Abstract
In a retrospective analysis, we previously reported that children whose leukemia cells harbored the TEL/AML1 gene rearrangement have excellent outcomes. From 1996 to 2000, we conducted a prospective study to determine the incidence and outcomes of children with TEL/AML1-positive acute lymphoblastic leukemia (ALL). Children with newly diagnosed ALL were treated on DFCI ALL Consortium Protocol 95-01. Patients were risk stratified primarily by current National Cancer Institute (NCI)–Rome risk criteria. With a median follow-up of 5.2 years, the 5-year event-free survival for TEL/AML1-positive patients was 89% compared with 80% for TEL/AML1-negative B-precursor patients (P = .05). The 5-year overall survival rate was 97% among TEL/AML-positive patients compared with 89% among TEL/AML1-negative patients (P = .03). However, in a multivariable analysis, risk group (age and leukocyte count at diagnosis) and asparaginase treatment group, but not TEL/AML1 status, were found to be independent predictors of outcome. We conclude that TEL/AML1-positive patients have excellent outcomes, confirming our previous findings. However, factors such as age at diagnosis and presenting leukocyte count should be taken into consideration when treating this group of patients.
Introduction
The TEL/AML1 gene rearrangement results from a balanced, reciprocal, t(12;21)(p12;q22) and occurs in 25% of children with B-lineage acute lymphoblastic leukemia (ALL).1,2 Current research focuses on determining the biologic function of the chimeric fusion protein, the cooperating events needed for leukemia development, the prognostic significance of this fusion gene, and the optimal therapy for these patients.
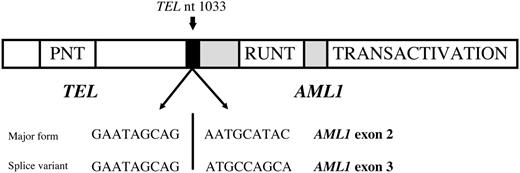
The TEL gene (also known as ETV6), first cloned in 1994 as a novel fusion partner to the PDGFRβ receptor in a patient with chronic myelomonocytic leukemia,3 is a member of the ETS family of transcription factors whose protein product is a nuclear phosphoprotein.4 Tel deficiency in mice results in early embryonic lethality in part caused by defective yolk sac angiogenesis and an inability to establish bone marrow hematopoeisis.5 The TEL gene was subsequently described to partner with RUNX1 (also known as CBFA2 or AML1) in pediatric B-precursor cell ALL (Figure 1).6,7
One of the most important predictors of failure in current risk models of ALL is the therapy received.8 Identifying cohorts of patients who can be treated with specific combinations of therapy has been one of the key goals of current clinical trials. The detection of recurrent somatic cytogenetic abnormalities has allowed investigators to more accurately predict the outcome of patients sharing these events. For instance, it has been well established that ALL patients with the BCR/ABL gene rearrangement have a poor prognosis with standard ALL therapy and often require allogeneic transplantation for optimal treatment.9
Several retrospective studies have divergent results with respect to the prognostic significance of the TEL/AML1 fusion gene.10 We previously reported that a subset of 22 TEL/AML1-positive patients treated on DFCI ALL Consortium protocols were 100% free of relapse at a median length of follow-up of 8.3 years.11 Investigators from St Jude Children's Research Hospital (St Jude) also reported an exceptionally favorable outcome for pediatric patients with TEL/AML1.1
However, subsequent reports noted that as many as 25% of children on Berlin-Frankfurt-Münster (BFM) protocols who had relapses were TEL/AML1-positive.12,13 In addition, another cooperative group could not confirm that the TEL/AML1 fusion gene conferred a favorable prognosis.14 Such differences in reported outcome may be explained by risk stratification and subsequent therapy received, and they indicated that a prospective analysis of outcome was warranted.
Schematic representation of the DNA sequence of the TEL/AML1 fusion genes detected in this assay. The most common form fuses exon 5 of TEL (ETV6) with exon 1 of AML1 (RUNX1). A frequent splice variant fuses exon 5 of TEL (ETV6) with exon 2 of AML1 (RUNX1), and both were detected in this assay.
Schematic representation of the DNA sequence of the TEL/AML1 fusion genes detected in this assay. The most common form fuses exon 5 of TEL (ETV6) with exon 1 of AML1 (RUNX1). A frequent splice variant fuses exon 5 of TEL (ETV6) with exon 2 of AML1 (RUNX1), and both were detected in this assay.
To address potential selection biases from previous retrospective reports, we conducted a prospective study on the prognostic significance of the TEL/AML1 fusion gene in children enrolled on Dana-Farber Cancer Institute ALL Consortium Protocol 95-01, a phase 3 clinical trial that accrued patients with newly diagnosed ALL. Protocol 95-01 stratified patients according to age and presenting white blood cell count using current National Cancer Institute (NCI)–Rome criteria, thereby eliminating risk stratification differences with many other cooperative groups.
Patients, materials, and methods
Patients and leukemia samples
Diagnostic bone marrow and peripheral blood samples were collected prospectively from patients eligible to participate on DFCI ALL Consortium Protocol 95-01 between January 1996 and September 2000. Four hundred ninety-one patients enrolled on DFCI 95-01. For the purposes of this analysis, any bone marrow or peripheral blood sample falling within 5 days of the date of registration on study was considered diagnostic. Bone marrow or peripheral blood samples at diagnosis were obtained from 396 (81%) patients. Of the 396 patients, 352 had B-precursor ALL and 44 had T-cell ALL. Informed consent was obtained according to the Declaration of Helsinki. Approval for laboratory studies performed on Protocol 95-01 was obtained from the Committees on Human Research at the Dana-Farber Cancer Institute and the University of California, San Francisco.
The 9 consortium institutions were Children's Hospital, Boston and the Dana-Farber Cancer Institute (Boston, MA), Le Centre Hospitalier de l'Universite Laval (Quebec, Quebec), McMaster University (Hamilton, Ontario, Canada), Maine Children's Cancer Center, (Portland, ME), Mt Sinai Hospital (New York, NY), Ochsner Health Clinic (New Orleans, LA), Ste Justine Hospital (Montreal, Quebec), San Jorge Children's Hospital (San Juan, Puerto Rico), and University of Rochester (Rochester, NY). The institutional review boards of each participating institution approved the protocol before patient enrollment.
Therapy
Risk group stratification. Children at standard risk (SR) ranged in age from 1 to younger than 10 years of age at the time of diagnosis and had presenting white blood cell (WBC) counts of less than 50 × 109/L and no CNS 2 (less than 5/μL WBCs but cytospin-positive for blasts) or CNS 3 more than 5/μL WBCs and cytospin-positive for blasts or any cranial nerve palsy disease. In addition, SR patients could not have a T-cell immunophenotype, anterior mediastinal mass, or presence of the Philadelphia chromosome. Patients with mature B-cell leukemia were excluded from this trial. All other patients were deemed at high risk (HR). Infants (younger than 12 months) were considered at HR but were treated with an additional cycle of postremission consolidation, including high-dose methotrexate and high-dose cytarabine.
Systemic therapy. Types of therapy are summarized in Table 1. Chemotherapy was continued for a total of 24 months from the date of complete clinical remission. Patients could participate in 2 randomizations related to systemic therapy: (1) a comparison of Escherichia coli and Erwinia asparaginase during induction and postremission intensification (all patients) and (2) doxorubicin delivered with or without dexrazoxane (HR patients only). The asparaginase randomization closed on December 12, 1998, because accrual goals were met. All subsequent patients received E coli asparaginase.
Therapy on DFCI ALL Consortium Protocol 95-01
Type of therapy, duration, and regimen . |
|---|
| Induction therapy, 4 wk |
| Vincristine 1.5 mg/m2 intravenously every week for 4 weeks |
| Prednisone 40 mg/m2 by mouth every day for 28 days |
| Doxorubicin 30 mg/m2 intravenously on days 0 and 1* |
| Methotrexate 4 g/m2 intravenously on day 2 |
| Leucovorin 200 mg/m2 intravenous push hour 36, then 24 mg/m2 intravenously every 6 hours until methotrexate level < 0.1 μM |
| Asparaginase 25 000 IU/m2 intramuscularly on day 4† |
| IT-Ara-C at diagnosis, dosed by age‡ |
| ITT on day 16 and 30, dosed by age§ |
| CNS treatment, 3 wk |
| Vincristine 2 mg/m2 intravenously for 1 dose |
| 6-mercaptopurine 50 mg/m2 by mouth for 14 days |
| Doxorubicin 30 mg/m2 intravenously for 1 dose (HR only)* |
| SR: 1800 cGy cranial x-ray therapy (XRT) with IT every 18 weeks vs no XRT and intensive ITT every 9 weeks for 6 doses, then every 18 weeks |
| HR: IT every 18 weeks with 1800 cGy cranial XRT |
| Intensification therapy, 5-6 mo |
| Vincristine 2 mg/m2 intravenously every 3 weeks |
| Prednisone 40 mg/m2 by mouth for 5 days every 3 weeks (SR) |
| Prednisone 120 mg/m2 by mouth for 5 days every 3 weeks (HR) |
| 6-mercaptopurine 50 mg/m2 by mouth for 14 days every 3 weeks |
| Asparaginase every week for 20 doses† |
| Methotrexate 30 mg/m2 intramuscularly every week (SR only) |
| Doxorubicin 30 mg/m2 intravenously every 3 weeks (HR only)* |
| Continuation therapy, 2 y of CCR |
| Vincristine as intensification |
| Prednisone as intensification |
| 6-mercaptopurine as intensification |
| Methotrexate as intensification |
Type of therapy, duration, and regimen . |
|---|
| Induction therapy, 4 wk |
| Vincristine 1.5 mg/m2 intravenously every week for 4 weeks |
| Prednisone 40 mg/m2 by mouth every day for 28 days |
| Doxorubicin 30 mg/m2 intravenously on days 0 and 1* |
| Methotrexate 4 g/m2 intravenously on day 2 |
| Leucovorin 200 mg/m2 intravenous push hour 36, then 24 mg/m2 intravenously every 6 hours until methotrexate level < 0.1 μM |
| Asparaginase 25 000 IU/m2 intramuscularly on day 4† |
| IT-Ara-C at diagnosis, dosed by age‡ |
| ITT on day 16 and 30, dosed by age§ |
| CNS treatment, 3 wk |
| Vincristine 2 mg/m2 intravenously for 1 dose |
| 6-mercaptopurine 50 mg/m2 by mouth for 14 days |
| Doxorubicin 30 mg/m2 intravenously for 1 dose (HR only)* |
| SR: 1800 cGy cranial x-ray therapy (XRT) with IT every 18 weeks vs no XRT and intensive ITT every 9 weeks for 6 doses, then every 18 weeks |
| HR: IT every 18 weeks with 1800 cGy cranial XRT |
| Intensification therapy, 5-6 mo |
| Vincristine 2 mg/m2 intravenously every 3 weeks |
| Prednisone 40 mg/m2 by mouth for 5 days every 3 weeks (SR) |
| Prednisone 120 mg/m2 by mouth for 5 days every 3 weeks (HR) |
| 6-mercaptopurine 50 mg/m2 by mouth for 14 days every 3 weeks |
| Asparaginase every week for 20 doses† |
| Methotrexate 30 mg/m2 intramuscularly every week (SR only) |
| Doxorubicin 30 mg/m2 intravenously every 3 weeks (HR only)* |
| Continuation therapy, 2 y of CCR |
| Vincristine as intensification |
| Prednisone as intensification |
| 6-mercaptopurine as intensification |
| Methotrexate as intensification |
Infants were treated as HR patients but received additional chemotherapy, including high-dose methotrexate and high-dose Ara-C.
HR patients: doxorubicin given with or without dexrazoxane 300 mg/m2. Total cumulative dose of doxorubicin was 300 mg/m2 for HR and 60 mg/m2 for SR patients.
Asparaginase type randomized: E coli or Erwinia each at 25 000 IU/m2 intramuscularly.
IT-Ara-C dosed by age: younger than 1 year, 15 mg; 1-1.99 years, 20 mg; 2-2.99 years, 30 mg; older than 3 years, 40 mg.
ITT: triple intrathecal chemotherapy dosed per age: Ara-C dosing above, younger than 1 year, methotrexate 6 mg and hydrocortisone 6 mg; 1-1.99 years, methotrexate 8 mg and hydrocortisone 9 mg; 2-2.99 years, methotrexate 10 mg and hydrocortisone 12 mg; older than 3 years, methotrexate 12 mg and hydrocortisone 15 mg.
Laboratory methods
After bone marrow or peripheral blood was collected, mononuclear cells were collected after density gradient centrifugation using Hypaque-Ficoll according to standard methods. A minimum of 5 million cells was placed directly into RNA-Stat 60 (January 1996 to November 1997) or into RNeasy (Qiagen, Valencia, CA) or RNAqueous buffer (November 1997 to 2001) (Ambion, Austin, TX). Total RNA was extracted according to the specific protocol and subjected to DNAse with DNAse I (Ambion) followed by inactivation by DNAse Inactivator (Ambion). Total RNA was reverse transcribed into cDNA using standard methods. An aliquot of cDNA was then used in duplicate control polymerase chain reaction (PCR) to verify the integrity of the RNA sample using TEL-specific primers 458 and 750R and were visualized on a 2.5% agarose gels, as previously described.11,15 Only duplicate-positive samples for TEL were analyzed for the presence of the TEL/AML1 fusion gene.
Amplification of the TEL/AML1 fusion gene was performed using TEL forward primer 958 5′CTGGCTTACATGAACCACATCA3′ and TEL/AML1 reverse primer 1058R 5′CGGCTCGTGCTGGCA3′. The 25-μL PCR reaction mixture included 2 μL cDNA, 10 × buffer (Applied Biosystems, Foster City, CA), 2.5 mM MgCl, 250 nM dNTPs (Boehringer Mannheim), 1000 nM primers, 0.75 U Amplitaq Gold (Applied Biosystems), and DEPC H2O (Ambion). After an initial denaturing step at 95° for 10 minutes, a 2-step PCR (95° for 15 seconds followed by 60° for 1 minute) was performed for 40 cycles to amplify either a 127-bp product or a 93-bp product representing the most common splice variant. Products were visualized on agarose gels.
Statistical methods
χ2 analysis or, where cell frequencies were small, Fisher exact tests were used to compare categorical variables between groups. Clinical and laboratory characteristics were categorized for analyses as shown in Table 2. Event-free survival (EFS) was measured from date of complete remission at the end of induction therapy to the first of any event, including induction failure, relapse, death from any cause, or censored at last contact; induction failure and induction death were considered events at time zero. Overall survival was defined as the time from randomization to death or censored at last contact. Survival distributions were estimated using the Kaplan-Meier method, and univariate associations were tested using log-rank tests. Cox proportional hazards regression was used to model the survival data on TEL /AML1 status while adjusting for known prognostic factors and treatment. Manual stepwise model selection was performed with likelihood ratio tests using the variables in Table 2; all 2-way interactions between variables in the final model were examined separately. Given that WBC count and age were found to be highly collinear with risk group, the final multivariable modeling used risk group and excluded WBC and age. All reported P values were from 2-sided tests, with 5% type 1 error rates. No adjustments were made for multiple comparisons. Analyses of the data set were current as of December 2004.
Presenting clinical features of B-precursor patients enrolled on DFCI 95-01 with TEL/AML1 status at diagnosis
. | TEL/AML1 diagnostic status . | . | . | |
|---|---|---|---|---|
| Presenting feature . | Negative, no. (%) . | Positive, no. (%) . | χ2P . | |
| Age | .05 | |||
| 0 y to less than 1 y | 10 (5) | 0 (0) | ||
| 1 y to less than 10 y | 174 (78) | 69 (90) | ||
| More than 10 y | 38 (17) | 8 (10) | ||
| Sex | .65 | |||
| Male | 119 (54) | 39 (51) | ||
| Female | 103 (46) | 38 (49) | ||
| Risk | .23 | |||
| Standard | 127 (57) | 50 (65) | ||
| High | 95 (43) | 27 (35) | ||
| WBC count | .08 | |||
| Less than 50 × 109/L | 186 (84) | 58 (75) | ||
| More than 50 × 109/L | 35 (16) | 19 (25) | ||
| Unknown | 1 (0) | 0 (0) | ||
| CNS disease | .57 | |||
| No | 179 (81) | 66 (86) | ||
| Yes | 30 (14) | 7 (9) | ||
| Traumatic | 10 (4) | 3 (4) | ||
| Unknown | 3 (1) | 1 (1) | ||
| Ethnicity | .75* | |||
| White | 179 (81) | 66 (86) | ||
| Black | 10 (4) | 3 (4) | ||
| Hispanic | 24 (11) | 7 (9) | ||
| Other | 9 (4) | 1 (1) | ||
| Asparaginase treatment | .14 | |||
| Randomized E coli | 59 (27) | 26 (34) | ||
| Randomized Erwinia | 56 (25) | 24 (31) | ||
| Assigned E coli | 107 (48) | 27 (35) | ||
. | TEL/AML1 diagnostic status . | . | . | |
|---|---|---|---|---|
| Presenting feature . | Negative, no. (%) . | Positive, no. (%) . | χ2P . | |
| Age | .05 | |||
| 0 y to less than 1 y | 10 (5) | 0 (0) | ||
| 1 y to less than 10 y | 174 (78) | 69 (90) | ||
| More than 10 y | 38 (17) | 8 (10) | ||
| Sex | .65 | |||
| Male | 119 (54) | 39 (51) | ||
| Female | 103 (46) | 38 (49) | ||
| Risk | .23 | |||
| Standard | 127 (57) | 50 (65) | ||
| High | 95 (43) | 27 (35) | ||
| WBC count | .08 | |||
| Less than 50 × 109/L | 186 (84) | 58 (75) | ||
| More than 50 × 109/L | 35 (16) | 19 (25) | ||
| Unknown | 1 (0) | 0 (0) | ||
| CNS disease | .57 | |||
| No | 179 (81) | 66 (86) | ||
| Yes | 30 (14) | 7 (9) | ||
| Traumatic | 10 (4) | 3 (4) | ||
| Unknown | 3 (1) | 1 (1) | ||
| Ethnicity | .75* | |||
| White | 179 (81) | 66 (86) | ||
| Black | 10 (4) | 3 (4) | ||
| Hispanic | 24 (11) | 7 (9) | ||
| Other | 9 (4) | 1 (1) | ||
| Asparaginase treatment | .14 | |||
| Randomized E coli | 59 (27) | 26 (34) | ||
| Randomized Erwinia | 56 (25) | 24 (31) | ||
| Assigned E coli | 107 (48) | 27 (35) | ||
For TEL/AML1-negative patients, n = 222; for TEL/AML1-positive patients, n = 77.
Fisher exact test.
Results
Of the 491 eligible patients enrolled on Protocol 95-01, 396 (81%) had a diagnostic peripheral blood or a bone marrow sample obtained for TEL/AML1 analysis. There was no difference in age, sex, risk group, immunophenotype, CNS involvement, or asparaginase treatment between patients whose diagnostic samples were submitted compared with those whose were not, though patients presenting with a higher leukocyte count (50 000/mm3 or higher) were more likely to have a diagnostic sample submitted than patients with a lower leukocyte count (less than 50 000 mm3) (P = .002). In addition, Hispanic patients were less likely to have submitted a diagnostic sample (P = .01) for unclear reasons (69% sample acquisition rate for Hispanic patients was similar at multiple consortium sites).
Reverse transcription–polymerase chain reaction (RT-PCR) analysis for the TEL/AML1 fusion was performed successfully in 341 patients (86% of available samples), of whom 299 had B-precursor ALL. Seventy-seven (26%) of 299 B-precursor ALL patients were TEL/AML1-positive. None of the 42 patients with T-cell leukemia were TEL/AML1-positive. There were no significant differences between the clinical characteristics of patients who had successful PCR and those who did not. Because none of the T-cell patients were TEL/AML1-positive, all analyses of the association of TEL/AML1 status with survival end points included only the 299 B-precursor patients with informative TEL/AML1 results. Table 2 outlines the clinical and laboratory characteristics for the B-precursor TEL/AML1-positive and -negative patients at diagnosis. As shown, TEL/AML1-positive and -negative patients did not differ significantly with respect to any of these variables, with the exception of age (P = .05); TEL/AML1-positive patients were more likely to be between 1 and 10 years of age.
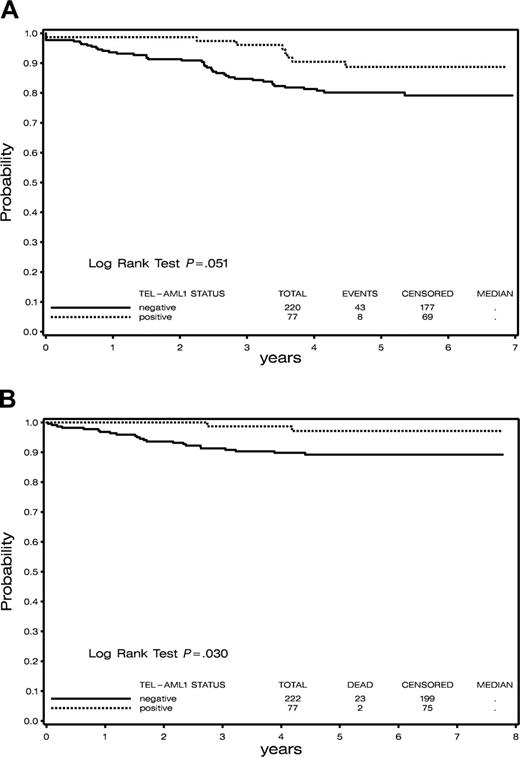
Follow-up remission data were lacking for 2 TEL/AML1-negative patients, and they were excluded from all EFS analyses. With a median follow-up of 5.2 years, 5-year EFS (± SE) for TEL/AML1-positive patients was 0.89 (± 0.04) compared with 0.80 (± 0.03) for TEL/AML1-negative B-precursor patients (P = .05; Figure 2A), indicating a marginally significant association of TEL/AML1 status with EFS. TEL/AML1 status was not associated with EFS within each risk group (P = .14 for high risk and P = .28 for standard risk). When infants (all of whom were TEL/AML1-negative) were excluded from the analysis, the trend toward inferior EFS in TEL/AML1-negative patients was still observed (5-year EFS, 0.82), though the difference in EFS between TEL/AML1-negative and -positive patients did not reach the level of statistical significance (P = .09).
The median relapse time among the 7 TEL/AML1-positive patients who had relapses was 42.8 months (range, 27-53.6 months), compared with 28.8 months (range, 5-64.2 months) among the 37 TEL/AML1-negative B-precursor ALL patients who had relapses. One TEL/AML1-positive patient and 3 TEL/AML1-negative patients experienced induction failure. No induction deaths occurred among the TEL/AML1-positive patients, but 2 TEL/AML1-negative patients died. One remission death occurred among the TEL/AML1-negative patients and none among the TEL/AML1-positive patients. Fourteen of the TEL/AML1-negative patients had relapses within the first year of treatment, whereas only one TEL/AML1-positive patient had a relapse within that time. The next failure among the TEL/AML1-positive patients occurred 2.25 years after the end of induction. Five-year overall survival was 0.97 (± .02) for TEL/AML1-positive patients compared with 0.89 (± 0.02) for TEL/AML1-negative patients (P = .03; Figure 2B).
There were no differences between TEL/AML1-positive and -negative patients with respect to the preparation of asparaginase received. Fifty-three (69%) TEL/AML1-positive patients and 166 (75%) TEL/AML1-negative patients received E coli asparaginase, either as a result of the randomization or direct assignment after the randomization was closed, compared with 24 (31%) TEL/AML1-positive patients and 56 (25%) of TEL/AML1-negative patients who received Erwinia asparaginase during the randomization (P = .31). When comparing patients who were assigned the same type of asparaginase (E coli or Erwinia), TEL/AML1 status was not significantly associated with EFS (P = .07 and P = .44 for E coli and Erwinia groups, respectively), though there was a trend toward fewer events among TEL/AML1-positive patients in each asparaginase group. Moreover, within TEL/AML1 status groups, the type of asparaginase assigned was not significantly associated with EFS (P = .80 comparing EFS between E coli- and Erwinia-assigned TEL/AML1-positive patients subset and P = .77 among the TEL/AML1-negative B-precursor patients).
In multivariable regression analyses examining the association of EFS with TEL/AML1 status and the other known prognostic factors and treatment groups shown in Table 1, none of the following were statistically significant predictors of outcome: TEL/AML1 status, sex, CNS status, or race. When controlling for other significant predictors of EFS in the final model, risk group (P = .009) and asparaginase treatment group (P = .04), but not TEL/AML1 status (P = .12), were significantly associated with EFS. None of the interactions between these variables were significant, indicating that the effect of TEL/AML1 status on EFS did not differ within treatment or risk groups. However, the power for these comparisons was low. When WBC count and age were included in the modeling instead of risk group, they were also highly statistically significant and overall results were similar. In all models, when controlling for risk group (or WBC count and age) and asparaginase treatment group, the effect of TEL/AML1 on EFS was not statistically significant.
Discussion
One of the most important prognostic factors for patients with ALL is the intensity of treatment received.15 Among the major determinants of treatment is the assignment of patients to risk groups at diagnosis. The criteria used to assign patients to groups stratified according to risk have varied among national and international cooperative groups. In 1995, the Cancer Treatment and Evaluation Program gathered investigators to discuss unifying the approach to risk stratification, thus increasing the efficiency of clinical research in ALL.16 In combination with data gathered from Rome, these joint efforts led to the widely and currently used NCI–Rome risk stratification, which uses the 2 parameters of age and white blood cell count at presentation as the basis for this grouping.
Survival in patients on Consortium Protocol DFCI 9501. (A) Event-free survival of TEL/AML1-positive compared with TEL/AML1-negative patients. (B) Overall survival of TEL/AML1-positive compared with TEL/AML1-negative patients.
Survival in patients on Consortium Protocol DFCI 9501. (A) Event-free survival of TEL/AML1-positive compared with TEL/AML1-negative patients. (B) Overall survival of TEL/AML1-positive compared with TEL/AML1-negative patients.
Therefore, when the outcome data for patients who have TEL/AML1-positive leukemia is interpreted, it is crucial to assess risk stratification and subsequent dose intensity received. The heterogeneity of reported outcomes of TEL/AML1-positive patients can be analyzed within this context. For instance, DFCI ALL Consortium protocols before 1995 used more stringent criteria for defining patients at lower risk (standard risk, age 2-9 years; presenting WBC count, less than 20 × 109/L; no CNS 2 or 3 disease; lack of T-cell immunophenotype; lack of anterior mediastinal mass).11,17 All other patients were considered at high risk for relapse and received intensive therapy, including a higher cumulative dose of anthracycline and, often, cranial radiation. In fact, half the TEL/AML1-positive patients in the first retrospective report from the DFCI, and many of the patients reported by St Jude (who placed them in a higher risk group in light of a diploid DNA index) received intensive therapy, potentially explaining the superior outcomes for these groups.1,11 In contrast, many of the patients reported to have relapsed disease in the initial BFM studies were classified as having low or intermediate risk features and might have had less intensive therapy. Indeed, findings from several studies by investigators in the Children's Cancer Group (CCG) and by institutions in Japan have supported this hypothesis indirectly; in these studies, the outcome of patients with TEL/AML1-positive disease has improved with more intensive therapy.18
A second factor to consider when evaluating the use of TEL/AML1 as a prognostic indicator is evidence that the type of chemotherapy received by patients can make a difference in outcome. For example, TEL/AML1-positive lymphoblasts are exquisitely sensitive to steroids, vincristine, and asparaginase in vitro.19,20 The mechanism for the increased sensitivity to asparaginase among TEL/AML1-positive lymphoblasts is unclear and does not appear to be related to the levels of asparagine synthetase, either at baseline or after exposure to the drug.21 The favorable outcome of TEL/AML1-positive patients on DFCI ALL Consortium protocols may be the result of protracted use of asparaginase during intensification and frequent pulses of vincristine and steroids during the 2 years of treatment. The exposure to weekly L-asparaginase during the intensification phase in DFCI clinical trials has been closely linked with EFS.22,23 In addition, investigators in Pediatric Oncology Group (POG) reported that patients with TEL/AML1-positive leukemia accumulate lower amounts of methotrexate polyglutamates. Such decreases have led to one speculation that these patients may be better treated by the increased doses of methotrexate used in these protocols.24
In the present report, there was no difference in EFS among patients who were TEL/AML1-positive with respect to the type of asparaginase preparation received. Erwinia asparaginase has a shorter half-life than E coli asparaginase and has been associated with higher relapse rates when used instead of E coli asparaginase.25 Similarly, when analyzing all children enrolled on Protocol 95-01, Erwinia asparaginase was associated with an inferior EFS.26 The lack of association between outcome and type of asparaginase used in TEL/AML1-positive patients supports that these patients have an increased sensitivity to asparaginase, resulting in adequate treatment even when a less effective preparation is used.
A third factor to consider when evaluating the outcome of TEL/AML1-positive patients is that relapsed disease might represent a novel “secondary” TEL/AML1-positive leukemia in which the original, preleukemic cell of origin was not completely eradicated by upfront chemotherapy. In support of this possibility, studies demonstrating the presence of low levels of the TEL/AML1 fusion gene in bloodspots from newborns who later had leukemia establish that the initiating event of the TEL/AML1 rearrangement occurs in utero and that subsequent events are required in early childhood to facilitate the full transformation to leukemia.27 The secondary events that occur at the time of diagnosis of TEL/AML1-positive leukemia often include deletion of the wild-type TEL allele in most cases28 and acquisition of trisomy 21.29
Analyses of cohorts of patients with relapsed TEL/AML1-positive leukemia indicate that patients remain sensitive to the same chemotherapeutic agents used at initial diagnosis.30 Using microsatellite markers to study TEL gene rearrangements, immunoglobulin or TCR rearrangements, and fluorescence in situ hybridization (FISH) analysis, a number of patients experiencing late relapses had TEL/AML1-positive leukemia derived from, but not identical to, the dominant clone at diagnosis.31,32 In addition, at least one patient who experienced relapse during therapy developed microsatellite instability in the relapsed subclone of the original leukemia. Taken together, these data support the hypothesis that preleukemic cells harboring the TEL/AML1 fusion gene are not eradicated by initial therapy in some patients who later have relapses. A second, independent transforming event may then give rise to another TEL/AML1-positive leukemia, still marked by excellent response to chemotherapy.
In summary, we have confirmed in a prospective analysis that the TEL/AML1 fusion gene occurs in approximately 26% of pediatric patients with B-precursor ALL and that these patients have excellent outcomes with currently available therapy from the DFCI ALL Consortium. The longer time to relapse for TEL/AML1-positive patients is consistent with other reports, and the excellent overall survival of these patients in this study suggests that those who have relapses can be successfully treated. Our results indicate that presenting age and leukocyte count, not TEL/AML1 status, were independent predictors of outcome, suggesting that NCI–Rome risk group status should be considered when treating TEL/AML1-positive patients. Because the association of TEL/AML1 status with EFS was a secondary objective, this study was not designed to guarantee sufficient power for examination of this end point. However, the lack of data in support of TEL/AML1 as a prognostic factor independent of risk group in this clinical trial provides a cautionary note to avoid future decreases in the intensity of therapy for TEL/AML1-positive patients otherwise at high risk because of age or presenting leukocyte count.
Prepublished online as Blood First Edition Paper, February 21, 2006; DOI 10.1182/blood-2005-08-3451.
Supported by grant K23 CA80195 from the National Cancer Institute (NCI) of the National Institutes of Health (NIH), the California Research Coordinating Committee, the Karen and Jeffery Peterson Foundation (M.L.L.), and National Cancer Institute grant PO1 CA 68484 (M.A.G., L.B.S., A.C., D.S.N., S.E.S., D.G.G.).
M.L.L. was responsible for the design and implementation of the project, sample processing, data integrity, and writing the manuscript; M.A.G. was responsible for data integrity, statistical analysis, and editing the manuscript; L.B.S. was responsible for statistical interpretation and editing the manuscript; W.M.P. and S.V. were responsible for analysis of samples; A.C. was responsible for processing of samples; D.S.N. was responsible for supervision of statistical analysis; K.M.S. was responsible for mentorship of M.L.L. and data integrity; S.E.S. was Principal Investigator of Program Project Grant and was responsible for editing the manuscript; and D.G.G. was responsible for data integrity and editing the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank all the patients and physicians (J. M. LeClerc, A. Moghrabi, B. Asselin, R. Barr, L. Clavell, C. Hurwitz, J. Lipton, Y. Samson, M. Schorin) who contributed samples to the investigators. We also thank members of the Gribben laboratory for their contributions to the collection of samples.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal