Abstract
Seventy-one patients with acute myeloid leukemia (AML), most of them (63/71) considered ineligible for conventional allogeneic hematopoietic stem cell transplantation (HSCT), were enrolled into a phase 2 study on reduced-intensity myeloablative conditioning with fractionated 8-Gy total body irradiation (TBI) and fludarabine (120 mg/m2). Patients received mobilized peripheral blood stem cells (n = 68) or bone marrow (n = 3) from siblings (n = 39) or unrelated donors (n = 32). Thirty-six patients received a transplant in complete remission (CR) and 35 had untreated or refractory disease (non-CR). Median patient age was 51 years (range, 20-66 years). Sustained engraftment was attained in all evaluable patients. With a median follow-up of 25.9 months (range, 3.7-61.2 months) in surviving patients, probabilities of overall survival for patients who received a transplant in CR and non-CR were 81% and 21% at 2 years, respectively. Relapse-free survival rates were 78% and 16%. The cumulative incidence of nonrelapse mortality (NRM) in CR patients was 8% at 2 years and beyond but amounted to 37% at 2 years in non-CR patients. Outcome data in this poor-risk population indicate that allogeneic HSCT from related or unrelated donors with 8-Gy TBI/fludarabine conditioning is feasible with low NRM and preserved antileukemic activity in AML patients in first or later CR.
Introduction
For patients with acute myeloid leukemias (AMLs), allogeneic hematopoietic stem cell transplantation (HSCT) is one of the most potent treatment options currently available.1-7 Its antileukemic activity results in part from the powerful graft-versus-leukemia (GvL) effect mediated by donor immune cells8-10 but is also attributed to myeloablative conditioning with high-dose chemoradiotherapy. However, the gains in long-term disease control achieved with conventional allogeneic HSCT are to be weighed against considerable nonrelapse mortality (NRM) secondary to treatment-related toxicity, severe graft-versus-host disease (GvHD), and infectious complications.1-7,11-14 In a recent registry report, 2-year probabilities of NRM with standard approaches were 23% to 24%, 31% to 33%, and 46% to 48% for AML patients who received a transplant from sibling donors in first and second complete remission (CR) and with advanced disease, respectively, regardless of whether the stem cell source was bone marrow or peripheral blood.15
In order to overcome the high risk of fatal treatment-related complications, reduced-intensity and nonmyeloablative conditioning regimens for allogeneic HSCT are currently being explored in various hematologic malignancies including AML.16-28 These novel transplantation strategies improve treatment safety and harness GvL effects rather than the eradication of the malignant clone by high-dose chemo-radiotherapy. The regimens proposed, however, differ considerably in dose intensity and myelosuppressive activity of cytotoxic agents. In a recent retrospective comparison of reduced-intensity and even lower-dose conditioning for allogeneic HSCT in patients with AML and high-risk myelodysplastic syndromes, higher dose intensity of the preparative regimen was shown to be relevant for long-term disease control though associated with increased morbidity and NRM. In the referenced study, both regimens consisted of combinations of fludarabine with cytostatic agents (ie, melphalan or cytarabine/idarubicin).25,29 Earlier studies comparing total body irradiation (TBI)-based myeloablative regimens found similar dosing effects with an inverse relation between long-term antileukemic activity and NRM.11,13,14,30,31 Thus, at least for allogeneic HSCT in AML, the optimal dose intensity of preparative regimens for disease control at an acceptable rate of treatment-related lethal complications has not been determined.
We therefore evaluated reduced-intensity myeloablative conditioning with 8-Gy TBI and fludarabine in AML patients considered ineligible for conventional conditioning. The results suggest that with this preparative regimen, related and unrelated donor transplantations can be performed in AML patients in first or second CR with a remarkably low 2-year NRM and satisfactory disease control.
Patients, materials, and methods
Patient eligibility and accrual
From May 1999 to March 2003, 50 patients with AML who had an HLA-compatible related or unrelated donor and were considered ineligible for conventional conditioning were enrolled on a prospective reduced-intensity transplantation protocol of the Cooperative German Transplant Study Group. From September 2003 to October 2004, 21 additional patients were treated on this protocol in order to validate outcome data particularly in CR patients. Pretransplantation comorbidities were assessed retrospectively by means of the weighted Charlson comorbidity index (CCI) as adapted recently for allogeneic HSCT.32-34 Patients were accrued at the Universities of Muenster, Dresden, and Marburg and at the Clinic for Bone Marrow Transplantation (BMT) and Hematology/Oncology in Idar-Oberstein, Germany. The protocol was approved by the institutional review boards and written informed consent was obtained from all patients. Results were analyzed as of February 1, 2005.
Treatment regimen
All patients were conditioned with 8 Gy-TBI applied in 4 fractions of 2 Gy with dose rates varying by centers from 8.9 to 30 cGy/min on 2 consecutive days. Fludarabine at a total dose of 120 mg/m2 was given intravenously on 4 consecutive days (30 mg/m2 over 60 minutes daily) in all except 3 patients. Two patients, one with dyskeratosis congenita and one 53-year-old patient with intense pretreatment, received a total dose of 90 mg/m2. In one patient with refractory AML, fludarabine was given at a total dose of 150 mg/m2. Additional pretreatment with antithymocyte globulin (ATG) was recommended per protocol but left to the decision of the participating centers. Forty-five patients received rabbit ATG (Fresenius, Bad Homburg, Germany) at a total dose of 40 mg/kg body weight (BW) and 4 patients received rabbit ATG (Merieux, Lyon, France) at doses of 1 to 10 mg/kg BW. Twenty-four of 36 patients (66%) with CR and 25 of 35 patients (71%) with active disease received ATG as well as 27 of 39 patients with sibling donors (69%) and 22 of 32 (69%) with unrelated donors (differences not significant).
All patients received cyclosporine (CSA) for acute GvHD prophylaxis starting with a dose of 3 mg/kg/d intravenously and were changed to oral administration as soon as possible. The dose of CSA was adjusted to maintain blood levels between 150 and 300 ng/mL during the first 50 days and then tapered if GvHD was absent or inactive according to the transplantation centers' policy. A short course of methotrexate (MTX) was given in 66 patients on days 1 (15 mg/m2), 3, 6 (and 11; 10 mg/m2) after transplantation. The dose of MTX was decreased for severe mucositis, extravascular fluid accumulation, or renal dysfunction. The 5 patients without MTX treatment had refractory disease. Acute and chronic GvHD were diagnosed and graded using established criteria.35,36
Donor selection and blood stem cell harvest
Related or unrelated donors were selected based on the compatibility for HLA-A, -B, and -C by serologic matching or intermediate resolution DNA typing and for DRB1 and DQB1 by high-resolution DNA typing. Sixtyeight patients received peripheral blood stem cells (PBSCs); 3 patients received a bone marrow graft. Mobilization of PBSCs with granulocyte colony-stimulating factor (G-CSF) and aphereses were performed according to the policy of the collection center. The median number of CD34+ stem cells per kg BW in the PBSC grafts was 7.3 × 106 (range, 2.5 × 106-15.9 × 106). PBSCs or bone marrow were infused on day 0 without further manipulation.
Supportive care
Infectious disease prophylaxis during the peritransplantation period consisted of pentamidine inhalation or administration of trimethoprimsulfamethoxazole for Pneumocystis carinii prophylaxis, systemic antibiotics, fluconazole, and acyclovir. Patients underwent at least weekly surveillance testing for cytomegalovirus (CMV) pp65 antigen and/or polymerase chain reaction (PCR) for CMV DNA with preemptive ganciclovir therapy instituted for positive assays. Thirty-four patients received 5 μg/kg filgrastim subcutaneously daily beginning on day 6 after transplantation until an absolute neutrophil count (ANC) of 1.5 × 109/L or higher was achieved for at least 3 consecutive days. All blood products were filtered and irradiated prior to transfusion.
Engraftment and toxicity
Neutrophil engraftment was defined as the first of 3 consecutive days with an ANC of at least 0.5 × 109/L. Platelet engraftment was defined as the first day that the platelet count exceeded 50 × 109/L without transfusion support. Hematopoietic chimerism was evaluated on bone marrow or peripheral blood cells by fluorescent in situ hybridization (FISH) studies in sex-mismatched cases for Y chromosome or analysis of DNA microsatellite polymorphisms by PCR as described.37,38 The sensitivity of chimerism analysis was 1% by FISH and 5% or less by PCR.37,38 Chimerism was assessed within 2 months of transplantation and subsequently at least every third month during the first 2 years. Regimen-related toxicity was graded according to the Common Toxicity Criteria Version 2.0 of the National Cancer Institute of the United States.39
Response, causes of death
CR prior to HSCT was defined by morphology of less than 5% blasts in marrow aspirates, the absence of blasts in the peripheral blood and of extramedullary manifestations, and ANC greater than 1.5 × 109/L. Relapse was defined by standard morphologic criteria, reappearance of informative cytogenetic abnormalities, or both. CR after transplantation was defined using the same criteria as before HSCI with donor cell engraftment.
Deaths after relapse were categorized as due to malignancy irrespective of any other causes. Early death was defined as death before day 15 after transplantation. Deaths in the absence of active AML, including early deaths, were categorized as NRM. Thus, the term NRM as used here determines the number of deaths with no relapse in relation to the number of patients evaluated in the study and is sometimes given as a percentage. Additionally, wherever the term mortality is used it relates to the study population.
Statistical analyses
The primary end points of this exploratory study were overall survival (OS), relapse-free survival (RFS), and the cumulative incidences of mortality from disease progression (MDP) and NRM. OS was calculated from the day of transplantation, with patients alive at the time of last follow-up being administratively censored. RFS was counted from the day of transplantation to relapse or death. Both, OS and RFS were estimated by Kaplan and Meier curves.40 Probabilities of relapse, MDP, GvHD, and NRM were calculated using cumulative incidence estimates to accommodate competing risks.41
For statistical comparisons of group characteristics (donor type, ATG treatment, GvHD incidence), chi-square analysis was used. Differences in estimated OS, RFS, relapse incidence, MDP, and NRM between subgroups were evaluated using the log-rank test. The Cox proportional hazards regression model was used to assess the ability of patient covariates to predict probabilities of relapse. All computations were carried out in SPSS (version 11.0.1; SPSS, Chicago, IL) or SAS (version 8.02; SAS Institute, Cary, NC) using standard program functions.
Results
Patients and disease characteristics
Patient, disease, and transplantation characteristics are summarized in Table 1. All 71 patients had primary or secondary AML. The first 50 patients enrolled in the protocol were considered ineligible for conventional allogeneic HSCT by the treating physicians because of age (≥ 60 years), comorbidities (eg, severe diabetes mellitus, chronic obstructive pulmonary disease, or congestive heart failure), pretreatment intensity (eg, prior autologous HSCT), pretreatment-related severe infections (eg, recent [< 30 days] but controlled invasive fungal infection with suspected residual activity), or prior organ toxicities (eg, chronic renal failure). Of the 21 additional patients who received a transplant per protocol between September 2003 and October 2004, 13 were ineligible for conventional conditioning therapy because of reasons outlined in Table 1. Thirty of these 63 patients fulfilled more than one of the exclusion criteria listed in Table 1. Invasive fungal infection was the sole reason for applying reduced-intensity conditioning according to the study protocol in 14 patients and a contributory criterion in an additional 13 patients.
Patient, disease, and donor characteristics
Characteristics . | Values . |
|---|---|
| Median age, y (range) | 51 (20-66) |
| Sex, no. (%) | |
| Male | 42 (59) |
| Female | 29 (41) |
| Disease diagnosis, no. (%) | |
| AML FAB M1 | 13 (18) |
| AML FAB M2 | 16 (23)* |
| AML FAB M3 | 1 (1)* |
| AML FAB M4 | 16 (23) |
| AML FAB M5 | 16 (23) |
| AML FAB M6 | 3 (4) |
| AML unclassified | 6 (8) |
| Secondary AML | 17 (24) |
| Karyotype | |
| Good prognosis | 5 (7)† |
| Standard risk | 47 (66) |
| Poor prognosis | 17 (24) |
| Not available | 2 (3) |
| Disease status at transplantation, no. (%) | |
| Complete remission | 36 (51) |
| First CR | 22 (31) |
| Second CR | 14 (20) |
| Non-CR | 35 (49) |
| Untreated primary disease | 1 (1) |
| Untreated relapse | 7 (10) |
| Refractory disease | 27 (38) |
| Primary induction failure | 15 (21) |
| Refractory relapse | 12 (17) |
| Median time from diagnosis to transplantation, mo. (range) | 6 (1-54) |
| Patients considered ineligible for conventional conditioning by main exclusion criteria, no. (%) | 63 (87) |
| Age, older than 60 y | 11 (16) |
| Invasive fungal infection | 27 (38) |
| Severe infections during prior therapy | 8 (11) |
| Multiple therapies prior to transplantation | 2 (3) |
| Prior autograft/allograft‡ | 10 (14) |
| Organ dysfunction/prior organ toxicity | 5 (7) |
| Recipient CMV positive, no. (%) | 44 (62) |
| Donor characteristics, no. (%) | |
| HLA-identical sibling | 38 (54) |
| Mismatched sibling | 1 (1) |
| Matched unrelated, 10/10 HLA match | 22 (31) |
| Mismatched unrelated, 9 or fewer/10 HLA match | 10 (14) |
| Source of stem cells, no (%) | |
| PBSCs | 68 (96) |
| BM | 3 (4) |
| Median no. CD34+ cells infused × 106/kg (range) | 7.2 (1.8-15.9) |
Characteristics . | Values . |
|---|---|
| Median age, y (range) | 51 (20-66) |
| Sex, no. (%) | |
| Male | 42 (59) |
| Female | 29 (41) |
| Disease diagnosis, no. (%) | |
| AML FAB M1 | 13 (18) |
| AML FAB M2 | 16 (23)* |
| AML FAB M3 | 1 (1)* |
| AML FAB M4 | 16 (23) |
| AML FAB M5 | 16 (23) |
| AML FAB M6 | 3 (4) |
| AML unclassified | 6 (8) |
| Secondary AML | 17 (24) |
| Karyotype | |
| Good prognosis | 5 (7)† |
| Standard risk | 47 (66) |
| Poor prognosis | 17 (24) |
| Not available | 2 (3) |
| Disease status at transplantation, no. (%) | |
| Complete remission | 36 (51) |
| First CR | 22 (31) |
| Second CR | 14 (20) |
| Non-CR | 35 (49) |
| Untreated primary disease | 1 (1) |
| Untreated relapse | 7 (10) |
| Refractory disease | 27 (38) |
| Primary induction failure | 15 (21) |
| Refractory relapse | 12 (17) |
| Median time from diagnosis to transplantation, mo. (range) | 6 (1-54) |
| Patients considered ineligible for conventional conditioning by main exclusion criteria, no. (%) | 63 (87) |
| Age, older than 60 y | 11 (16) |
| Invasive fungal infection | 27 (38) |
| Severe infections during prior therapy | 8 (11) |
| Multiple therapies prior to transplantation | 2 (3) |
| Prior autograft/allograft‡ | 10 (14) |
| Organ dysfunction/prior organ toxicity | 5 (7) |
| Recipient CMV positive, no. (%) | 44 (62) |
| Donor characteristics, no. (%) | |
| HLA-identical sibling | 38 (54) |
| Mismatched sibling | 1 (1) |
| Matched unrelated, 10/10 HLA match | 22 (31) |
| Mismatched unrelated, 9 or fewer/10 HLA match | 10 (14) |
| Source of stem cells, no (%) | |
| PBSCs | 68 (96) |
| BM | 3 (4) |
| Median no. CD34+ cells infused × 106/kg (range) | 7.2 (1.8-15.9) |
FAB indicates French-American-British; CMV, cytomegalovirus; PBSCs, peripheral blood stem cells; and BM, bone marrow.
Of the 16 patients with AML FAB M2, 5 received a transplant in CR1 (2 with secondary AML), 4 in CR2, 7 with non-CR. The patient with AML FAB M3 was in second CR
Four patients in second CR; one additional patient with complex karyotype and t(8;21) in first CR but with early relapse after transplantation
One patient with prior allograft
The median patient age was 51 years (range, 20-66 years). Thirty-six patients received a transplant in CR (CR1, 23; CR2, 13) and 35 patients in non-CR (refractory disease, 27; untreated relapse, 7; untreated primary disease, 1). Pretransplantation comorbidities as assessed by the adapted CCI were similar in the 2 patient groups regardless of whether or not age was included in the score. The proportions of patients who received a transplant in CR or in non-CR with a CCI score (including age) of 0 were 17% and 9%, with a CCI score of 1 to 2 were 66% and 82%, and with a CCI score of 3 or greater were 17% and 9%, respectively.
Thirty-eight patients received a transplant from HLA-identical sibling donors (CR1, 15; CR2, 7; non-CR, 16) and 32 from matched unrelated donors (CR1, 7; CR2, 7; non-CR, 18), 3 of them with an allele mismatch in HLA DRB1 (2 with an additional mismatch in HLA Cw) and 7 of them with an antigen mismatch in HLA Cw. One patient with untreated relapse received a transplant from a sibling donor with 2 allele mismatches in HLA DRB1 and DQB1.
The median follow-up of the surviving patients was 25.9 months (range, 3.7-61.2 months).
Engraftment and chimerism
All of the 67 evaluable patients achieved sustained neutrophil engraftment after a median of 18 days (range, 8-33 days). Of these patients, 56 (80%) reached a platelet count of greater than 50 × 109/L without transfusion support after a median of 22 days (range, 12-288 days). Four patients were not evaluable for engraftment because of early death. Within 2 months of transplantation, donor cell chimerism was at least 94% in 66 of 67 patients (median, 99%; range, 94%-100%). One patient who received a transplant for refractory AML showed a mixed donor chimerism of 67% on day 27 and died early from progressive disease on day 159 after transplantation. Thirty-five of the 37 surviving patients had follow-up chimerism analyses at the scheduled time points from 98 to 1597 days after transplantation (median, 531 days), showing a median donor cell chimerism of 99% (range, 2%-100%). Only one of those patients had a secondary graft failure of unknown cause with 2% donor chimerism one year after transplantation. This patient recovered with his own hematopoiesis without further treatment. Engraftment data are summarized in Table 2.
Patient outcome
. | Values . | . | |
|---|---|---|---|
. | CR patients . | Non-CR patients . | |
| No. patients | 36 | 35 | |
| Neutrophil engraftment | |||
| No. patients evaluable | 35 | 32 | |
| Median time to ANC greater than 0.5 × 109/L, d (range) | 18 (11-25) | 18 (8-33) | |
| Platelet engraftment | |||
| No. patients evaluable | 35 | 24 | |
| Median time to platelet count greater than 50 × 109/L, d (range) | 21 (12-76) | 26 (12-288) | |
| Donor cell chimerism, median % (range)* | 99 (95-100) | 99 (67-100) | |
| Acute GvHD, no. (%) | |||
| No. patients evaluable | 35 | 32 | |
| Grade II-IV | 8 (23) | 20 (63) | |
| Grade III-IV | 8 (23) | 8 (25) | |
| Chronic GvHD, no. (%) | |||
| No. patients evaluable | 34 | 25 | |
| Limited disease | 11 (32) | 2 (8) | |
| Extensive disease | 6 (18) | 8 (25) | |
| Early death, 0-15 d after transplantation, no. (%) | 1 (3) | 3 (9) | |
| Death not related to relapse or disease progression, no. (%) | 3 (8) | 13 (37) | |
| Relapse, no. (%) | 6 (17) | 16 (46) | |
| Death with relapsed or progressive disease, no. (%) | 3 (8) | 15 (43) | |
| Alive and well in CR, no. (%) | 30 (83) | 6 (17) | |
| Alive with controlled disease, no. (%) | 0 | 1 (3) | |
. | Values . | . | |
|---|---|---|---|
. | CR patients . | Non-CR patients . | |
| No. patients | 36 | 35 | |
| Neutrophil engraftment | |||
| No. patients evaluable | 35 | 32 | |
| Median time to ANC greater than 0.5 × 109/L, d (range) | 18 (11-25) | 18 (8-33) | |
| Platelet engraftment | |||
| No. patients evaluable | 35 | 24 | |
| Median time to platelet count greater than 50 × 109/L, d (range) | 21 (12-76) | 26 (12-288) | |
| Donor cell chimerism, median % (range)* | 99 (95-100) | 99 (67-100) | |
| Acute GvHD, no. (%) | |||
| No. patients evaluable | 35 | 32 | |
| Grade II-IV | 8 (23) | 20 (63) | |
| Grade III-IV | 8 (23) | 8 (25) | |
| Chronic GvHD, no. (%) | |||
| No. patients evaluable | 34 | 25 | |
| Limited disease | 11 (32) | 2 (8) | |
| Extensive disease | 6 (18) | 8 (25) | |
| Early death, 0-15 d after transplantation, no. (%) | 1 (3) | 3 (9) | |
| Death not related to relapse or disease progression, no. (%) | 3 (8) | 13 (37) | |
| Relapse, no. (%) | 6 (17) | 16 (46) | |
| Death with relapsed or progressive disease, no. (%) | 3 (8) | 15 (43) | |
| Alive and well in CR, no. (%) | 30 (83) | 6 (17) | |
| Alive with controlled disease, no. (%) | 0 | 1 (3) | |
ANC indicates absolute neutrophil count; GvHD, graft-versus-host disease.
Within 2 months of transplantation
Regimen-related toxicity
Regimen-related toxicities are specified and graded in Table 3. Grade III and IV toxicities were observed in 66% and 24%, respectively, of the total patient population. In patients who received a transplant in CR, reversible grade III toxicities, predominately infections, mucositis, and elevation of liver enzymes and bilirubin level, occurred in 72% (26/36). Seven patients in this group (19%) developed grade IV toxicities (3 reversible liver dysfunctions, 1 reversible infection, and 3 fatal infections).
Maximum organ toxicities
. | Organ toxicities, no. patients (%) . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade . | Renal . | Hepatic . | Pulmonary . | Cardiac . | GI . | Mucositis . | CNS . | Infection . | |||||||
| 0 | 42 (59) | 34 (48) | 51 (72) | 54 (76) | 32 (45) | 10 (14) | 57 (80) | 19 (27) | |||||||
| I | 11 (15) | 1 (1) | 6 (8) | 2 (3) | 15 (21) | 8 (11) | 4 (6) | 0 (0) | |||||||
| II | 10 (14) | 10 (14) | 2 (3) | 4 (6) | 16 (23) | 16 (23) | 6 (8) | 3 (4) | |||||||
| III | 4 (6) | 16 (23) | 8 (11) | 8 (11) | 7 (10) | 37 (52) | 4 (6) | 42 (59) | |||||||
| IV | 4 (6) | 10 (14)* | 4 (6) | 3 (4) | 1 (1) | 0 (0) | 0 (0) | 7 (10) | |||||||
. | Organ toxicities, no. patients (%) . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade . | Renal . | Hepatic . | Pulmonary . | Cardiac . | GI . | Mucositis . | CNS . | Infection . | |||||||
| 0 | 42 (59) | 34 (48) | 51 (72) | 54 (76) | 32 (45) | 10 (14) | 57 (80) | 19 (27) | |||||||
| I | 11 (15) | 1 (1) | 6 (8) | 2 (3) | 15 (21) | 8 (11) | 4 (6) | 0 (0) | |||||||
| II | 10 (14) | 10 (14) | 2 (3) | 4 (6) | 16 (23) | 16 (23) | 6 (8) | 3 (4) | |||||||
| III | 4 (6) | 16 (23) | 8 (11) | 8 (11) | 7 (10) | 37 (52) | 4 (6) | 42 (59) | |||||||
| IV | 4 (6) | 10 (14)* | 4 (6) | 3 (4) | 1 (1) | 0 (0) | 0 (0) | 7 (10) | |||||||
Toxicity grading according to NCI common toxicity criteria (CTC 2.0) with recommended adaptations for bone marrow transplantation.
Includes 2 suspected severe veno-occlusive diseases
Similarly, grade III and IV toxicities were documented in 60% and 29%, respectively, of the patients who received a transplant in non-CR. Most common toxicities were infections, mucositis, elevation of liver enzymes and bilirubin level, and pulmonary and cardiac dysfunctions. Of the 10 patients with grade IV toxicities, 6 had severe liver toxicity (2 veno-occlusive diseases, both fatal) in combination with other adverse events (renal, pulmonary, cardiac, or infectious), 1 developed fatal heart failure, 1 had a fatal pulmonary hemorrhage, and 2 patients died from septic multiorgan failure.
GvHD
Cumulative incidences of acute GvHD for the total cohort are depicted in Figure 1. Grade II, III, and IV acute GvHD occurred in 19%, 14%, and 3%, respectively, of 37 evaluable patients who received a transplant from sibling donors. The corresponding rates for the 30 evaluable patients who received a transplant from unrelated donors were 17%, 13%, and 7%, respectively. The cumulative incidence of grade II to IV GvHD was lower in patients who received a transplant in CR (23%) than in non-CR patients (50%; P < .05), although neither the percentage of unrelated donor transplantations (39% and 51%, respectively) nor the percentage of patients receiving ATG with the preparative regimen (67% and 71%, respectively) differed significantly between the groups.
Acute GvHD. Estimates of cumulative incidences of acute GvHD by severity grading.
Acute GvHD. Estimates of cumulative incidences of acute GvHD by severity grading.
Of the 36 patients who received a transplant in CR, 34 were evaluable for chronic GvHD. Among these patients, 17 developed chronic GvHD (11 limited and 6 extensive). Of those 17 CR patients with chronic GvHD, 10 had received ATG with the conditioning regimen. Because of the higher competing risks of NRM and MDP, the incidence of chronic GvHD tended to be lower in non-CR patients (25 evaluable patients, 2 limited and 8 extensive chronic GvHDs).
Disease response and relapse
Disease responses and outcomes after transplantation are shown in Table 2. Of the 35 evaluable patients who received a transplant in CR, 6 patients suffered a relapse between days 68 and 868 after transplantation (cumulative incidence 17%). All 3 patients with late relapse (> 1 year after transplantation) achieved a subsequent CR lasting 1301+, 1160+, and 1160+ days, respectively. Reinduction treatment consisted of cytoreductive therapy with cytarabine, additional blood stem cell transfusions from the original donor, and subsequent treatment with granulocyte-macrophage colony-stimulating factor (GM-CSF) in 2 patients42 or a second transplantation from the original donor in the third patient (conditioning therapy with fludarabine and melphalan).
Of the 35 non-CR patients, 32 were evaluable for disease response and relapse incidence, whereas 3 patients died early (days 9, 12, and 13, respectively). Relapse of AML occurred in 16 of 32 patients between days 27 and 315 after transplantation (median, 84 days; cumulative incidence, 50%). Two patients, one relapsing on day 155 and one on day 315, initially responded to donor lymphocyte and blood progenitor cell transfusions. One of these patients died 1583 days after transplantation from progressive disease and one is alive with controlled disease at 684+ days. Four non-CR patients have remained in CR after transplantation.
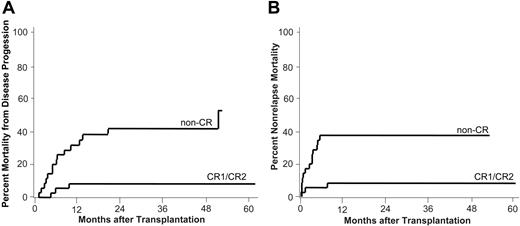
Mortality from disease progression and nonrelapse mortality. Estimates of the cumulative incidences of mortality from disease progression (A) and nonrelapse mortality (B) by disease status at transplantation are shown. CR1/CR2 indicates complete remission 1 (n = 22) or 2 (n = 14); non-CR, patients with refractory disease (n = 27), untreated relapse (n = 7), or untreated primary disease (n = 1).
Mortality from disease progression and nonrelapse mortality. Estimates of the cumulative incidences of mortality from disease progression (A) and nonrelapse mortality (B) by disease status at transplantation are shown. CR1/CR2 indicates complete remission 1 (n = 22) or 2 (n = 14); non-CR, patients with refractory disease (n = 27), untreated relapse (n = 7), or untreated primary disease (n = 1).
Mortality from disease progression
Cumulative incidence of MDP for patients who received a transplant in CR was 8% (3 patients) at 3 years. Fifteen of 35 patients with active disease (non-CR) who received a transplant died from disease progression at a median of 193 days after transplantation (range, 45-1583 days; cumulative incidence, 42%; Figure 2A).
Nonrelapse mortality
Notably, of 36 patients who received a transplant in CR, only 3 patients (8%) had died from causes other than relapse at 1 year and the cumulative rate of NRM remained at 8% by 2 years and beyond. Two of the 3 patients died from infections while in CR on day 42 and 232, respectively. One 64-year-old patient who received a transplant in CR2 with reduced renal function and invasive fungal infection died early on day 14 due to severe septicemia.
The cumulative rates of NRM for patients with active disease (non-CR) who received a transplant were 20% (n = 7) at day 100 and 37% (n = 13) at 1 and 2 years. In this group, patients died from causes other then progressive disease at a median of 78 days (range, 9-168 days) after transplantation (Figure 2B).
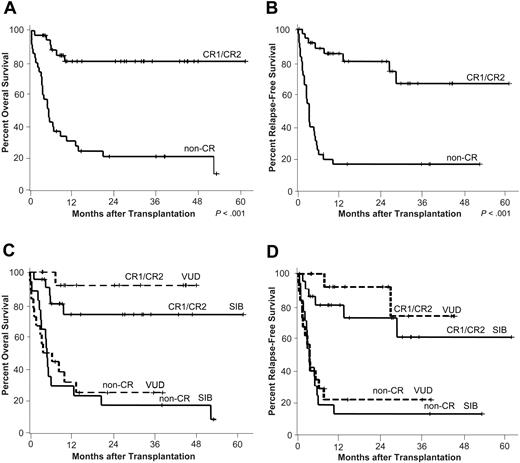
Overall and relapse-free survival. Kaplan-Meier estimates of overall (A) and relapse-free survival (B) by disease status at transplantation are depicted. CR1/CR2 indicates complete remission 1 (n = 22) or 2 (n = 14); non-CR, patients with refractory disease (n = 27), untreated relapse (n = 7), or untreated primary disease (n = 1). (C-D) Overall and relapse-free survival was similar for patients who received a transplant from siblings (SIB) or volunteer unrelated donors (VUD) (CR group: SIB n = 22, VUD n = 14; non-CR group: SIB n = 17, VUD n = 18).
Overall and relapse-free survival. Kaplan-Meier estimates of overall (A) and relapse-free survival (B) by disease status at transplantation are depicted. CR1/CR2 indicates complete remission 1 (n = 22) or 2 (n = 14); non-CR, patients with refractory disease (n = 27), untreated relapse (n = 7), or untreated primary disease (n = 1). (C-D) Overall and relapse-free survival was similar for patients who received a transplant from siblings (SIB) or volunteer unrelated donors (VUD) (CR group: SIB n = 22, VUD n = 14; non-CR group: SIB n = 17, VUD n = 18).
Relapse-free and overall survival
For patients who received a transplant in CR, RFS at 2 years was 78% (95% confidence interval [CI], 63%-93%; Figure 3B). Since 3 of the 6 relapsing patients achieved another long-lasting CR, OS at 2 years and beyond remained stable at 81% (95% CI, 67%-95%; Figure 3A).
The high rates of MDP and NRM in non-CR patients resulted in a RFS and OS of only 16% (95% CI, 3%-29%; Figure 3B) and 21% (95% CI, 7%-36%; Figure 3A), respectively, at 2 years.
OS and RFS were similar in patients who received a transplant from sibling or unrelated donors (Figure 3C-D).
Association among variables in univariate and Cox model analyses
Univariate analysis demonstrated that disease status before transplantation was significantly associated with the probabilities of relapse (P < .001), MDP (P < .001), NRM (P = .002), RFS (P < .001), and OS (P < .001). As another disease characteristic, unfavorable AML karyotype was associated with higher probabilities of relapse (P = .04) and shorter RFS (P = .02). Sex mismatch between host and donor significantly increased the probability of NRM (P = .016), whereas it did not influence RFS and OS. The use of ATG, patient age, primary or secondary AML, CMV status, related or unrelated donor, or occurrence of acute or chronic GvHD did not show significant associations with any of the above outcome parameters. Cox proportional hazards regression showed that only disease status at transplantation was independently related to OS (hazard ratio [HR] 6.87; 95% CI, 2.68-17.63; P < .001), RFS (HR 5.62; 95% CI, 2.49-12.68; P < .001), and NRM (HR 5.78; 95% CI, 1.56-21.67; P = .009).
Discussion
Despite improvements in treatment safety, the corridor of favorable benefit-risk ratios for conventional allogeneic HSCT has remained narrow, limiting indications to patients with a dismal disease prognosis who are young and otherwise medically fit. Thus, a majority of potential candidates in many hematologic malignancies including AML are still being excluded from the procedure because of its inherent vital risks. The present trial was aimed at defining a transplantation strategy in AML with an improved benefit-risk ratio (ie, maintained antileukemic activity with reduced treatment-related morbidity and lethality) and the disease or remission status in which patients would benefit most.
The study was designed as a phase 2 trial in patients with AML considered to have a transplantation indication but not eligible for allogeneic HSCT with conventionally dosed conditioning. Several reasons led us to evaluate this intermediate-dose TBI-based preparative regimen. In a meta-analysis on the efficacy and safety of conventional myeloablative conditioning with cyclophosphamide in combinations with either busulfan or TBI, both types of regimens were shown to yield similar survival outcomes in the overall study population.43 A nonsignificant 10% higher survival rate after cyclophosphamide/TBI compared with cyclophosphamide/busulfan in patients who received a transplant for AML reported in the referenced article43 is to be interpreted with caution. Notwithstanding, the 10-year overall and disease-free survival estimates for AML patients (mainly CR1) of 63% and 57%, respectively, demonstrate the potent antileukemic activity of TBI regimens. Likewise, a recent survey of the International Bone Marrow Transplant Registry (IBMTR) unveils a lower relapse risk for AML after allogeneic bone marrow transplantation with conventionally dosed TBI conditioning in comparison to busulfan/cyclophosphamide combinations.44 Increasing the TBI dose from 12 to 15.75 Gy tended to reduce the relapse rate in patients who received a transplant for AML, but a significantly higher NRM during the first 6 months after transplantation abolished potential survival advantages.11,31 Lowering the TBI dose to 9.9 Gy given along with cyclophosphamide appeared to be associated with a higher relapse rate while not reducing NRM.45,46 However, relapse rates may be dependent on not only total TBI dose but also dose rate and fractionation schema. Along these lines, evidence of an improved benefit-risk ratio is provided by a recent series of unrelated donor transplantations after conditioning with single-exposure TBI of 5.5 Gy and cyclophosphamide.27,28 Further reducing the TBI dose to 2 Gy has yielded promising results with regard to engraftment and substantially lowered treatment-related toxicity.20,21 However, for patients with AML, it remains to be shown whether this minimal conditioning is sufficiently potent for long-term disease control.23 With this background, we hypothesized that an intermediate-dose 8-Gy fractionated TBI-conditioning schedule combined with selectively lymphocytotoxic fludarabine would improve the benefit-risk ratio of allogeneic HSCT, at least for AML patients with controlled or sensitive disease who received a transplant.
The results certainly need to be interpreted with caution since the trial, though prospective, was primarily explorative and the numbers of patients treated within each group are still limited. As another limitation of the study, TBI dose rates and the policies of ATG treatment (actual dosing and numbers of patients receiving ATG) differed between the participating centers. However, subgroup analyses showed similar outcomes for engraftment, overall toxicities, and relapse or survival in CR patients and non-CR patients treated with or without ATG, and univariate analyses failed to demonstrate any significant association of these outcome parameters to ATG treatment. These findings indicate that a potential for inherent selection bias secondary to treatment with or without ATG did not alter patient outcome in the present study, although the data do not allow drawing any firm conclusions on the role of ATG as a part of conditioning regimens. On the other hand and in contrast to the majority of studies on reduced-intensity conditioning, the data are representative of a single disease entity (ie, AML) and represent data from a multicenter trial. With a median follow-up of 25.9 months for surviving patients, the observed trends are stabilizing hence allowing to draw conclusions and generate hypotheses for future testing in randomized studies.
The risk of grade III and IV organ toxicities with the 8-Gy regimen was substantial (66% an 24%, respectively), even though toxicities were reversible in most cases. It should be borne in mind that the combined 66% grade III toxicity rate according to National Cancer Institute (NCI) criteria (version 2.0) includes reversible grade III infections in 42 (59%) of 71 patients. Notwithstanding, rates of grade III/IV mucositis and hepatic toxicity (52% and 37%, respectively) were considerably higher than those reported for the 5.5-Gy (6%-9% and 3%-8%)27,28 and the 2-Gy regimens (2% and 18%-21%).20,21 Possibly, the risk of serious toxicity with the 8-Gy TBI dose could be reduced (eg, by uniform TBI dose rate or by novel supportive approaches preventing severe mucositis).47 However, in contrast to outcomes in patients with active and in most of our cases refractory disease who received a transplant, severe organ toxicities excluding infections were consistently reversible in CR patients (fatal organ toxicities, 0/36 CR patients versus 4/35 non-CR patients). Hence, although the regimen-related toxicity in CR patients was higher than that reported for the 2-Gy or 5.5-Gy regimens, it does appear to be manageable without translating into fatal events. Consequently, the 2-year or greater cumulative incidence of NRM was only 8% in patients who received a transplant with CR and, thus, in the lower range reported for 2-Gy conditioning (1-year NRM, 16%-20%), despite comparable pretransplantation comorbidity scores.33,34
However, the favorable survival data in this group of patients are not only attributable to the lowered NRM but also reflect the retained antileukemic activity of the study regimen and the improving immunocellular treatment options for relapses after transplantation that can provide long-lasting remissions and possibly cure.42,48 Finally, patients who received a transplant from siblings or unrelated donors had similar survival outcomes. This observation would suggest that the favorable benefit-risk ratio of the study regimen equally applies to related and unrelated donor transplantations with the improved donor-recipient matching currently in use. Similar observations have recently been reported for allogeneic HSCT after myeloablative conditioning in acute lymphoblastic leukemia.49 While our finding likely reflects small patient sample size, one might also speculate that particularly in the setting of reduced-intensity conditioning the use of unrelated donors potentially could be more efficacious secondary to an improved GvL effect.
The high rates of both NRM and relapse incidence for patients with active disease observed in the present study are comparable to those reported for conventional conditioning.15,50,51 In this subgroup of AML patients, conditioning with 8-Gy TBI/fludarabine does not confer a treatment advantage indicating the need for novel, probably more aggressive transplantation approaches.
In summary, reduced-intensity conditioning with TBI 8-Gy/fludarabine appears to be a safe and effective preparative regimen for related and unrelated donor stem cell transplantations in AML. This conclusion applies to patients in first or later CR considered medically unfit for conventional myeloablative conditioning. In addition, considering the favorable survival data in CR patients and the improving immunocellular salvage options for posttransplantation AML relapses as successfully used in the present trial, this regimen might also offer an improved benefit-risk ratio to AML patients in CR who are medically fit enough for standard conditioning regimens. To further substantiate this notion, 2 separate randomized trials in AML CR1 and CR2 or more are currently being conducted by the Cooperative German Transplant Study Group, both of which compare TBI 8-Gy/fludarabine to conventionally dosed conditioning with TBI 12-Gy/cyclophosphamide.
Appendix
Department of Hematology, Oncology, and Transfusion Medicine, Charite-Campus Benjamin Franklin, Berlin, Germany; Department of Hematology and Oncology, University Hospital Charite, Campus Virchow-Klinikum, Berlin, Germany; Klinik für Innere Medizin III, Klinikum Chemnitz, Chemnitz, Germany; Department of Hematology/Oncology, University of Dresden, Dresden, Germany; Department of Hematology, Oncology and Clinical Immunology, Heinrich-Heine University, Duesseldorf, Germany; Department of Bone Marrow Transplantation, University Hospital of Essen, Essen, Germany; Department of Haematology and Oncology, Johann Wolfgang Goethe University, Frankfurt/Main, Germany; Department of Hematology and Oncology, Albert Ludwigs University Medical Center, Freiburg, Germany; Department of Hematology/Oncology, Martin Luther University, Halle-Wittenberg, Germany; Bone Marrow Transplantation, University Hospital Hamburg, Hamburg, Germany; Hannover Medical School, Department of Hematology and Oncology, Hannover, Germany; Department of Internal Medicine V, University of Heidelberg, Heidelberg, Germany; Medizinische Klinik I, Universitatsklinikum des Saarlandes, Homburg, Germany; Clinic for BMT and Hematology/Oncology, Idar-Oberstein, Germany; Department of Internal Medicine II, Friedrich Schiller University, Jena, Germany; Second Medical Department, University of Schleswig-Holstein, Kiel, Germany; First Department of Internal Medicine, University Hospital Cologne, Cologne, Germany; Department of Medicine III, Johannes Gutenberg University, Mainz, Germany; Department of Medicine, Hematology/Oncology, University of Marburg, Marburg, Germany; Hematopoietic Cell Transplantation, Dept of Medicine III, Clinical University of Munich-Grosshadern, Munich, Germany; Department of Medicine/Hematology and Oncology, University of Muenster, Muenster, Germany; 5th Medical Department and Institute of Medical Oncology and Hamatology, Nuremberg, Germany; Department of Hematology/Oncology, University of Regensburg, Regensburg, Germany; Department of Hematology and Medical Oncology, Deaconess Hospital, Oncological Center of Stuttgart, Stuttgart, Germany; Department of Hematology/Oncology/Immunology and Rheumatology, University of Tubingen, Tubingen, Germany; Deutsche Klinik fuer Diagnostik, Wiesbaden, Germany; and Medizinische Poliklinik, Universitat Wurzburg, Wurzburg, Germany.
Prepublished online as Blood First Edition Paper, July 14, 2005; DOI 10.1182/blood-2005-04-1377.
Supported by the German Competence Network for Acute and Chronic Leukemias and a grant of the German Bundesminister für Bildung und Forschung (BMBF).
M.S. and M.B. contributed equally to this study.
A complete list of the members of the Cooperative German Transplant Study Group appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal