biol, Fu Zhou, China) and visualized under a light microscope (Olympus BX-51; Olympus Optical). The percentages of PCNA-positive cells were calculated from 500 cells in liver or spleen. Tissue sections were counterstained with hematoxylin before mounting. For the detection of in situ cell death, terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) assay was performed on paraffin sections, according to the manufacturer's instructions (Roche, Mannheim, Germany). All images were captured with an Olympus DP50 camera using Viewfinder Lite and Studio Lite 1.0 software (Pixera, Los Gatos, CA). Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA) was used for image processing. The same protocol was also used in the following immunofluorescence analysis.
Leukemic cell differentiation assay
Besides cell morphologic criteria, the differentiation of leukemic cells was also assessed by mouse granulocytic differentiation–related antigens Gr-1 and Mac-1 on flow cytometry. Briefly, BM and spleen cells were resuspended in 100 μL phosphate-buffered saline (PBS) and incubated with phycoerythrin (PE)–conjugated rat anti–mouse Gr-1 and fluorescein isothiocyanate (FITC)–conjugated rat anti–mouse Mac-1 monoclonal antibodies (BD Biosciences, San Diego, CA) for 30 minutes on ice in the dark. PE- or FITC-conjugated rat IgG2b,κ (BD Biosciences, San Diego, CA) was used as a nonspecific negative control. After rinsing, cell samples were analyzed on a flow cytometer (Beckman Coulter, Miami, FL). After creating a scattergram combining side scatter (SSC)/forward scatter (FSC) dot plots for the whole population, a region (higher size) that contains myeloid cells (R1 for BM, R2 for spleen) was drawn for assessing percentages of Gr-1+ and Mac-1+ cells. Data were acquired and processed on at least 10 000 events for each sample.
Immunofluorescence analysis for PML-RARα and Gr-1 proteins
Bone marrow and spleen cells were suspended in PBS and filtered through nylon mesh. Cytospins were prepared and fixed sequentially with 4% paraformaldehyde and –20°C methanol. Samples were blocked by 10% normal bovine serum in PBS for 15 minutes and incubated for 1 hour with a rabbit anti-PML antibody that recognizes both human and mouse PML (H-238; Santa Cruz Biotechnology), followed by 30 minutes of incubation with FITC-labeled bovine anti–rabbit IgG (SC-2365; Santa Cruz Biotechnology) or PE-labeled rat anti–mouse Gr-1 antibody. Fluorescence signals were examined by fluorescence microscopy (Olympus BX-51; Olympus Optical).
Apoptosis assay
In addition to TUNEL assay described in “Histologic and cytologic analyses,” apoptotic cells were detected by histogram distribution of cell cycle–related nuclear DNA content and annexin V assay on flow cytometry, as reported previously.32 Briefly, cell suspensions fixed overnight in 70% cold ethanol at –20°C were treated with Tris-HCl buffer (pH 7.4) supplemented with 1% RNase A and stained with 50 μg/mL propidium iodide (PI; Sigma). Cell cycle distribution was determined by flow cytometry (Beckman Coulter), and sub-G1 cells were regarded as apoptotic cells.32 Annexin V assay was performed by the ApoAlert Annexin V kit (BD Biosciences, Palo Alto, CA) on flow cytometry (Beckman Coulter).
Western blot
Tissue lysates were subjected to 8% to 12% sodium dodecyl sulfate (SDS)–polyacrylamide gels, electrophoresed, and transferred to a nitrocellulose membrane (Amersham Bioscience, Buckinghamshire, United Kingdom). Membranes were stained with 0.2% Ponceau S red to ensure equal protein loading. After blocking with 5% nonfat milk in PBS, the membranes were blotted with anticleaved caspase-3 (Cell Signaling, Beverly, MA), anti–poly ADP (adenosine diphosphate)–ribose polymerase (PARP; Santa Cruz Biotechnology), anti-RARα (a gift from Dr P. Chambon, Institute of Genetics and Cellular and Molecular Biology, CNRS-INSERM-ULP College of France, France), and anti–hypoxia-inducible factor 1 α (HIF-1α; Novus Biologicals, Littleton, CO), followed by HRP-linked secondary antibody (Cell Signaling). The signals were detected by a chemiluminescence phototope-HRP kit, according to manufacturer's instructions (Cell Signaling). All experiments were repeated at least 3 times with similar results.
Statistical analysis
The software program Microcal Origin (v.5.0; Microcal, Northampton, MA) was used to prepare Kaplan-Meier curves. Other statistical analyses were performed with Excel 2000 (Microsoft, Redmond, WA) using the Student t test.
Results
CoCl2 but not DFO slightly prolongs survival of leukemic mice
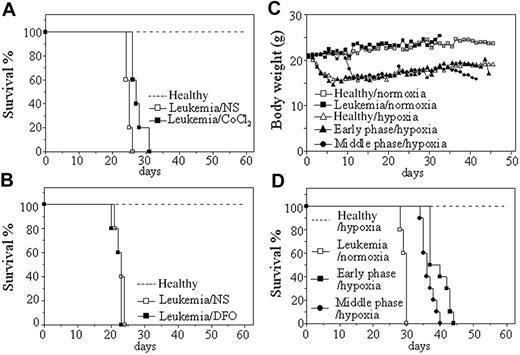
Leukemic cells from hMRP8–PML-RARα transgenic mice, which developed transplantable leukemias,31 were injected by tail vein into syngenic mice. Transplantation was always successful because all mice died with a narrow intraexperimental variation, generally in 26 to 30 days and 20 to 26 days in normal oxygen, respectively, for injection of 2 × 105 and 2 × 106 cells each mouse (Figure 1). Hence, we first evaluated the effect of hypoxia-mimetic agent CoCl2 on the survival of these leukemic mice with injection of 2 × 106 leukemic cells per mouse. In pilot toxicity experiments, 5, 10, 15, 20, and 25 μg/g body wt of CoCl2 or equal volume of normal saline were intraperitoneally injected into normal mice every 2 days for 1 month. The results showed that 20 and 25 μg/g body wt of CoCl2 led to many early deaths presumably due to toxicity because pathologic examination showed hepatic damage and pulmonary edema (data not shown), whereas all mice could endure the treatment of 15 μg/g body wt of CoCl2 with no evidence for toxicity. Therefore, 20 leukemic mice were randomly treated with 15 μg/g body wt of CoCl2 and normal saline every 2 days (10 mice per group) from day 7 after injection of leukemic cells. Our 3 independent experiments revealed that such a dose of CoCl2 could prolong survival of leukemic mice compared with normal saline–injected mice (P < .001; Figure 1A). Next, we also evaluated the effect of another hypoxia-simulating agent DFO at 50 μg/g body wt in a similar protocol to CoCl2 treatment. As shown in Figure 1B, no difference in survival existed between DFO-treated and normal saline–treated leukemic mice (P > .05), although these mice could tolerate such a dose of DFO.
Intermittent hypoxia significantly prolongs survival of leukemic mice
In the next phase of analysis, the potential effect of intermittent hypoxia on the survival of leukemic mice was evaluated. For this set of experiments, leukemic mice that received transplants were housed in a hypoxic chamber equivalent to an altitude of 6000 m for 18 hours each consecutive day, beginning on day 1 or day 7 after transplantation of 2 × 105 leukemic cells per mouse (hereafter called early-phase [day 1] and middle-phase [day 7] leukemic mice, respectively). Normal (mice that did not receive transplants) and leukemic mice in normal oxygen as well as normal mice in intermittent hypoxia were used as controls. Every treatment included 10 mice in each independent experiment. The results showed that both healthy and leukemic mice developed poor activity and anorexia with reduction of body weight (Figure 1C) in intermittent hypoxia. However, normal mice kept alive under hypoxia for 60 days did not show evidence for hypoxic damage by pathologic examination, indicating that mice could endure such an intermittent hypoxia. As can be seen in Figure 1D, hypoxia-treated early-phase (39.7 ± 3.02 days; P < .001 vs leukemic mice in normal oxygen) and middle-phase leukemic mice (36.5 ± 1.96 days; P < .001 vs leukemic mice in normal oxygen) had significantly longer survival than leukemic mice in normal oxygen (29.4 ± 0.84 days). Of note, early-phase leukemic mice also showed longer survival than middle-phase leukemic mice in hypoxia (P = .006).
Effects of hypoxia-mimetic agents and intermittent hypoxia on survival of leukemic mice that received transplants. (A-B) Leukemic cells (2 × 106) from BM and spleen of hMRP8–PML-RARα transgenic mice were injected intravenously into FVB/N mice. Seven days later, 15 μg/g body wt of CoCl2 (A) or 50 μg/g body wt of DFO (B) and equal volume of normal saline (NS) were intraperitoneally given every 2 days until first mice were moribund. Healthy mice (broken line) without treatment were used as controls. Survival curves are shown by Kaplan-Meier methods. (C-D) Leukemic cells (2 × 105) were transplanted into FVB/N mice by tail vein. Then, these mice were randomly placed into intermittent hypoxia on day 1 (early phase/hypoxia) or day 7 (middle phase/hypoxia) after transplantation or kept in normal oxygen (leukemia/normoxia). Normal mice that did not receive transplants in normal oxygen (Healthy/normoxia) and in intermittent hypoxia (Healthy/hypoxia) were used as controls. Dynamic changes of body weight (C) and Kaplan-Meier curves are shown (D). For all experiments, every treatment included 10 mice and every experiment was repeated 3 times with similar results.
Effects of hypoxia-mimetic agents and intermittent hypoxia on survival of leukemic mice that received transplants. (A-B) Leukemic cells (2 × 106) from BM and spleen of hMRP8–PML-RARα transgenic mice were injected intravenously into FVB/N mice. Seven days later, 15 μg/g body wt of CoCl2 (A) or 50 μg/g body wt of DFO (B) and equal volume of normal saline (NS) were intraperitoneally given every 2 days until first mice were moribund. Healthy mice (broken line) without treatment were used as controls. Survival curves are shown by Kaplan-Meier methods. (C-D) Leukemic cells (2 × 105) were transplanted into FVB/N mice by tail vein. Then, these mice were randomly placed into intermittent hypoxia on day 1 (early phase/hypoxia) or day 7 (middle phase/hypoxia) after transplantation or kept in normal oxygen (leukemia/normoxia). Normal mice that did not receive transplants in normal oxygen (Healthy/normoxia) and in intermittent hypoxia (Healthy/hypoxia) were used as controls. Dynamic changes of body weight (C) and Kaplan-Meier curves are shown (D). For all experiments, every treatment included 10 mice and every experiment was repeated 3 times with similar results.
Intermittent hypoxia induces tumor regression
To figure out the in vivo cellular effects of intermittent hypoxia on leukemia, leukemic mice were randomly assigned to hypoxia at day 1 or day 7 after transplantation or into normal oxygen with 3 or 4 mice per treatment. When leukemic mice in normal oxygen were moribund, all mice were killed. Peripheral blood and tissues including BM, spleen, and liver were collected for further examinations. The same experiments were repeated 4 times. As depicted in Figure 2A, unlike leukemic mice in normal oxygen that presented a marked elevation in WBC count and severe thrombocytopenia in peripheral blood, both early and middle-phase leukemic mice had normal WBC and platelet counts in hypoxia. Of note, all mice showed similar RBC counts, possibly due to the long half-life of RBCs. Furthermore, the WBCs in leukemic mice in normal oxygen were strictly monomorphic, immature, promyelocyte-like cells, which could rarely be seen in hypoxia-treated early and middle-phase leukemic mice under microscopic observations in peripheral blood smears (Figure 2B top). Similarly, the BM of leukemic mice in normal oxygen was filled with massive strictly monomorphic, promyelocyte-like cells, whereas these leukemic cells could rarely be seen in the BM of hypoxia-treated early and middle-phase leukemic mice (Figure 2B bottom). In parallel, the percentages of granulocytes in the BM of hypoxia-treated early and middle-phase leukemic mice were similar to those of normal oxygen or hypoxia-treated normal mice, as evidenced by cell size–based (FSC) and cell granularity–based (SSC) dot plots in flow cytometry (Figure 3A top). The similar phenomena could also be clearly seen in spleen (Figure 3A bottom). More intriguingly, leukemic mice in normal oxygen presented huge spleen, whereas both early and middle-phase leukemic mice in hypoxia almost completely normalized the macroscopic appearance of the organ without increased spleen weight (Figure 3B). In agreement, microscopic examination of cell suspensions (Figure 4A) and tissue sections (Figure 4B-C) also revealed that leukemic cells massively infiltrated into the spleen of leukemic mice in normal oxygen, whereas such an infiltration was significantly reduced in hypoxia-treated early and middle-phase leukemic mice. Reduced infiltration of leukemic cells could also be seen in livers of hypoxia-treated leukemic mice. As depicted in Figure 5A, only small remaining tumor masses existed mainly around vessels of the portal tracts or centrilobular veins of livers under microscopic examination in hypoxia-treated early and middle-phase leukemic mice. In total, these results indicate that intermittent hypoxia significantly reduces the infiltration of leukemic cells in peripheral tissues.
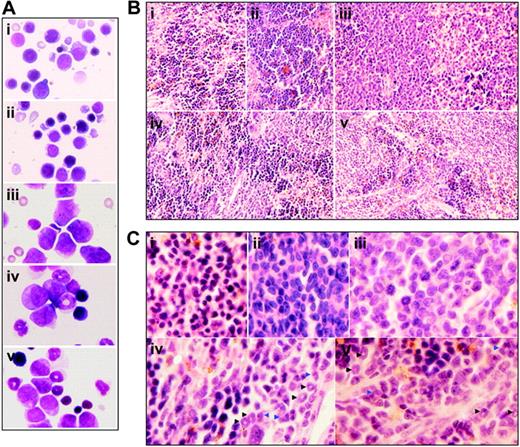
Cytologic changes of peripheral blood and bone marrow of leukemic mice that received transplants in intermittent hypoxia. FVB/N mice with or without transplantation of leukemic cells (2 × 105) were treated with methods described in Figure 1C. When leukemic mice in normal oxygen were moribund (approximately at day 30), all mice (i, normal mice in normal oxygen; ii, normal mice in hypoxia; iii, leukemic mice in normal oxygen; iv, early-phase leukemic mice in hypoxia; v, middle-phase leukemic mice in hypoxia) were killed. (A) WBCs, RBCs, and PLs in peripheral blood (PB) were counted. Every column represents mean of 3 or 4 mice; error bars represent standard deviation (SD). *P < .001, compared with other groups; #P > .05, compared with mice i and ii. (B) Cytologic analysis by Wright Giemsa staining of peripheral blood (top row) and bone marrow (bottom row). Images were observed under a microscope (original magnification × 100/1.30 numeric aperture [NA] oil objective lens).
Cytologic changes of peripheral blood and bone marrow of leukemic mice that received transplants in intermittent hypoxia. FVB/N mice with or without transplantation of leukemic cells (2 × 105) were treated with methods described in Figure 1C. When leukemic mice in normal oxygen were moribund (approximately at day 30), all mice (i, normal mice in normal oxygen; ii, normal mice in hypoxia; iii, leukemic mice in normal oxygen; iv, early-phase leukemic mice in hypoxia; v, middle-phase leukemic mice in hypoxia) were killed. (A) WBCs, RBCs, and PLs in peripheral blood (PB) were counted. Every column represents mean of 3 or 4 mice; error bars represent standard deviation (SD). *P < .001, compared with other groups; #P > .05, compared with mice i and ii. (B) Cytologic analysis by Wright Giemsa staining of peripheral blood (top row) and bone marrow (bottom row). Images were observed under a microscope (original magnification × 100/1.30 numeric aperture [NA] oil objective lens).
Inhibition of leukemic cell infiltration in bone marrow and spleen by intermittent hypoxia. (A) In the same experiments as those described in Figure 2, cells were isolated from bone marrow (top row) and spleen (bottom row) and suspended in RPMI 1640 medium. Then, scattergram of FSC versus SSC was created in flow cytometry to allow gating on the myeloid cells. R1 and R2 regions indicate myeloid cells, respectively, in BM and in spleen cells. (B) The spleen of every mouse in the same experiments as Figure 2 was weighed and data are shown as the ratio of spleen (mg) to body wt (g). Each column represents the mean of 3 or 4 mice in an independent experiment; error bars represent SD. *P < .001, compared with other groups; #P > .05, compared with mice i and ii. Symbols i to v represent mice with the same treatment as in Figure 2.
Inhibition of leukemic cell infiltration in bone marrow and spleen by intermittent hypoxia. (A) In the same experiments as those described in Figure 2, cells were isolated from bone marrow (top row) and spleen (bottom row) and suspended in RPMI 1640 medium. Then, scattergram of FSC versus SSC was created in flow cytometry to allow gating on the myeloid cells. R1 and R2 regions indicate myeloid cells, respectively, in BM and in spleen cells. (B) The spleen of every mouse in the same experiments as Figure 2 was weighed and data are shown as the ratio of spleen (mg) to body wt (g). Each column represents the mean of 3 or 4 mice in an independent experiment; error bars represent SD. *P < .001, compared with other groups; #P > .05, compared with mice i and ii. Symbols i to v represent mice with the same treatment as in Figure 2.
Intermittent hypoxia inhibits proliferation without apoptosis induction of leukemic cells
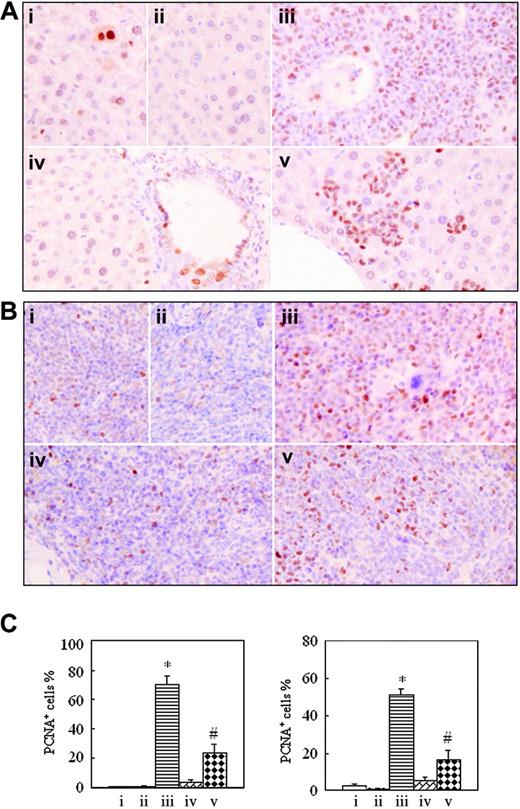
To understand whether intermittent hypoxia impacts in vivo cell proliferation and apoptosis, formalin-fixed, deparaffinized spleen and liver sections were histochemically stained, respectively, with anti-PCNA antibody and TUNEL assay. The positive PCNA and TUNEL signals indicate proliferating and apoptotic cells, respectively.32,33 As shown in Figure 6, the number of PCNA-positive cells in tissues of leukemic mice in normal oxygen (70.33% ± 5.51% for liver and 51.33% ± 3.21% for spleen) was far higher than those of hypoxia-treated early-phase leukemic mice (3.33% ± 1.53% for liver and 5.50% ± 1.80% for spleen; P < .001 vs leukemic mice in normal oxygen), the latter having no significant difference from those seen in normal mice in normal oxygen (0.47% ± 0.15% for liver and 2.83% ± 0.76% for spleen) and in hypoxia (0.33% ± 0.58% for liver and 0.93% ± 0.51% for spleen). The percentages of PCNA-positive cells in liver and spleen of hypoxia-treated middle-phase leukemic mice were, respectively, 23.33% ± 6.11% and 16.33% ± 5.13%, which were higher than those of normal mice and hypoxia-treated early-phase leukemic mice but were far lower than those of leukemic mice in normal oxygen (P < .001).
Histologic features of spleen of leukemic mice that received transplants under intermittent hypoxia. (A) In the same experiments as those described in Figure 2, splenocytes were collected onto slides by cytospin, stained by Wright Giemsa, and observed under a microscope (original magnification × 100/1.30 NA oil objective lens). (B-C) Histopathologic sections were stained with H&E (B, original magnification × 10/0.30 NA PH1 objective lens; C, original magnification × 100/1.30 NA oil objective lens). Arrowheads indicate mature myeloid cells with ring- (black) or horseshoe-shaped (blue) nuclei. Symbols i to v represent mice with the same treatment as in Figure 2.
Histologic features of spleen of leukemic mice that received transplants under intermittent hypoxia. (A) In the same experiments as those described in Figure 2, splenocytes were collected onto slides by cytospin, stained by Wright Giemsa, and observed under a microscope (original magnification × 100/1.30 NA oil objective lens). (B-C) Histopathologic sections were stained with H&E (B, original magnification × 10/0.30 NA PH1 objective lens; C, original magnification × 100/1.30 NA oil objective lens). Arrowheads indicate mature myeloid cells with ring- (black) or horseshoe-shaped (blue) nuclei. Symbols i to v represent mice with the same treatment as in Figure 2.
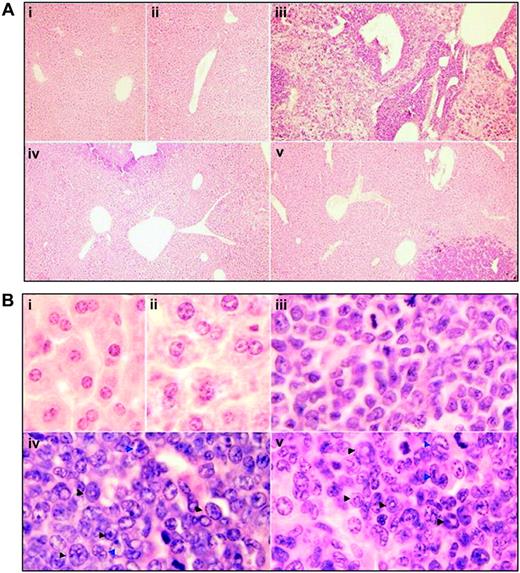
Histologic features of liver in leukemic mice that received transplants in intermittent hypoxia. In the same experiments as those described in Figure 2, histopathologic sections were stained with H&E for liver (A, original magnification × 10/0.30 NA PH1 objective lens; B, original magnification × 100/1.30 NA oil objective lens). Arrowheads indicate mature myeloid cells with ring- (black) or horseshoe-shaped (blue) nuclei. Symbols i to v represent mice with the same treatment as in Figure 2.
Histologic features of liver in leukemic mice that received transplants in intermittent hypoxia. In the same experiments as those described in Figure 2, histopathologic sections were stained with H&E for liver (A, original magnification × 10/0.30 NA PH1 objective lens; B, original magnification × 100/1.30 NA oil objective lens). Arrowheads indicate mature myeloid cells with ring- (black) or horseshoe-shaped (blue) nuclei. Symbols i to v represent mice with the same treatment as in Figure 2.
Immunohistochemical detection of proliferating cell nuclear antigen on formalin-fixed, deparaffinized mouse liver and spleen sections. In the same experiments as those described in Figure 2, histopathologic sections of liver (A) and spleen (B) were stained with anti-PCNA antibody as described in “Materials and methods.” Images were observed under a microscope (original magnification × 40/0.75 NA PH2 objective lens). (C) PCNA-positive cell percentages are shown for liver (left) and spleen (right). Each column represents mean of 3 or 4 mice in an independent experiment; error bars represent SD. *P < .001, compared with other groups; #P < .05, compared with mice i, ii, and iv. Symbols i to v represent mice with the same treatment as in Figure 2.
Immunohistochemical detection of proliferating cell nuclear antigen on formalin-fixed, deparaffinized mouse liver and spleen sections. In the same experiments as those described in Figure 2, histopathologic sections of liver (A) and spleen (B) were stained with anti-PCNA antibody as described in “Materials and methods.” Images were observed under a microscope (original magnification × 40/0.75 NA PH2 objective lens). (C) PCNA-positive cell percentages are shown for liver (left) and spleen (right). Each column represents mean of 3 or 4 mice in an independent experiment; error bars represent SD. *P < .001, compared with other groups; #P < .05, compared with mice i, ii, and iv. Symbols i to v represent mice with the same treatment as in Figure 2.
On the other hand, few TUNEL-positive cells were seen in all mice with different treatments (Figure 7A). In agreement with this, annexin V+ cells and sub-G1 cells were rarely found on flow cytometry in splenic suspensions and BM of all mice (Figure 7B; data not shown). Additionally, PARP cleavage (Figure 7C) and activated caspase-3 (data not shown) were also undetectable, the latter being measured by Western blot against cleaved caspase-3 antibody.32 Then, we also evaluated in vivo effects of short-term hypoxia on cell proliferation and apoptosis. For this purpose, leukemic mice were permitted to be fed in normal oxygen for 24 days after transplantation of leukemic cells and then were housed into intermittent hypoxia for 1 to 3 days. As shown in Figure 7D, intermittent hypoxia increased G1-phase cells with significant reduction of S-phase cells and without the appearance of sub-G1 cells. These results strongly indicate that hypoxia can inhibit proliferation but does not induce apoptosis of leukemic cells.
Intermittent hypoxia induces differentiation of leukemic cells
There were mainly ringlike terminal differentiated cells except for a low percentage of leukemic cells in the BM of hypoxia-treated early- and middle-phase leukemic mice (Figure 2B). Furthermore, splenocyte suspensions of hypoxia-treated early and middle-phase leukemic mice were filled with maturating myeloid cells that presented differentiation-related morphologic features such as condensed chromatin with indented, distorted, horse-shoed, or donut-shaped nuclei, which were significantly different from those seen in normal mice and leukemic mice in normal oxygen (Figure 4A). In accord with this, the few infiltrated leukemic cells in the spleen also predominantly consisted of maturating myeloid cells that presented multimorphic, horse-shoed, or donut-shaped nuclei (Figure 4C arrowheads). This similar phenomenon could also be seen in the liver of hypoxia-treated early- and middle-phase leukemic mice (Figure 5B). Moreover, we also measured mouse granulocytic differentiation–related antigens Gr-1 and Mac-1 in the regions after gating for myeloid cells by FSC and SSC on flow cytometry. The results demonstrated that in these myeloid cells, the BM and spleen had similar percentages of Gr-1+ and Mac-1+ cells in hypoxia-treated early- and middle-phase leukemic mice as those of normal mice, which were much higher than those of leukemic mice in normal oxygen (Figure 8A). Furthermore, we also detected Gr-1+ cells by immunofluorescent staining with anti–mouse Gr-1 antibody. As can be seen in Figure 8B, Gr-1+ cells were hardly seen in BM of leukemic mice in normal oxygen but they significantly increased in those of hypoxia-treated early- and middle-phase leukemic mice. Similarly, increased Gr-1+ cells also appeared in the spleen of hypoxia-treated leukemic mice.
To ascertain that these maturating cells came from leukemic cells, BM and spleen cells were immunofluorescent stained with anti–human PML antibody. As depicted in Figure 8C, PML speckles of normal appearance (known as PML oncogenic domains [PODs] or nuclear bodies),34 about 3 to 6 in each cell nucleus, could be seen in BM cells of normal mice, indicating that the anti–human PML antibody cross-reacted with mouse PML protein. Almost all BM cells of leukemic mice in normal oxygen, which exhibited negative Gr-1 staining (Figure 8B), presented hundreds of micropunctates with anti-PML antibody in the nuclei, corresponding to the previously described abnormal staining pattern caused by PML-RARα and/or PML-RARα/PML heterodimers in APL cells,35-37 further supporting the infiltration of leukemic cells in BM. Of great importance, PML-RARα–related micropunctates tended to disappear and were replaced by normal POD appearance in BM cells of hypoxia-treated early- and middle-phase leukemic mice. On the other hand, splenocytes of normal mice were PML- and Gr-1–negative, indicating that splenic lymphocytes had undetectable PML expression (Figure 8C). However, alterations of PML-RARα (Figure 8C) similar to those of BM cells could also be seen in spleen cells of leukemic mice in normal oxygen and hypoxia-treated early- and middle-phase leukemic mice.
Effects of intermittent hypoxia on PML-RARα and HIF-1α protein levels in vivo
Finally, we compared PML-RARα protein levels in spleen tissue of normal and leukemic mice with different treatments. The results revealed that normal mice in normal oxygen (Figure 8D lane 1) or in hypoxia (Figure 8D lane 2) had undetectable PML-RARα protein that could be clearly seen in NB4 cell line from APL with “physiologic” PML-RARα expression (Figure 8D lane 6),32,38 indicating the specificity of PML-RARα detection by anti-RARα antibody. Accordingly, leukemic mice in normal oxygen expressed a higher level of PML-RARα protein (Figure 8D lane 3) than those of hypoxia-treated early- (Figure 8D lane 4) and middle-phase (Figure 8D lane 5) leukemic mice. For the detection of HIF-1α protein, leukemic cells were injected into mice. One day later, mice were housed into intermittent hypoxia for different days. At these times, HIF-1α protein of liver extracts was detected by Western blot. As consistent with previous report,39 HIF-1α protein presented a triplet at approximately 120 kDa (Figure 8E). Liver tissue of mice in normal oxygen (day 0) expressed detectable but few HIF-1α proteins, which were induced remarkably and progressively in hypoxia (Figure 8E). Of note, CoCl2 and DFO at doses used in this work failed to remarkably increase HIF-1α protein (Figure 8F).
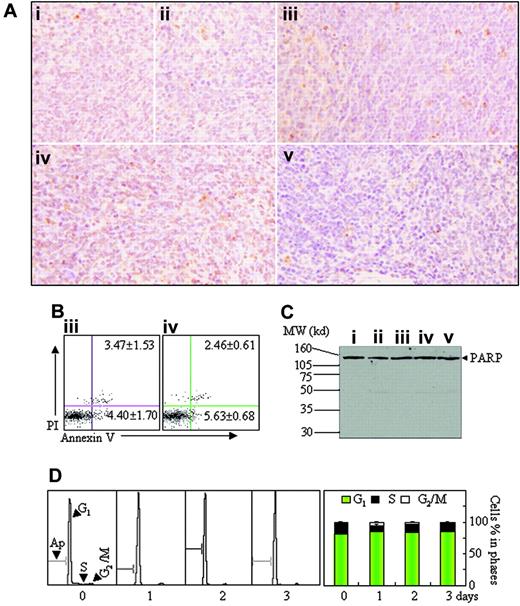
Apoptosis assay in spleen tissue. (A-C) Histopathologic sections of spleen in the same experiments as those described in Figure 2 were stained with TUNEL kit as described in “Materials and methods” (A, original magnification × 40/0.75 PH2 objective lens). Splenocytes were isolated from mice ii and iv, and annexin V+ cells were detected on flow cytometry (B). Splenocytes were isolated from all mice and PARP proteins were detected by Western blots (C). MW indicates molecular weight. (D) Leukemic mice, which were fed in normal oxygen for 24 days after injection of leukemic cells, were housed into intermittent hypoxia for 1 to 3 days, and the distribution of cell cycle–related nuclear DNA contents of splenocytes were analyzed in flow cytometry (left) and the percentages of G1-, S-, and G2/M-phase cells were shown (right). Symbols i to v represent mice with the same treatment as in Figure 2. In addition, BM cells were also analyzed in the same experiments, and similar results were obtained (data not shown).
Apoptosis assay in spleen tissue. (A-C) Histopathologic sections of spleen in the same experiments as those described in Figure 2 were stained with TUNEL kit as described in “Materials and methods” (A, original magnification × 40/0.75 PH2 objective lens). Splenocytes were isolated from mice ii and iv, and annexin V+ cells were detected on flow cytometry (B). Splenocytes were isolated from all mice and PARP proteins were detected by Western blots (C). MW indicates molecular weight. (D) Leukemic mice, which were fed in normal oxygen for 24 days after injection of leukemic cells, were housed into intermittent hypoxia for 1 to 3 days, and the distribution of cell cycle–related nuclear DNA contents of splenocytes were analyzed in flow cytometry (left) and the percentages of G1-, S-, and G2/M-phase cells were shown (right). Symbols i to v represent mice with the same treatment as in Figure 2. In addition, BM cells were also analyzed in the same experiments, and similar results were obtained (data not shown).
Discussion
AML, a common heterogenous group of hematopoietic malignancies, is characterized by maturation/differentiation block at specific stages during hematopoietic development.40 Significant advances in understanding the biologic, molecular, and cytogenetic aspects of this malignancy have been achieved over the past 2 decades.41-43 Meanwhile, the cellular and molecular mechanism by which leukemic cells undergo differentiation has become a “hot topic” in hematology. We reported that mild hypoxia and hypoxia mimetics induce the differentiation of human AML cells in association with an increase in HIF-1α protein.27-29 Moreover, hypoxia-mimetic agents also enhance As2O3-induced differentiation in the APL cell line NB4 but not in the promonocytic leukemic U937 cell line. Based on these findings, here we investigate the possible in vivo effects of CoCl2, DFO, and intermittent hypoxia on AML mice. Due to the relative unavailability of non-APL leukemic models, a transgenic mouse model with human APL, which has been used to evaluate the efficacy of combined treatment of ATRA and As2O3 in APL,44 was employed. As documented,45-48 oral CoCl2 has been used to treat aplastic anemia, refractory anemia of chronic renal failure, and patients undergoing long-term hemodialysis from the 1960s to the 1970s. Iron chelators have also presented promising therapeutic potential in cancer therapy,49,50 and the safety of subcutaneous bolus injection of DFO was also reviewed in adult patients with iron overload.51 Here, we showed that CoCl2 did prolong survival of leukemic mice, although this treatment regime failed to cure the disease. However, DFO had no effect on the survival of the mouse model, which was consistent with the previous report that showed the failure of subcutaneous DFO to alter the course of AML in the rat.52 It was proposed that the short plasma half-life of DFO, so as not to achieve effective drug concentration in vivo, was a potential reason for this lack of protection. Moreover, our results showed that CoCl2 and DFO failed to increase HIF-1α protein, arguing against the in vivo hypoxic effects of these 2 agents at doses used in this work. Therefore, potential effects of CoCl2 and novel iron chelators in the treatment of AML deserve to be further evaluated in the context of pharmacokinetic and pharmacodynamic analyses.
In spite of the limited effects of CoCl2 and DFO in this model, we still investigated the possible in vivo effects of hypoxia. Our results showed that intermittent hypoxia could markedly prolong the survival of either early-phase or middle-phase leukemic mice. Because it was too difficult to culture in vitro leukemic blasts from PML-RARα transgenic mice for more than 2 days, especially in hypoxia, we directly observed the possible in vivo cellular effects of intermittent hypoxia on leukemic mice. Histologic and cytologic analyses showed that intermittent hypoxia significantly inhibited the in vivo infiltration of leukemic blasts in peripheral blood, BM, spleen, and liver with the restraint of the proliferation of leukemic blasts, the latter being evidenced by immunohistochemical detection of PCNA. The reduced PML-RARα protein correlated with the inhibition of leukemic cell infiltration in peripheral tissues of hypoxia-treated leukemic mice. Of note, such an inhibitory effect of hypoxia on leukemic mice was not because of hypoxia toxicity, as all mice could endure hypoxic conditions used in this work without evidence of tissue damage. Moreover, long-term and short-term hypoxia also failed to induce apoptosis in vivo, as evidenced by TUNEL assay and flow cytometric analysis for annexin V+ cells and sub-G1 cells, important indications of apoptotic cells.32 Additionally, the activated caspase-3, a critical executioner for apoptosis initiation,53 and the cleavage of its substrate PARP could also not be found in the peripheral tissues such as spleen and liver of hypoxia-treated mice.
Detection of differentiation-related antigens, PML-RARα, and HIF-1α proteins. (A-D) When leukemic mice in normal oxygen were moribund in the same experiments as those described in Figure 2, all mice were killed and cells from BM and spleen were harvested. (A) Myeloid cells in R1 or R2 region as shown in Figure 3A were analyzed for Gr-1 and Mac-1 expression on flow cytometry. Each column represent the mean percentages of Gr-1+ and Mac-1+ cells of 3 or 4 mice in an independent experiment; error bars represent SD. Compared with leukemic mice in normal oxygen (iii): *P = 6.746 × 10–5, **P = 5.303 × 10–5 for Gr-1+ cells; #P = .002, ##P = .014 for Mac-1+ cells in BM; &P = .023, &&P = .024 for Gr-1+ cells; and P = .002 for Mac-1+ cells in spleen cells. (B-C) Isolated cells were collected onto slides by cytospin and stained, respectively, with anti–mouse Gr-1 (B) and anti–human PML antibodies (C) as described in “Materials and methods.” Immunofluorescence was observed under a microscope (original magnification × 100/1.30 NA oil objective lens). Red staining beside cytoplasms (B) and green staining in the nuclei (C) represent Gr-1 and PML, respectively. (D) Splenocytes from the indicated mice were extracted and PML-RARα proteins were detected by Western blot with NB4 cells as positive control. Symbols i to v represent mice with the same treatment as in Figure 2. (E-F) On the first day after transplantation of leukemic cells, mice were housed into intermittent hypoxia (E), or on the seventh day after transplantation of leukemic cells, mice were treated with 15 μg/g body wt of CoCl2 or 50 μg/g body wt of DFO (F). HIF-1α protein of liver extracts was dynamically detected by Western blots. For panels D-F, equal protein loading was confirmed by Ponceau S staining.
Detection of differentiation-related antigens, PML-RARα, and HIF-1α proteins. (A-D) When leukemic mice in normal oxygen were moribund in the same experiments as those described in Figure 2, all mice were killed and cells from BM and spleen were harvested. (A) Myeloid cells in R1 or R2 region as shown in Figure 3A were analyzed for Gr-1 and Mac-1 expression on flow cytometry. Each column represent the mean percentages of Gr-1+ and Mac-1+ cells of 3 or 4 mice in an independent experiment; error bars represent SD. Compared with leukemic mice in normal oxygen (iii): *P = 6.746 × 10–5, **P = 5.303 × 10–5 for Gr-1+ cells; #P = .002, ##P = .014 for Mac-1+ cells in BM; &P = .023, &&P = .024 for Gr-1+ cells; and P = .002 for Mac-1+ cells in spleen cells. (B-C) Isolated cells were collected onto slides by cytospin and stained, respectively, with anti–mouse Gr-1 (B) and anti–human PML antibodies (C) as described in “Materials and methods.” Immunofluorescence was observed under a microscope (original magnification × 100/1.30 NA oil objective lens). Red staining beside cytoplasms (B) and green staining in the nuclei (C) represent Gr-1 and PML, respectively. (D) Splenocytes from the indicated mice were extracted and PML-RARα proteins were detected by Western blot with NB4 cells as positive control. Symbols i to v represent mice with the same treatment as in Figure 2. (E-F) On the first day after transplantation of leukemic cells, mice were housed into intermittent hypoxia (E), or on the seventh day after transplantation of leukemic cells, mice were treated with 15 μg/g body wt of CoCl2 or 50 μg/g body wt of DFO (F). HIF-1α protein of liver extracts was dynamically detected by Western blots. For panels D-F, equal protein loading was confirmed by Ponceau S staining.
More interestingly, there was a low percentage of leukemic blastlike cells in the BM of leukemic mice under hypoxia, which tended to morphologic maturation. Most infiltrated leukemic cells in the spleen and liver of hypoxia-treated leukemic mice predominantly consisted of maturating myeloid cells. In agreement, the BM and spleen had much higher percentages of differentiation-related antigen Gr-1+ and Mac-1+ cells than those of leukemic mice in normal oxygen. These more mature cells were not due to a stress response of normal myeloid precursors to hypoxia because increasing mature cells could not be seen in normal mice that had been subjected to hypoxia. It should be pointed out that although normal granulocytes as well as granulocytes derived from differentiating myeloid leukemia cells in the BM can both give a POD-like nuclear staining pattern with anti-PML antibody, spleen cells in normal mice had undetectable PML staining whereas infiltrated leukemic cells in the spleen of leukemic mice in normal oxygen exhibited Gr-1– staining and hundreds of PML-RARα–related micropunctates in the nuclei. Furthermore, cells in spleen of hypoxia-treated early and middle-phase leukemic mice presented Gr-1+ staining and normal and even disappeared PML speckles. These results suggest that intermittent hypoxia could induce in vivo differentiation of leukemic cells. Of note, it also remains to be further confirmed whether intermittent hypoxia might also modulate and degrade PML-RARα protein of APL cells in vivo, like both ATRA and As2O3,54-56 although this work showed that cells in the BM and spleen of leukemic mice in hypoxia exhibited a low PML-RARα protein level and normal and even disappeared PML speckles with Gr-1+ staining.
In addition, here we showed that intermittent hypoxia remarkably and progressively increased HIF-1α protein in peripheral tissues, supporting the efficacy of hypoxia used in this work. Our previous in vitro works proposed that HIF-1α protein, a critical transcriptional factor, mediated hypoxia-induced leukemic cell differentiation.27-29 Recently, we also showed that inducible expression of HIF-1α protein directly induced leukemic U937 cells to undergo differentiation (our unpublished data). Whether HIF-1α protein contributes hypoxia-induced leukemic cell differentiation in vivo remains to be further confirmed, possibly by assessing the response to low oxygen levels of leukemias from mice lacking the HIF-1A gene.
The therapeutic approach to AML patients has evolved toward new frontiers. A potentially less cytotoxic therapeutic strategy known as differentiation therapy has been developed with the introduction of ATRA in APL.57-59 To date, ATRA and anthracycline-based chemotherapy as well as the recent addition of As2O3 have made APL potentially curable in most patients.60 Although the success of differentiation therapy is still limited to the application of ATRA and maybe As2O3 in APL,61 the development of many mechanism-based agents such as histone deacetylase inhibitors,62-64 novel retinoids,65,66 and new therapies with defined molecular targets (eg, monoclonal antibodies, hypomethylating agents, tyrosine kinase inhibitors)67-69 are promising and have renewed enthusiasm and optimism among patients and healthcare providers. Despite these advances, the majority of AML patients still die of this disease. To our knowledge, this study represents the first attempt to show that intermittent hypoxia prolongs survival in a leukemic mouse model via tumor arrest and differentiation induction. Although the generality to other AML subtypes remains to be confirmed with additional in vivo models with non-APL leukemia, for which new therapies are more urgent, this work coupled with our previous in vitro experiments27-29 elucidates a new hypoxia-mediated signaling mechanism for differentiation induction of leukemic cells. With deeper understanding, these discoveries may lead to exploration of new targets for differentiation-inducing drugs. In addition, it would be of interest to investigate whether there is a lower incidence of leukemia and better prognosis in populations who live at very high altitudes than ones who live near sea level.70 Additional investigations may uncover ways to mimic the differentiative effects of hypoxia in a manner that will benefit human patients with AML.
Prepublished online as Blood First Edition Paper, September 15, 2005; DOI 10.1182/blood-2005-03-1278.
Supported in part by National Key Program (973) for Basic Research of China (NO2002CB512805, NO2002CB512806), National Natural Science Foundation of China (90408009, 30400184), International Collaborative Items of Ministry of Science and Technology of China (2003DF000038), and Grants from Science and Technology Committee of Shanghai. S.C.K. was a Burroughs Wellcome Fund Career Award Recipient and is a scholar of the Leukemia & Lymphoma Society.
W.L., M.G., and Y.-B.X. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We appreciate Dr P. Chambon for generously providing us with the anti-RARα antibody. We are also grateful to Dr Xu-Cheng Jiang and Ms Lin Zheng of the Department of Pathology, Shanghai Jiao-Tong University School of Medicine for their technological assistance of pathologic examinations.

![Figure 2. Cytologic changes of peripheral blood and bone marrow of leukemic mice that received transplants in intermittent hypoxia. FVB/N mice with or without transplantation of leukemic cells (2 × 105) were treated with methods described in Figure 1C. When leukemic mice in normal oxygen were moribund (approximately at day 30), all mice (i, normal mice in normal oxygen; ii, normal mice in hypoxia; iii, leukemic mice in normal oxygen; iv, early-phase leukemic mice in hypoxia; v, middle-phase leukemic mice in hypoxia) were killed. (A) WBCs, RBCs, and PLs in peripheral blood (PB) were counted. Every column represents mean of 3 or 4 mice; error bars represent standard deviation (SD). *P < .001, compared with other groups; #P > .05, compared with mice i and ii. (B) Cytologic analysis by Wright Giemsa staining of peripheral blood (top row) and bone marrow (bottom row). Images were observed under a microscope (original magnification × 100/1.30 numeric aperture [NA] oil objective lens).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/2/10.1182_blood-2005-03-1278/4/m_zh80020689260002.jpeg?Expires=1765919683&Signature=V-c1hOrXgZZMuord-NGXogmrr3hK3qUip569U8VfmCNtwuP3M2UuiQ9H2kwiUfUL73rWDWXgAt4AqgqLLoigug2XVN-tfR0sSZgdvZf8ZjWy2NMVBMZqyJji2VILB3keHgLigDHe9p-36FWOwetejsOdiwvXK8Uwc9vsN7lEmZE3ILiHDP5RImaqBj0mssNehJQ9gDYDxD5ZVGTrnFukxp2b7ZGf-iQltmf8ux4Suh~fkEx8y2WGZJndGPGZr2apFRLSi19C1mBDLXdSAjUxKAYyW0tky6qCAWiYONE759GD8jrFhrigcFUK78Rc5tfxVLypBILJxoeX2bllXBZgvw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal