Abstract
Efficient bone marrow (BM) homing is a prerequisite for successful engraftment of transplanted hematopoietic cells (HPCs). Contradictory conclusions about the contribution of SDF-1/CXCR4 have clouded our understanding of its role within the molecular pathway cooperation needed for BM homing, particularly with the well-defined hierarchic network of adhesion molecules. In the present study we sought to unravel cooperative and compensatory molecular pathways guiding BM homing. Fresh BM-HPCs, rendered either SDF-1 unresponsive or Gi-signaling refractory, homed quite efficiently, because of compensation by α4-integrin interacting with VCAM-1. The contribution of SDF-1/CXCR4- or Gi-protein-mediated signals to BM homing became apparent after their blockade was combined with deletion of α4-integrin, leading to dramatic reduction in BM homing. Similar conclusions were revealed when VCAM-1-deficient hosts were used. Cytokine incubation changed the functional properties of BM-HPCs and hierarchy of molecular pathway usage in homing, by shifting the dominance among the homing mediators: loss of CXCR4 or Gi-signaling now significantly reduced BM homing, with only partial compensation through α4/VCAM-1 and endothelial selectins. These studies depict a flexible hierarchy of cooperating homing pathways, in which dominant players are repositioned with changing cytokine milieu, and possibly source of HPCs.
Introduction
Bone marrow (BM) homing and engraftment are sequential steps in hematopoietic reconstitution after transplantation. The molecular mechanisms of BM homing have been under investigation for several years, and a host of contributing molecular players have been described, including integrins and endothelial selectins with their respective ligands, CD44, complement, certain lipid mediators, and intracellular signaling molecules.1-9 Recently, the chemokine SDF-1 and its receptor CXCR4 have garnered much interest because of their dominant role as a homing-defining molecule in several tissues.10,11 SDF-1 is expressed by a wide variety of cells, including hematopoietic cells (HPCs) and BM stromal cells, and is displayed as cell-bound, extracellular matrix-bound, or in soluble form. It is greatly up-regulated in many tissues, including BM, by “stress,” such as injury or irradiation. CXCR4 is expressed predominantly intracellularly in immature hematopoietic cells, where it can be rapidly surface-expressed via clathrin-coated pits, to function as SDF-1 receptor.12 All known functions of SDF-1 (antiapoptosis, proliferation, adhesion, and migration) are mediated through binding to its receptor, CXCR4, which signals through Gi-protein second messengers. Downstream signals involve IP3 and PKC,13 and, although a Gi-independent contribution of the Jak/Stat pathway to IP3 activation has been shown, in the absence of Gi-signaling Jak/Stat activation could not elicit functional SDF-1 downstream signaling events.14-16 In contrast to its well-established and generally accepted role in BM retention of HPCs and in long-term maintenance of hematopoiesis, evidence of a role of SDF-1/CXCR4/Gi-protein-dependent signaling in BM homing is unsettled because of discrepant data.17-25 The purpose of the experiments described here was to explore possible reasons for disparity in findings about the role of Gi-signals in BM homing and to further define hierarchic pathway usage in homing. Our data suggest that BM homing of immature HPCs is guided by cooperating molecules with significant functional overlap. Fresh steady-state BM-HPCs efficiently compensate for the loss of SDF-1/CXCR4 or Gi-signaling through engagement of α4-integrin by VCAM-1. However, cytokine incubation shifts the dominance among the homing molecules toward SDF-1/CXCR4/Gi-protein signals and provides at least a partial explanation for previously reported discrepant findings.
Materials and methods
Animals
B6/129 mice (Jackson Laboratories, Bar Harbor, ME), or mice deficient in α4-integrin, β2-integrin, or L-selectin (CD62L) served as BM donors.3,26 Donor BM was collected from long bones and pooled, as described.21 Adult B6/129 recipients or VCAM-1-/- or endothelial selectin-/- (CD62-/-) recipients of B6/129 background1,27 were lethally irradiated with a single dose of 1150 cGy using a cesium source (at a dose rate of > 100 cGy/min), and transplanted within 1 to 2 hours of irradiation. Donor BM cells were injected in a volume of 1 mL into the lateral tail vein. Mice were housed and bred in the specific pathogen-free facility at the University of Washington, Seattle, with water and chow ad libitum. All procedures were approved by the institutional animal care and use committee (IACUC).
Reagents
Recombinant murine stem cell factor (rmuSCF), recombinant murine stromal-cell-derived factor 1α (SDF-1) and recombinant human SCF (rhuSCF) were purchased from PeproTech (Rocky Hill, NJ). Murine TPO-conditioned medium was made as described,28 using baby hamster kidney (BHK) cells generously given to us by Dr K. Kaushansky, University of California San Diego (UCSD), San Diego, CA. Pertussis Toxin holotoxin (PTX) was from List Biological Laboratories (Campbell, CA). The small molecule CXCR4 antagonist AMD3100 was purchased from Sigma (St Louis, MO). X-Vivo20 serum-free medium is a product of BioWhittacker (Walkersville, MD). Methyl cellulose medium for mouse colony-forming cells in culture (CFU-C) was purchased from StemCell Technologies (Vancouver, BC, Canada), methyl cellulose medium for human CFU-C was from Miltenyi Biotech (Auburn, CA).
Human cells
Peripheral-blood CD34+ cells, procured by apheresis of G-CSF-mobilized (Neupogen; Amgen, Thousand Oaks, CA) healthy volunteer donors and immunomagnetic purification (CliniMacs [magnetic activated cell sorting]; Miltenyi), were received from the National Institutes of Health (NIH) repository at the Fred Hutchinson Cancer Research Center (FHCRC; Shelly Heimfeld), with approval from the FHCRC institutional review board (IRB). Transplantation of these cells into mice was approved by the University of Washington Human Subjects Division and by the University of Washington Institutional Animal Care and Use Committee.
Migration assay
BM cells were suspended in serum-free medium (for rmuSCF or muTPO-incubated cells, medium was enriched with an identical concentration of the respective cytokine) as previously described; spontaneous and SDF-1-directed migration through 5-μm transwells was quantified after 4 hours, as described.29
CFU-C assay
CFU-C assays were performed as previously described.21 After 7 days (14 days for human CFU-Cs), colonies were counted on the basis of morphologic criteria.
Homing assay
BM cells used for homing assays were processed as follows: to block Gi-signaling, adult BM cells were incubated at 37°C under standard conditions in X-Vivo20, either short term without cytokines for 4 hours, or overnight with rmuSCF (100 ng/mL) or muTPO (2% conditioned medium), with or without PTX. Previous experiments had identified 4 hours to be sufficient to block migration,21 and BM cells survived 4 hours without addition of growth factors and without loss of colony-forming activity or BM homing efficiency. To desensitize the cells to SDF-1, adult BM cells were incubated in PBS/BSA 0.5% with AMD3100 (100 μg/mL) for 15 minutes at 37°C prior to transplantation and coinjected with 100 μg/mL AMD3100/recipient, as previously described,18 except that a higher dose of AMD3100 was used by us. Frozen human CD34+ cells were thawed and incubated overnight in serum-free medium with 25 ng/mL rhuSCF, with or without PTX.
A fraction of the injected cells was plated in colony assays to quantify the number of injected CFU-Cs. Recipients were irradiated, and cells were transplanted. Animals receiving AMD3100-treated cells were killed 3 hours after transplantation, to accommodate for the short half-life of AMD3100. All other transplant recipients were killed 18 hours after transplantation. Fractions of BM were plated in methylcellulose medium in duplicate to provide a direct quantitative assessment of functional CFU-Cs that have lodged to BM. CFU-C number homed per femur was calculated from the number of CFU-Cs in the colony assays and expressed as fraction of the input CFU-C/femur. Graphs show mean + SEM for all mice that were tested for a particular condition.
Homing of Lin-c-kit+ cells
A population enriched for HSCs was isolated from murine wild-type (WT) BM by subsequent selection of c-kit+ cells, using immunomagnetic sorting (Miltenyi), followed by staining of the positive fraction with PE-conjugated antibodies (BD Pharmingen, San Diego, CA) against lineage markers CD3, CD11b, B220, Ter119, and GR-1, and flow sorting of lin- cells on the FACSAria (fluorescence activated cell sorting; BD Immunocytometry Systems, San Jose, CA), similar to what was previously described.30 Approximately 5% of c-kit+ cells were thus collected. Lin-c-kit+ cells were incubated overnight in SCF ± PTX at the usual dosage, stained with CFDA/SE (Molecular Probes, Eugene, OR) as described,31 and then injected into lethally irradiated WT recipients. Eighteen hours after transplantation, bone marrow was harvested, red cells were briefly lysed in hypotonic buffer, and BM suspensions were analyzed by flow cytometry (FACSCalibur; BD).
Short-term engraftment
Lethally irradiated B6/129 mice received injections containing 1.5 × 106 total BM cells, incubated as described in “Homing assay,” and input CFU-Cs were quantified. Recipients were killed 8 days after transplantation, and CFU-C content in BM was assessed. Two additional values were calculated based on these figures: calculated number of initially homed CFU-Cs/femur = (number of injected CFU-Cs) × (percentage of homed CFU-Cs) as assessed by 18-hour homing assay. From these numbers, fold expansion = (number of recovered CFU-Cs/femur after 8 days) / (calculated number of initially homed CFU-Cs/femur).
Flow cytometry
Flow cytometry was performed according to standard protocols, using directly conjugated specific or isotype control antibodies (BD). Samples were acquired on the FACSCalibur (BD) and analyzed with CellQuest software (BD Immunocytometry Systems) for OS10.
Statistical analysis
Descriptive statistics and t tests were calculated using Excel (Microsoft, Redmond, WA). A P value less than .05 was considered statistically significant.
Results
Sequential use of α4-integrin and Gi-protein signals in BM homing
The aim of our initial studies was to compare the role of SDF-1/CXCR4 signaling or Gi-signaling for in vitro migration with that for in vivo homing. To render fresh steady-state (ss)BM-HPCs refractory to SDF-1, cells were exposed to the CXCR4 antagonist AMD3100. To inhibit Gi-protein signals in fresh BM-HPCs, cells were incubated short term with PTX (4 hours without serum or cytokines). Similarly incubated samples without the antagonists served as controls. In keeping with previously reported data,21,32 treatment with AMD3100 or PTX completely blocked chemotactic migration of c-kit+ cells in vitro (Figure 1A). The small spontaneous migration of fresh BM-c-kit+ cells was not PTX sensitive (Figure 1A). To test the contribution of SDF-1 or Gi-protein-mediated signaling in BM homing, fresh BM cells were incubated briefly with AMD3100 or for 4 hours with PTX, prior to injection into lethally irradiated hosts, and BM homing was quantified by enumerating CFU-Cs in the transplanted cell suspension and in the recipient BM. We found that BM homing of AMD3100- or PTX-treated fresh BM-CFU-Cs was no different from concurrently used controls not treated with inhibitors (Figure 1B,D). Thus, HPCs unable to migrate toward SDF-1 in vitro were nevertheless able to home efficiently to BM, consistent with previous reports.18,20,22,33
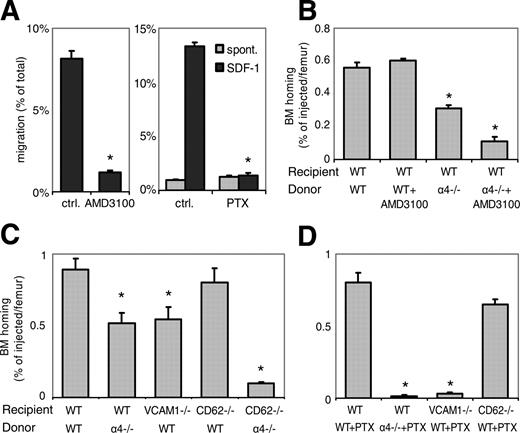
Dominant role of α4-integrin/VCAM-1 interaction in BM homing of fresh BM-HPCs. (A) AMD3100 or PTX block SDF-1-induced chemotaxis. Chemokinetic (▦) or chemotactic (SDF-1, ▪) migration of fresh c-kit+ WT BM-HPCs through 5-μm transwells was tested in untreated control BM cells, in the presence of AMD3100 (100 μg/mL) or after PTX treatment (100 ng/mL, 4 hours). AMD3100 (left) significantly attenuated SDF-1-directed migration. PTX incubation (right) completely blocked SDF-1-directed migration, but low-level spontaneous migration was maintained in the presence of PTX (*P < .05). (B-D) BM homing of fresh BM-HPCs was tested 3 hours (B) or 18 hours (C,D) after transplantation of lethally irradiated recipients. Donor-cell strain/treatment and recipient strain are indicated below the respective bars. BM homing is given as the percentage of injected CFU-Cs recovered per femur. Depicted are mean plus SEM of all mice tested with this modality. (B) Three-hour BM homing of fresh BM cells treated with or without AMD3100. BM homing of fresh WT BM cells was not affected by AMD3100 incubation/coinjection. BM homing of α4-/- BM cells in WT recipients was significantly decreased compared with WT-to-WT (*P < .05). AMD3100 treatment of α4-/- BM cells additionally reduced BM homing (*P < .05 compared with untreated α4-/--to-WT transplantation). (C) Eighteen-hour BM homing of fresh BM cells. BM homing of α4-/- cells in WT recipients, or WT cells in VCAM-1-/- recipients was significantly reduced compared with WT-to-WT (*P < .05). BM homing of WT BM in CD62-/- recipients, which are deficient in endothelial selectins, was no different from WT-to-WT. BM homing of α4-/- BM-HPCs in CD62-/- recipients was significantly less efficient than that of α4-/- in WT (*P < .05). (D) Eighteen-hour BM homing of fresh PTX-treated BM cells. BM homing of PTX-treated WT cells in WT recipients was no different from untreated WT-to-WT, but BM homing of PTX-treated α4-/- cells in WT recipients, or BM homing of PTX-treated WT cells in VCAM-1-/- hosts were almost completely abrogated (*P < .05 compared with untreated α4-/--to-WT or WT-to-VCAM-1 transplantation). In contrast, PTX-treated WT cells homed normally in CD62-/- hosts.
Dominant role of α4-integrin/VCAM-1 interaction in BM homing of fresh BM-HPCs. (A) AMD3100 or PTX block SDF-1-induced chemotaxis. Chemokinetic (▦) or chemotactic (SDF-1, ▪) migration of fresh c-kit+ WT BM-HPCs through 5-μm transwells was tested in untreated control BM cells, in the presence of AMD3100 (100 μg/mL) or after PTX treatment (100 ng/mL, 4 hours). AMD3100 (left) significantly attenuated SDF-1-directed migration. PTX incubation (right) completely blocked SDF-1-directed migration, but low-level spontaneous migration was maintained in the presence of PTX (*P < .05). (B-D) BM homing of fresh BM-HPCs was tested 3 hours (B) or 18 hours (C,D) after transplantation of lethally irradiated recipients. Donor-cell strain/treatment and recipient strain are indicated below the respective bars. BM homing is given as the percentage of injected CFU-Cs recovered per femur. Depicted are mean plus SEM of all mice tested with this modality. (B) Three-hour BM homing of fresh BM cells treated with or without AMD3100. BM homing of fresh WT BM cells was not affected by AMD3100 incubation/coinjection. BM homing of α4-/- BM cells in WT recipients was significantly decreased compared with WT-to-WT (*P < .05). AMD3100 treatment of α4-/- BM cells additionally reduced BM homing (*P < .05 compared with untreated α4-/--to-WT transplantation). (C) Eighteen-hour BM homing of fresh BM cells. BM homing of α4-/- cells in WT recipients, or WT cells in VCAM-1-/- recipients was significantly reduced compared with WT-to-WT (*P < .05). BM homing of WT BM in CD62-/- recipients, which are deficient in endothelial selectins, was no different from WT-to-WT. BM homing of α4-/- BM-HPCs in CD62-/- recipients was significantly less efficient than that of α4-/- in WT (*P < .05). (D) Eighteen-hour BM homing of fresh PTX-treated BM cells. BM homing of PTX-treated WT cells in WT recipients was no different from untreated WT-to-WT, but BM homing of PTX-treated α4-/- cells in WT recipients, or BM homing of PTX-treated WT cells in VCAM-1-/- hosts were almost completely abrogated (*P < .05 compared with untreated α4-/--to-WT or WT-to-VCAM-1 transplantation). In contrast, PTX-treated WT cells homed normally in CD62-/- hosts.
Cooperation of Gi-signals with integrins was previously described.34-38 Therefore, the consequences of combining blockade of the SDF-1/CXCR4/Gi-protein pathway and of α4β1-integrin/VCAM-1 were next tested. As was previously shown, VCAM-1 is the relevant receptor for α4β1-integrin. Accordingly, deficiencies in α4-integrin or VCAM-1 resulted in very similar alterations of BM homing, in that fresh donor cells deficient in α4-integrin (α4-/-) or hosts lacking VCAM-1 (VCAM-1-/-) provided approximately 40% reduced BM homing compared with WT-to-WT transplantation.3,27,39 These data were confirmed here (Figure 1C). To test potential cooperation of α4/VCAM-1 pathway with Gi-signals or SDF-1/CXCR4 in BM homing, fresh BM cells from α4-/- donors were rendered Gi-signaling incompetent by PTX, or SDF-1 refractory by AMD3100 incubation. Both treatments significantly reduced BM homing compared with α4 deficiency alone, to less than 5% (PTX; Figure 1D) or 20% (AMD3100; Figure 1B) of appropriate WT-to-WT controls. The same was true for fresh PTX-treated WT BM-HPCs administered to VCAM-1-/- recipients, which was less than 5% of WT-to-WT control (Figure 1D). These data thus uncover contributions of Gi-protein-mediated signaling in BM homing, not evident when WT cells were tested. Consequently, under steady-state conditions the contribution of SDF-1/CXCR4/Gi-protein signaling is completely masked or compensated by functional α4β1-integrin/VCAM-1 binding.
To see whether cooperation with Gi-protein signals is extended to endothelial selectins, BM homing was similarly studied in recipients with a genetic deficiency of endothelial selectins (E- and P-selectin). BM homing of fresh untreated WT BM-HPCs in endothelial selectin-null recipients (CD62-/-) was normal (Figure 1C), in keeping with earlier published work.1,20,22 Homing of fresh PTX-treated WT BM cells in CD62-/- recipients was also normal (Figure 1D), that is, no different from WT + PTX-to-WT (Figure 1D) or from WT-to-CD62-/- controls (Figure 1C). Thus, Gi-protein signals did not cooperate with endothelial selectins in BM homing of fresh steady-state BM-HPCs. In contrast, cooperation of α4-integrin and endothelial selectins is suggested by the fact that BM homing of fresh α4-/- cells in CD62-/- recipients was almost 90% reduced (Figure 1C).
In summary, efficient BM homing is apparently possible for cells incapable of SDF-1-mediated chemotaxis in vitro, possibly by virtue of SDF-1/CXCR4/Gi-independent migratory signals.40 Furthermore, in fresh WT BM cells the significant contributions of Gi-signals/the SDF-1/CXCR4 pathway and of endothelial selectins to BM homing were fully masked, or compensated, by the dominant presence of functional α4-integrin/VCAM-1.
Cytokine incubation shifts the hierarchy of pathway usage in BM homing
Effects of HPC incubation with SCF on in vitro functional behavior and on surface expression of adhesion molecules and receptors have been intensively studied, albeit mostly with human cells.41 The analysis of the consequences of these on in vitro engraftment have yielded variable results, likely the result of differences in dosage, duration of exposure, and cytokine combinations.42,43
We first studied the effect of short-term (overnight) SCF treatment on in vitro migration and expression of CXCR4, endothelial selectin ligands, and α4-integrin. As seen in Figure 2A, SCF incubation significantly increased migration, both spontaneous and chemotactic. Chemotaxis in fresh and in incubated BM-HPCs and the increase in chemokinesis of incubated over fresh BM-HPCs were entirely PTX sensitive. In contrast, the spontaneous migration of fresh BM-HPCs and the residual migration of PTX-treated cytokine-incubated BM-HPCs were not affected by PTX, indicating the capacity of BM-HPCs for a modest degree of Gi-signaling-independent migration (Figure 2A).
To test whether this effect on migration was specific to SCF, migration of cells incubated overnight in TPO, serum, or IL-3 was also tested. An at least similar increase in migration was seen in c-kit+ cells incubated under any of these conditions (data not shown), suggesting lack of specificity of this effect. Flow cytometric analysis revealed up-regulated CXCR4 on SCF-incubated c-kit+ BM-HPCs as a potential mechanism for the increase in chemotactic migration (data not shown), in keeping with earlier published data from human CD34+ cells.17 Differences in expression of E-selectin ligands, as detected by CLA (HECA452) antibody, or of α4-integrin were negligible after overnight cytokine incubation (data not shown).
We next sought to study whether the functional changes noted in our in vitro studies were of consequence for BM homing. WT-to-WT transplantation of overnight SCF-incubated BM-HPCs was first tested, after 3 hours and after 18 hours. Overall BM homing of overnight SCF-incubated cells was not statistically different from that of fresh cells (Figure 2B-C). Overnight incubation with SCF + PTX reduced BM homing by 75% (Figure 2D) compared with similarly incubated WT BM-HPCs without PTX, in keeping with our earlier data.21 Overnight SCF-incubated BM-HPCs were also treated short term with or without AMD3100, and BM homing was quantified after 3 hours. Similarly to PTX, AMD3100 significantly reduced BM homing of cytokine-incubated cells (Figure 2B). To address potential effects of different length of PTX incubation (4 hours for fresh, overnight for SCF-incubated BM-HPCs) in additional experiments, BM-HPCs were incubated with SCF overnight, and PTX was added for the last 4 hours of the incubation. PTX treatment of SCF-incubated BM-HPCs for the final 4 hours of the incubation reduced BM homing by almost 75% (data not shown), similarly to cells treated with PTX throughout, alleviating these concerns.
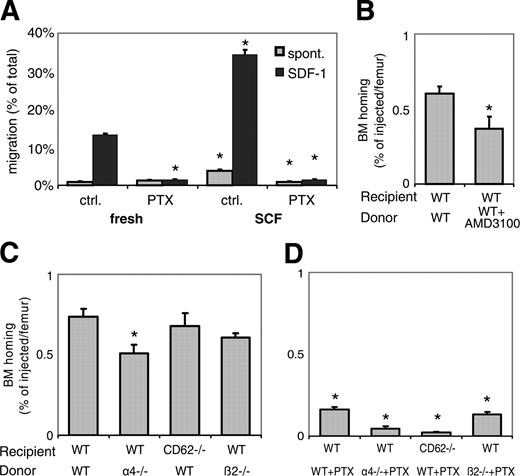
Dominant role of CXCR4/SDF-1 and Gi-signals in BM homing of ex vivo cytokine-incubated BM-HPCs. (A) SCF incubation increases cell motility. Spontaneous migration (▦) and SDF-1-directed chemotactic migration (▪) of fresh or overnight SCF-incubated cells, with or without PTX, was quantified after 4 hours. Depicted are mean plus SEM. Spontaneous and SDF-1-directed migration were both significantly increased by SCF incubation. This increase in migration was completely blocked by PTX, to levels of spontaneous migration of fresh BM, which apparently represents Gi-protein-independent migration (*P < .05). (B-D) BM homing of overnight SCF-incubated BM-HPCs. BM homing of SCF-incubated BM-HPCs was tested 3 hours (B) or 18 hours (C,D) after transplantation of lethally irradiated recipients. Donor-cell source/treatment and recipient strain are indicated below the respective bars. BM homing is given as percentage of injected CFU-Cs recovered per femur. Depicted are mean plus SEM of all mice tested with this donor/host constellation. (B) Three-hour BM homing of SCF-incubated cells treated with or without AMD3100. BM homing of fresh WT BM cells was significantly reduced by AMD3100 incubation/coinjection (*P < .05). (C) Eighteen-hour BM homing of SCF-incubated BM cells. BM homing of α4-/- cells in WT recipients was significantly reduced compared with WT-to-WT (*P < .05). BM homing of WT cells in CD62-/- recipients or of β2-/- cells in WT recipients were no different from WT-to-WT. (D) Eighteen-hour BM homing of SCF plus PTX-treated BM cells. BM homing of SCF plus PTX-treated WT cells in WT recipients was 75% reduced compared with SCF-treated WT-to-WT without PTX (P < .05). BM homing of SCF plus PTX-treated α4-/- cells in WT recipients or BM homing of SCF plus PTX-treated WT cells in VCAM-1-/- hosts was almost completely abrogated, significantly more strongly than SCF plus PTX-treated WT-to-WT (*P < .05). The inhibition of homing of SCF plus PTX-incubated β2-/- cells in WT recipients was similar to that of SCF plus PTX-treated WT donor cells.
Dominant role of CXCR4/SDF-1 and Gi-signals in BM homing of ex vivo cytokine-incubated BM-HPCs. (A) SCF incubation increases cell motility. Spontaneous migration (▦) and SDF-1-directed chemotactic migration (▪) of fresh or overnight SCF-incubated cells, with or without PTX, was quantified after 4 hours. Depicted are mean plus SEM. Spontaneous and SDF-1-directed migration were both significantly increased by SCF incubation. This increase in migration was completely blocked by PTX, to levels of spontaneous migration of fresh BM, which apparently represents Gi-protein-independent migration (*P < .05). (B-D) BM homing of overnight SCF-incubated BM-HPCs. BM homing of SCF-incubated BM-HPCs was tested 3 hours (B) or 18 hours (C,D) after transplantation of lethally irradiated recipients. Donor-cell source/treatment and recipient strain are indicated below the respective bars. BM homing is given as percentage of injected CFU-Cs recovered per femur. Depicted are mean plus SEM of all mice tested with this donor/host constellation. (B) Three-hour BM homing of SCF-incubated cells treated with or without AMD3100. BM homing of fresh WT BM cells was significantly reduced by AMD3100 incubation/coinjection (*P < .05). (C) Eighteen-hour BM homing of SCF-incubated BM cells. BM homing of α4-/- cells in WT recipients was significantly reduced compared with WT-to-WT (*P < .05). BM homing of WT cells in CD62-/- recipients or of β2-/- cells in WT recipients were no different from WT-to-WT. (D) Eighteen-hour BM homing of SCF plus PTX-treated BM cells. BM homing of SCF plus PTX-treated WT cells in WT recipients was 75% reduced compared with SCF-treated WT-to-WT without PTX (P < .05). BM homing of SCF plus PTX-treated α4-/- cells in WT recipients or BM homing of SCF plus PTX-treated WT cells in VCAM-1-/- hosts was almost completely abrogated, significantly more strongly than SCF plus PTX-treated WT-to-WT (*P < .05). The inhibition of homing of SCF plus PTX-incubated β2-/- cells in WT recipients was similar to that of SCF plus PTX-treated WT donor cells.
To test whether the observed dependence on Gi-protein signals for BM homing of SCF-incubated BM-HPCs was specific to SCF, or rather a more general characteristic of cytokine-incubation, BM-HPCs were incubated with TPO instead of SCF, in the presence or absence of PTX. Just like SCF incubation, overnight TPO + PTX incubation led to 75% reduced BM homing compared with incubation with TPO alone (Figure 3A).
The cooperation of Gi-protein signals with α4-integrin remained demonstrable in SCF-incubated cells, because BM homing of SCF + PTX-treated α4-/- HPCs was almost completely inhibited to less than 10% of WT-to-WT (Figure 2D). BM homing was significantly poorer than that of SCF + PTX-incubated WT HPCs (Figure 2D) or SCF-incubated α4-/- HPCs in WT hosts (Figure 2C). Potential interactions between Gi-proteins and endothelial selectins in SCF-incubated donor cells were next investigated. BM homing of SCF-incubated BM-HPCs in selectin-deficient hosts (CD62-/-) was normal (Figure 2C), but BM homing of SCF + PTX-incubated WT BM-HPCs in CD62-/- hosts was significantly more strongly impaired (> 95%) than that of similarly treated cells in WT recipients (Figure 2D), in contrast to fresh BM cells (Figure 1D). These data demonstrate increased contributions of Gi-protein signals and endothelial selectin ligands and decreased contributions of α4/VCAM-1 to BM homing of cytokine-incubated cells compared with fresh BM-HPCs.
Additional studies addressed the role of β2-integrin in this network of homing molecules, because cooperation with α4-integrin had previously been demonstrated, but cooperation with Gi-signaling pathways was never tested.1 In keeping with previous studies,1 β2-/- HPCs homed as efficiently as WT HPCs (Figure 2C). PTX-treatment reduced homing of β2-/- cells no more strongly than WT cells, that is, to about 25% of normal (Figure 2D). Cooperation of endothelial selectins with β2 integrins was additionally addressed, but BM homing of β2-/- cells in CD62-/- hosts, which are deficient in endothelial selectins, was entirely normal (data not shown). Thus, β2-integrins neither play a dominant role in BM homing nor do they cooperate with Gi-protein or endothelial selectin pathways. In addition, the potential role of L-selectin in BM homing was tested in the context of Gi-signaling inhibition. As was earlier shown, homing of SCF-incubated L-selectin-/- cells (BM cells from CD62-/- donors) in WT recipients was no different from similarly treated WT cells, and BM homing of SCF + PTX-incubated L-selectin-/- cells was also no different from that of SCF + PTX-incubated WT cells (data not shown); that is, there was no indication of a cooperation of L-selectin with Gi-signaling-mediated homing pathways.
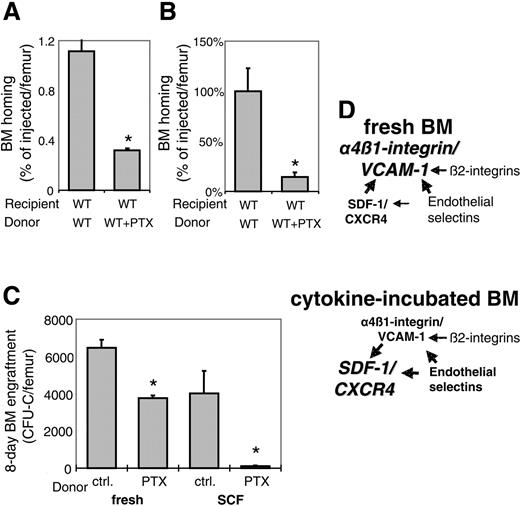
Gi-protein signals in BM homing and short-term engraftment. (A) PTX-sensitive signals guide BM homing of TPO-incubated murine CFU-Cs. Murine WT BM cells, incubated overnight in TPO ± PTX, was transplanted and analyzed as described above. As with SCF plus PTX-treated BM cells, 18-hour homing of TPO plus PTX-incubated CFU-Cs was significantly decreased compared with TPO alone (*P < .05). BM homing is given as percentage of injected CFU-Cs recovered per femur (mean plus SEM). (B) Effect of PTX on lin-c-kit+ BM-cell homing. BM homing of SCF ± PTX-incubated lin-c-kit+ cells, a population enriched in HSCs, was tested. The efficiency of 18-hour BM homing was significantly reduced by PTX (*P < .05), similar to identically treated CFU-Cs. The mean of cells recovered from BM of recipients of untreated control cells was considered 100%; BM homing is given as percentage thereof (percentage of mean of control plus SEM). (C) Equal numbers of BM cells, which contained similar numbers of CFU-Cs, either incubated with cytokine-free ± PTX for 4 hours (left) or with SCF ± PTX overnight (right), were injected into lethally irradiated recipients. Mean plus SEM of recovered CFU-C/femur 8 days after transplantation of fresh (left) or SCF-incubated (right) BM cells are depicted. Short-term engraftment of PTX-treated samples was significantly poorer than that of PTX untreated samples (*P < .05), in excess of the effect of PTX on BM homing under the respective conditions. (D) Schematic representation of the cooperative model of homing molecules between α4β1-integrin/VCAM-1, SDF-1/CXCR4/Gi-proteins, endothelial selectins, and β2-integrins, suggested by the data presented in this study. Cytokine incubation shifts the dominance among the molecular homing pathways from α4β1/VCAM-1 (top) toward SDF-1/CXCR4 (bottom).
Gi-protein signals in BM homing and short-term engraftment. (A) PTX-sensitive signals guide BM homing of TPO-incubated murine CFU-Cs. Murine WT BM cells, incubated overnight in TPO ± PTX, was transplanted and analyzed as described above. As with SCF plus PTX-treated BM cells, 18-hour homing of TPO plus PTX-incubated CFU-Cs was significantly decreased compared with TPO alone (*P < .05). BM homing is given as percentage of injected CFU-Cs recovered per femur (mean plus SEM). (B) Effect of PTX on lin-c-kit+ BM-cell homing. BM homing of SCF ± PTX-incubated lin-c-kit+ cells, a population enriched in HSCs, was tested. The efficiency of 18-hour BM homing was significantly reduced by PTX (*P < .05), similar to identically treated CFU-Cs. The mean of cells recovered from BM of recipients of untreated control cells was considered 100%; BM homing is given as percentage thereof (percentage of mean of control plus SEM). (C) Equal numbers of BM cells, which contained similar numbers of CFU-Cs, either incubated with cytokine-free ± PTX for 4 hours (left) or with SCF ± PTX overnight (right), were injected into lethally irradiated recipients. Mean plus SEM of recovered CFU-C/femur 8 days after transplantation of fresh (left) or SCF-incubated (right) BM cells are depicted. Short-term engraftment of PTX-treated samples was significantly poorer than that of PTX untreated samples (*P < .05), in excess of the effect of PTX on BM homing under the respective conditions. (D) Schematic representation of the cooperative model of homing molecules between α4β1-integrin/VCAM-1, SDF-1/CXCR4/Gi-proteins, endothelial selectins, and β2-integrins, suggested by the data presented in this study. Cytokine incubation shifts the dominance among the molecular homing pathways from α4β1/VCAM-1 (top) toward SDF-1/CXCR4 (bottom).
In an additional experiment, we tested whether PTX impaired homing of lin-c-kit+ cells, a population enriched for HSCs.30 These cells were incubated overnight in SCF ± PTX as described, labeled with CFDA/SE,31 and then injected into lethally irradiated recipients. Accumulation of the cells in recipient BM was enumerated by flow cytometry 18 hours after transplantation. BM homing of PTX-treated lin-c-kit+ cells was similarly reduced as BM homing of CFU-Cs, suggesting similar pathway requirements for BM homing of lin-c-kit+ cells and CFU-Cs (Figure 3B).
To test whether human CFU-Cs had similar requirements for Gi-protein signals in BM homing, previously frozen human CD34+ peripheral-blood cells from G-CSF-mobilized donors were incubated overnight in serum-free medium with rhuSCF ± PTX prior to transplantation into lethally irradiated nonobese diabetic/severe combined immunodeficient (NOD/SCID) β2 microglobulin-/- recipients. These conditions, rather than short-term cytokine-free incubation, were chosen because this was the single modality where effects of Gi-signaling blockade alone resulted in marked decrease of BM homing of murine BM-HPCs. BM homing was quantified 18 hours after transplantation. Of the not PTX-incubated CFU-Cs, 1.66% ± 0.19% of the injected cells had homed to 1 femur, whereas of the PTX-treated cells only 0.38% ± 0.06% had homed. This was equivalent to a greater than 75% inhibition. Thus, after cytokine-incubation, the same potent contribution of Gi-signaling to BM homing was seen for human cells as for mouse cells.
Short-term engraftment of PTX cells is inferior to controls
Lethally irradiated recipients receiving transplants of fresh or SCF-incubated murine BM-HPCs, treated with or without PTX, were assessed for short-term engraftment. Each mouse received 1.5 × 106 BM cells, which contained very similar numbers of CFU-Cs (5700 ± 162 fresh, 6066 ± 97 fresh + PTX, 5940 ± 126 SCF, 5880 ± 119 SCF + PTX CFU-C/recipient), thus ruling out nonspecific toxicity of PTX, or loss of colony-forming activity after overnight incubation. After 8 days, recipients were killed, and BM CFU-Cs were quantified. Despite quantitatively normal BM homing of fresh PTX-treated WT BM-HPCs, the CFU-C content of femurs 8 days after transplantation was about 40% reduced compared with non-PTX-treated controls (Figure 3C, left). Eightday BM engraftment of SCF + PTX-treated WT BM-HPCs was reduced by 97% compared with relevant controls (Figure 3C, right). From 18-hour homing data, we calculated that on average 51 (fresh), 49 (fresh + PTX), 44 (SCF), and 9 (SCF + PTX) of the initially injected CFU-Cs had homed to each femur. If the number of recovered CFU-C/femur after 8 days is divided by the calculated number of homed CFU-Cs, an approximate index of progenitor expansion from surviving preprogenitors can be assessed. The average fold expansion was 127 and 77 (fresh) and 92 and 12 (SCF) for control and PTX-treated transplants, respectively. Thus, after this correction for differential BM homing, progenitor expansion of the PTX-incubated BM-HPCs was only 60% for fresh and less than 15% of SCF-incubated controls; that is, in both cases the PTX-induced engraftment deficit exceeded the homing deficit, indicating roles of Gi-protein signals in engraftment of both fresh and SCF-incubated BM-HPCs.
Discussion
SDF-1/CXCR4/Gi-protein signaling and BM homing
In vitro, the inhibitory effects of the Gi-protein inhibitor PTX or of the CXCR4 antagonist AMD3100 on migration of immature HPCs are potent and highly reproducible.21,32 However, these in vitro observations, contrary to expectation, were not consistently found to predict their in vivo BM homing behavior. The notion of SDF-1/CXCR4 pathway contribution to BM homing has gained significant momentum since the initial publication showing that incubation of human cord-blood CD34+ cells with mouse anti-human CXCR4 antibodies virtually completely abrogated BM homing in NOD/SCID mice.20 The investigators later reported similarly derived data about primary human acute myelocytic leukemia (AML) cells, which, however, were not reproduced.24,25 In several subsequent studies, direct testing of the effects of Gi-protein signaling using the Gi-inhibitor PTX yielded equally controversial results.20-22 Inhibition of an intracellular signaling molecule downstream of Gi-proteins, protein kinase C ζ, did not affect BM homing.23 Likewise, BM homing of murine HPCs made refractory to SDF-1 by incubation and coinjection with a CXCR4 receptor antagonist was normal or only mildly reduced.18 A recent study demonstrated inhibition of BM homing of leukemic but not of normal lymphocytes by PTX, AMD3100, and SDF-1 pretreatment.33 Additional indirect data also strongly advocate that BM homing of fresh HPCs is not dominated by SDF-1/CXCR4-dependent events: CXCR4-/- fetal liver cells engrafted lethally irradiated WT recipients, even though the efficiency of BM homing was not directly assessed in these studies.44-46 Similarly, HPCs in which CXCR4 had been knocked down by means of SDF-1 intrakine engrafted successfully; again, quantitative analysis of BM homing was not undertaken.47 Finally, human or nonhuman primate CXCR4 cells engrafted NOD/SCID mice with similar efficiency as CXCR4+ cells.48,49 In the latter case it was proposed, but not directly tested, that the intracellular stores of CXCR4 could efficiently and rapidly up-regulate CXCR4 surface expression to guide BM homing of these cells.50 Disparate data about the role of SDF-1 pretreatment on homing or engraftment have also been reported.19,20,51 These controversial outcomes have questioned the dependence of initial events of BM homing of immature cells like CFU-C on SDF-1/CXCR4 signaling. In contrast to BM homing, the essential requirement of SDF-1/CXCR4 for long-term maintenance of hematopoiesis is undisputed.
The studies presented in this manuscript were initiated to further explore the specific requirements of SDF-1/CXCR4/Gi-protein signaling and to attempt to reconcile divergence in data outcomes. In keeping with previous data,20,22 homing of fresh, steady-state BM-HPCs unable to signal through the Gi-protein pathway was quantitatively normal.
A functional crosstalk between α4-integrin and SDF-1 has repeatedly been pointed out. SDF-1, via Gi-protein-dependent signaling pathways, can increase adhesion through α4-integrin,34-38 and α4-integrin/VCAM-1 engagement facilitates SDF-1-induced in vitro migration.52 The dominant role of α4-integrin/VCAM-1 among the adhesion molecules guiding BM homing and engraftment has been reported previously,1 but cooperation between the SDF-/CXCR4/Gi-protein and α4/VCAM-1 pathways had not previously been studied in vivo. As shown, when SDF-1-refractory or Gi-signaling-deficient α4-integrin-/- cells were transplanted into WT hosts, or when Gi-signaling-incompetent WT cells were transplanted into VCAM-1-/- recipients, BM homing was virtually completely abrogated. Thus, α4-integrin/VCAM-1 can compensate for the loss of SDF-1/CXCR4 signaling, establishing α4/VCAM-1 as the most upstream ligand/receptor pair for BM homing of fresh steady-state BM cells. One striking conclusion from these or previous data is that efficient BM homing is possible in cells largely unable to migrate in vitro. Is in vivo migration then entirely redundant for BM homing? More likely, the residual cell motility, activated through ligands other than SDF-1 and mediated through non-Gi second messengers is sufficient to guide immature BM cells to BM.40
Homing of cytokine-incubated BM-HPCs
As previously demonstrated, SCF and SDF-1 activate overlapping intracellular signaling pathways, resulting in functional synergism on chemokinesis.53 Our data suggest that this functional synergism may not be exclusive to SCF, because similar data were observed in our studies using TPO instead of SCF. Cytokine-mediated increase in cell migration and CXCR4 expression of murine BM-HPCs are in keeping with earlier studies of human HPCs.17,54 This increase in cell motility was entirely Gi-signal dependent.
BM homing of overnight cytokine-incubated cells was not quantitatively different from that of fresh BM cells. When, however, cytokine-incubated cells were treated with PTX and transplanted, their BM homing was greater than 75% reduced. From our studies, using α4-/- cells, we concluded that in fresh BM-HPCs a functional α4-integrin/VCAM-1 pathway can completely compensate for the loss of CXCR4/SDF-1/Gi-protein signaling. However, this compensation fails in cytokine-incubated BM-HPCs, suggesting a shift from dominance of α4/VCAM-1 to dominance of the Gi-protein pathway.
One of the functional modifications of cytokine-incubated cells is increased motility or enhanced sensitivity to SDF-1. Whether this enhancement of motility brings the SDF-1/CXCR4 pathway into prominence is unclear. By the same vein, whether cells with inherent increase in motility, that is, fetal-liver cells or cord-blood HPCs,55,56 follow the same hierarchy in pathway usage as cytokine-incubated cells remains to be seen.
No significant cooperation between endothelial selectins and Gi-protein signals in BM homing of fresh BM-HPCs was observed in this study. In contrast, BM homing of SCF + PTX-treated cells given to selectin-deficient recipients was inhibited in great excess of the effect of SCF + PTX treatment alone. The reasons for this difference are not obvious. We did not find evidence of selectin-ligand up-regulation after SCF incubation, which might have indicated an increased participation of this pathway, but other untested ligands, which might be affected by SCF incubation, cannot be excluded. It is alternatively possible that a relatively weakened integrin-mediated function might have unmasked contributions of endothelial selectins in BM homing.
Homing and engraftment are differentially regulated
An accepted definition of homing refers to early events that lodge and firmly retain cells in BM prior to their proliferation and expansion.57 These early events could include transendothelial migration, transmarrow trafficking, and cell compartmentalization into specific niches, whereas engraftment is dominated by proliferation and differentiation events. Clearly, some degree of BM homing is required for engraftment, but homing and engraftment are independently regulated. Engraftment can be relatively less or more efficient than BM homing. For example, fetal liver cells and cord-blood cells homed inefficiently to BM, but their proliferative prowess nevertheless afforded more rapid engraftment than adult hemopoietic cells.58,59 A decrease of short-term engraftment in BM was observed for fresh PTX-treated CFU-Cs despite quantitatively normal BM homing, and a decrease in excess of that of BM homing was shown for cytokine-incubated CFU-Cs (Figure 3A) and for α4-/- grafts.3 Our data thus provide an additional example of independent regulation of homing versus engraftment. Several possibilities can be entertained to explain these findings: Gi-signaling-incompetent cells might be impaired in their responsiveness to cytokines and other mediators needed for engraftment; lack of firm adhesion may lead to continued egress from BM of contributing progenitor cells. Furthermore, the assay used here to gauge BM homing does not address potential differences in compartmental (or niche) distribution of transplanted cells, so that the possibility that HPCs are retained in BM but fail to be recruited to appropriate sites cannot be ruled out.60 Such a mechanism was described for PTX-treated lymphocytes, which seeded quantitatively to spleen but failed to migrate to the white pulp.61 It is thus conceivable that, although cells could be retained (ie, homed) somewhat efficiently, the cells' recruitment to sites mediating proliferation, or guarding survival, could be impaired. How relevant such a mechanism is in the context of a severely radiation-damaged and altered BM microenvironment with leaky endothelia has not been adequately explored.
In summary, the data presented herein enhance our understanding of BM homing by constructing a hierarchic model of major molecular pathways in BM homing. Two major upstream pathways, those of VLA-4/VCAM-1 and of SDF-1/CXCR4, are operative in BM homing of adult BM-HPCs, with secondary contributions by β2-integrins and endothelial selectins. As we are showing, the hierarchy among these homing pathways is not fixed, and it can change depending on culture conditions, as depicted by the diagram in Figure 3C. This flexible hierarchy of homing molecules provides an explanation for some of the contradictory findings in the literature as to contributions of individual pathways, particularly with regards to the role of SDF-1/CXCR4/Gi-signaling.17-25
Prepublished online as Blood First Edition Paper, September 1, 2005; DOI 10.1182/blood-2005-05-2023.
Supported by an American Society of Hematology (ASH) Fellow Scholar Award (H.B.) and the National Institutes of Health (grant HL58734) (T.P.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Tsvee Lapidot for insightful discussions about the role of SCF in sensitizing cells to SDF-1, and we are grateful to P. Koni and A. Beaudet for the use of their mice.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal