Abstract
The lack of donor availability is a major limitation to the widespread use of allogeneic hematopoietic stem cell transplantation, and therefore it would be beneficial to identify less immunogenic HLA mismatches. The maternal and fetal antigens that are transmitted through the bidirectional transplacental passage during pregnancy may induce tolerance to noninherited maternal antigens (NIMAs) in offspring and to inherited paternal antigens (IPAs) in the mother. Using mouse models of bone marrow transplantation (BMT), we found that a “child-to-mother” BMT from a NIMA-exposed donor reduced the morbidity and mortality of graft-versus-host disease in an antigen-specific manner; however, a “mother-to-child” BMT from an IPA-exposed donor did not. The NIMA-complementary BMT preserved the graft-versus-leukemia effects and favored the immune reconstitution, thus resulting in a marked improvement of the outcome after BMT. These tolerogenic NIMA effects were completely abolished by the depletion of CD4+CD25+ cells from the donor inocula, thus suggesting the involvement of CD4+CD25+ regulatory T cells in the tolerogenic NIMA effects. Our findings may therefore have profound implications on the performance of clinical BMT while also potentially helping to develop new strategies for using a NIMA-mismatched donor in the absence of an HLA-identical donor.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) cures various hematologic malignant tumors, bone marrow failures, and congenital metabolic disorders. However, its widespread application is limited due to lack of histocompatible donors in proportion to the patients who have a rare HLA haplotype. For these patients, transplantation from an HLA-mismatched relative donor is complicated, with high rates of severe graft-versus-host disease (GVHD).
Several decades ago, Owen1 and Billingham et al2 provided evidence that the exposure of neonates to allogeneic cells induced a long-lasting tolerance specific to alloantigens of the cell donor. Since then, accumulating evidence has suggested the beneficial effects of hematopoietic mixed chimerism for the induction of donor-specific tolerance.3,4 Not only allogeneic transplantation but also the natural fetal-maternal transmission of hematopoietic cells during pregnancy can lead to persistent microchimerism associated with an antigen-specific suppression of the T-cell responses.5,6 Maternal antigens and fetal antigens transmitted through the bidirectional transplacental passage during pregnancy may induce tolerance to noninherited maternal antigens (NIMAs) in the offspring and inherited paternal antigens (IPAs) in the mother.
Studies in experimental and clinical transplantation have suggested the hypothesis that the offspring and mother may be hyporesponsive to NIMAs and IPAs, respectively. In animal models, the transplantation of heart or skin grafts from the mother to NIMA-exposed offspring induces acquired tolerance.7,8 In clinical transplantation, renal transplantation from a NIMA-mismatched sibling donor has been reported to increase graft survival in comparison with a donor mismatched for noninherited paternal antigens (NIPAs).9 In HSCT, the recipients of transplants from siblings mismatched for NIMAs had a significantly lower incidence of GVHD than those mismatched for NIPAs.5 Although there is a body of evidence suggesting the existence of the tolerogenic NIMA effects in transplantation, the impact of IPA exposure has not yet been well studied, and little is known about the mechanisms by which the transmission of maternal and fetal antigens drives the immune response to the corresponding antigens toward tolerance. Furthermore, it is also important to determine the influence of the NIMA effects on immune reconstitution and graft-versus-leukemia (GVL) effects. In this study, we addressed these issues in mouse models of allogeneic HSCT.
Materials and methods
Mice
Female C57BL/6 (B6, H-2b/b), B6D2F1 (H-2b/d), and B6C3F1 (H-2b/k) mice were purchased from Charles River Japan (Yokohama, Japan). The offspring of B6D2F1 backcross breeding pairs were nursed by the mother, weaned after 3 weeks, and typed for the H-2 locus by flow cytometry using monoclonal antibodies (mAbs) specific for H-2Kb and H-2Kd (BD Pharmingen, San Diego, CA). All experiments involving animals were performed under the auspices of the Institutional Animal Care and Research Advisory Committee, Okayama University Advanced Science Research Center.
Bone marrow transplantation
Mice received transplants according to a standard protocol described previously.10 Briefly, B6D2F1 mice received lethal total body irradiation (TBI) (x-ray) split into 2 doses separated by 3 hours to minimize gastrointestinal toxicity. Recipient mice were injected with 5 × 106 T-cell–depleted bone marrow (TCD BM) cells plus 2 × 106 T cells or 1 × 106 CD4+ T cells from the donors. T-cell depletion of donor BM cells was performed using anti-CD90–Microbeads and an AutoMACS system (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. Splenic CD4+ T cells were isolated above 90% purity using a CD4+ T-cell isolation kit (Miltenyi Biotec) and an AutoMACS system. Depletion of CD25+ cells was performed using PE-conjugated anti-CD25 mAbs and Microbeads (Miltenyi Biotec) and an AutoMACS system. The purity levels of the CD25-CD4+ T-cell fractions were above 95%. Donor cells were injected intravenously into the recipients on day 0.
Assessment of GVHD and GVL effects
Survival after bone marrow transplantation (BMT) was monitored daily, and the degree of clinical GVHD was assessed weekly by a scoring system that evaluates the changes in 5 clinical parameters: weight loss, posture, activity, fur texture, and skin integrity (maximum index = 10) as described.11 This score is a more sensitive index of GVHD severity than weight loss alone in multiple murine models. P815 (H-2d) is a mastocytoma derived from a DBA/2 mouse. In GVL experiments, 2.5 × 104 and 5 × 105 P815 cells were intravenously injected into BMT recipients on day 0. The cause of each death after BMT was determined by a postmortem examination to be either GVHD or tumor death as previously described.12 Briefly, leukemia death was defined by the occurrence of either hepatosplenomegaly, macroscopic tumor nodules in the liver and/or spleen, or hind-leg paralysis. GVHD death was defined as the absence of leukemia and by the presence of clinical signs of GVHD, as assessed by a clinical scoring system.12
Flow cytometric analysis
The mAbs used were FITC- or PE-conjugated antimouse CD4, CD8, H-2Kb, and H-2Kd (BD Pharmingen). Cells were preincubated with 2.4G2 mAb for 10 minutes at 4°C and then were incubated with the relevant mAbs for 15 minutes on ice. Finally, the cells were washed twice with 0.2% BSA in PBS and fixed. After lysis of red blood cells with fluorescence-activated cell sorter (FACS) lysing solution (BD Pharmingen), the cells were analyzed using a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA). 7-Amino-actinomycin D–positive dead cells were excluded from the analysis. At least 5000 live events were acquired for the analysis.
Cell cultures
All culture media and incubation conditions have been described previously.13 Dendritic cells (DCs) were generated by culturing BM cells in the presence of 20 ng/mL granulocyte macrophage-colony-stimulating factor (GM-CSF) and 20 ng/mL interleukin-4 (IL-4) (Peprotec, London, United Kingdom) for 7 days. The cultured DCs were activated by the addition of 1 μg/mL lipopolysaccharide (LPS) (Sigma-Aldrich, St Louis, MO) during the final 24 hours of culture. The numbers of cells were normalized for CD4+ T cells and then were cultured in the wells of a 96-well plate at a concentration of 5 × 104 CD4+ T cells per well with 2.5 × 103 irradiated (20 Gy) DCs per well, or with 5 μg/mL platebound anti-CD3ϵ mAbs and 2 μg/mL anti-CD28 mAbs. Seventy-two hours after the initiation of culture, the supernatants were collected for the measurement of the cytokine levels, and proliferation was determined by a thymidine uptake assay as previously described.13 An enzyme-linked immunosorbent assay (ELISA) for interferon-γ (IFN-γ) was performed according to the manufacturer's protocol (R&D Systems, Minneapolis, MN) as previously described.13 The sensitivity of the assays was 31.25 pg/mL.
Statistical analysis
The Mann-Whitney U test was used for the analysis of the cytokine data and the clinical scores. We used the Kaplan-Meier product limit method to obtain the survival probability, and the log-rank test was applied to compare the survival curves. We defined P < .05 as statistically significant.
Results
NIMA exposure reduces allogeneic T-cell responses to NIMAs
Fetal and maternal antigens transmit each other through bidirectional transplacental passage during pregnancy. To generate IPA/NIMA-exposed mice, we used the F1 × P backcross breeding schema.7 A B6 male and a B6D2F1 female were mated to generate H-2b/b offspring that were exposed to NIMA-H-2d in utero. The controls were H-2b/b offspring of a B6 mother and a B6D2F1 father and were NIMA-H-2d nonexposed. We first examined the influence of NIMA exposure on the alloreactive T-cell responses to NIMAs in vitro. CD4+ T cells isolated from spleens of NIMA-exposed B6 and control B6 mice were cultured with B6D2F1 DCs or with anti-CD3 mAbs and anti-CD28 mAbs. Proliferation and IFN-γ production of T cells from NIMA-exposed mice in response to NIMAs were significantly reduced in comparison to those from the controls (Figure 1A-B), although the responses to CD3/CD28 stimulation were equivalent between NIMA-exposed T cells and the control T cells (data not shown). Next, to confirm hyporesponsiveness of NIMA-exposed T cells in vivo, 5 × 105 CD4+ T cells isolated from spleens of NIMA-H-2d–exposed and control mice were adoptively transferred into lethally irradiated B6D2F1 mice. Five days later, the donor CD4+ T-cell expansion was determined in the mesenteric lymph nodes (MLNs). Donor CD4+ T-cell expansion as determined by CD4+ H-2d- decreased significantly more in mice receiving NIMA-exposed T cells than those receiving control T cells (Figure 1C). When adoptively transferred into lethally irradiated third-party B6C3F1 (H-2b/k) mice, donor T-cell expansion was not impaired (Figure 1D).
NIMA exposure reduces T-cell proliferative and cytokine responses to NIMAs. CD4+ T cells isolated from spleens of NIMA-exposed and control B6 mice were cultured with B6D2F1 DCs for 72 hours. Supernatants were collected for the measurement of IFN-γ levels, and the cells were pulsed with 3[H]thymidine to determine cell proliferation. Proliferation (A) and IFN-γ levels (B) are shown as mean ± SD. (C-D) A total of 5 × 105 CD4+ T cells isolated from spleens from NIMA-exposed and control mice were adoptively transferred into lethally irradiated B6D2F1 mice (C) or third-party B6C3F1 mice (D). Five days later, donor CD4+ T-cell expansion as determined by H-2d- CD4+ in MLNs was determined. *P < .05.
NIMA exposure reduces T-cell proliferative and cytokine responses to NIMAs. CD4+ T cells isolated from spleens of NIMA-exposed and control B6 mice were cultured with B6D2F1 DCs for 72 hours. Supernatants were collected for the measurement of IFN-γ levels, and the cells were pulsed with 3[H]thymidine to determine cell proliferation. Proliferation (A) and IFN-γ levels (B) are shown as mean ± SD. (C-D) A total of 5 × 105 CD4+ T cells isolated from spleens from NIMA-exposed and control mice were adoptively transferred into lethally irradiated B6D2F1 mice (C) or third-party B6C3F1 mice (D). Five days later, donor CD4+ T-cell expansion as determined by H-2d- CD4+ in MLNs was determined. *P < .05.
BMT from a NIMA-exposed donor reduces acute GVHD
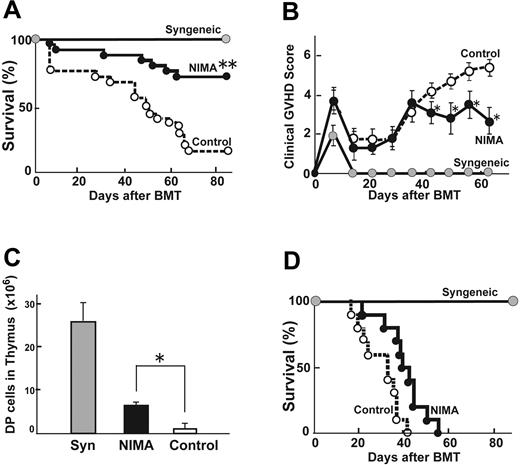
We next investigated whether the use of a NIMA-exposed donor could produce less severe GVHD after allogeneic BMT. Lethally irradiated (13 Gy) B6D2F1 mice received transplants with 5 × 106 TCD BM from the control B6 mice together with 2 × 106 T cells from either 8-week-old NIMA-exposed B6 mice or control B6 donors. GVHD was severe in allogeneic controls, with only a 16% survival at day 80. Transplantation from a NIMA-exposed donor significantly improved survival to 72% (P < .01) (Figure 2A). Allogeneic control mice developed significantly more severe clinical GVHD compared with the syngeneic controls, as assessed by clinical GVHD scores (Figure 2B). Clinical scores in the recipients of NIMA-exposed donors were initially equivalent to those in the allogeneic controls but were significantly less on day 42 and thereafter, although the scores remained higher than in the syngeneic controls. In a separate experiment with very high-dose TBI conditioning, which induces a high GVHD mortality, 100% of the controls died within 7 days after BMT, whereas all mice in the NIMA group survived this period until day 14, thus demonstrating a potential survival benefit not reflected by the clinical scores (data not shown). Immunodeficiency, another cardinal feature of GVHD after allogeneic BMT, was observed with a profound reduction in the number of double-positive (DP) thymocytes (Figure 2C). The number of DP thymocytes was significantly higher in the recipients of NIMA-exposed donors than in those of the controls. An analysis of donor cell engraftment at day 60 after BMT in the spleens showed that more than 98% was donor derived in the recipients of NIMA-exposed donors, thus ruling out rejection or mixed chimerism as a potential cause of GVHD suppression. When transplanted to a third-party B6C3F1 (H-2b/k), no protective NIMA effect was observed (Figure 2D), thus demonstrating the antigen specificity of the NIMA effect.
BMT from a NIMA-exposed donor reduces acute GVHD in an antigen-specific fashion. Lethally irradiated (13 Gy) B6D2F1 mice received transplants with 5 × 106 TCD BM from control B6 mice together with 2 × 106 T cells from either a NIMA-exposed B6 or control B6 donor. (A) Survivals of recipients of syngeneic BMT ( , n = 15), allogeneic BMT from a control donor (○, n = 25), and allogeneic BMT from a NIMA-exposed B6 (•,n = 25) are shown. Data from 5 similar experiments were combined. (B) Clinical scores of these recipients are shown as the mean ± SE. (C) Forty days after BMT, thymuses were harvested and DP cells were enumerated. Data are shown as mean ± SD. (D) Survival rates of third-party B6C3F1 (H-2b/k) mice receiving 2 × 106 T cells from an H-2d-NIMA–exposed B6 donor along with 5 × 106 TCD BM from a control B6 mouse. *P < .05, **P < .005 versus controls.
, n = 15), allogeneic BMT from a control donor (○, n = 25), and allogeneic BMT from a NIMA-exposed B6 (•,n = 25) are shown. Data from 5 similar experiments were combined. (B) Clinical scores of these recipients are shown as the mean ± SE. (C) Forty days after BMT, thymuses were harvested and DP cells were enumerated. Data are shown as mean ± SD. (D) Survival rates of third-party B6C3F1 (H-2b/k) mice receiving 2 × 106 T cells from an H-2d-NIMA–exposed B6 donor along with 5 × 106 TCD BM from a control B6 mouse. *P < .05, **P < .005 versus controls.
BMT from a NIMA-exposed donor reduces acute GVHD in an antigen-specific fashion. Lethally irradiated (13 Gy) B6D2F1 mice received transplants with 5 × 106 TCD BM from control B6 mice together with 2 × 106 T cells from either a NIMA-exposed B6 or control B6 donor. (A) Survivals of recipients of syngeneic BMT ( , n = 15), allogeneic BMT from a control donor (○, n = 25), and allogeneic BMT from a NIMA-exposed B6 (•,n = 25) are shown. Data from 5 similar experiments were combined. (B) Clinical scores of these recipients are shown as the mean ± SE. (C) Forty days after BMT, thymuses were harvested and DP cells were enumerated. Data are shown as mean ± SD. (D) Survival rates of third-party B6C3F1 (H-2b/k) mice receiving 2 × 106 T cells from an H-2d-NIMA–exposed B6 donor along with 5 × 106 TCD BM from a control B6 mouse. *P < .05, **P < .005 versus controls.
, n = 15), allogeneic BMT from a control donor (○, n = 25), and allogeneic BMT from a NIMA-exposed B6 (•,n = 25) are shown. Data from 5 similar experiments were combined. (B) Clinical scores of these recipients are shown as the mean ± SE. (C) Forty days after BMT, thymuses were harvested and DP cells were enumerated. Data are shown as mean ± SD. (D) Survival rates of third-party B6C3F1 (H-2b/k) mice receiving 2 × 106 T cells from an H-2d-NIMA–exposed B6 donor along with 5 × 106 TCD BM from a control B6 mouse. *P < .05, **P < .005 versus controls.
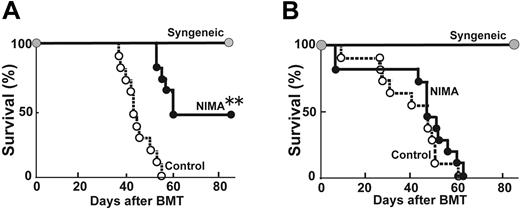
IPA exposure did not reduce T-cell responses to IPAs. (A) CD4+ T cells isolated from spleens of IPA-exposed B6 and control B6 mice were cultured with B6D2F1 DCs for 72 hours, and the cells were pulsed with 3[H]thymidine. Proliferation is shown as mean ± SD. (B) Lethally irradiated B6D2F1 mice received transplants with 5 × 106 TCD BM from control B6 mice together with 2 × 106 T cells from either an IPA-exposed (IPA×1), an IPA–double-exposed (IPA×2), or a control B6 donor. Survival rates of recipients of syngeneic BMT (•, n = 6), allogeneic BMT from a control donor (○, n = 11), allogeneic BMT from an IPA×1 donor (•, n = 11), and allogeneic BMT from an IPA×2 donor (▪, n = 11) are shown. Data from 2 similar experiments were combined.
IPA exposure did not reduce T-cell responses to IPAs. (A) CD4+ T cells isolated from spleens of IPA-exposed B6 and control B6 mice were cultured with B6D2F1 DCs for 72 hours, and the cells were pulsed with 3[H]thymidine. Proliferation is shown as mean ± SD. (B) Lethally irradiated B6D2F1 mice received transplants with 5 × 106 TCD BM from control B6 mice together with 2 × 106 T cells from either an IPA-exposed (IPA×1), an IPA–double-exposed (IPA×2), or a control B6 donor. Survival rates of recipients of syngeneic BMT (•, n = 6), allogeneic BMT from a control donor (○, n = 11), allogeneic BMT from an IPA×1 donor (•, n = 11), and allogeneic BMT from an IPA×2 donor (▪, n = 11) are shown. Data from 2 similar experiments were combined.
IPA exposure of donors did not reduce acute GVHD
IPAs in the fetus can be exposed to the mother during pregnancy. To study the influence of IPA exposure on the mother's allogeneic response, a female B6 mouse was mated with a male B6D2F1 mouse and was exposed to IPA-H-2d from her H-2b/d offspring during pregnancy. A control B6 female mouse was mated with a male B6 mouse, and therefore it was not exposed to IPAs. CD4+ T cells were isolated from the spleens of the IPA-exposed B6 and control B6 mice and were stimulated with B6D2F1 DCs. In contrast to NIMA exposure, proliferation of T cells isolated from the IPA-exposed mice did not decrease in response to the corresponding antigens (Figure 3A). Furthermore, BMT from an IPA-exposed donor at 8 weeks after delivery into lethally irradiated B6D2F1 mice did not reduce GVHD in comparison to transplantation from a control donor. In additional experiments, an IPA-exposed female B6 mouse was again mated with a male B6D2F1 mouse to be reexposed to IPAs. However, transplantation from an IPA–double-exposed donor did not decrease the GVHD mortality (Figure 3B).
Depletion of CD4+CD25+ cells from the donor inocula abolished the tolerogenic NIMA effects
A recent study demonstrated that CD4+CD25+ regulatory T cells mediated maternal tolerance to the fetus.14 We therefore examined whether CD4+CD25+ regulatory T cells are involved in the tolerogenic NIMA effects. Lethally irradiated B6D2F1 mice received transplants with 5 × 106 TCD BM from the control B6 mice together with 1 × 106 CD4+ T cells or 1 × 106 CD25-CD4+ T-cell fractions from a NIMA-exposed B6 or control B6 donor. The doses of CD25-CD4+ cells were comparable between the groups. Transplantation from a NIMA-exposed donor again significantly improved the survival (P < .01) (Figure 4A). However, when CD4+CD25+ cells were depleted from the donor inocula, the tolerogenic NIMA effects were completely abolished (Figure 4B). These results suggest that CD4+CD25+ regulatory T cells are primarily involved in the tolerogenic NIMA effects.
NIMA-induced allotolerance requires CD4+CD25+ T cells. Lethally irradiated B6D2F1 mice received transplants with 5 × 106 TCD BM from control B6 mice together with 1 × 106 CD4+ T cells from either a NIMA-exposed B6 or control B6 donor. (A) Survival rates of syngeneic BMT ( , n = 6), allogeneic BMT from a control donor (○, n = 11), and allogeneic BMT from a NIMA-exposed B6 (•, n = 11) are shown. Data from 2 similar experiments were combined. (B) CD25+ T cells were depleted from CD4+ T cells of a NIMA-exposed or NIMA-nonexposed B6 donor. Lethally irradiated B6D2F1 mice were injected with 5 × 106 TCD BM from a control B6 mouse together with 1 × 106 CD25-depleted CD4+ T cells from a NIMA donor or a control donor. Survival rates of syngeneic BMT (•, n = 6), allogeneic BMT from a control donor (○, n = 11), and allogeneic BMT from a NIMA-exposed B6 (•, n = 11) are shown. Data from 2 similar experiments were combined. **P < .005 versus controls.
, n = 6), allogeneic BMT from a control donor (○, n = 11), and allogeneic BMT from a NIMA-exposed B6 (•, n = 11) are shown. Data from 2 similar experiments were combined. (B) CD25+ T cells were depleted from CD4+ T cells of a NIMA-exposed or NIMA-nonexposed B6 donor. Lethally irradiated B6D2F1 mice were injected with 5 × 106 TCD BM from a control B6 mouse together with 1 × 106 CD25-depleted CD4+ T cells from a NIMA donor or a control donor. Survival rates of syngeneic BMT (•, n = 6), allogeneic BMT from a control donor (○, n = 11), and allogeneic BMT from a NIMA-exposed B6 (•, n = 11) are shown. Data from 2 similar experiments were combined. **P < .005 versus controls.
NIMA-induced allotolerance requires CD4+CD25+ T cells. Lethally irradiated B6D2F1 mice received transplants with 5 × 106 TCD BM from control B6 mice together with 1 × 106 CD4+ T cells from either a NIMA-exposed B6 or control B6 donor. (A) Survival rates of syngeneic BMT ( , n = 6), allogeneic BMT from a control donor (○, n = 11), and allogeneic BMT from a NIMA-exposed B6 (•, n = 11) are shown. Data from 2 similar experiments were combined. (B) CD25+ T cells were depleted from CD4+ T cells of a NIMA-exposed or NIMA-nonexposed B6 donor. Lethally irradiated B6D2F1 mice were injected with 5 × 106 TCD BM from a control B6 mouse together with 1 × 106 CD25-depleted CD4+ T cells from a NIMA donor or a control donor. Survival rates of syngeneic BMT (•, n = 6), allogeneic BMT from a control donor (○, n = 11), and allogeneic BMT from a NIMA-exposed B6 (•, n = 11) are shown. Data from 2 similar experiments were combined. **P < .005 versus controls.
, n = 6), allogeneic BMT from a control donor (○, n = 11), and allogeneic BMT from a NIMA-exposed B6 (•, n = 11) are shown. Data from 2 similar experiments were combined. (B) CD25+ T cells were depleted from CD4+ T cells of a NIMA-exposed or NIMA-nonexposed B6 donor. Lethally irradiated B6D2F1 mice were injected with 5 × 106 TCD BM from a control B6 mouse together with 1 × 106 CD25-depleted CD4+ T cells from a NIMA donor or a control donor. Survival rates of syngeneic BMT (•, n = 6), allogeneic BMT from a control donor (○, n = 11), and allogeneic BMT from a NIMA-exposed B6 (•, n = 11) are shown. Data from 2 similar experiments were combined. **P < .005 versus controls.
Enhanced immune reconstitution and the preservation of the GVL effect after transplantation from a NIMA-exposed donor
It is essential to determine whether transplantation from a NIMA donor is compatible with immune reconstitution and the GVL effects. We tested the effects of NIMA-complementary transplantation on subsequent T-cell reconstitution by evaluating the number of splenic T cells. Reconstitution of both CD4+ and CD8+ T cells was significantly improved in NIMA recipients in comparison to that in the allogeneic controls (Table 1). We next studied the influence of NIMA exposure to GVL effects. Animals received transplants with the addition of 2.5 × 104 and 5 × 105 host-type P815 leukemia cells to the donor inocula. Syngeneic BMT recipients receiving transplants with P815 all died from leukemia by day 20 (Table 2). Allogeneic BMT from a NIMA-exposed donor significantly delayed the leukemia relapse, thus demonstrating a GVL effect equivalent to that seen after allogeneic BMT from a control donor.
BMT from NIMA-exposed donor favored immune reconstitution
. | . | CD4+ T cells, × 106 . | . | CD8+ T cells, × 106 . | . | ||
|---|---|---|---|---|---|---|---|
| Donor . | Host . | Average . | SE . | Average . | SE . | ||
| BDF1 | BDF1 | 6.53 | 0.65 | 3.24 | 0.35 | ||
| NIMA-B6 | BDF1 | 1.38* | 0.31 | 1.25* | 0.05 | ||
| Control-B6 | BDF1 | 0.16 | 0.03 | 0.12 | 0.03 | ||
. | . | CD4+ T cells, × 106 . | . | CD8+ T cells, × 106 . | . | ||
|---|---|---|---|---|---|---|---|
| Donor . | Host . | Average . | SE . | Average . | SE . | ||
| BDF1 | BDF1 | 6.53 | 0.65 | 3.24 | 0.35 | ||
| NIMA-B6 | BDF1 | 1.38* | 0.31 | 1.25* | 0.05 | ||
| Control-B6 | BDF1 | 0.16 | 0.03 | 0.12 | 0.03 | ||
B6D2F1 mice were irradiated with 13 Gy TBI and received transplants with 5 × 106 TCD BM cells from control B6 mice together with 2 × 106 T cells from either a NIMA donor or a control donor. The numbers of CD4+ and CD8+ T cells in spleen 40 days after BMT are shown.
SE indicates standard error.
P < .05 versus controls
BMT from NIMA-exposed donor maintained GVL activity
. | . | P815 cells, 2.5 × 104 . | . | P815 cells, 5.0 × 105 . | . | ||
|---|---|---|---|---|---|---|---|
| Donor . | Host . | MST, d . | SE . | MST, d . | SE . | ||
| BDF1 | BDF1 | 14.2 | 0.4 | 10.3 | 2.9 | ||
| NIMA-B6 | BDF1 | 51.2* | 2.6 | 48.0* | 3.1 | ||
| Control-B6 | BDF1 | 55.7* | 12.0 | 48.3* | 5.7 | ||
. | . | P815 cells, 2.5 × 104 . | . | P815 cells, 5.0 × 105 . | . | ||
|---|---|---|---|---|---|---|---|
| Donor . | Host . | MST, d . | SE . | MST, d . | SE . | ||
| BDF1 | BDF1 | 14.2 | 0.4 | 10.3 | 2.9 | ||
| NIMA-B6 | BDF1 | 51.2* | 2.6 | 48.0* | 3.1 | ||
| Control-B6 | BDF1 | 55.7* | 12.0 | 48.3* | 5.7 | ||
B6D2F1 mice were irradiated with 13 Gy TBI and received transplants with 5 × 106 TCD BM cells from a control B6 mouse and 2.5 × 104 P815 cells and 5 × 105 together with 2 × 106 T cells from either a NIMA donor or a control donor.
MST indicates mean survival time; SE, standard error.
P < .005 versus syngeneic controls
Discussion
We first confirmed that the exposure of the fetus to NIMAs during the perinatal period suppresses the T-cell responses to the corresponding antigens in a mouse model of NIMA exposure. T cells isolated from NIMA-exposed mice showed hyporesponsiveness to NIMAs in vitro as previously shown in humans and animals.8,15-17 This tolerogenic NIMA effect was further confirmed in vivo in adoptive transfer experiments. Furthermore, donor T-cell expansion did not decrease in third-party recipients of NIMA-exposed T cells, thus demonstrating that the tolerogenic NIMA effect was antigen specific in vivo. As a consequence of reduced allogeneic T-cell responses, BMT from a NIMA-exposed donor significantly decreased the mortality and morbidity of acute GVHD. However, this needs to be investigated in different strain combinations because in a recent study using a transgenic mouse model, exposure to NIMAs was shown to either down-regulate or upregulate the allogeneic B-cell responses depending on the NIMA haplotypes.18
In contrast to the NIMA effects, it remains controversial whether the maternal exposure to IPAs induces tolerance in clinical transplantation.19 Survival rates after BMT from a maternal donor were better than those after BMT from a paternal donor.20 On the other hand, the allograft survival rates in previously pregnant females were not different from those in male recipients in renal transplantation from an offspring donor to a parent recipient.21 Furthermore, the impact of IPA exposure has not yet been well studied in animal models. We tested whether IPA exposure influences the outcome after allogeneic HSCT. T cells isolated from IPA-exposed mice were not hyporesponsive to IPAs, and BMT from an IPA-exposed donor did not result in a clinically significant reduction of acute GVHD compared with transplantation from a control donor. These results demonstrate that NIMA exposure induces T-cell hyporesponsiveness toward the corresponding antigens more potently than IPA exposure. Our results are well in line with the clinical observations that the incidence of severe acute GVHD was significantly lower in HSCT from a NIMA-mismatched donor than from an IPA-mismatched donor.5,19
Although the underlying mechanisms by which fetal exposure to NIMAs drives the immune system toward tolerance more potently than maternal exposure to IPAs remain to be elucidated, there are several explanations for this difference. First, NIMAs are exposed to the fetus perinatally when the immune system is immature and tolerance can be easily achieved,22,23 whereas IPAs are exposed during adulthood when the immune system has matured and exposure to foreign antigens induces immunity. Second, NIMAs can also be exposed to a child after birth via breast-feeding in addition to in utero exposure, while IPAs are only exposed in utero. In murine models of skin transplantation, foster nursing experiments have demonstrated the requirements of both pregnancy and breast-feeding to achieve long-term skin graft survival.7,8 Breast milk has been shown to be rich in maternal major histocompatibility complex (MHC) antigens in both cellular and soluble forms.8,24,25 Exposure to NIMAs both in utero and by breast-feeding appears to generate higher levels of maternal microchimerism than each exposure alone, and the degree of microchimerism has been reported to show a positive correlation with a prolonged survival of maternal skin grafts.8 As a result, breast-feeding may contribute to the induction of the NIMA effects. Third, a mother might become sensitized to paternal minor histocompatibility antigens (mHAs) during pregnancy.26,27 Indeed, multiparity promotes priming to male-specific mHAs.28
The cellular mechanisms of the tolerogenic NIMA effects are still poorly understood. We found that CD4+CD25+ T cells in the donor inocula were primarily responsible for the tolerogenic NIMA effects. Recently, it was shown that CD4+CD25+ regulatory T cells contribute to the maintenance of pregnancy and experimental neonatal tolerance.14,29 The CD4+CD25+ regulatory T-cell pool expands during pregnancy and mediates maternal tolerance to the fetus.14 Recent reports have shown that CD4+CD25+ regulatory T cells play an important role in tolerance induction to allogeneic HSCT.30-35
We showed that the tolerogenic NIMA effect was antigen specific. Although the antigen-nonspecific suppressive effects of CD4+CD25+ regulatory T cells have been well recognized,36 in vitro experiments have shown that suppressive functions require the activation of CD4+CD25+ regulatory T cells through their T-cell receptor (TCR),36 thus indicating that the activation and function of CD4+CD25+ regulatory T cells is controlled by the specificity of the TCR. Indeed, several studies have demonstrated that alloantigen-specific regulatory T cells could be generated.29,34,37,38 The antigen-specific expansion of regulatory T cells in vivo requires a T-cell–deficient environment and stimulation with the corresponding antigens in the presence of IL-2.34,39,40 Therefore, prenatal lymphopenic condition of the fetus may help antigen-specific regulatory T cells to expand. Similarly, lymphopenia induced by myeloablative conditioning may allow for the expansion of these cells following allogeneic HSCT.35 Currently, calcineurin inhibitors such as cyclosporine and tacrolimus are almost exclusively used for GVHD prevention in allogeneic HSCT. These agents inhibit T-cell production of IL-2, which is required for activation and expansion of CD4+CD25+ regulatory T cells and thereby may suppress the NIMA effects. Immunosuppressants such as sirolimus and mycophenolate mofetil, which do not interfere with these regulatory cells, may therefore be of use for NIMA-associated HSCT.29,41
Although the possibility of inhibiting GVHD with the use of a NIMA donor has been shown, its effects on the immune reconstitution and GVL effects remain to be investigated. We herein demonstrated that NIMA-complementary HSCT favors T-cell immune reconstitution and permits the GVL effect at least to some extent. A recent study of experimental BMT showed the infusion of CD4+CD25+ regulatory T cells to reduce GVHD while preserving the GVL effect after allogeneic HSCT because regulatory T cells did not completely inhibit the activation of alloreactive donor T cells.35 Trenado et al also demonstrated that the infusion of CD4+CD25+ T cells specific for recipient's alloantigens was compatible with some form of GVL activity.34 In our experiments, the use of a NIMA-exposed donor did not completely inhibit the activation of alloreactive T cells and the development of GVHD. This may thus permit the induction of the GVL effect, which is primarily mediated by both donor CD4+ and CD8+ T cells.12
In summary, our study demonstrates that transplantation from a NIMA-mismatched donor, but not an IPA-mismatched donor, reduces GVHD while maintaining a GVL effect and favoring the T-cell immune reconstitution, thus resulting in an improved outcome after HSCT. The tolerogenic NIMA effects are at least partly mediated by CD4+CD25+ T cells. Our findings should have profound implications on clinical HSCT; they suggest that the use of a NIMA-mismatched donor in the absence of an HLA-identical donor may be a potentially effective treatment strategy.
Prepublished online as Blood First Edition Paper, September 8, 2005; DOI 10.1182/blood-2005-07-3045.
Supported by research funds from the Ministry of Education, Culture, Sports, Science, and Technology (no. 17390280) (T.T.); the Health and Labor Science Research Grants (T.I., T.T.); and the Japan Leukemia Research Fund (T.T.).
K.-i.M. performed research and wrote the paper; T.I. designed the research; D.H., S.A., and M.T. performed research; and T.T. designed and organized the research.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. NIMA exposure reduces T-cell proliferative and cytokine responses to NIMAs. CD4+ T cells isolated from spleens of NIMA-exposed and control B6 mice were cultured with B6D2F1 DCs for 72 hours. Supernatants were collected for the measurement of IFN-γ levels, and the cells were pulsed with 3[H]thymidine to determine cell proliferation. Proliferation (A) and IFN-γ levels (B) are shown as mean ± SD. (C-D) A total of 5 × 105 CD4+ T cells isolated from spleens from NIMA-exposed and control mice were adoptively transferred into lethally irradiated B6D2F1 mice (C) or third-party B6C3F1 mice (D). Five days later, donor CD4+ T-cell expansion as determined by H-2d- CD4+ in MLNs was determined. *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/1/10.1182_blood-2005-07-3045/4/m_zh80010688850001.jpeg?Expires=1769147476&Signature=t6WNvJba64ZeutKuMsVkE5ynQaCBp6DxQXAE9aAJSuz-MBXHwpDvM2~DI~kL2f5zyw9DYIwZB8lmpBSaKFkbSc7HCfvhXESzeWxldHLjYYJvW8WUWCEMVs5TDg~zOZRR-EZ8HpBIY8PARXLx0RjCVylWQcMXvwjKmvbdzMx8C3cBmlrLJmajiylYaD7qJOutIA1RlzIaN-5XrYFa9v1ToAR3bagamFb5PdyPuvAWFnuX3MvCR2FHbGsKPKxHY8eALFTWBhnjGmW~XQUuF5aNIEVtT-pccaQR1soY8isvtYtpxuwZdc0~4xDpAeZS9x620s7H4gvEm9hxzdEwHjsWJw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. IPA exposure did not reduce T-cell responses to IPAs. (A) CD4+ T cells isolated from spleens of IPA-exposed B6 and control B6 mice were cultured with B6D2F1 DCs for 72 hours, and the cells were pulsed with 3[H]thymidine. Proliferation is shown as mean ± SD. (B) Lethally irradiated B6D2F1 mice received transplants with 5 × 106 TCD BM from control B6 mice together with 2 × 106 T cells from either an IPA-exposed (IPA×1), an IPA–double-exposed (IPA×2), or a control B6 donor. Survival rates of recipients of syngeneic BMT (•, n = 6), allogeneic BMT from a control donor (○, n = 11), allogeneic BMT from an IPA×1 donor (•, n = 11), and allogeneic BMT from an IPA×2 donor (▪, n = 11) are shown. Data from 2 similar experiments were combined.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/1/10.1182_blood-2005-07-3045/4/m_zh80010688850003.jpeg?Expires=1769147476&Signature=t-ol7S1F7ulWsApgjWMkj8Bvs2UU1foG-Nb4rkvbIhKBv8oZ0F0-QX4UV2nCtH2~m9VD~FOEectfSZPePpbpj0NwkaPX-yttau0NwLkN77E1~O41AjIzkS0EoMFUiRl3Wowj1nDzFl75kjetBBBPT4P2bEMsKLXa~h44OhJbmUKshtOlwycQvVIM3ZvxxxbYeLZw~skge6C7rnH2oGY7C2h65AaYMsc5RVvloUl1GTNACNhvWOrJAUHEbWymsuQ5pewYH5~H94NMj~WtlBYYgvOO5or2hBnSSKZ6w-6p37i0gInbWYZYSRfFWn3lBqwaHB907IHBIGzAi8BklmPHlw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal