Abstract
Rho family GTPases are key signal transducers in cell regulation. Although a body of literature has implicated the Rho family members Rac1 and Rac2 in multiple hematopoietic-cell functions, the role of Cdc42 in hematopoiesis remains unclear. Here we have examined the hematopoietic properties and the hematopoietic stem/progenitor cell (HSP) functions of gene-targeted mice carrying null alleles of cdc42gap, a negative regulator of Cdc42. The Cdc42GAP-/- fetal liver and bone marrow cells showed a 3-fold increase in Cdc42 activity but normal Rac and RhoA activities, indicating that Cdc42GAP knockout resulted in a gain of Cdc42 activity in the hematopoietic tissues. Cdc42GAP-/- mice were anemic. The cellularity of fetal liver and bone marrow, the number and composition percentage of HSPs, and the erythroid blast-forming unit and colony-forming unit (BFU-E/CFU-E) activities were significantly reduced in the homozygous mice. The decrease in HSP number was associated with increased apoptosis of the Cdc42GAP-/- HSPs and the activation of JNK-mediated apoptotic machinery. Moreover, homozygous HSPs showed impaired cortical F-actin assembly, deficiency in adhesion and migration, and defective engraftment. These results provide evidence that Cdc42 activity is important for erythropoiesis and for multiple HSP functions, including survival, adhesion, and engraftment.
Introduction
The Rho family GTPases function as intracellular molecules to cycle between the GTP-bound, active and the GDP-bound, inactive states to transduce diverse signals in cells.1-5 Upon ligand binding to a variety of cell receptors, guanine nucleotide exchange factors are activated to mediate Rho GTPase activation and to turn on an array of cellular responses by endowing the active Rho GTPases with the ability to engage specific downstream effectors. In parallel, Rho GTPase-activating proteins (GAPs) serve to stimulate the intrinsic GTP-hydrolytic activities of Rho GTPases and to help return the active Rho proteins to the inactive conformation. This GTP-binding/GTP-hydrolysis cycle of Rho GTPases is tightly regulated by the interplay of these regulatory molecules that control the level of GTP-bound Rho proteins to ensure proper signaling output in many cell types, including hematopoietic cells.1,2
One prominent member of the Rho family, Cdc42, was first identified in the budding yeast Saccharomyces cerevisiae as a key bud-site assembly protein.3 It has since been shown to play an essential role in the growth of lower eukaryotes and in mammalian actin cytoskeleton-based functions, DNA synthesis, intracellular trafficking, and transcription regulation.4,5 Our current knowledge of Cdc42 function in most mammalian cells is obtained by overexpressing the constitutively active or dominant-negative Cdc42 mutants, which may have significant drawbacks. For example, constitutively active mutants of Cdc42 lack specificity for effectors because overexpression of the constitutively GTP-bound Cdc42 could bind many effectors in a non-spatiotemporal manner. It is now known that multiple Cdc42 effectors exist in the cell and that dynamic binding of Cdc42 to each effector is necessary for optimal Cdc42 signaling.4 Thus, it is highly desirable to use genetic approaches with minimal perturbation of endogenous cellular environment to study Cdc42 function. Using homologous recombination in mice, 2 related Rho GTPases, Rac1 and Rac2, have been characterized in various hematopoietic-cell functions, including hematopoietic stem/progenitor cell (HSP) adhesion and migration,6,7 mast-cell survival,8,9 neutrophil superoxide generation,7,10,11 and B-, T-, and dendritic-cell development.12-14 To date, limited information is available about the contribution of Cdc42 to hematopoiesis and its lineage-specific blood-cell functions, partly because genetic deletion of Cdc42 led to early embryonic lethality in mice.15 However, studies of one of the Cdc42 effectors, the Wiskott-Aldrich syndrome protein (WASP), have provided evidence that upon activation by GTP-bound Cdc42, WASP can bind the actin-associated complex Arp2/3 to promote actin polymerization, and this is essential for HSP migration and homing.16 Therefore, it is anticipated that Cdc42 may play a unique and important role in mammalian hematopoiesis, possibly in regulating HSP functions.
As the first identified RhoGAP family member, Cdc42GAP (also termed p50RhoGAP)17,18 contains a RhoGAP domain that has been shown to specifically catalyze GTP-hydrolysis of Cdc42 and a BCH/Sec14 domain that is also capable of interacting with Cdc42,19,20 suggesting that this molecule functions as a Cdc42-specific negative regulator. To define the role of Cdc42 in regulating hematopoiesis, we examined Cdc42GAP knockout mice and found that the hematopoietic cells of the Cdc42GAP-/- genotype contain constitutively elevated Cdc42 activity. Our phenotypic analysis of this gain of Cdc42 activity-mouse model, combined with in vitro and in vivo characterizations of the Cdc42GAP-/- HSP activities, provide strong evidence that Cdc42 is important for erythropoiesis and for multiple HSP functions, including survival, adhesion, migration, and engraftment.
Materials and methods
Mice
Cdc42GAP knockout mice were generated by a conventional homologous recombination gene-targeting strategy described elsewhere.34 The mice were housed in pathogen-free conditions according to the guidelines of the Institutional Animal Care and Use Committee. Gestational age of heterozygous pregnancies was determined by detection of the vaginal plug (as embryonic day 0.5 [E0.5]), and E14.5 embryos were harvested for fetal livers. PCR genotyping was performed as described elsewhere.34 Tail vein or retro-orbital blood was collected, and the blood counts were performed on a Hemavet 850 hematology analyzer (Drew Scientific, Oxford, CT).
Fluorescence-activated cell sorting purification of HSPs
Fetal livers derived from Cdc42GAP heterozygous crossed mice were dissected from E14.5 embryos. Fetal livers were gently homogenized and filtered through a 40-μm filter to generate single-cell suspensions. The cells were laid on top of Histopaque-1081 (Sigma, St Louis, MO) and centrifuged at 400g for 15 minutes at room temperature (RT). Isolated mono-nuclear low-density fetal liver cells were washed in PBS and stained for 15 minutes at room temperature with a cocktail of FITC-conjugated antimouse antibodies specific for the mature cell-lineage antigens, including CD45R (B220; clone RA3-6B2), Gr-1 (Ly-6G and Ly-6C; clone RB6-8C5), CD4 (L3T4; clone RM4-5), CD8a (Ly-2; clone 53-6.7), CD3e (clone 145-2C11), and a biotinylated antibody for Ter119 (Ly-76) according to the manufacturer's recommendations (PharMingen, San Diego, CA).7,21 After washing with PBS, the cells were labeled for another 15 minutes with APC-conjugated anti-mouse CD117 (c-Kit; Clone 2B8) and streptavidin-FITC (PharMingen). In some cases, the PE-Sca1 (Ly-6A/E; clone D7; PharMingen) antibody was also included in the latter labeling step. Stained cells were then washed with red blood cell (RBC) lysis buffer (BD Biotechnology) and were further incubated with 7-AAD (PharMingen), and the viable Lin-c-Kit+7-AAD-, designated as HSPs or Lin-c-Kit+Sca1+7-AAD- cells, were isolated by a FACStar Plus sorter (BD Biotechnology) under sterile conditions.
Colony-formation assays
Fetal liver or bone marrow cells (5 × 104 cells) were cultured in 1 mL methylcellulose medium (1% methylcellulose, 30% FBS, 2% penicillin and streptomycin, 1% BSA, and 10-4 M β-mercaptoethanol) containing 4 U/mL EPO, 100 ng/mL rrSCF, 100 ng/mL G-CSF, and 100 ng/mL IL-3 for 7 to 10 days and granulocyte macrophage colony-forming unit (CFU-GM), erythroid burst-forming unit (BFU-E), and granulocyte, erythroid, macrophage, megakaryocyte CFU (CFU-GEMM) colonies were counted under an inverted microscope. For erythroid CFU (CFU-E) assays, 2 × 105 fetal liver or bone marrow cells were cultured in 1 mL methylcellulose medium supplemented with 100 ng/mL rrSCF and 4 U/mL EPO for 2 days.
F-actin fluorescence microscopy
To characterize F-actin assembly, 1 × 104 HSPs were seeded onto plastic chamber slides (Nalge Nunc International, Naperville, IL) in HBSS for 1 hour at 37°C before stimulation with 100 ng/mL SDF-1 or rrSCF for different lengths of time. The cells were fixed with 2% paraformaldehyde (pH 7.4; Sigma) and permeabilized with 0.1% Triton X-100 (Sigma). After blocking in 2% BSA, the cells were stained with TRITC-conjugated phalloidin (Molecular Probes, Eugene, OR) and DAPI and mounted for fluorescence imaging analysis on a Leica DMIRB (Wetzlar, Germany) fluorescence microscope equipped with a 63× oil-immersion CORRPH2 0.1-1.3/c objective lens and a Hamamatsu C4742 digital camera (Hamamatsu Photonics, Hamamatsu City, Japan) and deconvolution software (Improvision, Lexington, MA).
Cell adhesion and migration assays
Isolated low-density fetal liver cells from E14.5 embryos were subjected to adhesion and migration assays in vitro. For adhesion assays, the cells were incubated on plates coated with recombinant fibronectin fragment H-296 containing the integrin α4β1 binding site7 for 1 hour in Iscove modification of DMEM (IM; Mediatech, Herndon, VA) and were washed with PBS 3 times before the adherent cells were harvested by using the Cell Dissociation Buffer (BD Biotechnology). For migration assays, the cells were plated in the upper well of a Transwell chamber separated with a filter containing 5-μm pore size (Corning, Corning, NY) in IM medium with 2% BSA. After 4-hour incubation against an SDF-1α gradient at the lower chamber, the cells that migrated through the filter were harvested. They were then assayed for colony-forming activity in methylcellulose medium containing rrSCF, IL3, EPO, and G-CSF. The percentage of adherent or migrated HSPs was calculated and expressed as a ratio of formed colony numbers from the adherent or migrated cells and from the total input cells.
Proliferation, apoptosis, and cell-cycle progression assays
Fetal liver-derived HSPs (Lin-c-Kit+) were cultured in the IM medium containing 1% FBS for 6 hours before stimulation with 100 ng/mL rrSCF. Cell numbers at the indicated times were counted. To determine the cell population that was apoptotic, 2 × 104 cells were stained with 7-AAD and APC-conjugated annexin-V per manufacturer's instructions (Pharmingen) and were analyzed by flow cytometry at different time points. For cell-cycle analysis, 2 × 104 cells were labeled by PI/RNase at the indicated time points and were analyzed by flow cytometry.
In vivo BrdU labeling and TUNEL assays
To examine HSP cell-cycle progression and apoptosis in vivo, female Cdc42GAP+/- mice were timed for pregnancy after crossing with male heterozygous mice. At E14.5, the pregnant mice were injected intraperitoneally with 100 μg BrdU/g body weight. BrdU incorporation and terminal deoxynucleotidyl transferase nick-end labeling (TUNEL) staining were performed as described.22 One hour or 12 hours after BrdU injection, the mice were killed and transcardial perfusion was performed. Fetal livers of the embryos were dissected and embedded in paraffin. Five-micrometer sections were deparaffinized, and DIG-11-dUTP was incorporated into the sections by terminal deoxynucleotidyl transferase treatment. Sections were subsequently treated with 0.07 N NaOH to expose single-stranded DNA and were labeled with AP-conjugated sheep anti-DIG Fab fragments and mouse anti-BrdU monoclonal antibody (Sigma). The sections were stained with secondary biotinylated antisheep antibody followed by labeling with Texas Red-streptavidin and fluorescein-conjugated antimouse antibody before mounting on Vectashield (Vector Laboratories, Burlingame, CA) for immunofluorescence examination.
Western blotting, JNK kinase inhibitor, and JNK siRNA assays
Low-density fetal liver cells were lysed and immunoblotted with mouse monoclonal anti-Cdc42GAP antibody (BD Biotechnology) to examine the expression of Cdc42GAP in the homozygous and wild-type (WT) mice. To examine the JNK and BID (Biosources, Camarillo, CA) activities, isolated HSPs were cultured in the presence or absence of 10 μM JNK inhibitor SP600125, serum starved for 6 hours, and stimulated with 100 ng/mL SCF for 10 minutes before cells were lysed in the RIPA buffer for immunoblotting with anti-BID, phosphor-JNK, JNK, Bax, and Bcl-xL antibodies (Cell Signaling Technology, Beverly, MA) or for the analysis of cell-cycle progression or apoptotic-cell populations by flow cytometry. For JNK siRNA assays, HSPs were transduced twice with retrovirus expressing pRS-ctrl or pRS-JNK siRNA, as described,23 followed by selection with puromycin (2 μg/mL) for 2 days before apoptosis analysis with annexin-V/7-AAD staining.
Rho GTPase effector domain pull-down assays
Rho GTPases (RhoA, Rac1, Rac2, and Cdc42) activities were examined by an effector domain, GST-fusion pull-down protocol, as previously described.24 In brief, low-density fetal liver cells were lysed in a buffer containing 1% Triton X-100 and incubated with the glutathione bead-bound GST-Rhotekin (for RhoA activity) or GST-PAK1 (for Cdc42, Rac1, and Rac2 activities). The bead-immobilized, GTP-bound Rho GTPases and the Rho GTPases in the lysates were probed by immunoblotting with respective antibodies, and the relative amount of proteins bound to the beads were quantified by densitometry measurements.
Transplantation and engraftment assays
A total of 2 × 106 donor fetal liver cells from genetically matched WT or homozygous donor mice (H-2KbCD45.2+) were injected into the tail vein of each 6- to 8-week-old, sublethally irradiated (3.5 Gy [total body irradiation],137Cs source; average dose rate, 62 Gy/min) NOD/SCID (H-2Kd) or lethally irradiated (12-Gy) B6.SJL/BoyJ (CD45.1+) recipient mouse. For competitive repopulation analysis, 1 × 106 donor fetal liver cells were mixed with 1 × 106 B6.SJL/BoyJ WT fetal liver cells and were injected into the tail veins of lethally irradiated B6.SJL/BoyJ recipient mice. Chimerism (percentage CD45+ H-2Kb+ or percentage CD45.2+) was determined by FACS analysis on peripheral blood leukocytes or bone marrow of the recipient mice at 1, 3, and 6 months after transplantation. Blood or bone marrow cells were lysed with RBC lysis buffer and subsequently were incubated with anti-CD45-APC, anti-H-2Kb-PE (Phar-Mingen), or CD45.2-PE, and 7-AAD for analysis of H-2Kb or CD45.2 expression in the viable cells.
Statistical analysis
Statistical significance of the difference between sample groups was calculated by using the Student t test and was defined as a P value less than or equal to .05.
Results
Cdc42GAP deletion causes a gain of Cdc42 activity in hematopoietic tissues, decreased hematopoietic cellularity, and anemia
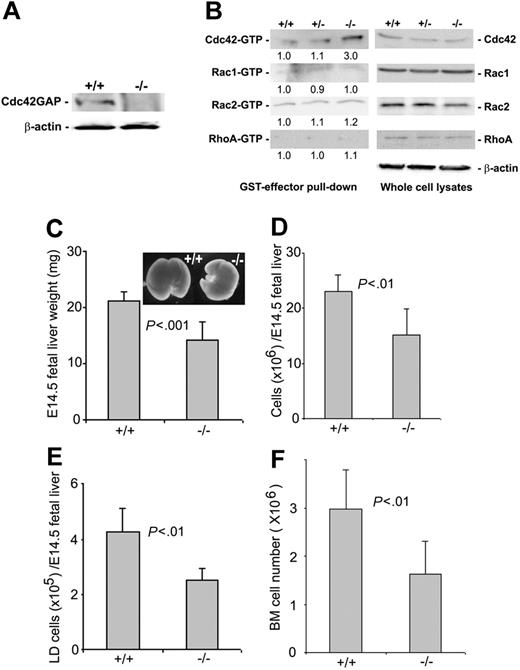
Cdc42 is ubiquitously expressed in mammalian tissues, including hematopoietic cells. Cdc42GAP has been shown to biochemically favor Cdc42 over other Rho GTPases as substrates and is widely distributed in various tissues, including fetal liver and adult bone marrow (Figure 1A; data not shown). Gene targeting of Cdc42GAP in mice by homologous recombination resulted in a deletion of the cdc42gap gene as detected by a PCR-based genotyping assay (data not shown) and a loss of Cdc42GAP protein expression in all tissue examined, including the E14.5 fetal liver (Figure 1A). Deletion of Cdc42GAP did not affect the expression of the Rho GTPases Cdc42, Rac1, Rac2, or RhoA, nor did it affect the level of active Rac1-GTP, Rac2-GTP, or RhoA-GTP in low-density fetal liver cells. However, it selectively increased the Cdc42-GTP content by 3-fold, as determined by the effector domain pull-down assay (Figure 1B), suggesting Cdc42GAP knockout causes a specific gain of Cdc42 activity. Consistent with the observation that deletion of Cdc42GAP was associated with reduced nonhematopoietic organ sizes in mice,34 the weight of Cdc42GAP-/- E14.5 fetal livers was reduced by approximately one third compared with that of WT age-matched embryos (Figure 1C). Both the total-cell number and the mononuclear-cell number of Cdc42GAP-/- fetal livers were correspondingly decreased (Figure 1D-E). Bone marrow cellularity of Cdc42GAP-/- mice was also significantly decreased (Figure 1F). These results indicate that the deletion of Cdc42GAP leads to an up-regulation of Cdc42 activity in hematopoietic cells associated with a reduction of hematopoietic cellularity in multiple anatomic sites.
Genetic deletion of Cdc42GAP and effects on Rho GTPase activities in fetal liver-derived hematopoietic cells and on hematopoietic organ cellularity. (A) Low-density fetal liver cells from WT (+/+) or homozygous (-/-) mice were immunoblotted with anti-Cdc42GAP monoclonal antibody for the detection of Cdc42GAP expression. (B) Low-density cells from E14.5 fetal livers of WT, heterozygous, or homozygous mice were subjected to effector domain pull-down assays, and the activities of Cdc42, Rac1, Rac2 (detected by GST-PAK1), and RhoA (detected by GST-Rhotekin) were examined and compared in the anti-Cdc42, Rac1, Rac2, or RhoA immunoblots. Blotting of the respective total-cell lysates was carried out in parallel. Relative amounts of GTP-bound form of the GTPases were quantified by densitometry measurements and normalized to those of the WT cells. (C-E) E14.5 embryos of WT (n = 20) or homozygous (n = 40) mice were compared for their fetal-liver weights (C), total fetal liver-cell numbers (D), and low-density fetal liver-cell numbers (E). (F) Bone marrow cell numbers from 3-day-old pups were quantified for the WT (n = 5) and homozygous (n = 5) mice.
Genetic deletion of Cdc42GAP and effects on Rho GTPase activities in fetal liver-derived hematopoietic cells and on hematopoietic organ cellularity. (A) Low-density fetal liver cells from WT (+/+) or homozygous (-/-) mice were immunoblotted with anti-Cdc42GAP monoclonal antibody for the detection of Cdc42GAP expression. (B) Low-density cells from E14.5 fetal livers of WT, heterozygous, or homozygous mice were subjected to effector domain pull-down assays, and the activities of Cdc42, Rac1, Rac2 (detected by GST-PAK1), and RhoA (detected by GST-Rhotekin) were examined and compared in the anti-Cdc42, Rac1, Rac2, or RhoA immunoblots. Blotting of the respective total-cell lysates was carried out in parallel. Relative amounts of GTP-bound form of the GTPases were quantified by densitometry measurements and normalized to those of the WT cells. (C-E) E14.5 embryos of WT (n = 20) or homozygous (n = 40) mice were compared for their fetal-liver weights (C), total fetal liver-cell numbers (D), and low-density fetal liver-cell numbers (E). (F) Bone marrow cell numbers from 3-day-old pups were quantified for the WT (n = 5) and homozygous (n = 5) mice.
Homozygous embryos were born in the predicted Mendelian ratio. However, more than 93% of Cdc42GAP-/- pups died within 1 week of birth. The surviving Cdc42GAP-/- animals were able to grow to adulthood. The homozygous mice were pale compared with WT littermates, prompting us to determine whether the blood counts were normal in these mice. Peripheral blood counts using a hematology analyzer revealed that 20-week-old Cdc42GAP-/- mice had normal counts of total leukocytes, neutrophils, and monocytes but minor yet significant decreases in platelet counts. Erythrocyte parameters, including RBC count, hemoglobin concentration, hematocrit level, mean corpuscular volume, and mean corpuscular hemoglobin level, were severely reduced compared with those of WT or heterozygous mice (Table 1). These results indicate that loss of Cdc42GAP causes anemia in mice.
Hematologic parameters of WT, heterozygous, and homozygous mice
Parameter . | Cdc42GAP+/+ . | Cdc42GAP+/– . | Cdc42GAP–/– . |
|---|---|---|---|
| White blood cell count, × 109/L | 8.0 ± 1.8 | 7.5 ± 2.8 | 9.9 ± 3.3 |
| Neutrophil count, × 109/L | 4.9 ± 5.3 | 4.5 ± 4.5 | 2.4 ± 1.8 |
| Monocyte count, × 109/L | 2.7 ± 2.5 | 3.6 ± 3.3 | 4.8 ± 2.7 |
| Red blood cell count, × 1012/L | 7.4 ± 1.3* | 6.8 ± 1.8 | 6.1 ± 1.6* |
| Hemoglobin level, g/L | 126 ± 17† | 106 ± 31‡ | 72 ± 24† |
| Hematocrit | .446 ± .06‡ | .414 ± .105 | .342 ± .087‡ |
| Mean corpuscular volume, fL | 60.9 ± 4.2* | 60.8 ± 3.5‡ | 56.6 ± 2.0* |
| Mean corpuscular hemoglobin level, pg/cell | 17.3 ± 2.0* | 16.1 ± 4.2 | 13.0 ± 5.5* |
| Platelet count, × 109/L | 586 ± 116* | 544 ± 229 | 424 ± 178* |
Parameter . | Cdc42GAP+/+ . | Cdc42GAP+/– . | Cdc42GAP–/– . |
|---|---|---|---|
| White blood cell count, × 109/L | 8.0 ± 1.8 | 7.5 ± 2.8 | 9.9 ± 3.3 |
| Neutrophil count, × 109/L | 4.9 ± 5.3 | 4.5 ± 4.5 | 2.4 ± 1.8 |
| Monocyte count, × 109/L | 2.7 ± 2.5 | 3.6 ± 3.3 | 4.8 ± 2.7 |
| Red blood cell count, × 1012/L | 7.4 ± 1.3* | 6.8 ± 1.8 | 6.1 ± 1.6* |
| Hemoglobin level, g/L | 126 ± 17† | 106 ± 31‡ | 72 ± 24† |
| Hematocrit | .446 ± .06‡ | .414 ± .105 | .342 ± .087‡ |
| Mean corpuscular volume, fL | 60.9 ± 4.2* | 60.8 ± 3.5‡ | 56.6 ± 2.0* |
| Mean corpuscular hemoglobin level, pg/cell | 17.3 ± 2.0* | 16.1 ± 4.2 | 13.0 ± 5.5* |
| Platelet count, × 109/L | 586 ± 116* | 544 ± 229 | 424 ± 178* |
Peripheral blood samples were collected from Cdc42GAP+/+ (n = 12), Cdc42GAP+/– (n = 26), and Cdc42GAP–/– (n = 9) mice when they were 20 weeks old. Blood counts were performed with a hematology analyzer. Data are mean ± SE.
P < .05
P < .001
P < .01
Cdc42GAP deletion leads to decreased hematopoietic stem/progenitor colony-forming activities
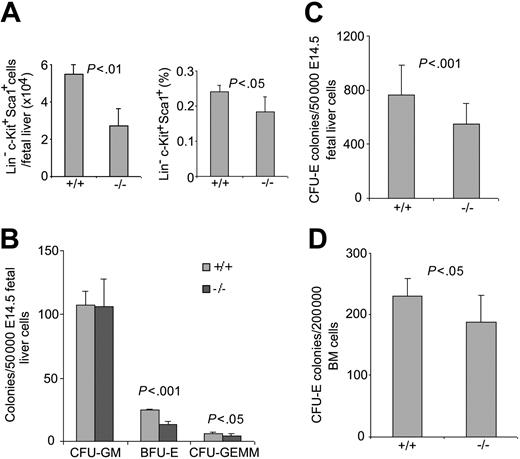
To determine whether Cdc42GAP deletion affects hematopoiesis, we next examined the cellularity and progenitor content of Cdc42GAP-/- mice. As shown in Figure 2A, both the number and the frequency of Lin-Sca1+c-Kit+ (LSK) cells of E14.5 fetal liver were significantly decreased in the Cdc42GAP-/- mice compared with those of WT mice. In vitro culturing of WT and homozygous HSPs in the presence of different cytokines in progenitor assays revealed further differences in multiple lineages. Although no difference was observed between WT and homozygous HSPs to form CFU-GM colonies, both BFU-E, a measure of primitive erythrocyte progenitor, and CFU-E, a measure of a later erythrocyte progenitor, were significantly reduced. CFU-GEMM, a multi-lineage progenitor, was also reduced (Figure 2B-C). The reduction in CFU-E was apparent in the bone marrow-derived Cdc42GAP-/- HSPs (Figure 2D). Morphologically, the progenitor colonies derived from the Cdc42GAP-/- mice were indistinguishable from those of WT mice (data not shown), suggesting that the differences in the colony-forming activities were mostly the result of defects in frequency or proliferative potential of the Cdc42GAP-/- hematopoietic stem/progenitors under the culture conditions. Combined with the observed reduction in cellularity of fetal liver and bone marrow in the Cdc42GAP-/- mice, these data suggest that hematopoiesis in general and erythropoiesis in particular are regulated by Cdc42GAP, likely by controlling the Cdc42 activity.
Effects of Cdc42GAP deletion on phenotypic HSP number and erythropoietic progenitor activities. (A) WT or homozygous E14.5 fetal liver (n = 20 for each group) cells were stained with lineage-FITC, Sca1-PE, and c-Kit-APC and were examined by flow cytometry for HSP number and percentage composition. (B-D) E14.5 fetal liver (n = 20 for each group) cells (B-C) or 3-day-old pup (n = 5 for each group)-derived bone marrow cells (D) were cultured in methylcellulose medium supplemented with 100 ng/mL SCF, 100 ng/mL IL-3, 4 U/mL EPO, and 100 ng/mL G-CSF for 7 days for the development of CFU-GM, BFU-E, or CFU-GEMM colonies (B) or with 100 ng/mL SCF and 4 U/mL EPO for 2 days for the growth of CFU-E colonies (C-D).
Effects of Cdc42GAP deletion on phenotypic HSP number and erythropoietic progenitor activities. (A) WT or homozygous E14.5 fetal liver (n = 20 for each group) cells were stained with lineage-FITC, Sca1-PE, and c-Kit-APC and were examined by flow cytometry for HSP number and percentage composition. (B-D) E14.5 fetal liver (n = 20 for each group) cells (B-C) or 3-day-old pup (n = 5 for each group)-derived bone marrow cells (D) were cultured in methylcellulose medium supplemented with 100 ng/mL SCF, 100 ng/mL IL-3, 4 U/mL EPO, and 100 ng/mL G-CSF for 7 days for the development of CFU-GM, BFU-E, or CFU-GEMM colonies (B) or with 100 ng/mL SCF and 4 U/mL EPO for 2 days for the growth of CFU-E colonies (C-D).
Effect of Cdc42GAP deletion on HSP proliferation and survival
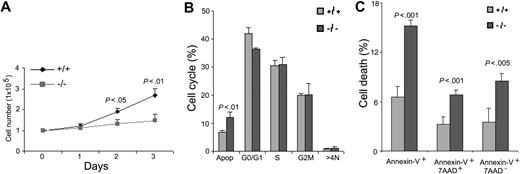
Previous studies in lower eukaryotes and in clonal fibroblast cells have shown that Cdc42 is critical for cell-growth control.25,26 However, Cdc42 activity does not appear to be required for mouse embryonic stem-cell proliferation.15 We next examined the effect of Cdc42GAP deletion on HSP (Lin-c-Kit+) proliferation, cell-cycle progression, and survival. In liquid culture supplemented with 100 ng/mL SCF, the proliferation rate of Cdc42GAP-/- HSPs was significantly lower than that of WT cells (Figure 3A). To determine whether the altered growth rate of these cells was associated with defective cell-cycle progression or survival, we performed cell cycle analysis by PI/RNase staining. Cdc42GAP-/- cells did not show significant changes in the percentage of cells in either the G1-S or the S-G2/M phase of the cell cycle (Figure 3B). On the other hand, apoptosis analysis of these cells revealed that there was a 2-fold increase in apoptotic-cell population as measured by annexin-V and 7-AAD-staining in the Cdc42GAP-/- HSPs compared with WT cells (Figure 3C), consistent with increases in the sub-G0/G1 peak in the PI/RNase-stained samples (Figure 3B). These results suggest that Cdc42GAP regulates HSP survival and apoptosis, at least in the in vitro culture system.
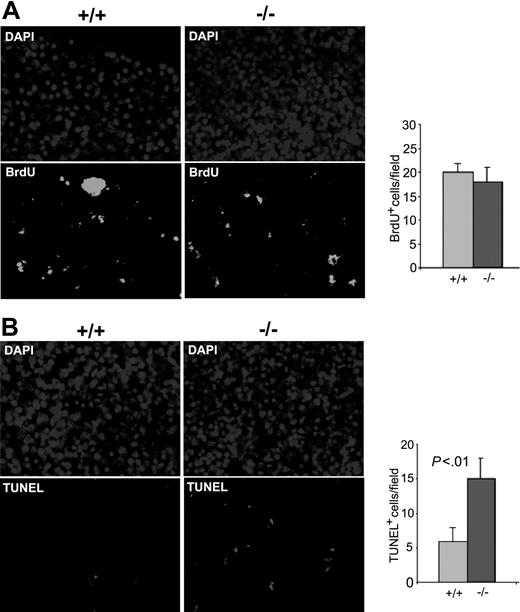
Next we investigated the effect of Cdc42GAP knockout on HSP proliferation and survival in vivo in the E14.5 fetal liver. BrdU labeling assays performed in Cdc42GAP-/- E14.5 fetal livers showed no significant change in BrdU incorporation after injection of BrdU into pregnant heterozygous females (Figure 4A). TUNEL staining of the homozygous fetal liver showed a 2-fold increase in apoptosis signals compared with that of WT (Figure 4B). The increased apoptosis in the fetal liver was seen in other hematopoietic organs, including spleen, thymus, and bone marrow of the Cdc42GAP-/- mice (data not shown). These data provide a likely explanation for the observed decrease in the cellularity in the hematopoietic organs (Figure 1C, F). Taken together, these results imply that Cdc42GAP impacts on hematopoiesis by modulating HSP numbers in various hematopoietic tissues through a survival-mediated mechanism.
Increased apoptosis of Cdc42GAP-/- HSPs is associated with elevated JNK activity
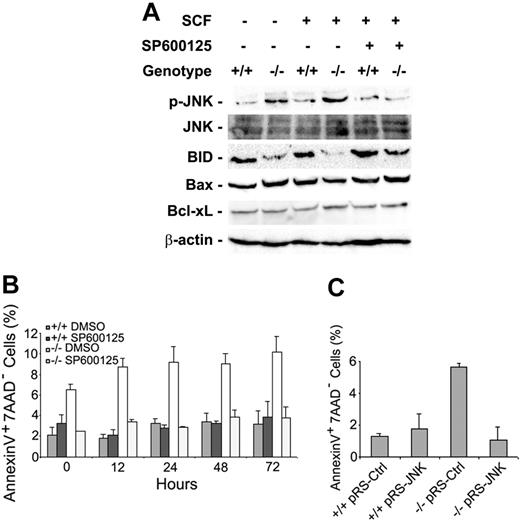
Cdc42 is known to regulate multiple MAPK signaling pathways that might have an impact on cell growth and survival. To understand the mechanism of increased apoptosis seen in the Cdc42GAP-/- HSPs (Lin-c-Kit+), we examined the MAPK pathways that might be altered by Cdc42GAP disruption. Isolated HSPs were serum starved and then stimulated for 10 minutes with 100 ng/mL SCF. Cell lysates were subjected to Western blot analysis. We observed that the Cdc42GAP-/- HSPs demonstrated significantly increased phospho-JNK and decreased full-length BID, a survival factor regulated by JNK, compared with WT cells (Figure 5A). In contrast, phospho-ERK and phospho-p38 levels of the homozygous cells were similar to those of WT cells (data not shown). Such an effect of JNK-BID up-regulation was previously shown to be involved in regulating mitochondria membrane integrity and cytochrome c release in fibroblasts.27,28 In contrast, the expression level of Bax and Bcl-xL, two known cell-survival factors, did not change in the Cdc42GAP-/- cells (Figure 5A). Treatment of the HSPs with a JNK inhibitor, SP600125, at 10 μM concentration almost completely reversed the apoptosis phenotype in the Cdc42GAP-/- cells under conditions in which WT cells were not affected, as analyzed by annexin-V and 7-AAD staining (Figure 5B). These data correlate with the down-regulated phospho-JNK level of the homozygous cells (Figure 5A). On the other hand, the JNK inhibitor treatment did not alter the cell-cycle progression profiles of either Cdc42GAP-/- or WT cells (data not shown). Moreover, transduction of the Cdc42GAP-/- cells with a recombinant retrovirus expressing an siRNA targeting JNK1/JNK2 that previously has been shown to effectively suppress JNK expression and activities,23 but not a control siRNA, led to a reversal of the apoptosis phenotype (Figure 5C). These results suggest that the increased Cdc42 activity in Cdc42GAP-/- HSPs causes JNK activation among multiple effector pathways regulated by Cdc42 that in turn induces the JNK-mediated apoptotic response. However, it remains a possibility that additional effector pathways of Cdc42, such as NF-κB or Akt, also contribute to the apoptotic effect.
Cdc42GAP regulates HSP proliferation and survival. Lin-c-Kit+ HSPs from WT (n = 8) and homozygous (n = 9) mice were isolated from the respective low-density fetal liver cells by flow cytometry. After 6-hour starvation, the cells were stimulated with 100 ng/mL SCF. (A) At the indicated time points, the cell numbers of each genotype were quantified in parallel. (B) Cell-cycle progression after 24-hour stimulation by SCF was analyzed by PI/RNase staining and FACS. (C) Apoptotic-cell populations were determined by annexin-V and 7-AAD staining and FACS analysis 24 hours after SCF stimulation.
Cdc42GAP regulates HSP proliferation and survival. Lin-c-Kit+ HSPs from WT (n = 8) and homozygous (n = 9) mice were isolated from the respective low-density fetal liver cells by flow cytometry. After 6-hour starvation, the cells were stimulated with 100 ng/mL SCF. (A) At the indicated time points, the cell numbers of each genotype were quantified in parallel. (B) Cell-cycle progression after 24-hour stimulation by SCF was analyzed by PI/RNase staining and FACS. (C) Apoptotic-cell populations were determined by annexin-V and 7-AAD staining and FACS analysis 24 hours after SCF stimulation.
Increased spontaneous apoptosis in the Cdc42GAP-deficient fetal livers. Female heterozygous mice were timed for pregnancy and intraperitoneally injected with 100 μg BrdU/g body weight at E14.5. One hour after BrdU incorporation, the mice were killed and then underwent transcardial perfusion. Fetal livers of the embryos were dissected and embedded in paraffin while genotyping was carried out in parallel. Five-micrometer sections were deparaffinized and examined by anti-BrdU (A) or TUNEL (B) immunofluorescence microscopy. Right panels show quantification of at least 6 fields of the anti-BrdU or TUNEL fluorescence section shown in the left panels. Data are representative of 3 independent measurements.
Increased spontaneous apoptosis in the Cdc42GAP-deficient fetal livers. Female heterozygous mice were timed for pregnancy and intraperitoneally injected with 100 μg BrdU/g body weight at E14.5. One hour after BrdU incorporation, the mice were killed and then underwent transcardial perfusion. Fetal livers of the embryos were dissected and embedded in paraffin while genotyping was carried out in parallel. Five-micrometer sections were deparaffinized and examined by anti-BrdU (A) or TUNEL (B) immunofluorescence microscopy. Right panels show quantification of at least 6 fields of the anti-BrdU or TUNEL fluorescence section shown in the left panels. Data are representative of 3 independent measurements.
Cdc42GAP deletion leads to JNK activation and increased apoptosis of HSPs. HSPs were cultured in the presence or absence of 10 μM JNK inhibitor SP600125 and were starved for 6 hours. Cells were stimulated with 100 ng/mL SCF for 10 minutes before they were subjected to Western blot analysis (A) or were analyzed for apoptosis by annexin-V- and 7-AAD-based FACS at different time points (B). (C) JNK siRNA-expressing retrovirus or the control retrovirus-treated HSPs were selected with puromycin (2 μg/mL) for 2 days to enrich the transduced cell population before they were subjected to apoptosis analysis.
Cdc42GAP deletion leads to JNK activation and increased apoptosis of HSPs. HSPs were cultured in the presence or absence of 10 μM JNK inhibitor SP600125 and were starved for 6 hours. Cells were stimulated with 100 ng/mL SCF for 10 minutes before they were subjected to Western blot analysis (A) or were analyzed for apoptosis by annexin-V- and 7-AAD-based FACS at different time points (B). (C) JNK siRNA-expressing retrovirus or the control retrovirus-treated HSPs were selected with puromycin (2 μg/mL) for 2 days to enrich the transduced cell population before they were subjected to apoptosis analysis.
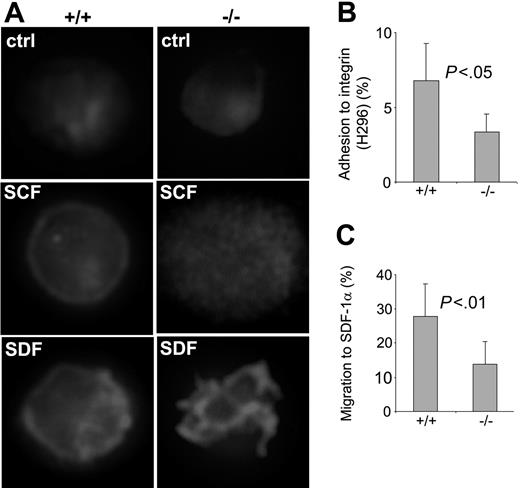
Cdc42GAP regulates HSP F-actin assembly, adhesion, and migration. (A) WT or homozygous LSK E14.5 fetal liver cells were isolated by FACS and cultured overnight in the chamber slides. Cells were stimulated with 100 ng/mL SCF or 100 ng/mL SDF-1α for 10 minutes and stained for F-actin with TRITC-conjugated phalloidin. (B-C) WT or homozygous low-density fetal liver (n = 10 for each group) cells were subjected to adhesion and migration assays in vitro. The percentages of the cells that adhered to an H-296 fragment of fibronectin after 1-hour incubation (B) and that migrated toward a 100 ng/mL SDF-1α gradient in 4 hours in a Transwell migration chamber (C) were calculated based on the colony-forming activities of the total input cells and the adherent or migrated cells assayed in a methylcellulose medium containing 100 ng/mL SCF, 100 ng/mL IL-3, 4 U/mL EPO, and 100 ng/mL G-CSF.
Cdc42GAP regulates HSP F-actin assembly, adhesion, and migration. (A) WT or homozygous LSK E14.5 fetal liver cells were isolated by FACS and cultured overnight in the chamber slides. Cells were stimulated with 100 ng/mL SCF or 100 ng/mL SDF-1α for 10 minutes and stained for F-actin with TRITC-conjugated phalloidin. (B-C) WT or homozygous low-density fetal liver (n = 10 for each group) cells were subjected to adhesion and migration assays in vitro. The percentages of the cells that adhered to an H-296 fragment of fibronectin after 1-hour incubation (B) and that migrated toward a 100 ng/mL SDF-1α gradient in 4 hours in a Transwell migration chamber (C) were calculated based on the colony-forming activities of the total input cells and the adherent or migrated cells assayed in a methylcellulose medium containing 100 ng/mL SCF, 100 ng/mL IL-3, 4 U/mL EPO, and 100 ng/mL G-CSF.
Effect of Cdc42GAP deletion on HSP F-actin assembly, adhesion, and migration
Cdc42 is critical to the regulation of actin cytoskeleton in embryonic stem cells and fibroblasts.15,29 One of the downstream effectors of Cdc42, WASP, has been shown to be required for HSP actin reorganization, adhesion, and migration.16 To determine the effect of the gain of Cdc42 activity on HSP actin assembly and actin-associated cellular processes, we next examined WT and Cdc42GAP-/- HSP actin structure, adhesion, and migration properties in vitro. In the absence of stimuli, the F-actin distribution of WT and homozygous LSK cells appeared similar, with diffused TRITC-phalloidin staining (Figure 6A). Within 10 minutes of stimulation with 100 ng/mL SCF or 100 ng/mL SDF-1α, the WT cells formed a ringlike cortical actin structure around the cell surface, as previously described.7 In contrast, the homozygous cells displayed a diffuse, dotlike distribution of F-actin under SCF stimulation or formed actin protrusions around the cell surface after SDF-1α stimulation (Figure 6A). Adhesion of the homozygous HSPs to the recombinant fibronectin fragment H-296 through integrin α4β1 also appeared to be defective, with more than a 2-fold decrease in HSPs adherent to the H-296-coated tissue culture surface after 1-hour incubation (Figure 6B). In a Transwell migration assay, the Cdc42GAP-/- HSPs displayed more than a 2-fold decrease in migration to an SDF-1α gradient (Figure 6C). These results indicate that Cdc42GAP deletion causes defective F-actin reorganization in HSPs in response to SCF or SDF-1α and results in impaired adhesion to fibronectin and directional migration.
Cdc42GAP regulates HSP engraftment
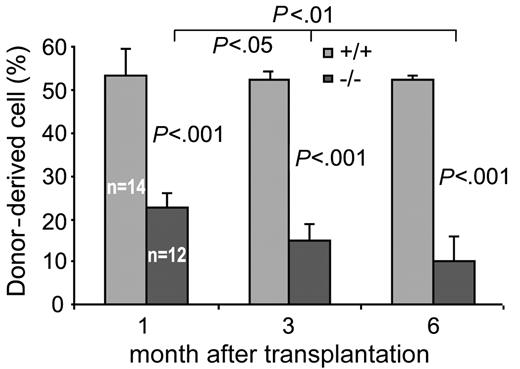
The above in vitro data suggest defects in Cdc42GAP-/- HSP survival, adhesion, and migration. The phenotypes may be predicted to have a collective impact on HSP engraftment in vivo. To investigate the engraftment activity of the Cdc42GAP-deficient HSPs, we used a mouse bone marrow/fetal liver transplantation protocol to assess the ability of the donor-derived HSPs to engraft and to contribute to active hematopoiesis in recipient mice. On transplantation to sublethally irradiated NOD/SCID or lethally irradiated B6.SJL/BoyJ mice, the Cdc42GAP-/- donor cells (CD45+H-2Kb+ or CD45.2+) showed only a modest, but statistically significant, decrease of donor chimerism at 6 weeks after transplantation in the peripheral blood and bone marrow (data not shown), suggesting that homozygous HSP cells are capable of engraftment. However, in a competitive repopulation assay in which the donor-derived fetal liver cells competed with WT HSPs at a 1:1 ratio, we observed markedly decreased chimerism of the Cdc42GAP-/- HSPs in the B6.SJL/BoyJ recipient mice in the peripheral blood and bone marrow. This defect in engraftment of the Cdc42GAP-/- HSPs was evident at 1 month and persisted at 3 months and 6 months after transplantation (Figure 7). The extent of loss of the donor-derived Cdc42GAP-/- blood and bone marrow in the competitive repopulation assay is much more pronounced than the reduction in the fetal liver HSC composition of the homozygous mice (Figure 2A). These data indicate that the engraftment ability of Cdc42GAP-/- HSPs is decreased compared with that of WT. We conclude that Cdc42GAP is important for HSP engraftment in vivo.
Discussion
The Rho GTPase family belongs to the Ras superfamily and consists of more than 20 structurally related proteins.30 Rho GTPases have been shown to be involved in regulating a variety of cellular functions, mostly in nonhematopoietic cell types, such as actin cytoskeleton reorganization, adhesion, migration, endocytosis, cell-cycle regulation, and transcriptional activation.4,5 Among the Rho family members, Rac2 is hematopoietic lineage-specific and has been shown to play roles in HSP adhesion, migration and survival.6,7 The closely related Rac1 GTPase appears to have functionally unique roles in regulating HSP cell-cycle progression and together with Rac2, controls HSP mobility and engraftment.7 Recently, another hematopoietic specific Rho GTPase, RhoH, has been found to regulate HSP proliferation, migration, and engraftment, possibly through counteracting Rac1 and Rac2 activities in the cells.31 It is anticipated that individual members of the Rho family may contribute uniquely to hematopoiesis.
Cdc42GAP regulates HSP engraftment. One million donor-derived fetal liver cells mixed with 1 million recipient-derived fetal liver cells from B6.SJL/BoyJ mice were injected into lethally irradiated B6.SJL/BoyJ mice. Chimerism in the peripheral blood of the recipients was examined at indicated times after transplantation. Numbers of each group of animals that underwent transplantation are indicated. Results are representative of 2 independent experiments.
Cdc42GAP regulates HSP engraftment. One million donor-derived fetal liver cells mixed with 1 million recipient-derived fetal liver cells from B6.SJL/BoyJ mice were injected into lethally irradiated B6.SJL/BoyJ mice. Chimerism in the peripheral blood of the recipients was examined at indicated times after transplantation. Numbers of each group of animals that underwent transplantation are indicated. Results are representative of 2 independent experiments.
Cdc42 shares approximately 70% sequence homology with Rac1 and Rac2. Much knowledge of Cdc42 function has been acquired by using dominant-negative or constitutively active mutants expressed in clonal cell lines. To date, the lack of a suitable animal model for either loss or gain of Cdc42 function has hindered a direct study of its role in mammalian hematopoiesis, despite implications from studies of Rac2 and one of the Cdc42 effectors, WASP, in knockout mice, that potential crosstalk between Rac2 and Cdc42 is involved in regulating HSP adhesion and migration and that the Cdc42-WASP pathway is critical for cell-actin assembly and proper homing of HSPs.16 To begin to dissect the function of Cdc42 in hematopoiesis, we examined a gain of Cdc42 activity mouse model generated by gene targeting a ubiquitously expressed negative regulator of Cdc42, Cdc42GAP. The Cdc42GAP-/- embryos were able to develop to term, but most homozygous pups died within 1 week of birth. The reason for the neonatal death of the mice remains unclear. Nonetheless, as we show here, Cdc42GAP deletion causes a specific increase of Cdc42 activity in low-density fetal liver and bone marrow and in other blood-cell types, allowing a direct examination of the consequences of Cdc42 activation in hematopoiesis and in HSP functions.
Using the Cdc42GAP homozygous mice, we determined the effect of elevated Cdc42 activity on various hematopoietic phenotypes. Globally, the deletion of Cdc42GAP resulted in decreased cellularity in various hematopoietic tissues, including fetal liver and bone marrow, and caused anemia in adult mice. Examination of the phenotypic LSK cells and of the colony-forming activities of the blood progenitor cells indicated that the deletion of Cdc42GAP led to reduced LSK-cell numbers and percentages in fetal liver and to significantly reduced BFU-E and CFU-E activities. The deficiencies in HSP cellularity and erythropoietic proliferation capability may provide partial explanation for the observed defective hematologic parameters, including reduced RBC counts and hemoglobin concentration, of the homozygous mice.
Previous studies by overexpression of Cdc42-activating or dominant-negative mutants in fibroblasts have shown that Cdc42 plays an essential role in cell-growth control by regulating G1-S cell-cycle transition.32,33 In embryonic stem cells, however, gene targeting of Cdc42 did not affect cell growth or replication.15 Interestingly, we found that the Cdc42 gain-of-function HSPs experienced a growth disadvantage compared with WT cells in the liquid culture and that the slower growth rate did not correlate with the apparently unaltered cell-cycle profiles. Rather, the mechanism of reduced growth potential of the Cdc42GAP-/- HSPs was associated with the increased apoptosis rate. Among the many downstream components related to apoptosis regulated by Cdc42, the activity of JNK, but not ERK or p38, was significantly elevated by Cdc42GAP deletion. One of the JNK-controlled effector molecules, BID, was correspondingly reduced as a full-length survival factor. Given the previously demonstrated role of the JNK-BID pathway in regulating mitochondria integrity and apoptosis induction,27,28 we hypothesize that the increased Cdc42-JNK-BID signaling axle in the Cdc42GAP-/- cells might be involved in the apoptosis phenotype. In support of this possibility, application of a JNK inhibitor or JNK-specific siRNA to the Cdc42GAP homozygous HSPs mostly reversed the apoptosis phenotype, whereas the protein levels of the Bcl-2 family members Bax and Bcl-xL did not change. It is, therefore, possible that the Cdc42-regulated JNK pathway plays a role in mediating apoptosis of the Cdc42GAP-/- HSPs, though we cannot exclude the potential contributions from other Cdc42 effector pathways such as NF-κB or Akt in this effect. To examine whether increased spontaneous apoptosis occurs in the hematopoietic tissues in vivo, we have carried out a set of TUNEL and BrdU incorporation assays in fetal liver and adult bone marrow. The results are consistent with the observations made in liquid culture conditions that homozygous hematopoietic cells display significantly increased spontaneous apoptosis but not cell-cycle alteration. They provide a possible mechanistic explanation for the reduction of hematopoietic cellularity in Cdc42GAP-/- mice.
Cdc42 is known for its ability to regulate actin cytoskeletal organization and the actin structure-related adhesion and migration of fibroblasts.29 To determine whether the gain of Cdc42 activity by Cdc42GAP deletion affects HSP function, we examined F-actin reorganization on cell stimulation by SCF or SDF-1α and observed marked differences between Cdc42GAP-/- and WT HSPs. Although WT cells could rearrange cortical actin to a uniformly ringlike structure, the homozygous cells displayed a dotlike, locally concentrated F-actin assembly with actin protrusions around the cell membrane, consistent with hyperactivation of Cdc42 in the cell. Although we have yet to fully understand the mechanism and molecular consequences of the actin-response defect of the Cdc42GAP-/- HSPs, including how it relates to the previously reported WASP-associated F-actin assembly defect,16 our measurements of adhesion and migration activities suggest a functional outcome of the actin organization defect: the gain of Cdc42 activity HSPs are deficient in adherence to the fibronectin H-296 fragment through α4β1 integrin binding and are reduced in ability to migrate toward an SDF-1α-directed gradient. These results indicate that well-maintained Cdc42 activity is important for F-actin assembly, adhesion, and migration of HSPs, consistent with observations made in Cdc42GAP-deleted neutrophils that show a defective F-actin response to fMLP and consequently impaired movement toward the chemoattractant gradient (M.-D.F. and D.A.W., unpublished results, July 2005).
HSP engraftment is an important aspect of hematopoiesis and is determined by multiple events that occur in a hematopoietic microenvironment, including adhesion, homing, migration, and proliferation. The adhesion, migration, and apoptosis properties of the Cdc42GAP-/- HSPs suggest that the deletion of Cdc42GAP may affect HSP engraftment. Using a competitive repopulation assay, we determined that Cdc42GAP null HSPs are indeed defective in engraftment and repopulation in the recipient mice after transplantation. These data strongly support a role of Cdc42 activity in HSP function in vivo. Further investigation into the mechanism of the specific contributions by actin-based adhesion and migration and by the Cdc42-JNK pathway-mediated cell survival to the HSP engraftment is under way so as to fully decipher the input of Cdc42GAP to hematopoiesis.
In summary, our studies of the Cdc42GAP knockout mice provide the first clue that the regulator of Cdc42, and by inference the Cdc42 activity, plays a role in hematopoiesis. Significantly, Cdc42GAP regulates hematopoietic tissue cellularity and HSP survival, actin cytoskeleton structure, adhesion, and migration. Normal Cdc42 activity is required for erythropoiesis and HSP engraftment. Given the pleiotropic nature of Cdc42 in cellular signaling, we fully expect that Cdc42 may have additional functions in HSP regulation and in hematopoiesis that are not revealed by present studies of the gain-of-activity mouse model. Future studies of Cdc42 loss-of-function mice by hematopoietic specific, inducible knockout strategies are likely to provide further insight into the complex role this signaling molecule plays in hematopoiesis.
Prepublished online as Blood First Edition Paper, September 22, 2005; DOI 10.1182/blood-2005-05-2171.
Supported in part by National Institutes of Health grants R01 GM53943 and R01 CA105117 (Y.Z.) and R01DK62757 (to D.A.W.). L.W. was supported by an Illick Fellowship (Albert J. Ryan Foundation).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal