Abstract
Interactions between the tyrphostin adaphostin and proteasome inhibitors (eg, MG-132 and bortezomib) were examined in multiple human leukemia cell lines and primary acute myeloid leukemia (AML) specimens. Cotreatment of Jurkat cells with marginally toxic concentrations of adaphostin and proteasome inhibitors synergistically potentiated mitochondrial damage (eg, cytochrome c release), caspase activation, and apoptosis. Similar interactions occurred in other human leukemia cell types (eg, U937, HL-60, Raji). These interactions were associated with a marked increase in oxidative damage (eg, ROS generation), down-regulation of the Raf/MEK/ERK pathway, and JNK activation. Adaphostin/MG-132 lethality as well as mitochondrial damage, down-regulation of Raf/MEK/ERK, and activation of JNK were attenuated by the free-radical scavenger NAC, suggesting that oxidative damage plays a functional role in antileukemic effects. Ectopic expression of Raf-1 or constitutively active MEK/ERK or genetic interruption of the JNK pathway significantly diminished adaphostin/MG-132-mediated lethality. Interestingly, enforced Raf or MEK/ERK activation partially diminished adaphostin/MG-132-mediated ROS generation, suggesting the existence of an amplification loop. Finally, the adaphostin/MG-132 regimen displayed similar toxicity toward 5 primary AML samples but not normal hematopoietic progenitors (eg, bone marrow CD34+ cells). Collectively, these findings suggest that potentiating oxidative damage by combining adaphostin with proteasome inhibitors warrants attention as an antileukemic strategy.
Introduction
The tyrphostin family of tyrosine kinase inhibitors comprise a group of small molecules that interfere with peptide binding rather than the kinase ATP binding site.1 The tyrphostin AG957 was originally developed as an alternative to STI571 (imatinib mesylate) as an inhibitor of the Bcr/Abl kinase,2 dysregulation of which is central to the pathogenesis of chronic myelogenous leukemia (CML).Adaphostin (NSC 680410) is an adamantyl ester of AG957 that is more potent on a molar basis than AG957 in vitro and in vivo and is currently undergoing preclinical development. Recent studies demonstrated that adaphostin induces apoptosis more rapidly than imatinib mesylate in Bcr/Abl-positive cells in association with Bcr/Abl down-regulation as well as STAT5 inactivation and triggers cell death in certain imatinib mesylate-resistant cells.3 However, the actions of adaphostin are not restricted to CML cells, inasmuch as this agent also induces apoptosis in Bcr/Abl-negative human leukemia lines (eg, Jurkat, U937) as well as glioblastoma cells.4 Recently, reports from several laboratories including our own have shown that adaphostin initiates apoptosis in various human leukemia cells in association with generation of reactive oxygen species (ROS).5,6 Furthermore, these events are associated with perturbations in multiple survival and stress-related signal transduction pathways, including inactivation of extracellular signal-regulated kinase1/2 (ERK1/2) and Akt and activation of c-Jun N-terminal kinase (JNK).6 Together, these findings suggest a possible therapeutic role for adaphostin in CML and potentially other leukemias.
Proteasome inhibitors consist of a diverse group of agents that inhibit the 26S proteasome, a multiprotein complex responsible for the degradation and intracellular disposition of multiple proteins implicated in signal transduction, cell-cycle regulation, cell survival, and other critical cellular functions.7 These include cyclins, CDK inhibitors, tumor suppressors such as p53, and transcriptional inhibitors such as IκBα.8 The proteasome consists of a 20S catalytic unit, which exhibits various proteolytic activities (eg, chymotryptic) and two 19S regulatory units. Following ubiquitination by a series of specific ubiquitin ligases, unwanted proteins are shuttled into the catalytic chamber of the proteasome where they are proteolytically degraded.9 In neoplastic cells, interference with proteasome function (eg, by proteasome inhibitors) results in dysregulated expression of numerous proteins, which culminates in cell death.10 This phenomenon has been attributed to multiple mechanisms, including accumulation of proapoptotic proteins (eg, p53, Bax), activation of stress-associated signaling pathways (eg, JNK), and disruption of cell-cycle-related events, among others.11,12 In addition, inhibition of the proteasome system results in accumulation of the IκBα protein, which binds to and disables the cytoprotective transcription factor NF-κB.13 Recently, the lethal actions of proteasome inhibitors, including the clinically relevant dipeptidyl boronic acid bortezomib (Velcade; previously known as PS-341), in epithelial14 and hematopoietic15 malignancies has been related to induction of oxidative damage (ie, ROS generation) as well as endoplasmic reticulum (ER)-related stress.16 Notably, proteasome inhibitors appear to be relatively less toxic to normal than neoplastic cells17 and are able to trigger apoptosis in otherwise resistant cells.18 Significantly, the proteasome inhibitor bortezomib has shown substantial activity in patients with multiple myeloma resistant to standard chemotherapeutic regimens.18 Based on these considerations, efforts to extend the activity of bortezomib to other hematologic malignancies are currently the focus of intense interest.
In previous studies, our group and others have shown that, like adaphostin, proteasome inhibitor-induced cell death in human leukemia cells is associated with inactivation of cytoprotective (eg, ERK1/2, Akt) and activation of stress-related (eg, JNK) signaling pathways.19,20 In addition, both adaphostin and proteasome inhibitors appear to kill leukemia cells through an ROS-related mechanism.6,14-16 It therefore appeared plausible that combining these agents might lead to synergistic antileukemic effects. Furthermore, the relative lack of toxicity of proteasome inhibitors toward normal cells17 as well as evidence that adaphostin selectively spares normal hematopoietic progenitors21 could provide a basis for therapeutic selectivity of this regimen. The present studies were undertaken to determine whether combined exposure of human leukemia cells to adaphostin and proteasome inhibitors would lead to synergistic induction of cell death and, if so, what events might be responsible for this interaction. Our results indicate that adaphostin interacts in a highly synergistic manner with proteasome inhibitors such as MG-132 and bortezomib in human leukemia cells through a process that involves induction of oxidative injury, inactivation of cytoprotective signaling pathways, and a reciprocal activation of the stress-related JNK cascade.
Materials and methods
Cells
U937, Jurkat, HL-60, and Raji human leukemia cells were purchased from American Type Culture Collection (Manassas, VA). All cells were cultured in RPMI 1640 supplemented with sodium pyruvate, MEM essential vitamins, l-glutamate, penicillin, streptomycin, and 10% heat-inactivated FCS (Hyclone, Logan, UT). They were maintained in a fully humidified incubator at 37°C with 5% CO2 and prepared for experimental procedures when in log-phase growth (cell density 4 × 105/mL). Tet-on (Clontech, Mountain View, CA)-inducible constitutively active MEK1 and Raf-1 Jurkat clones were maintained and utilized as previously described.6 U937 cells stably overexpressing constitutively active MEK1 and their empty-vector counterpart (pUSE amp) were also described earlier.22 Stable U937 transfectants expressing TAM67 (a c-Jun transactivation domain-deficient mutant that retains normal DNA binding and dimerization functions) along with their empty-vector counterparts (pMM) have been previously described.23
Reagents
MG-132 was purchased from Calbiochem (San Diego, CA). Adaphostin was kindly provided by Dr Edward Sausville (Division of Cancer Treatment and Diagnosis, National Cancer Institute). Boc-fmk was purchased from Enzyme Products (Livermore, CA). All chemicals were formulated in sterile DMSO before use. Annexin V/PI was supplied by BD Pharmingen (San Diego, CA), and formulated as per the manufacturer's instructions. NAC was purchased from Sigma (St Louis, MO). 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, di(acetoxymethyl ester) was obtained from Molecular Probes (Eugene, OR). Bortezomib was provided by Millennium Pharmaceuticals (Cambridge, MA). All other cell culture products including sodium pyruvate, MEM essential vitamins, and l-glutamate were obtained from Invitrogen (Carlsbad, CA).
Experimental format
Logarithmically growing cells were placed in sterile plastic T flasks (Corning, Corning, NY) to which the designated drugs were added, and the flasks were placed in the incubator for the indicated intervals. At the end of the incubation period, cells were transferred to sterile centrifuge tubes, pelleted by centrifugation at 400g for 10 minutes at room temperature, and prepared for analysis.
Assessment of apoptosis
After drug exposure, cells were stained with annexin V/PI as described previously.22 In short, cells were washed with 1 × PBS and stained with annexin V/PI (BD Pharmingen) for 30 minutes at room temperature. Cells were then processed and analyzed using a Becton Dickinson FACScan cytofluorometer (Mansfield, MA) with the use of Cell Quest software (Becton Dickinson, Palo Alto, CA). Cells were considered to be apoptotic if they were either annexin V+/PI- (early apoptotic) or annexin V+/PI+ (late apoptotic).
Measurement of ROS production
Cells were treated with 20 μM 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, di(acetoxymethyl ester) for 30 minutes at 37°C, and fluorescence was measured by flow cytometry on a fluorescence-activated cell-sorting scan and analyzed with Cell Quest software (Becton Dickinson).
Quantification of glutathione levels in cells
Glutathione levels in cells were measured using a glutathione assay kit provided by Cayman Chemicals (Ann Arbor, MI) as per the manufacturer instructions. Briefly, cells were exposed to the designated agent for the specified interval. Cells were then pelleted by centrifugation and homogenized by sonication in cold buffer (50 mM phosphate, 1 mM EDTA, pH 6 to 7). Cell lysates were centrifuged at 10 000g for 15 minutes, and the protein content of the supernatant was determined. Protein levels of the samples were normalized with the phosphate buffer and subsequently deproteinated using metaphosphoric acid and triethanolamine. Samples were subjected to GSH estimation spectrophotometrically through reaction with DTNB at 405 nm against a GSSG standard.
Preparation of S-100 fractions and assessment of cytochrome c, AIF, Smac release
Cytosolic fractions of cells were prepared as described previously.22 Briefly, cells were centrifuged at 600g for 10 minutes at 4°C and washed with PBS. Cells (4 × 106) were then lysed by incubating for 3 minutes in 100 μL lysis buffer containing 75 mM NaCl, 8 mM Na2HPO4, 1 mM NaH2PO4, 1 mM EDTA, 250 mM sucrose, and 350 μg/mL digitonin. The lysates were centrifuged at 12 000g for 1 minute, and samples were denatured with 4 × loading buffer and separated by 4% to 12% gradient Bis-Tris Gel (Invitrogen, Carlsbad, CA).
Collection and processing of primary cells
Patient-derived leukemic blasts were obtained with informed consent from the peripheral blood of patients with acute myeloid leukemia (AML) (French-American-British [FAB] classification M2 in 4 cases and M5 in 1 case). The percentage of blasts was more than 70% for all samples. CD34+ cells were isolated in an immunomagnetic bead separation technique from the bone marrow of patients undergoing routine bone marrow aspirations for nonmyeloid hematologic disorders.24 Normal peripheral-blood mononuclear cells were obtained from healthy volunteers. These studies have been approved by the investigational review board of Medical College of Virginia/Virginia Commonwealth University (MCV/VCU). Blood and bone marrow samples were collected in sterile syringes containing heparin and processed/treated as described earlier.24
Immunoblot analysis
Immunoblotting was performed as described previously.6 In brief, cells were homogenized after appropriate drug treatment and separated by 4% to 12% Gradient Bis-Tris Gel and probed with appropriate antibodies. The sources of primary antibodies were as follows: AIF, cytochrome c, p-JNK, JNK1, JNK2, p-ERK, Raf1, HA-MEK, Akt, p-Akt were from Santa Cruz Biotechnology (Santa Cruz, CA); cleaved caspase-3, cleaved caspase-9, p-p38, p-c-Jun, c-Jun, p-MEK1 were from Cell Signaling Technology (Beverly, MA); MEK1 was from Transduction Laboratories (Lexington, KY); PARP (C-2-10) was from BioMol Research Laboratories (Plymouth, MA); Smac was from Upstate Biotechnology (Lake Placid, NY); caspase-8 was from Alexis (San Diego, CA); tubulin was from Oncogene (San Diego, CA).
Statistical analysis
The significance of differences between experimental conditions was determined using the 2-tailed Student t test. Analysis of synergism and antagonism was performed using median dose effect analysis in conjunction with a commercially available software program (Calcusyn; Biosoft, Ferguson, MO) as we have previously described.25
Results
Combined treatment with adaphostin and MG-132 synergistically induces apoptosis in multiple leukemia cell types
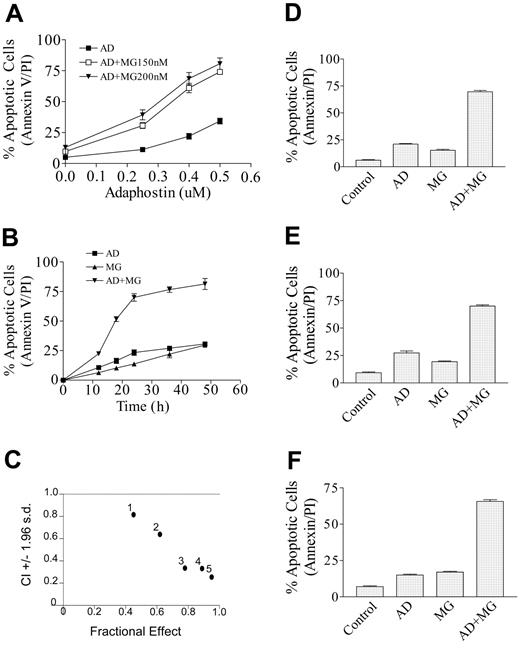
Interactions between adaphostin and proteosome inhibitors were first assessed in Jurkat lymphoblastic leukemia cells. Individual exposure (24 hours) to low concentrations of adaphostin (400 nM) or MG-132 (150 or 200 nM) resulted in only modest toxicity (eg, about 10% to 20% apoptotic cells, reflected by annexin V/PI staining) (Figure 1A). In contrast, combined treatment resulted in a synergistic induction of cell death (eg, about 70% to 80%) (Figure 1A). The increase in cell death/apoptosis was first noted at 12 hours and approached plateau levels by 24 hours (Figure 1B). It was also observed that combined treatment of Jurkat cells with adaphostin (400 nM) and MG-132 (200 nM) induced a progressive decline in the uptake of DiOC6, reflecting loss of mitochondrial membrane potential (ΔΨm) as early as 6 hours (25.0% ± 2.8%) and reaching plateau levels by 20 hours (75.0% ± 4.2%), whereas treatment with either adaphostin or MG-132 alone had either no or only modest effects (data not shown). Such findings suggest that alterations in mitochondrial membrane potential may represent an early marker of apoptosis induced by this drug combination. Median dose effect analysis of apoptosis induction by adaphostin and MG-132 administered at a fixed ratio (adaphostin-MG-132, 2:1) yielded combination index (CI) values ranging from 0.3 to 0.8, signifying a strong synergistic interaction (Figure 1C). To verify whether synergistic interactions could be extended to other leukemic cell types, studies were performed in U937 myelomonocytic leukemia, HL-60 promyelocytic leukemia, and Raji B-lymphoblastic leukemia cells. In each case, greater than additive effects were observed (Figures 1D-F), which were confirmed by median dose effect analysis (data not shown). Finally, comparable results were obtained when adaphostin was combined with other proteasome inhibitors such as ALLN and the clinically relevant bortezomib (Table 1). These findings indicate that adaphostin interacts synergistically with proteasome inhibitors to induce cell death in a variety of human leukemia cell types.
Extent of apoptotic cells after exposure of Jurkat and U937 cells to the indicated drugs
. | Cell type . | . | |
|---|---|---|---|
| Name of drug . | Jurkat . | U937 . | |
| Control | 8 ± 1.2 | 7 ± 0.98 | |
| AD | 20 ± 2.4 | 18 ± 2.0 | |
| Bortezomib | 22 ± 1.98 | 19 ± 1.9 | |
| ALLN | 19 ± 2.5 | 20 ± 1.7 | |
| AD + bortezomib | 72 ± 4.2 | 74 ± 3.2 | |
| AD + ALLN | 70 ± 2.8 | 68 ± 3.0 | |
. | Cell type . | . | |
|---|---|---|---|
| Name of drug . | Jurkat . | U937 . | |
| Control | 8 ± 1.2 | 7 ± 0.98 | |
| AD | 20 ± 2.4 | 18 ± 2.0 | |
| Bortezomib | 22 ± 1.98 | 19 ± 1.9 | |
| ALLN | 19 ± 2.5 | 20 ± 1.7 | |
| AD + bortezomib | 72 ± 4.2 | 74 ± 3.2 | |
| AD + ALLN | 70 ± 2.8 | 68 ± 3.0 | |
Combined treatment with adaphostin (AD) and proteosome inhibitors other than MG-132 (eg, bortezomib and ALLN) interact synergistically to induce cell death in Jurkat and U937 cells. Jurkat and U937 cells were exposed to the indicated drugs alone and in combination for 24 hours, after which the extent of apoptotic cells was determined by flow cytometric analysis after annexin V/PI staining as described in “Materials and methods.” Values represent the means plus or minus SD for 3 separate experiments performed in triplicate. Drug concentrations were as follows: Jurkat: AD, 400 nM; bortezomib, 4.0 nM; ALLN, 4.0 μM; U937: AD, 750 nM; bortezomib, 4.0 nM, ALLN, 4.0 μM.
Cotreatment of adaphostin with MG-132 synergistically induces cell death in human leukemia cells. (A) Jurkat cells were treated alone or in combination with the indicated concentrations of adaphostin and MG-132 (150 or 200 nM) for 24 hours, after which the percentage of apoptotic cells was monitored by annexin V/PI staining as described in “Materials and methods.” (B) Jurkat cells were treated with adaphostin (400 nM) or MG-132 (200 nM) individually as well as in combination, after which induction of apoptosis was monitored at intervals from 0 to 48 hours. (C) Jurkat cells were exposed to a range of adaphostin and MG-132 concentrations alone and in combination at a fixed ratio (eg, 2:1) simultaneously for 24 hours. At the end of this period, the percentage of cells undergoing apoptosis (reflected by annexin V/PI positivity) was determined for each condition. Fractional effect values were determined by comparing results with those of untreated controls, and median dose effect analysis was employed to characterize the nature of the interaction. Combination index values less than 1.0 denote a synergistic interaction. Two additional studies yielded equivalent results. (D) U937 cells were treated with 750 nM adaphostin plus or minus 250 nM MG-132 for 24 hours. (E) HL-60 cells were treated with adaphostin (1.0 μM) plus or minus MG-132 (300 nM) for 48 hours. (F) Raji cells were treated with adaphostin (1.0 μM) plus or minus MG-132 (225 nM) for 36 hours, after which the percentage of apoptotic cells was monitored by annexin V/PI staining and flow cytometry. For panels A, B, D, E, and F, values represent the means plus or minus strandard deviation (SD) for 3 separate experiments performed in triplicate.
Cotreatment of adaphostin with MG-132 synergistically induces cell death in human leukemia cells. (A) Jurkat cells were treated alone or in combination with the indicated concentrations of adaphostin and MG-132 (150 or 200 nM) for 24 hours, after which the percentage of apoptotic cells was monitored by annexin V/PI staining as described in “Materials and methods.” (B) Jurkat cells were treated with adaphostin (400 nM) or MG-132 (200 nM) individually as well as in combination, after which induction of apoptosis was monitored at intervals from 0 to 48 hours. (C) Jurkat cells were exposed to a range of adaphostin and MG-132 concentrations alone and in combination at a fixed ratio (eg, 2:1) simultaneously for 24 hours. At the end of this period, the percentage of cells undergoing apoptosis (reflected by annexin V/PI positivity) was determined for each condition. Fractional effect values were determined by comparing results with those of untreated controls, and median dose effect analysis was employed to characterize the nature of the interaction. Combination index values less than 1.0 denote a synergistic interaction. Two additional studies yielded equivalent results. (D) U937 cells were treated with 750 nM adaphostin plus or minus 250 nM MG-132 for 24 hours. (E) HL-60 cells were treated with adaphostin (1.0 μM) plus or minus MG-132 (300 nM) for 48 hours. (F) Raji cells were treated with adaphostin (1.0 μM) plus or minus MG-132 (225 nM) for 36 hours, after which the percentage of apoptotic cells was monitored by annexin V/PI staining and flow cytometry. For panels A, B, D, E, and F, values represent the means plus or minus strandard deviation (SD) for 3 separate experiments performed in triplicate.
Coexposure of leukemia cells to adaphostin and MG-132 results in a marked increase in release of proapoptotic mitochondrial proteins and activation/cleavage of caspases and PARP
Western blot analysis of lysates from Jurkat cells exposed to 400 nM adaphostin or 200 nM MG-132 individually for 8 hours had little effect on release of proapoptotic mitochondrial protein cytochrome c, Smac/DIABLO, or AIF into the cytoplasmic S-100 fraction, whereas coadministration led to very pronounced increases (Figure 2A). Similar effects were noted when cleavage of caspases-3, -8, and -9 and PARP were monitored. Essentially identical results were observed in U937 cells (Figure 2B) or when adaphostin was combined with bortezomib (data not shown). These data indicate that combined exposure of leukemia cells to adaphostin in combination with proteasome inhibitors potently induces mitochondrial injury and caspase activation. In separate studies, administration of these agents either alone or in combination for the same interval had little or no effect on expression of various Bcl-2 family members, including Bcl-2, Mcl-1, XIAP, and Bad (data not shown).
Exposure of human leukemia cells to adaphostin and proteasome inhibitors shifts the balance toward stress-related and away from survival-related signal transduction pathways
The effects of adaphostin and MG-132 were then examined in relation to activation status of various signal transduction pathways. Whereas individual exposure of Jurkat cells to 400 nM adaphostin or 200 nM MG-132 alone for 8 hours had little effect on phosphorylation/activation of the stress-related kinase JNK1/2, combined treatment resulted in a pronounced increase in JNK phosphorylation (Figure 2C). No changes in levels of total JNK1 or JNK2 were observed. Consistent with these findings, combined but not individual exposure to these agents led to a marked increase in phosphorylation of c-Jun in absence of changes in total c-Jun levels. Similar findings were noted in cases of the stress-related kinase p38 MAPK.
Effects of these agents alone and in combination were then examined in relation to survival-related signaling pathways. Exposure of Jurkat cells to adaphostin alone (8 hours) modestly reduced the expression of MEK1 (mitogen-activated protein kinase kinase 1), while MG-132 had little effect. However, combined treatment resulted in a marked decrease in MEK1 expression (Figure 2D). Parallel results were noted in the case of phospho-MEK levels. Similarly, combined but not individual treatment with these agents reduced levels of Raf-1. In addition, expression of phospho-ERK1/2 was only diminished in cells exposed to both agents, whereas total levels of ERK1/2 remained unchanged with any treatment. Expression of phospho-Akt was slightly reduced in cells exposed to the combination of adaphostin and MG-132. Finally, a similar pattern of perturbations in signaling pathways was observed in U937 cells (Figure 2E) and in Jurkat cells exposed to adaphostin and bortezomib (Figure 2F). Thus, combined exposure of leukemia cells to adaphostin and protesome inhibitors resulted in increased output from the stress-related JNK and p38 MAPK pathways and a reciprocal reduction in output from the cytoprotective Raf/MEK/ERK and Akt pathways.
Simultaneous exposure of leukemic cells to adaphostin and proteasome inhibitors results in a marked increase in ROS-dependent cell death
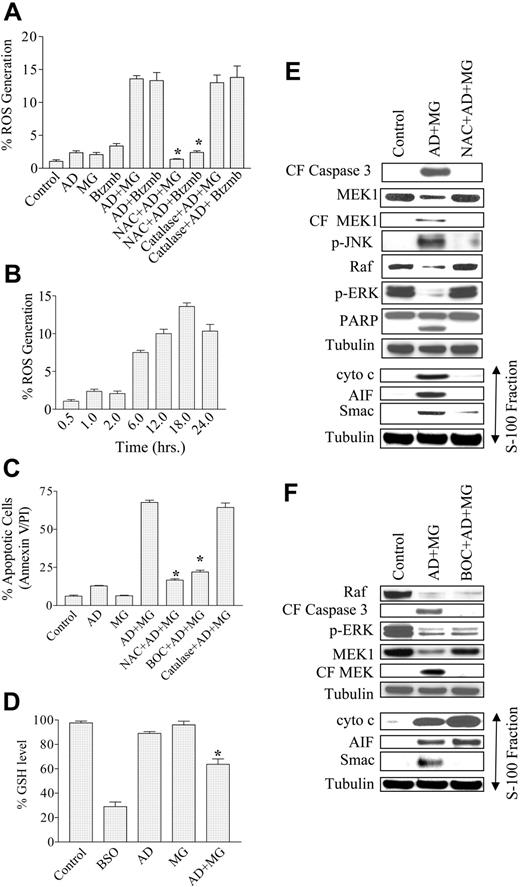
The lethal effects of both adaphostin and proteasome inhibitors have been related to oxidative damage.5,14,20 Consequently, an attempt was made to ascertain what role, if any, ROS generation might play in the lethality of this drug combination. When Jurkat cells were exposed individually for 18 hours to 400 nM adaphostin, 200 nM MG-132, or 4.0 nM bortezomib, concentrations associated with minimal lethality, no increase in ROS generation was observed (Figure 3A). However, when adaphostin was combined with MG-132 or bortezomib, a significant increase in ROS generation was detected. As anticipated, the increase in ROS was abrogated by the free-radical scavenger L-N-acetylcysteine (NAC). In contrast, pretreatment with catalase did not significantly inhibit ROS generation, indicating that ROS production was largely an intracellular event and arguing against the involvement of ROS generation related to peroxides (Figure 3A). The time course study shown in Figure 3B revealed that an increase in ROS generation was observed as early as 6 hours after treatment with adaphostin/MG-132 and reached plateau values by 18 hours (Figure 3B). Furthermore, pretreatment with 6 mM NAC for 3 hours essentially abrogated the increase in apoptosis induced by the adaphostin/MG-132 (Figure 3C) or adaphostin/bortezomib regimens (data not shown). In fact, NAC was at least as effective as the pancaspase inhibitor Boc-D-fmk in blocking adaphostin/MG-132-induced lethality (Figure 3C). Similar results were observed in U937 cells (data not shown). However, as anticipated, pretreatment with catalase failed to offer significant protection from adaphostin/MG-132-induced apoptosis in Jurkat cells (Figure 3C). Similar findings were observed in U937 cells or treatment of Jurkat cells with adaphostin and bortezomib (data not shown).
Combined treatment with adaphostin and MG-132 or bortezomib induces apoptosis in leukemia cells through induction of mitochondrial injury, caspase activation, down-regulation of Raf/MEK/ERK, and activation of JNK. Jurkat cells were treated with 400 nM adaphostin plus or minus 200 nM MG-132 or 4.0 nM bortezomib, while U937 cells were treated with 750 nM adaphostin plus or minus 250 nM MG-132, each for 8 hours. (A-B) Cytosolic (S-100) fractions were obtained as described in “Materials and methods,” and expression of cytochrome c, AIF, and Smac/DIABLO was monitored by Western blot. (C-F) At the end of the drug exposure (8 hours), cells were lysed, sonicated, and the proteins denatured and subjected to Western blot analysis using the indicated primary antibodies. For panels A and B, each lane was loaded with 20 μg protein, whereas for panels C-F, 30 μg protein was loaded in each lane. Blots were stripped and reprobed with antitubulin antibodies to ensure equal loading and transfer of protein. Results are representative of 3 separate studies.
Combined treatment with adaphostin and MG-132 or bortezomib induces apoptosis in leukemia cells through induction of mitochondrial injury, caspase activation, down-regulation of Raf/MEK/ERK, and activation of JNK. Jurkat cells were treated with 400 nM adaphostin plus or minus 200 nM MG-132 or 4.0 nM bortezomib, while U937 cells were treated with 750 nM adaphostin plus or minus 250 nM MG-132, each for 8 hours. (A-B) Cytosolic (S-100) fractions were obtained as described in “Materials and methods,” and expression of cytochrome c, AIF, and Smac/DIABLO was monitored by Western blot. (C-F) At the end of the drug exposure (8 hours), cells were lysed, sonicated, and the proteins denatured and subjected to Western blot analysis using the indicated primary antibodies. For panels A and B, each lane was loaded with 20 μg protein, whereas for panels C-F, 30 μg protein was loaded in each lane. Blots were stripped and reprobed with antitubulin antibodies to ensure equal loading and transfer of protein. Results are representative of 3 separate studies.
Combined treatment of leukemia cells with adaphostin and MG-132 or bortezomib leads to an increase in ROS generation and lethality, events that are attenuated by the free-radical scavenger NAC. (A) Jurkat cells preteated with or without 6 mM NAC for 3 hours or 1000 U/mL catalase for 1 hour were exposed to 400 nM adaphostin plus or minus 200 nM MG-132 or 4.0 nM bortezomib (Btzmb), and ROS generation was monitored by flow cytometry using DHCF as the dye following 18 hours of drug exposure. (B) Jurkat cells were treated with 400 nM adaphostin plus or minus 200 nM MG-132, after which levels of ROS generation were measured at the indicated interval (eg, 0.5 to 24 hours). (C) Jurkat cells preteated with or without 6 mM NAC for 3 hours or 10 μM Boc-fmk for 30 minutes or 1000 U/mL catalase for 1 hour were exposed to 400 nM adaphostin plus or minus 200 nM MG-132 for 24 hours, after which apoptotic cells were monitored by annexin V/PI staining and flow cytometry as described previously. (D) Jurkat cells were treated with 400 nM adaphostin plus or minus 200 nM MG-132 for 16 hours or with 1 mM BSO for 24 hours. Then cells were harvested and homogenized. The samples were then deproteinated, and GSH level was determined as described in “Materials and methods.” Jurkat cells were (E) pretreated with or without 6 mM NAC for 3 hours or (F) pretreated with or without 10 μM Boc-fmk for 30 minutes followed by exposure to 400 nM adaphostin plus or minus 200 nM MG-132 for 8 hours. Following drug treatment, cytosolic (S-100) fractions and whole-cell lysates were obtained as described in “Materials and methods.” Protein samples were subjected to Western blot analysis using the indicated primary antibodies. Blots were stripped and reprobed with antitubulin antibodies to ensure equal loading and transfer of protein. *Significantly less than values for cells exposed to drugs in the absence of NAC or Boc-fmk; P < .005.
Combined treatment of leukemia cells with adaphostin and MG-132 or bortezomib leads to an increase in ROS generation and lethality, events that are attenuated by the free-radical scavenger NAC. (A) Jurkat cells preteated with or without 6 mM NAC for 3 hours or 1000 U/mL catalase for 1 hour were exposed to 400 nM adaphostin plus or minus 200 nM MG-132 or 4.0 nM bortezomib (Btzmb), and ROS generation was monitored by flow cytometry using DHCF as the dye following 18 hours of drug exposure. (B) Jurkat cells were treated with 400 nM adaphostin plus or minus 200 nM MG-132, after which levels of ROS generation were measured at the indicated interval (eg, 0.5 to 24 hours). (C) Jurkat cells preteated with or without 6 mM NAC for 3 hours or 10 μM Boc-fmk for 30 minutes or 1000 U/mL catalase for 1 hour were exposed to 400 nM adaphostin plus or minus 200 nM MG-132 for 24 hours, after which apoptotic cells were monitored by annexin V/PI staining and flow cytometry as described previously. (D) Jurkat cells were treated with 400 nM adaphostin plus or minus 200 nM MG-132 for 16 hours or with 1 mM BSO for 24 hours. Then cells were harvested and homogenized. The samples were then deproteinated, and GSH level was determined as described in “Materials and methods.” Jurkat cells were (E) pretreated with or without 6 mM NAC for 3 hours or (F) pretreated with or without 10 μM Boc-fmk for 30 minutes followed by exposure to 400 nM adaphostin plus or minus 200 nM MG-132 for 8 hours. Following drug treatment, cytosolic (S-100) fractions and whole-cell lysates were obtained as described in “Materials and methods.” Protein samples were subjected to Western blot analysis using the indicated primary antibodies. Blots were stripped and reprobed with antitubulin antibodies to ensure equal loading and transfer of protein. *Significantly less than values for cells exposed to drugs in the absence of NAC or Boc-fmk; P < .005.
Attempts were also made to determine whether the sequence of drug administration influenced ROS generation. When MG-132 was added 6 hours following adaphostin, the extent of ROS generation after an additional 10 hours of combined drug exposure was essentially equivalent to that observed following simultaneous drug exposure (P > .05 versus values for concomitant administration; data not shown). However, when the agents were added in the reverse order (ie, adaphostin for 10 hours following 6 hours of MG-132 exposure) there was a modest but statistically significant decrease in ROS generation (P < .05; data not shown). In both cases, the extent of lethality closely paralleled ROS generation (data not shown).
It has previously been reported5 that ROS-mediated cytotoxicity induced by various drugs induces depletion of endogenous levels of GSH. To determine whether a similar phenomenon occurred in cells exposed to this drug combination, GSH levels were monitored in Jurkat cells treated with adaphostin plus or minus MG-132 versus untreated controls. As depicted in Figure 3D, treatment with adaphostin alone resulted in a slight decrease in endogenous GSH levels while MG-132 had little effect. However, combined treatment with adaphostin and MG-132 produced a significant reduction in the level of GSH (34% ± 3.5% of control; P < .02), a finding consistent with earlier reports involving drug-induced oxidative injury.5 Treatment with BSO, a known inhibitor of GSH synthesis, served as a positive control in this experiment. Collectively, these findings suggest that enhanced oxidative injury plays a significant functional role in synergistic antileukemic interactions between these agents.
Adaphostin/MG-132 signaling perturbations are variably ROS- and caspase-dependent
To gain insights into the hierarchy of adaphostin/MG-132-mediated events, Western blot analysis was performed on lysates from cells exposed to these agents for 8 hours in the presence or absence of either NAC (Figure 3E) or Boc-D-fmk (Figure 3F). As shown in Figure 3D, caspase-3 cleavage, PARP degradation, and MEK1 cleavage induced by adaphostin/MG-132 were blocked by NAC. In addition, NAC largely prevented JNK activation, Raf-1 down-regulation, and ERK inactivation. Last, NAC abrogated adaphostin/MG-132-mediated cytochrome c and AIF release into the cytosolic S-100 fraction and substantially reduced the release of Smac/DIABLO. Similarly, Boc-D-fmk blocked caspase-3 cleavage, MEK1 degradation, and release of Smac/DIABLO into the cytosol. However, in marked contrast to NAC, Boc-D-fmk failed to block Raf-1 down-regulation, ERK inactivation, or mitochondrial release of cytochrome c and AIF. Together, these findings suggest that adaphostin/MG-132-mediated perturbations in mitochondrial integrity and signaling pathways primarily reflect oxidative injury and that a subset of these events represent caspase-dependent phenomena.
Enforced activation of MEK/ERK attenuates adaphostin/MG-132 lethality. (A) Jurkat cells inducibly expressing a constitutively active MEK1 construct were incubated in medium in the presence or absence of 1 μM doxycycline for 30 hours, followed by exposure to 500 nM adaphostin and 250 nM MG-132 for 24 hours. At the end of 24 hours of drug exposure, apoptosis was monitored by annexin V/PI staining and flow cytometry. (B) Cells treated as in panel A were monitored for ROS generation after 16 hours of drug exposure. (C) Following 8 hours of drug exposure, Western blot analysis was employed to monitor the effect of drugs on expression of the indicated proteins. Blots were stripped and reprobed with antitubulin antibodies to ensure equal loading and transfer of protein. (D) U937 cells expressing a constitutively active MEK1 construct and stably transfected empty-vector cells were treated with 750 nM adaphostin plus or minus 250 nM MG-132. At the end of 24 hours, apoptotic cells were monitored. (E) U937 cells treated identically were assayed for ROS generation after 2 hours of drug treatment. (F) Following 8 hours of exposure of U937 cells to adaphostin and MG-132 as in panel D, Western blot analysis was employed to monitor effects on protein expression. Blots were stripped and reprobed with antitubulin antibodies to ensure equal loading and transfer of protein. *Significantly less than values for cells exposed to drugs in the absence of doxycycline (A,B) or control pMM cells (D,E); P < .05.
Enforced activation of MEK/ERK attenuates adaphostin/MG-132 lethality. (A) Jurkat cells inducibly expressing a constitutively active MEK1 construct were incubated in medium in the presence or absence of 1 μM doxycycline for 30 hours, followed by exposure to 500 nM adaphostin and 250 nM MG-132 for 24 hours. At the end of 24 hours of drug exposure, apoptosis was monitored by annexin V/PI staining and flow cytometry. (B) Cells treated as in panel A were monitored for ROS generation after 16 hours of drug exposure. (C) Following 8 hours of drug exposure, Western blot analysis was employed to monitor the effect of drugs on expression of the indicated proteins. Blots were stripped and reprobed with antitubulin antibodies to ensure equal loading and transfer of protein. (D) U937 cells expressing a constitutively active MEK1 construct and stably transfected empty-vector cells were treated with 750 nM adaphostin plus or minus 250 nM MG-132. At the end of 24 hours, apoptotic cells were monitored. (E) U937 cells treated identically were assayed for ROS generation after 2 hours of drug treatment. (F) Following 8 hours of exposure of U937 cells to adaphostin and MG-132 as in panel D, Western blot analysis was employed to monitor effects on protein expression. Blots were stripped and reprobed with antitubulin antibodies to ensure equal loading and transfer of protein. *Significantly less than values for cells exposed to drugs in the absence of doxycycline (A,B) or control pMM cells (D,E); P < .05.
Enforced activation of Raf-1 or MEK1/ERK1/2 protects cells from adaphostin/MG-132-mediated lethality
Attempts were then made to assess the contribution of down-regulation of the Raf/MEK/ERK axis to synergistic interactions between these agents. To this end, Jurkat cells inducibly expressing constitutively active MEK1 or Raf-1 constructs under the control of a doxycycline-responsive promoter were employed. As shown in Figure 4A, addition of doxycycline to MEK1-inducible cells significantly, albeit partially, attenuated the lethality of the adaphostin/MG-132 regimen (24 hours; P < .05). Induction of MEK1 also partially but significantly diminished adaphostin/MG-132-mediated ROS generation at 16 hours (Figure 4B). The Western blot in Figure 4C documents the ability of doxycycline to induce HA-tagged MEK1 and to block adaphostin/MG-132-mediated ERK inactivation (8 hours). Enforced expression of constitutively active MEK1 did not, however, prevent adaphostin/MG-132-mediated JNK activation. Essentially equivalent results were obtained in U937 cells stably transfected with a constitutively active (myristolated) MEK1 construct.22 Expression of this construct prevented down-regulation/inactivation of ERK following an 8-hour adaphostin/MG-132 exposure and resulted in significant protection from lethality as well as ROS generation (P < .02 versus empty-vector controls; Figure 4D-E). Western blot analysis documented prevention of ERK inactivation at 8 hours by adaphostin/MG-132 in U937/MEK cells (Figure 4F).
Parallel studies were performed using a Raf-1-inducible Jurkat cell line. In these cells, addition of doxycycline to the medium resulted in a partial but statistically significant (P < .02) reduction in adaphostin/MG-132-mediated apoptosis at 24 hours (Figure 5A) as well as ROS generation at 16 hours (Figure 5B). As shown by the Western blot (Figure 5C), addition of doxycycline prevented adaphostin/MG-132-mediated Raf-1 down-regulation and reduced, albeit partially, down-regulation/inactivation of ERK (8 hours). As in the case of MEK1-inducible cells, enforced expression of Raf-1 failed to prevent adaphostin/MG-132-mediated JNK activation. Finally, cells ectopically expressing a constitutively active form of Akt22 failed to display resistance to adaphostin/MG-132-mediated ROS generation or apoptosis (data not shown). Together, these findings suggest that inactivation of the Raf-1/ERK1/2/MEK1 axis, but not the Akt pathway, by the adaphostin/MG-132 regimen plays a significant functional role in the lethality of these agents.
Ectopic expression of constitutively active Raf-1 or a c-Jun dominant-negative mutant significantly protects cells from adaphostin/MG-132 induced lethality. (A) Jurkat cells inducibly expressing a constitutively active Raf-1 construct were incubated in medium in the presence or absence of 1 μM doxycycline for 30 hours, followed by exposure to 500 nM adaphostin plus 250 nM MG-132. After 24 hours of drug exposure, apoptotic cells were monitored by annexin V/PI staining and flow cytometry. (B) Alternatively, levels of ROS generation were determined after 16 hours of drug treatment. (C) Following 8 hours of drug exposure as in panel A, Western blot analysis was employed to monitor protein expression of Raf, phospho-ERK, and phospho-JNK. (D) U937 cells ectopically expressing a c-Jun dominant-negative construct (TAM67) or stably transfected empty-vector controls (pMM) were treated with 750 nM adaphostin plus 250 nM MG-132. At the end of 24 hours of drug exposure, apoptotic cells were monitored by annexin V/PI staining followed by flow cytometric analysis. (E) Following 8 hours of drug exposure, Western blot analysis was employed to monitor protein expression of phospho-c-Jun and phospho-ERK. Blots were stripped and reprobed with antitubulin antibodies to ensure equal loading and transfer of protein (20 μg each lane). *Significantly less than values for control cells; P < .05.
Ectopic expression of constitutively active Raf-1 or a c-Jun dominant-negative mutant significantly protects cells from adaphostin/MG-132 induced lethality. (A) Jurkat cells inducibly expressing a constitutively active Raf-1 construct were incubated in medium in the presence or absence of 1 μM doxycycline for 30 hours, followed by exposure to 500 nM adaphostin plus 250 nM MG-132. After 24 hours of drug exposure, apoptotic cells were monitored by annexin V/PI staining and flow cytometry. (B) Alternatively, levels of ROS generation were determined after 16 hours of drug treatment. (C) Following 8 hours of drug exposure as in panel A, Western blot analysis was employed to monitor protein expression of Raf, phospho-ERK, and phospho-JNK. (D) U937 cells ectopically expressing a c-Jun dominant-negative construct (TAM67) or stably transfected empty-vector controls (pMM) were treated with 750 nM adaphostin plus 250 nM MG-132. At the end of 24 hours of drug exposure, apoptotic cells were monitored by annexin V/PI staining followed by flow cytometric analysis. (E) Following 8 hours of drug exposure, Western blot analysis was employed to monitor protein expression of phospho-c-Jun and phospho-ERK. Blots were stripped and reprobed with antitubulin antibodies to ensure equal loading and transfer of protein (20 μg each lane). *Significantly less than values for control cells; P < .05.
Ectopic expression of a dominant-negative c-Jun transactivation domain-defective mutant (TAM67) protects U937 cells from adaphostin/MG-132-mediated lethality
To determine the functional significance of JNK activation in adaphostin/MG-132-mediated lethality, U937 cells expressing a dominant-negative c-Jun N-terminal transactivation domain-deficient mutant (TAM67) were employed.23 When exposed to adaphostin/MG-132 for 24 hours, TAM67 cells were significantly more resistant to adaphostin/MG-132 than their pMM counterparts (P < .01; Figure 5D). Similar results were obtained when the cells were coexposed to the JNK inhibitor SP600125 (data not shown). Notably, empty-vector controls displayed a very substantial increase in JNK activation, manifested by phosphorylation of c-Jun, whereas the response in TAM67 cells was clearly attenuated (Figure 5E). On the other hand, almost complete down-regulation of phospho-ERK was observed in pMM empty-vector cells exposed to adaphostin/MG-132, whereas in TAM67 cells this response was attenuated. Together, these findings argue that enhanced activation of the stress-related JNK pathway plays a functional role in antileukemic interactions between these agents.
Simultaneous exposure of AML blasts but not normal CD34+ cells to adaphostin and MG-132 results in a marked increase in cell death
To determine whether similar events might occur in primary human leukemia cells, blasts were obtained from the peripheral blood of 5 patients with AML (FAB M2 subtype in 4 cases; M5 in 1 case) and exposed for 24 hours to adaphostin (750 nM) and MG-132 (300 nM) alone and in combination. As shown in Figure 6A, exposure of blasts to adaphostin or MG-132 individually resulted in relatively little toxicity, whereas combined exposure was associated with a marked increase in cell death (eg, 60% to 75%) in each of the samples. Western blot analysis revealed that combined exposure of blasts from one of these patients (no. 2) to the agents in combination for 18 hours resulted in a marked increase in PARP cleavage accompanied by JNK activation, MEK1 cleavage, and down-regulation of Raf-1 (Figure 6B), analogous to results observed in Jurkat and U937 cells. Finally, parallel studies were performed in normal bone marrow CD 34+ cells and in normal peripheral-blood mononuclear-cell samples. In contrast to results obtained in primary and continuously cultured human leukemia cells, individual and combined exposure to adaphostin and MG-132 resulted in relatively little toxicity toward these normal hematopoietic cells (Figure 6C).
Discussion
Evidence of a role for oncogenic tyrosine kinases in neoplastic transformation in general, and in leukemogenesis in particular,26 provides a strong rationale for the development of tyrosine kinase inhibitors27 as antineoplastic agents such as adaphostin.3 However, the spectrum of activity of adaphostin is almost certainly not restricted to inhibition of the Bcr/Abl kinase. Significantly, several groups, including our own, have reported that adaphostin induces apoptosis in other Bcr/Abl-negative (such as U937, Jurkat, and others) cells through an oxidative injury-related mechanism.4-6 Interestingly, the mechanism of lethality of the clinically relevant proteasome inhibitor bortezomib in both malignant epithelial and hematologic cells has been linked to oxidative injury and ROS generation.14,15 In view of the possibility that a common mechanism may underly the antileukemic actions of these agents, such findings provide a rational basis for combination approaches involving oxidative damage to enhance antileukemic activity.
Synergistic interactions between MG-132 and adaphostin were associated with a reciprocal down-regulation of the survival-related Raf/MEK/ERK cascade and activation of the stress-related JNK pathway. It has been widely reported that the net balance between the Raf/MEK/ERK and SAPK/JNK modules governs survival decisions in various neoplastic cell types.28 In this context, ERK1/2 activation represents a well-described protective response to oxidative stress29 and, conversely, JNK activation is known to play an important role in oxidative damage-related lethality.30,31 The mechanisms by which these pathways exert opposing effects on apoptosis are not known with certainty. However, JNK activation has been implicated in cytochrome c release whereas ERK has been shown to inactivate caspase-9 32,33 as well as several proapoptotic Bcl-2 family members including Bad and Bim.34 It is unlikely to be coincidental that the lethal effects of both proteasome inhibitors and adaphostin have been associated with qualitatively similar effects on these MAP kinase pathways,6,15,20 suggesting that combining these agents further shifts the balance away from survival and toward stress-related signaling. This phenomenon may have particular relevance for proteasome inhibitors in view of evidence that interruption of the MEK/ERK pathway enhances the susceptibility of neoplastic cells to these agents.35 The findings that ectopic activation of Raf and MEK1 as well as pharmacologic or genetic disruption of the JNK pathway significantly attenuated adaphostin/MG-132 lethality provide further support for this hypothesis.
Combined treatment with adaphostin and MG-132 markedly induces death in primary AML cells while exhibiting little toxicity toward normal hematopoietic cells. (A) Primary human AML blasts (FAB M2 subtype in 4 samples; M5 in 1 sample) were suspended in medium containing 10% FCS at a cell density of 0.8 × 106/mL in the presence of 750 nM adaphostin plus or minus 300 nM MG-132 for 24 hours. At the end of drug exposure, apoptotic cells were monitored by annexin V/PI staining. Cell death for control blasts was generally less than 15% to 25%. (B) Primary AML blasts (sample 2) were exposed to drugs alone and in combination as in panel A for 18 hours, after which cell lysates were obtained and Western blot analysis performed to monitor PARP and MEK1 cleavage and levels of phospho-JNK and Raf-1. Each lane was loaded with 30 μg protein, and blots were stripped and reprobed with antitubulin antibodies to ensure equal loading and transfer of protein. (C) CD34+ cells obtained from the bone marrow of a patient undergoing a routine diagnostic procedure for a nonmyeloid hematologic disorder (eg, thrombocytopenia) were isolated by an immunomagnetic bead separation technique as described in “Materials and methods” and exposed to adaphostin (1.0 μM) ± MG-132 (300 nM) for 24 hours. At the end of this period, the percentage of apoptotic cells was determined by annexin V/PI staining and flow cytometry. A parallel experiment was also performed with normal peripheral-blood mononuclear cells (NPBMNC) from a healthy donor. Values represent the means plus or minus SD for triplicate determinations.
Combined treatment with adaphostin and MG-132 markedly induces death in primary AML cells while exhibiting little toxicity toward normal hematopoietic cells. (A) Primary human AML blasts (FAB M2 subtype in 4 samples; M5 in 1 sample) were suspended in medium containing 10% FCS at a cell density of 0.8 × 106/mL in the presence of 750 nM adaphostin plus or minus 300 nM MG-132 for 24 hours. At the end of drug exposure, apoptotic cells were monitored by annexin V/PI staining. Cell death for control blasts was generally less than 15% to 25%. (B) Primary AML blasts (sample 2) were exposed to drugs alone and in combination as in panel A for 18 hours, after which cell lysates were obtained and Western blot analysis performed to monitor PARP and MEK1 cleavage and levels of phospho-JNK and Raf-1. Each lane was loaded with 30 μg protein, and blots were stripped and reprobed with antitubulin antibodies to ensure equal loading and transfer of protein. (C) CD34+ cells obtained from the bone marrow of a patient undergoing a routine diagnostic procedure for a nonmyeloid hematologic disorder (eg, thrombocytopenia) were isolated by an immunomagnetic bead separation technique as described in “Materials and methods” and exposed to adaphostin (1.0 μM) ± MG-132 (300 nM) for 24 hours. At the end of this period, the percentage of apoptotic cells was determined by annexin V/PI staining and flow cytometry. A parallel experiment was also performed with normal peripheral-blood mononuclear cells (NPBMNC) from a healthy donor. Values represent the means plus or minus SD for triplicate determinations.
The bulk of evidence suggests that adaphostin/MG-132-mediated oxidative injury plays an important functional role in the lethality of this regimen. For example, subtoxic or marginally toxic concentrations of adaphostin and MG-132, administered individually, had little effect on ROS levels, whereas combined treatment of cells with these agents resulted in a marked potentiation of ROS generation. Notably, coadministration of the free-radical scavenger NAC blocked MG-132/adaphostin-mediated oxidative injury and significantly attenuated lethality. ROS generation leads to disruption of the mitochondrial respiratory chain, which can lead to release of proapoptotic mitochondrial molecules such as cytochrome c.36 Consistent with this model, NAC blocked MG-132/adaphostin-mediated cytochrome c release as well as that of AIF. The ability of oxidative injury to trigger down-regulation of the MEK/ERK pathway has also been described,29,37 as has been ROS-induced activation of the SAPK/JNK pathway.38 In accord with such findings, NAC also attenuated Raf/MEK/ERK down-regulation and JNK activation. It is nevertheless interesting that enforced activation of Raf-1 and MEK/ERK attenuated, although modestly, adaphostin/MG-132-induced ROS generation. Activation of the Raf/MEK/ERK cascade has also been reported to operate upstream of oxidative damage and to limit free-radical production.22,29 Thus, it is conceivable that inactivation of the Raf/MEK/ERK cascade in adaphostin/proteasome inhibitor-treated cells serves to amplify oxidative injury and, by extension, the apoptotic response.
While most of the observed events in adaphostin/proteasome inhibitor-treated cells (eg, ROS generation, Raf-1 down-regulation, ERK inactivation, cytochrome c and AIF release) were unaffected by the pancaspase inhibitor Boc-D-fmk, others (eg, MEK1 cleavage and Smac/DIABLO release) were inhibited. Previous studies have shown that multiple signaling proteins, including MEK1, are themselves susceptible to caspase-dependent degradation.6 Analogously, Smac/DIABLO release has been shown under some circumstances to be caspase mediated.39 Thus, while MG-132/adaphostin-mediated ROS generation in all likelihood represents the initial stimulus for activation of the apoptotic cascade, it remains possible that caspase-mediated events also amplify the cell-death process.
Notably, combined treatment with adaphostin and MG-132 resulted in marked toxicity toward multiple leukemic cell lines and primary AML samples but exerted relatively modest toxicity toward primary normal human hematopoietic cells. While the ability of adaphostin to inhibit the Bcr/Abl kinase2,21 might explain its targeted activity against Bcr/Abl-dependent leukemia cells, the basis for selectivity in Bcr/Abl-negative leukemia cells remains to be determined. Nevertheless, adaphostin has previously been reported to be relatively nontoxic toward normal human hematopoietic cells.21 Similarly, proteasome inhibitors have been shown to exert selective toxicity toward neoplastic cells at concentrations that are relatively sparing toward their normal counterparts.10,17 This phenomenon may be particularly true in the case of multiple myeloma cells, which are highly susceptible to the lethal actions of the proteasome inhibitor bortezomib.40 Thus, it is possible that the in vitro selectivity of the MG-132/adaphostin regimen may reflect selective potentiation of adaphostin lethality by proteasome inhibition. In this regard, the lethality of proteasome inhibitors such as bortezomib has been related to inhibition of the NF-κB pathway,41,42 which is known to play an important role in protecting cells from oxidative injury.43 It will therefore be interesting to determine whether proteasome inhibitors selectively enhance adaphostin-mediated oxidative injury in leukemic cells through an NF-κB-dependent process.
Whatever the mechanism(s) underlying the current observations, the ultimate relevance of this approach for leukemia will depend upon the further clinical development of tyrphostins such as adaphostin, which is ongoing,2,44 and whether these in vitro findings can be extended to the in vivo setting. In addition, the unique susceptibility of multiple myeloma cells to bortezomib suggests that the strategy of combining proteasome inhibitors with adaphostin may be well suited for plasma-cell dyscrasias. Accordingly, efforts to test this hypothesis in multiple myeloma and related cells are currently under way. Finally, recent studies have highlighted the ability of several novel agents including 2-methoxyestradiol (2-ME)45 and histone deacetylase inhibitors46 to induce apoptosis in neoplastic cells through induction of oxidative injury. Notably, transformed cells appear to be more susceptible to the lethal consequences of induction of oxidative injury than their normal counterparts.45,46 If validated, this notion could provide an explanation for the relative sparing of normal hematopoietic cells from adaphostin/proteasome inhibitor-mediated toxicity. Taken in conjunction with earlier findings, the present results raise the possibility that the rational combination of such agents may represent an effective strategy against malignant hematopoietic cells and also offer the potential for therapeutic selectivity.
Prepublished online as Blood First Edition Paper, September 15, 2005; DOI 10.1182/blood-2005-06-2302.
Supported by awards CA 63753, CA 100866, and CA 93738 from the National Institutes of Health, award 6045-03 from Leukemia and Lymphoma Society of America, an award from the Department of Defense (DAMD-17-03-1-0209), and a Translational Research Award from the V Foundation.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal