Abstract
The combination of high levels of β2-microglobulin (β2-m) and chromosome 13 deletion allows identification of a high-risk subgroup of patients with de novo multiple myeloma (MM). In this population of patients, we have evaluated the impact of a murine anti–interleukin 6 (anti–IL-6) monoclonal antibody (BE-8) as part of the second conditioning regimen in a multicenter prospective randomized trial of tandem autologous stem cell transplantation (ASCT). Conditioning for the first ASCT was accomplished with melphalan 200 mg/m2 and for the second one with melphalan 220 mg/m2 plus dexamethasone with or without BE-8 infusion. This trial included 219 patients, of whom 166 were randomized, 85 without BE-8 (arm A) and 81 with BE-8 (arm B). The median overall survival (OS) and event-free survival (EFS) times of the whole group of patients were 41 and 30 months, respectively. Response rates, OS, and EFS were not different between the 2 arms of the trial. OS at 54 months was 46% in arm A and 51% in arm B (P = .90); median EFS was 35 months in arm A and 31 in arm B (P = .39). In high-risk patients the dose intensity of melphalan at 420 mg/m2 led to encouraging results, but the addition of anti–IL-6 monoclonal antibody to the second conditioning regimen did not improve either OS nor EFS.
Introduction
Autologous stem cell transplantation (ASCT) is currently considered as the standard of care for younger patients with multiple myeloma (MM).1,2 Melphalan, at a dose of 200 mg/m2 (mel200), is considered to be the optimal conditioning regimen.3 The randomized Intergroupe Francophone du Myélome 94 (IFM94) trial showed that double transplantation significantly improved both overall survival (OS) and event-free survival (EFS) compared with single ASCT.4
In patients with newly diagnosed MM, a number of biologic markers or genetic abnormalities can adversely influence the outcome after ASCT. These include high levels of β2-microglobulin (β2-m), C-reactive protein (CRP), or lactic dehydrogenase (LDH); increased plasma-cell labeling index; hypodiploidy; chromosome 13 deletion (Δ13); translocation (4;14); or a combination of these factors.2,4-8 In a retrospective trial of 110 patients treated with high-dose therapy (HDT) followed by ASCT, Facon et al7 have shown that patients presenting with both high β2-m and Δ13 (identified by fluorescent in situ hybridization [FISH]) at the time of diagnosis had a poor outcome with a median survival and progression-free survival (PFS) of 25 and 15 months, respectively. On the other hand, when Δ13 was not documented and the β2-m level was low, the median survival time was not reached at 111 months and the median PFS time was 37 months. Patients with either a high β2-m or a Δ13 had an intermediate outcome, with median survival and PFS times of 47 and 26 months, respectively. Thus, the combination of these 2 biologic markers at diagnosis allowed identification of a high-risk subgroup of patients, representing approximately 25% to 30% of the patients younger than 65 years with de novo disease.
To treat this subgroup of high-risk patients presenting with both Δ13 and high β2-m levels at the time of diagnosis, the Intergroupe Francophone du Myélome (IFM) study group designed 2 specific trials in 1999. When an HLA-identical sibling donor was identified at diagnosis, patients were offered dose-reduced allogeneic stem cell transplantation after a single melphalan-based (melphalan 200 mg/m2 [mel200]) ASCT; this was the IFM 99-03 trial. Those with no donor underwent tandem autologous transplantation with mel200 followed by further dose-increased melphalan 220 mg/m2 (mel220) and dexamethasone (DXM) with or without anti–interleukin 6 (anti–IL-6) monoclonal antibody (mAb), which was the IFM 99-04 trial.
The use of mel220 has been previously reported by us in a series of patients with advanced MM who had a relapse after prior HDT with a high response rate of 87.5%.9,10 B-E8 is a murine anti–IL-6 mAb that can suppress the proliferation of myeloma cells in vivo.11 To improve the response achieved with mel220 without increasing the toxicity of HDT, we previously conducted a phase 2 trial evaluating the combination of B-E8 with DXM and mel220 followed by ASCT in 16 patients with MM.12 The conditioning regimen was feasible, and a strong inhibition of IL-6 activity evaluated by quantification of CRP was observed in all patients and was correlated with the high complete remission (CR) rate achieved with this combination therapy. Thus, to study the impact of B-E8 therapy as part of second conditioning regimen, we have conducted in high-risk MM a multicenter prospective randomized trial of tandem autologous transplantation with mel200 followed by mel220 and DXM with or without B-E8 infusion, the IFM 99-04 trial. We present the results of this trial in 220 previously untreated patients.
Patients, materials, and methods
Eligibility
The IFM 99-04 trial was conducted from April 2000 to August 2004. Patients younger than 65 years of age, with Durie-Salmon stage I (one bone lesion), II, or III myeloma, who had both initial biologic features Δ13 (FISH analysis) and β2-m level greater than 3 mg/L were eligible. FISH analysis7 and β2-m studies were carried out centrally at the University of Nantes (H. Avet-Loiseau). The criteria for exclusion were prior treatment for myeloma, another cancer, abnormal cardiac function (indicated by a systolic ejection fraction < 50%), chronic respiratory disease (indicated by a vital capacity or carbon monoxide diffusing capacity < 50% of predicted), abnormal liver function (indicated by a serum bilirubin level > 2 mg/dL [35 μM] or an alanine aminotransferase or aspartate aminotransferase level more than 4 times the upper limit of normal), psychiatric disease, and availability of an HLA-identical sibling (inclusion criterion in the IFM 99-03 trial). The study was carried out in accordance with the Declaration of Helsinki, approved prior to initiation by the local Institutional Ethics Committee of the University Hospital of Nantes, then by the Institutional Review Board of each participating center (listed in the “Appendix”), and approved and registered by the official French agency for health security; patients gave written informed consent.
Study protocol
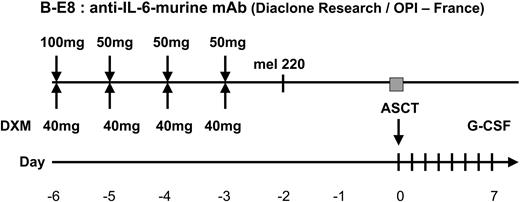
After registration in the study (Figure 1), patients were initially treated with a continuous intravenous infusion of 0.4 mg vincristine and doxorubicin 9 mg/m2 over a 24-hour period for 4 consecutive days, with 40 mg oral DXM/d on days 1 through 4 (VAD regimen). Three or 4 cycles of VAD were administered at 4-week intervals. After initial chemotherapy, patients with a performance status below World Health Organization (WHO) grade III and adequate cardiopulmonary, hepatic, and renal functions underwent peripheral-blood stem-cell (PBSC) collection. Stem cells were collected after priming with granulocyte colony-stimulating factor (G-CSF; 10 μg/kg/d for 6 days). Daily apheresis was continued until at least 5 × 106 CD34 cells/kg were collected to perform the tandem transplantation program. After PBSC collection, patients underwent a first ASCT conditioned by mel200. After this first ASCT, patients were then randomly assigned to one of the 2 HDT groups. Randomization was stratified according to the center and carried out by fax. In arm A, patients underwent a second ASCT after conditioning by the combination of DXM 40 mg/d during 4 days plus mel220 infused over 30 minutes 48 hours before stem-cell reinfusion. In arm B, patients underwent a second ASCT after conditioning by the combination of mel220, DXM, and the addition of B-E8 administered as previously described12 (Figure 2). No maintenance therapy was given after the second ASCT.
IFM 99-04 trial profile. VAD indicates vincristine-doxorubicin-dexamethasone [VAD] regimen.
IFM 99-04 trial profile. VAD indicates vincristine-doxorubicin-dexamethasone [VAD] regimen.
Assessment of response
The response criteria have been defined previously.4 A complete response was defined as the lack of detectable paraprotein by serum and urine electrophoresis and 5% or fewer plasma cells with normal morphologic features in a bone marrow aspirate. A very good partial response was defined as a 90% decrease in the serum paraprotein level; a partial response was defined as a 50% decrease in the paraprotein level or a 90% decrease in the level of Bence Jones protein (including patients with Bence Jones protein alone) or both; a minimal response was defined as a 25% decrease in the paraprotein level; stable disease was defined as no change in the paraprotein level; progressive disease was defined as a 25% increase in the paraprotein level; and a relapse was defined as the reappearance of paraprotein, the recurrence of bone marrow infiltration, or both in a patient who had had a complete response and as a 50% increase above the plateau level of paraprotein in 2 samples obtained 4 weeks apart in a patient who had had a partial response.
FISH analysis
Statistical analysis
The primary end point was to compare the CR rates achieved by 2 HDT modalities, the first one using mel220 plus DXM (arm A) and the second one using mel220 plus DXM plus anti–IL-6 mAb (arm B). Secondary end points were to compare both arms regarding OS and EFS and to study the feasibility and the toxicity of a tandem transplantation with 2 different dosages of melphalan (mel200 and mel220). Assuming the complete response rate to be 25% in the DXM plus mel220 arm, the study required 200 patients to have 80% power to detect an absolute improvement of 15% in the complete response rate in the mel220 plus DXM plus anti–IL-6 mAb arm. The recruitment target was 200 randomized patients. Two interim analyses were planned, the first one after the first 50 patients to check feasibility and toxic death rate, and the second after 140 randomized patients to check OS and EFS. The board of the IFM group agreed to stop the trial in September 2004 when a total of 165 patients had been randomized, considering the total lack of difference regarding primary and secondary end points of the study.
OS was calculated from the date of diagnosis to the date of death from any cause. Data on patients who were alive at the time of analysis were censored in the survival analysis on the last date they were known to be alive. EFS was calculated from the date of diagnosis to the date of progression, relapse, or death. Data on patients who had not shown progression or relapse were censored on the last date they were known to be alive and event-free. Analysis of prognostic factors for survival was performed including usual clinical (age, sex), biologic (isotype and β2-m, CRP, creatinine, hemoglobin, albumin, and calcium levels), and cytogenetic (14q32 rearrangement) characteristics at presentation. Comparison of frequencies between groups were performed using the χ2 and Fisher exact tests. Median values were compared by the Wilcoxon rank sum test. Survival was estimated by the Kaplan-Meier product-limit method, and curves were compared by the stratified log-rank test. A cut-off date of May 15, 2005 was used for survival analysis.
Results
Overall results
From April 2000 to September 2004, 219 patients from 48 centers met eligibility criteria and received at least one course of VAD. Table 1 shows the baseline characteristics of these 219 patients. A total of 53 (24.2%) enrolled patients did not proceed to randomization because of disease progression (n = 17), the patient's decision (n = 10), death during VAD therapy (n = 6), death during the first ASCT (n = 2), protocol violation (n = 4), severe ongoing infection (n = 9), inadequate stem-cell collection (n = 1), cardiac failure (n = 2), and pulmonary failure (n = 2). Thus, 166 (75.8%) patients were randomized (85 patients in arm A and 81 patients in arm B) and were treated according to the whole protocol.
Patient characteristics
. | IFM 99-04; N = 219 . | Arm A; n = 85 . | Arm B; n = 81 . | P . |
|---|---|---|---|---|
| Age at diagnosis, y (range) | 58 (28-65) | 56 (34-65) | 59 (28-65) | .05 |
| Sex, M/F | 114/105 | 41/44 | 47/34 | .28 |
| Isotype, G/A/BJ | 121/62/36 | 45/23/17 | 42/24/15 | .93 |
| Stage, I/II/III | 2/24/193 | 1/9/75 | 0/10/71 | .98 |
| β2-m level, mg/L (range) | 4.9 (3.03-39.4) | 4.6 (3.1-28.5) | 4.8 (3.1-37.2) | .35 |
| β2-m level greater than 5.5 mg/L, no. (%)* | 88 (40.2) | 29 (34.1) | 32 (39.5) | .26 |
| Albumin level, g/L (range) | 38 (16.2-54) | 38 (22.5-54) | 37 (16.2-52) | .62 |
| Albumin level greater than 35 g/L, no. (%) | 134 (61) | 51 (60) | 51 (63) | .47 |
| Platelet count, g/L (range) | 211 (20-500) | 216 (60-462) | 204 (84-469) | .63 |
| Hemoglobin level, g/dL (range) | 9.8 (4.7-14.7) | 9.5 (5.1-14.7) | 10.2 (4.7-14.4) | .14 |
| Calcium level, mM (range) | 2.43 (1.87-4.5) | 2.42 (1.96-4.5) | 2.42 (1.87-4.03) | .85 |
| CRP level, mg/L (range) | 6 (1-279) | 7 (1-244) | 5 (1-137) | .22 |
| t(11;14), yes/no/missing | 21/124/74 | 5/55/25 | 11/57/13 | .11 |
| t(4;14), yes/no/missing | 26/116/77 | 10/47/28 | 10/58/13 | 1 |
| Interval between VAD and 1st ASCT, d (range) | – | 130 (95-252) | 126 (99-199) | .61 |
| Interval between 1st and 2nd ASCT, d (range) | – | 90 (50-206) | 89 (45-242) | .78 |
. | IFM 99-04; N = 219 . | Arm A; n = 85 . | Arm B; n = 81 . | P . |
|---|---|---|---|---|
| Age at diagnosis, y (range) | 58 (28-65) | 56 (34-65) | 59 (28-65) | .05 |
| Sex, M/F | 114/105 | 41/44 | 47/34 | .28 |
| Isotype, G/A/BJ | 121/62/36 | 45/23/17 | 42/24/15 | .93 |
| Stage, I/II/III | 2/24/193 | 1/9/75 | 0/10/71 | .98 |
| β2-m level, mg/L (range) | 4.9 (3.03-39.4) | 4.6 (3.1-28.5) | 4.8 (3.1-37.2) | .35 |
| β2-m level greater than 5.5 mg/L, no. (%)* | 88 (40.2) | 29 (34.1) | 32 (39.5) | .26 |
| Albumin level, g/L (range) | 38 (16.2-54) | 38 (22.5-54) | 37 (16.2-52) | .62 |
| Albumin level greater than 35 g/L, no. (%) | 134 (61) | 51 (60) | 51 (63) | .47 |
| Platelet count, g/L (range) | 211 (20-500) | 216 (60-462) | 204 (84-469) | .63 |
| Hemoglobin level, g/dL (range) | 9.8 (4.7-14.7) | 9.5 (5.1-14.7) | 10.2 (4.7-14.4) | .14 |
| Calcium level, mM (range) | 2.43 (1.87-4.5) | 2.42 (1.96-4.5) | 2.42 (1.87-4.03) | .85 |
| CRP level, mg/L (range) | 6 (1-279) | 7 (1-244) | 5 (1-137) | .22 |
| t(11;14), yes/no/missing | 21/124/74 | 5/55/25 | 11/57/13 | .11 |
| t(4;14), yes/no/missing | 26/116/77 | 10/47/28 | 10/58/13 | 1 |
| Interval between VAD and 1st ASCT, d (range) | – | 130 (95-252) | 126 (99-199) | .61 |
| Interval between 1st and 2nd ASCT, d (range) | – | 90 (50-206) | 89 (45-242) | .78 |
– indicates not applicable.
ISS 3
Response to therapy and toxic death rate
Table 2 shows the response after the VAD induction regimen and after the first and the second ASCT. At each step of the protocol, the CR and very good partial response (VGPR) rates increased: CR plus VGPR 16% after VAD induction therapy, 34% after the first ASCT, and 51% after the second ASCT.
Disease response
Response . | VAD, no. (%); N = 219 . | ASCT no. 1, no. (%); n = 182 . | ASCT no. 2 Arm A, no. (%); n = 85 . | ASCT no. 2 Arm B, no. (%); n = 81 . | P . |
|---|---|---|---|---|---|
| CR | 9 (4.1) | 26 (14.3) | 26 (30.6) | 28 (34.6) | .62 |
| VGPR | 26 (11.9) | 36 (19.8) | 16 (18.8) | 14 (17.3) | .84 |
| PR | 104 (47.5) | 98 (53.8) | 39 (45.9) | 31 (36.5) | .44 |
| Stable or MR | 49 (22.3) | 16 (8.8) | 2 (2.4) | 4 (4.9) | .43 |
| Progressive | 25 (11.4) | 4 (2.2) | 1 (1.2) | 2 (2.5) | .61 |
| Death | 6 (2.7) | 2 (1.1) | 1 (1.2) | 2 (2.5) | .61 |
Response . | VAD, no. (%); N = 219 . | ASCT no. 1, no. (%); n = 182 . | ASCT no. 2 Arm A, no. (%); n = 85 . | ASCT no. 2 Arm B, no. (%); n = 81 . | P . |
|---|---|---|---|---|---|
| CR | 9 (4.1) | 26 (14.3) | 26 (30.6) | 28 (34.6) | .62 |
| VGPR | 26 (11.9) | 36 (19.8) | 16 (18.8) | 14 (17.3) | .84 |
| PR | 104 (47.5) | 98 (53.8) | 39 (45.9) | 31 (36.5) | .44 |
| Stable or MR | 49 (22.3) | 16 (8.8) | 2 (2.4) | 4 (4.9) | .43 |
| Progressive | 25 (11.4) | 4 (2.2) | 1 (1.2) | 2 (2.5) | .61 |
| Death | 6 (2.7) | 2 (1.1) | 1 (1.2) | 2 (2.5) | .61 |
CR indicates complete response; VGPR, very good partial response; PR, partial response; MR minimal response.
The treatment-related mortality rate was 5%; 6 patients (3%) died during the induction therapy with VAD, 2 patients died during the first ASCT, and 3 patients (2%) died during the second ASCT (1 in arm A and 2 in arm B).
OS and EFS
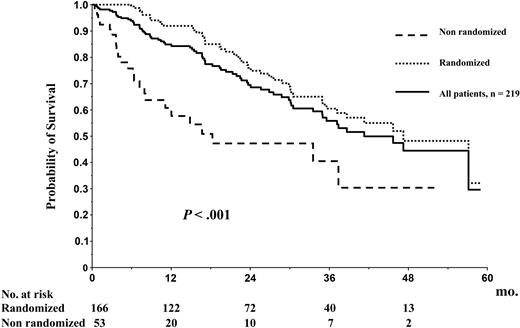
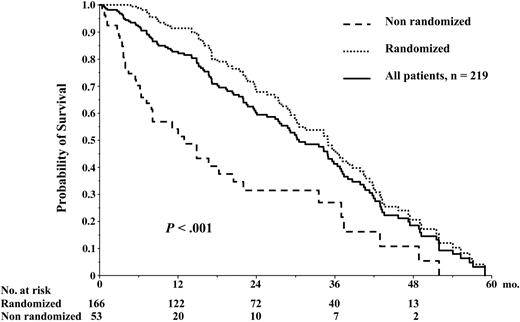
Figure 3 depicts OS from diagnosis for the whole group of 219 patients enrolled in the trial. At the reference date of May 15, 2005, the median OS was 41 months, and the 56-month survival rate was 44.4%. The median EFS from diagnosis for the whole group of 219 patients was 30 months, and the 5-year EFS was 0% (Figure 4). OS and EFS of the 166 patients who were randomized were better as compared with those of the 53 patients who could not proceed to randomization (median, 47 and 35 months, respectively, versus 17 and 12 months, respectively, P < .001; Figures 3, 4).
Randomized patients
Baseline characteristics were identical in the 2 treatment arms, except for age at diagnosis, which was a median age of 56 years in arm A and 59 years in arm B, P = .05 (Table 1).
Toxicity of the second conditioning regimen
Table 3 shows the toxicity of mel220 plus DXM with or without anti–IL-6 mAb. The median duration of hospitalization was identical, 22 days, in both arms of the trial. No adverse side effects were reported due to the anti–IL-6 mAb infusion. No veno-occlusive disease was reported. The duration of neutropenia and the number of transfusions were identical in both groups. The incidence of grades III and IV mucositis (WHO scale) was similar in both groups, 54% in arm A versus 49% in arm B. One patient in arm A and 2 patients in arm B died of infectious complications.
Toxicity of the second conditioning regimen
. | Arm A; n = 85 . | Arm B; n = 81 . | P . |
|---|---|---|---|
| Duration of G-CSF, d, range (median) | 4-13 (6) | 2-11 (6) | .70 |
| Duration of neutropenia, d, range (median) | 4-15 (8) | 4-19 (7) | .88 |
| Duration of thrombocytopenia, d, range (median) | 2-100 (8) | 0-200 (8) | .71 |
| No. of platelet transfusions, range (median) | 0-8 | 0-29 | .63 |
| No. of red blood cell transfusions, range (median) | 0-10 (1) | 0-9 (2) | .45 |
| Duration of hospitalization, d, range (median) | 17-120 (22) | 13-82 (22) | .68 |
| Anti–IL-6 toxicity | – | 0 | – |
| Cardiac toxicity grades III-IV, no. (%) | 1 (1.2) | 2 (2.5) | .61 |
| Mucositis grades III-IV, no. (%) | 46 (54.1) | 40 (49.4) | .64 |
| Pulmonary toxicity grades III-IV, no. (%) | 3 (3.5) | 3 (3.7) | > .99 |
| Renal toxicity grades III-IV, no. (%) | 3 (3.5) | 2 (2.5) | > .99 |
| Liver toxicity grades III-IV, no. (%) | 2 (2.4) | 2 (2.5) | > .99 |
| Toxic death, no. (%) | 1 (1.2) | 2 (2.5) | .61 |
. | Arm A; n = 85 . | Arm B; n = 81 . | P . |
|---|---|---|---|
| Duration of G-CSF, d, range (median) | 4-13 (6) | 2-11 (6) | .70 |
| Duration of neutropenia, d, range (median) | 4-15 (8) | 4-19 (7) | .88 |
| Duration of thrombocytopenia, d, range (median) | 2-100 (8) | 0-200 (8) | .71 |
| No. of platelet transfusions, range (median) | 0-8 | 0-29 | .63 |
| No. of red blood cell transfusions, range (median) | 0-10 (1) | 0-9 (2) | .45 |
| Duration of hospitalization, d, range (median) | 17-120 (22) | 13-82 (22) | .68 |
| Anti–IL-6 toxicity | – | 0 | – |
| Cardiac toxicity grades III-IV, no. (%) | 1 (1.2) | 2 (2.5) | .61 |
| Mucositis grades III-IV, no. (%) | 46 (54.1) | 40 (49.4) | .64 |
| Pulmonary toxicity grades III-IV, no. (%) | 3 (3.5) | 3 (3.7) | > .99 |
| Renal toxicity grades III-IV, no. (%) | 3 (3.5) | 2 (2.5) | > .99 |
| Liver toxicity grades III-IV, no. (%) | 2 (2.4) | 2 (2.5) | > .99 |
| Toxic death, no. (%) | 1 (1.2) | 2 (2.5) | .61 |
– indicates not applicable.
Overall survival. OS from diagnosis for the entire group of 219 patients enrolled in the study.
Overall survival. OS from diagnosis for the entire group of 219 patients enrolled in the study.
Response rate
The response rates after the second ASCT were identical in both arms of the study (Table 2): CR 31% in arm A and 35% in arm B, P = .62, and CR plus VGPR 50% in arm A and 53% in arm B, P = .42. The number of patients with a partial response was also similar in both arms (P = .44).
OS and EFS
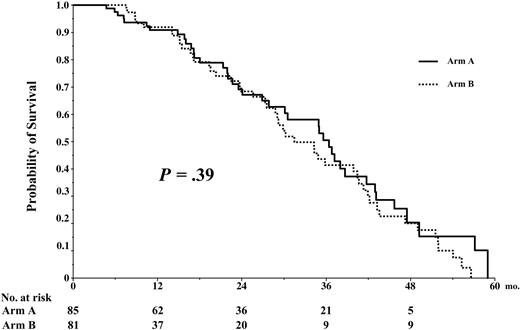
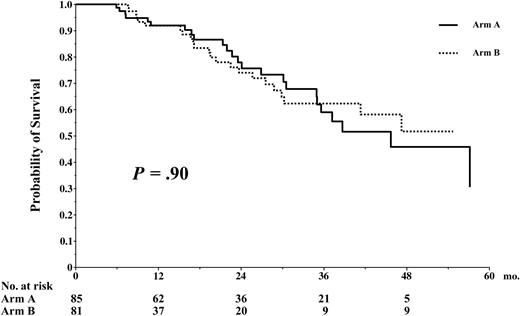
The median follow-up time for living patients who were randomized was 24 months (range, 9-59 mo). The EFS was identical in both arms of the study, median 35 months in arm A versus 31 in arm B, and 0% at 59 and 57 months, respectively, P = .39 (Figure 5). The OS was not statistically different in arm A versus arm B, 46% versus 51% at 54 months, respectively, P = .90 (Figure 6).
Prognostic factors for survival
In a statistical analysis of all 219 patients, a single factor was associated with an adverse outcome for both OS and EFS: a β2-m level greater than 8 mg/L (median survival 30 months in the group of 47 patients with β2-m > 8 versus 47 months for the 172 remaining patients, P = .002 and median EFS 22 months versus 35, P = .01). When the analysis was performed on the group of 166 randomized patients, this parameter remains statistically significant for both OS and EFS (median survival 30 months in the group of 30 patients with β2-m > 8 versus not reached for the 136 remaining patients, P = .04 and median EFS 24 months versus 36, P = .08). On the other hand, in this population with high-risk disease, albumin level, platelet count, or other presenting features did not statistically influence survival as single parameters. Neither t(4;14) nor t(11;14), the main translocations involving the 14q32 chromosomal region, significantly modified the outcome of the whole group of patients. As compared with patients who did not present with t(4;14) at diagnosis, the median OS and EFS of patients with t(4;14) were 37 and 23 months, respectively, versus 45 (P = .41) and 35 months (P = .34), respectively. Results were similar when the analysis was performed on the group of 166 randomized patients; the median OS and EFS of patients with t(4;14) were 37 and 27 months, respectively, versus 46 (P = .61) and 35 months (P = .46), respectively. The International Staging System13 (ISS) was not predictable for survival, but the inclusion criterion β2-m greater than 3 mg/L interferes with the definition of ISS1 (in which patients must present with β2-m level < 3.5 mg/L).
Discussion
Until now, no study has been specifically designed to study the impact of HDT on a subset of patients with high-risk de novo MM. Barlogie et al14 were the first to report among 229 patients treated with tandem transplants (Total Therapy I) that a subgroup of 23 patients presenting with the combination of unfavorable karyotype (Δ11/13) and elevated β2-m levels (> 4) experienced both shorter OS and EFS (median 2.1 and 1.7 years, respectively, dated from the time of the first cycle of VAD) as compared with others (median, 7.0+ and 4.2 years, respectively, P = .001, for the remaining 206 patients, 161 with β2-m levels less than 4 mg/L and 45 with β2-m levels greater than 4 mg/L but absence of Δ11/13), as shown in Table 4. More recently, the same group reported the outcome of 1475 MM patients scheduled to receive tandem transplants and showed in a multivariate analysis that Δ13/hypodiploid karyotype, a pretransplant level of β2-m greater than 2.5 mg/L, and pretransplant levels of albumin less than 35 g/L were the most important negative factors for both EFS and OS (from the time of the first transplant).15 The application of these factors identified 3 groups of patients with very different outcomes. The median EFS for patients with none (596 patients), one (562 patients), and at least 2 (317 patients, 21%) poor prognostic factors were 30, 22, and 11 months (P < .001), respectively, and the OS for the same groups of patients were 59, 41, and 16 months respectively (P < .001; Table 4). The IFM group has shown similar prognostic implications for the combination of β2-m and Δ13 detected by FISH analysis (Table 4).7 These findings led to the risk-adapted IFM99 protocols, with 2 specific trials for high-risk patients with both high β2-m and Δ13. The IFM 99-04 trial is the first prospective randomized trial of tandem ASCT in such patients.
Results of studies in high-risk MM patients and of tandem ASCT
Study in high-risk MM patients . | Criteria . | No. patients . | Median EFS, mo . | Median OS, mo . |
|---|---|---|---|---|
| IFM7 | β2-m ≥ 2.5 and Δ13* | 22/168 | 15† | 25 |
| Arkansas14 | β2-m > 4 and Δ11/13‡ | 23/229 | 20.4 | 25.2 |
| Arkansas15 | β2-m > 2.5 and Δ13/hypodiploid‡ and albumin <35 | 317/1475 | 11 | 16 |
| IFM 99-04 | β2-m > 3 and Δ13 | 219 | 30 | 41 |
| Tandem ASCT | ||||
| IFM 944 | de novo, < 61 y | 200 | 30 | 58 |
| MAG 9516 | de novo, < 59 y | 115 | 33 | 73 |
| Bologna17 | de novo, < 61 y | 113 | 31 | 60 |
Study in high-risk MM patients . | Criteria . | No. patients . | Median EFS, mo . | Median OS, mo . |
|---|---|---|---|---|
| IFM7 | β2-m ≥ 2.5 and Δ13* | 22/168 | 15† | 25 |
| Arkansas14 | β2-m > 4 and Δ11/13‡ | 23/229 | 20.4 | 25.2 |
| Arkansas15 | β2-m > 2.5 and Δ13/hypodiploid‡ and albumin <35 | 317/1475 | 11 | 16 |
| IFM 99-04 | β2-m > 3 and Δ13 | 219 | 30 | 41 |
| Tandem ASCT | ||||
| IFM 944 | de novo, < 61 y | 200 | 30 | 58 |
| MAG 9516 | de novo, < 59 y | 115 | 33 | 73 |
| Bologna17 | de novo, < 61 y | 113 | 31 | 60 |
FISH analysis
Progression-free survival
Conventional cytogenetic
The aims of the IFM 99-04 trial were first to check the interest of anti–IL-6 mAb as part of the second conditioning regimen in a tandem transplantation program, and second, to address the issue of a dose escalation of melphalan using mel200 for the first transplantation and mel220 for the second one.
Our study shows that the addition of anti–IL-6 mAb to the second conditioning regimen did not improve either OS or EFS. Even if we did not evaluate IL-6 or CRP levels (the surrogate marker of IL-6 production) in patients immediately before the infusion of the antibody, the main explanation for these negative results is probably that the majority of patients had responsive disease immediately before the second ASCT, with an 85% response rate after the induction chemotherapy and the first ASCT, without elevated in vivo IL-6 production in patients. This antibody, which has a true activity in vivo in high proliferative disease such as plasma-cell leukemia,11 should be used preferentially in patients at the time of relapse, when elevated serum levels of IL-6 are detectable.
Event-free survival. EFS from diagnosis for the whole group of 219 patients enrolled in this study.
Event-free survival. EFS from diagnosis for the whole group of 219 patients enrolled in this study.
The major findings of our study are the encouraging OS and EFS rates, superior to the 2-year and 18-month survival and EFS rates previously described in high-risk patients.7,14,15 This could be attributed to the dose intensity of mel420 (mel200 plus mel220), a tandem sequence that is tolerable. A clear relationship between dose and response in patients treated with melphalan for MM was described almost 20 years ago.18,19 In a previous work we showed that pharmacokinetic parameters of mel220 were the same as those of mel140 or mel200, except for area under the plasma concentration curve which was, as expected, higher with mel220 as compared with mel140 or mel200.9 When we used mel220 in patients with primary refractory disease or patients who had a relapse after a prior HDT, the response rate was 85%, with a 2-year survival rate of 67%.10 The escalation of melphalan dosage has also been investigated by another group in North America who reported in an abstract the use in patients with MM of an increased dose of melphalan 280 mg/m2 followed by a single ASCT performed as part of initial therapy along with amifostine to reduce toxicities to nonhematopoietic tissues.20 Forty patients with de novo MM responding to induction therapy received this conditioning regimen. No toxic death was reported, and with a short median follow-up of 13 months, 85% of the patients were alive without progressive disease. Of note, in our trial, despite the inclusion of poor-prognosis patients only, the median EFS for the whole group of 219 patients was 30 months, strictly identical to the median EFS of the double-transplant arm (200 patients) of the IFM94 trial, indicating that the tandem mel200, mel220 compares favorably with the tandem mel140, mel140 plus 8 Gy total body irradiation of this latter study.4 A similar median EFS of 33 and 31 months has also been described in the double-transplant arm of the MAG95 trial,16 and of the Bologna trial17 , respectively. In these 3 latter studies, IFM94, MAG95, and Bologna trials, patients with de novo MM were included regardless β2-m level or chromosome 13 abnormality. Nevertheless, in these 3 trials, the median OS ranged from 58 to 73 months, which is much longer than the median OS of 41 months described in the present study (Table 4). The treatment of relapse was not standardized in our trial and OS data should be interpreted cautiously; nevertheless, this probably indicates that the duration of survival after relapse is short and that salvage treatments, despite the availability of agents such as thalidomide and more recently bortezomib, are less frequently active in this subgroup of patients with poor-risk MM, in relation with disease severity. In such patients relapse after tandem HDT is explosive and often refractory, particularly in patients with chromosome 13 abnormality and a β2-m level greater than 8 mg/L. The role of a maintenance therapy should be explored in this situation.
The 27-month EFS and 37-month OS rates of patients with t(4;14) who received the whole procedure look slightly better than those previously described by our group.6 These results, using a tandem dose-intensified approach, are also apparently better than those recently reported by the group of Princess Margaret Hospital.8 In their series of 128 patients treated with a single ASCT prepared by mel200, t(4;14) was identified as the only adverse prognostic factor for both progression-free and OS (median 9.9 and 18.3 months, respectively). Our data indicate that patients with t(4;14) should not be excluded from double transplantation programs.
In conclusion, in the first prospective trial of tandem ASCT in patients with high-risk MM, we have shown that the addition of anti–IL-6 mAb as part of the second conditioning regimen in a tandem transplantation program does not improve survival. In this situation dose intensity of 420 mg/m2 melphalan leads to a median survival of 41 months. Innovative, more effective treatment approaches are warranted, or under evaluation, such as modification of induction regimen using thalidomide or proteasome inhibitor,21-25 or maintenance therapy using thalidomide analogs.
Appendix
The following additional centers and investigators from the Intergroupe Francophone du Myélome participated in this study: Amiens, Centre Hospitalier Général, V. Salles; Angers, Centre Hospitalier Régional et Universitaire, M. Dib; Avignon, Centre Hospitalier Général, G. Lepeu; Bourg-en-Bresse, Centre Hospitalier Général, M. C. Perrin; Brussels, Belgium, Centre Universitaire Saint Luc, A. Ferrant; Clermont-Ferrand, Centre Hospitalier Régional et Universitaire, C. Chaleteix; Dijon, Centre Hospitalier Régional et Universitaire, D. Caillot; Dunkerque, Centre Hospitalier Général, M. Wetterwald; Genève, Switzerland, Hôpital Universitaire, T. Matthès; Haine Saint-Paul, Belgium, Centre Hospitalier Jolimont, P. Delannoy; La Roche sur Yon, Centre Hospitalier Général, M. Tiab; Le Havre, Centre Hospitalier Général, M. Zarnitsky; Lyon-Sud, Centre Hospitalier Régional et Universitaire, C. Dumontet; Marseille, Centre Paoli-Calmette, A. M. Stoppa; Metz, Centre Hospitalier Général, V. Dorvaux; Pau, Clinique des Pyrénées, D. Schlaiffer; Percy-Clamart, Hôpital des Armées, B. Souleau; Perpignan, Centre Hospitalier Général, X. Vallantin; Poitiers, Centre Hospitalier Régional et Universitaire, F. Guilhot; Quimper, Centre Hospitalier Général, J. P. Vilque; Reims, Centre Hospitalier Régional et Universitaire, B. Kolb; Rennes-Sud, Centre Hospitalier Régional et Universitaire, B. Grosbois; Rennes-Ponchaillou, Centre Hospitalier Régional et Universitaire, C. Dauriac; Saint-Etienne, Centre Hospitalier Régional et Universitaire, J. Jaubert; Strasbourg, Centre Hospitalier Régional et Universitaire, F. Maloisel; Suresnes, Hôpital Foch, M. Janvier; Vannes, Centre Hospitalier Général, H. Jardel; Annecy, Centre Hospitalier Général, C. Martin; Institut Curie, Centre Anti-cancéreux, J. Decaudin; Laval, Centre Hospitalier Général, M. Jacomi; Boulogne-sur-mer, Centre Hospitalier Général, X. Agape; Caen, Centre Anti-cancéreux, A. M. Pény; Colmar, Centre Hospitalier Général, B. Audhuy; Villejuif, Institut Gustave Roussy, J. H. Bourhis; Brussels, Belgium, Institut Bordet, P. Bron; Bruges, Belgium, M. Lauvagie; Paris, Hôpital Saint-Louis, P. Brice; Draguignan, Centre Hospitalier Général, B. Valenza; Caen, Polyclinique du Parc, X. Levaltier; Le Mans, Centre Hospitalier Général, M. Duguet; and Blois, Centre Hospitalier Général, P. Rodon.
Prepublished online as Blood First Edition Paper, September 13, 2005; DOI 10.1182/blood-2005-06-2573.
A complete list of the members of the Intergroupe Francophone du Myélome (IFM) study group appears in the “Appendix.”
Supported by a major grant from the Programme Hospitalier de Recherche Clinique.
All the authors are members of IFM and were involved in conception and design of the study, provision of study patients, and manuscript review and approval. P.M. and J.L.H. were involved in data analysis and manuscript writing.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors want to thank Hervé Avet-Loiseau for FISH analysis and reviewing the manuscript. BE-8 was kindly provided by OPI (6 chemin de l'industrie, Dardilly, 69570, France).

![Figure 1. IFM 99-04 trial profile. VAD indicates vincristine-doxorubicin-dexamethasone [VAD] regimen.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/1/10.1182_blood-2005-06-2573/4/m_zh80010688680001.jpeg?Expires=1767806763&Signature=36bObw8Y30daTscxGSeafPGsH7lbarF3ebomqUPv-V3QZXQbdA8q~gm4ENpsv2Gahq7niwknUiF-wRRxXAtd65YSTyV9cXzssmxMJiRDsFPFo0FJKlctWLxmh~D0IeMODpEtpvWyB0J9ghFDAL7enjDW7VV7mrhmD3naWESWiF3uhLjJMbZeAZEsnDggo4UYA-oLwi6HLF4CJT5Up9l7iVSvSMNDGt1hLlUh3QN7ZH5NdC~oXx~4Ln8CAHq3qwSC2a6eJDkoZWqF9kL5OuQVUkKDGteFl4fGYhGOrzj8NFjxHPMwbUoaVZgwcfPa0mPbN8~lJocZ9tdCZFWrp3QqpQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal